Abstract
Apoptosis not only plays a key role in physiological demise of defunct hepatocytes, but is also associated with a plethora of acute and chronic liver diseases as well as with hepatotoxicity. The present paper focuses on the modelling of this mode of programmed cell death in primary hepatocyte cultures. Particular attention is paid to the activation of spontaneous apoptosis during the isolation of hepatocytes from the liver, its progressive manifestation upon the subsequent establishment of cell cultures and simultaneously to strategies to counteract this deleterious process. In addition, currently applied approaches to experimentally induce controlled apoptosis in this in vitro setting for mechanistic research purposes and thereby its detection using relevant biomarkers are reviewed.
Keywords: Cell death, Apoptosis, Primary hepatocyte, In vitro culture, Assays, Biomarkers
Introduction
Like in all multicellular systems, liver homoeostasis relies on the critical balance between cell growth and cell death. The latter is mainly accomplished by the programmed mode of apoptosis. In normal liver, the incidence of spontaneous apoptosis is very low, affecting only 0.1 and 0.05 % of all liver cells in mice and rats, respectively (Qiao and Farrell 1999), and particularly taking place in hepatocytes in the perivenous acinar region (Baier et al. 2006; Ni et al. 1994). However, in several acute and chronic liver diseases, including fulminant hepatic failure, alcoholic hepatitis, autoimmune hepatitis, acute and chronic viral hepatitis, non-alcoholic steatohepatitis, cholestasis, fibrosis and cirrhosis, apoptotic activity strongly increases (Guicciardi and Gores 2005; Malhi et al. 2006; Schattenberg et al. 2006; St-Pierre and Dufour 2012). Moreover, it has become clear in recent years that apoptosis predominates liver cell death induced by toxicants (Gomez-Lechon et al. 2002; Orrenius et al. 2011).
A number of protocols have been described to study hepatocellular apoptosis in vivo, including the direct administration of cell death-evoking toxicants to animals (Furukawa et al. 2000), the application of genetically modified subjects (Guicciardi et al. 2005) and the use of partially hepatectomised rodents (Baier et al. 2006). Such experiments not only raise serious ethical questions, but are also of rather limited scientific value. Indeed, apoptotic cells are barely detectable in vivo, as they are rapidly engulfed by neighbouring phagocytes. During in vitro experimentation, where phagocytosis does not take place, the full course of apoptosis can be monitored, whereby the late apoptotic phase is typically followed by secondary necrosis (Gomez-Lechon et al. 2002; Raffray and Cohen 1997). Cell lines are frequently applied experimental tools in in vitro apoptosis research, in casu in a hepatology context. However, these cells, such as HepG2 cells, are often derived from cancers and have typically acquired high resistance against apoptosis. Primary cells, directly isolated from healthy tissue, may offer a better alternative, as they display in vivo-like sensitivity to apoptosis, at least during short-term cultivation regimes (Schulze-Bergkamen et al. 2006).
In the present paper, a state-of-the-art overview of the use of primary hepatocytes and their cultures in liver apoptosis research is provided. Following a synopsis of the mechanisms that drive programmed cell death in the liver, the activation of spontaneous apoptosis during isolation of hepatocytes from the liver as well as strategies to counteract this process in vitro is discussed in the first part. Experimental approaches to induce controlled apoptosis and biomarkers for its unequivocal detection in primary hepatocyte cultures are addressed in the second part.
Mechanisms of hepatocyte apoptosis
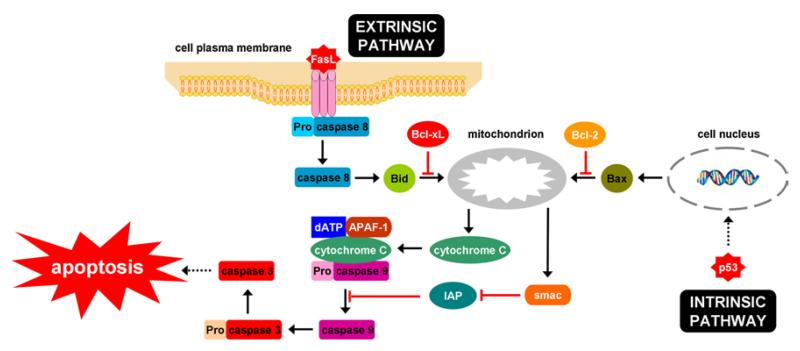
Apoptosis relies on the proteolytic activity of an evolutionary conserved family of cysteinyl aspartate-specific proteinases or caspases. Yet, 2 major apoptotic pathways have been described, namely the extrinsic signalling cascade and the intrinsic pathway (Fig. 1). The latter is initiated by stimulating the release of cytochrome C from mitochondria, a process that is controlled by proapoptotic and anti-apoptotic B cell lymphoma 2 (Bcl-2) proteins. Liberated cytochrome C forms an apoptosome with apoptotic protease activating factor 1, deoxyadenosine triphosphate and procaspase 9. Following activation of caspase 9, the apoptosome enrols and cleaves procaspase 3. In the extrinsic pathway, specific ligands, including Fas/CD95 ligand, tumour necrosis factor α (TNFα) and TNFα-related apoptosis-inducing ligand (TRAIL), bind to their corresponding receptors at the cell plasma membrane surface, which induces the recruitment and cleavage of procaspase 8 (Decrock et al. 2009; Feldmann 1997; Jaeschke et al. 2002; Malhi and Gores 2008; Malhi et al. 2006; Orrenius et al. 2011; Raffray and Cohen 1997; Schulze-Bergkamen et al. 2006). In type I cells, sufficient amounts of caspase 8 are produced through this route and caspase 3 becomes directly activated. However, in type II cells, such as hepatocytes, only minimal quantities of active caspase 8 can be generated and the execution of the apoptotic response requires mitochondrial amplification. In this scenario, caspase 8 activates the proapoptotic Bid protein, which then translocates to the mitochondria, where it leads to the release of cytochrome C and apoptosome formation (Malhi and Gores 2008; Malhi et al. 2006; Orrenius et al. 2011; Schulze-Bergkamen et al. 2006). The overall outcome of the apoptotic pathways is the activation of caspase 3, being the main executor of apoptosis. In fact, caspase 3 cleaves a broad spectrum of cellular proteins, such as cytoskeletal proteins, which gives rise to the typical apoptotic phenotype, involving blebbing, cell shrinkage, cytoplasmic and nuclear condensation, DNA fragmentation and the formation of apoptotic bodies (Decrock et al. 2009; Feldmann 1997; Jaeschke et al. 2002; Malhi and Gores 2008; Malhi et al. 2006; Raffray and Cohen 1997; Schulze-Bergkamen et al. 2006).
Fig. 1.
Mechanisms of hepatocyte apoptosis. In the intrinsic pathway, which can be activated by p53 upon DNA damage, cytochrome C is released from mitochondria. This process is mediated by B cell lymphoma 2 (Bcl-2) proteins which can act either proapoptotic (e.g. Bid and Bax) or anti-apoptotic (e.g. Bcl-xL and Bcl-2). Liberated cytochrome C forms an apoptosome with apoptotic protease activating factor 1 (APAF-1), deoxyadenosine triphosphate (dATP) and procaspase 9. The subsequent activation of caspase 9 can be counteracted by the inhibitor of apoptosis (IAP) family, which itself can be blocked by the smac protein. Caspase 9 then triggers caspase 3, which is a major executor of apoptosis by cleavage of a broad spectrum of cellular proteins. In the extrinsic pathway, specific ligands, such a Fas ligand, bind to their corresponding receptors at the cell plasma membrane surface, which induces the recruitment and cleavage of procaspase 8. In hepatocytes, this pathway can as such not lead to activation of caspase 3 and needs mitochondrial amplification, a process that relies on the actions of Bid
Activation of spontaneous apoptosis during hepatocyte isolation and cultivation
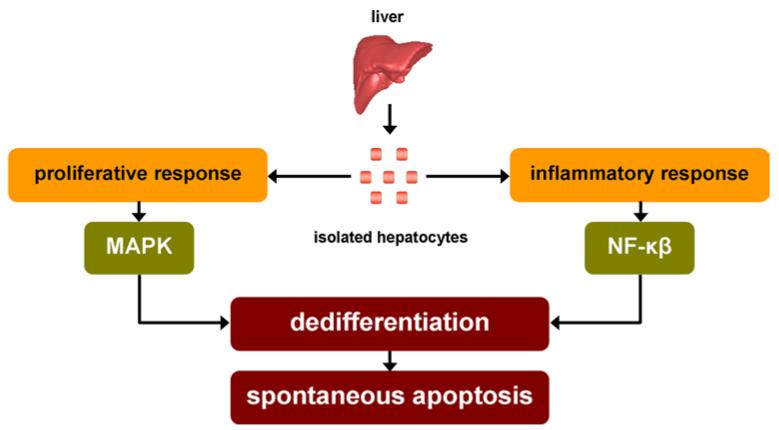
The 2-step collagenase perfusion technique is the most commonly used procedure to isolate hepatocytes form liver tissue of rodent and human origin (Papeleu et al. 2006; Seglen 1976). It is based upon the notion that calcium ions are critical for maintaining cellular adhesion. Thus, the freshly removed liver is perfused with a calcium-free medium, often supplemented with a calcium chelator, in a first run in order to disrupt calcium-dependent cell–cell junctions. In a second step, the liver is further perfused with a collagenase-containing buffer to eradicate cell–extracellular matrix (ECM) interactions (Alpini et al. 1994; Berry et al. 1997; Papeleu et al. 2006; Seglen 1976). The abolishment of the entire repertoire of cellular contacts triggers a proliferative response mediated by mitogen-activated protein kinase (MAPK). The hepatocytes are hereby forced to re-enter the cell cycle from their quiescent G0 status into the G1 phase, a process evidenced by the production of proto-oncogenes, such as c-jun and c-fos. Simultaneously, an inflammatory reaction is induced, with nuclear factor-kappa beta (NF-κβ) as master regulator, and is associated with the production of a plethora of cytokines. This is equally promoted by the inevitable occurrence of an ischaemia/reperfusion event as well as by the presence of impurities in the collagenase, such as lipopolysaccharide (LPS), used during the hepatocyte isolation procedure (Fig. 2) (Elaut et al. 2006; Paine and Andreakos 2004).
Fig. 2.
Activation of spontaneous apoptosis in hepatocyte cultures. During 2-step collagenase perfusion isolation of hepatocytes from liver tissue, cellular contacts are abolished and ischaemia/reperfusion occurs. These deleterious events trigger an inflammatory reaction, mediated by nuclear factor-kappa beta (NF-κβ), and a proliferative response, mediated by mitogen-activated protein kinase (MAPK). In turn, this results in the progressive deterioration of the differentiated phenotype, which ultimately burgeons into the onset of spontaneous apoptosis (adapted from Paine and Andreakos 2004; Vinken et al. 2012b)
Collectively, the deleterious processes activated during hepatocyte isolation not only negatively affect the differentiated hepatocyte phenotype, but also burgeon into the onset of cell death. Indeed, expression of Bax increases already 15 min after the start of the hepatocyte isolation procedure (Kucera et al. 2006). In addition to Bax, caspase 9 translocates from the cytoplasm to the nuclei of the hepatocytes shortly after isolation (Nipic et al. 2010). When seeded in a conventional monolayer configuration on a plastic culture dish, the apoptotic response becomes progressively manifested, with high production of Bax, Bid and caspase 3 shortly after the establishment of cell cultures (Vinken et al. 2011). Thereafter, increased cleavage of caspase 3, caspase 8 and caspase 9 is observed (Bailly-Maitre et al. 2002), resulting in minimal cell viability after 4 days of cultivation (Vinken et al. 2011). Furthermore, LPS is known to induce the expression of the Fas receptor (Muschen et al. 1998) and its ligand (Yao et al. 2000) in cultured hepatocytes. It should be stressed, however, that apoptosis is not the only mode of cell death that underlies cell demise in primary hepatocyte cultures (Kucera et al. 2006; Vinken et al. 2011). In fact, 2 peaks of cell death can be discerned in this in vitro setting, both which involve apoptosis and necrosis. The cell death wave during early time points of cultivation reflects cell damage underwent during the drastic hepatocyte isolation procedure (Vinken et al. 2011). Anoikis, a particular type of apoptosis activated by cell detachment from the ECM, is likely to play an important role in this process (Elaut et al. 2006; Smets et al. 2002). When the cell culture medium is renewed on a daily basis, and thus defunct hepatocytes are removed, apoptotic and necrotic biomarkers reach minimal values around the second day of cultivation. Subsequently, a second cell death peak is noticed, which probably is a manifestation of the poor and unfavourable accommodation of the hepatocytes to the artificial cultivation conditions and thus the absence of the vital in vivo micro-environment (Vinken et al. 2011). Recently, the spontaneous cell death phenomenon taking place in primary hepatocyte cultures was found to depend on connexin43 signalling, which, similar to the in vivo situation, becomes specifically activated in hepatocellular stress conditions (Vinken et al. 2012a).
A pivotal determinant of spontaneous cell death progression in primary hepatocyte cultures is cell density (Bresgen et al. 2008; Maeda et al. 1993; Qiao and Farrell 1999; Shinzawa et al. 1995). In this respect, hepatocytes plated at low density, i.e. 0.35 × 105 cells per cm2, display less apoptotic activity than their counterparts seeded at high density, i.e. 1.4 × 105 cells per cm2, which is about half of the in vivo density, i.e. 2–3 × 105 cells per cm2 liver tissue (Qiao and Farrell 1999). However, unlike cell survival, cell density positively correlates with the functional status of the cultured hepatocytes (Hamilton et al. 2001). Another critical parameter of cell survival in freshly isolated hepatocytes relates to the composition of the cell culture medium. A comparison of a number of commonly used and commercially available hepatocyte culture media revealed that Williams’ E medium and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer offer the lowest background of spontaneous apoptotic and necrotic cell death for freshly isolated rat hepatocytes in suspension culture (Elaut et al. 2005). More specific cell culture media, such as the University of Wisconsin solution, also are efficient in counteracting self-generated cell death in isolated hepatocytes (Fu et al. 2001).
Strategies to counteract spontaneous apoptosis in hepatocyte cultures
The addition of anti-apoptotic molecules to the cell culture medium
Epidermal growth factor and hepatocyte growth factor
Foetal bovine serum is typically added to freshly established hepatocyte cultures in order to promote cell attachment to the culture plate (Vinken et al. 2006). Foetal bovine serum enhances cell survival, yet it also negatively impacts liver-specific functionality (Tuschl et al. 2009). For this reason, and because it is a complex mixture of undefined constituents, the tendency exists to use single compounds. Among those are a set of growth factors, represented by hepatocyte growth factor (HGF) and epidermal growth factor (EGF) (Vinken et al. 2006). Growth factors are well known to protect cells from a variety of apoptotic stimuli (Ethier et al. 2003). HGF indeed significantly inhibits Fas-mediated cell death in cultures of primary human hepatocytes by reducing cleavage of caspase 8 and through induction of myeloid cell leukaemia 1 protein, an anti-apoptotic Bcl-2 protein. This is associated with the activation of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway, the MAPK/extracellular signal-regulated kinase (ERK) cascade and the signal transducer and activator of transcription 3 pathway (Schulze-Bergkamen et al. 2004). HGF also upregulates the production of Bcl-2 and Bcl-xL and protects against oxidative stress (Gomez-Quiroz et al. 2008). Similarly, EGF reduces Fas-mediated apoptosis in primary mouse hepatocyte cultures by downregulation of Bid expression (Ethier et al. 2003) and through upregulation of Bcl-xL production (Musallam et al. 2001), with both processes depending on kinase activity (Ethier et al. 2003; Musallam et al. 2001). Furthermore, EGF enables the G1/S cell cycle transition in primary hepatocyte cultures, and its effect on the Fas-mediated pathway is a permissive step in this event (Gilot et al. 2005).
Insulin and glucagon
Insulin and glucagon control glucose levels in blood, and their actions depend on cyclic adenosine monophosphate signalling. Both hormones are frequently added to standard hepatocyte culture media, as they enhance functional hepatocellular features (Vinken et al. 2006). Insulin, together with EGF, was reported to reduce spontaneous cell death in primary hepatocyte cultures (Bresgen et al. 2008). It also decreases the number of apoptotic cells in experimentally induced cell death in primary hepatocyte cultures by increasing Bcl-xL expression (Bilodeau et al. 2004), via inhibition of c-jun N-terminal kinase (JNK) (Pagliassotti et al. 2007) and through activation of Akt (Bilodeau et al. 2004; Pagliassotti et al. 2007). Likewise, Fas-mediated apoptosis in suspension cultures of primary rat hepatocytes is inhibited by glucagon by involving cyclic adenosine monophosphate-dependent protein kinase (Fladmark et al. 1997).
Dexamethasone
Glucocorticosteroids, such as dexamethasone, are known to retard dedifferentiation in primary hepatocyte cultures by positively affecting both cell morphology and functionality. In general, glucocorticosteroids perform these actions by interfering with gene transcription (Vinken et al. 2006). As such, dexamethasone inhibits cleavage of caspase 3, caspase 8 and caspase 9 as well as the expression of Bax and cytochrome C in primary cultures of human and rat hepatocytes. In addition, it prevents the loss of the anti-apoptotic Bcl-2 and Bcl-xL proteins and negatively affects Bad translocation to mitochondria (Bailly-Maitre et al. 2001, 2002; Bresgen et al. 2008). The stabilising effect of dexamethasone on Bcl-2 and Bcl-xL depends on the pregnane X receptor (Zucchini et al. 2005).
Dimethylsulphoxide
The beneficial effects of dimethylsulphoxide (DMSO) on the maintenance of the differentiated status in primary hepatocyte cultures have been described by several investigators. In general, concentrations ranging from 0.5 to 2 % DMSO are added to the hepatocyte culture medium and result in the strong promotion of liver-specific functionality (Arterburn et al. 1995; Isom et al. 1985; Vinken et al. 2006). When supplementing the hepatocyte culture medium with 1 % DMSO immediately after isolation, migration of caspase 9 into the nuclei is temporarily prevented. Activities of caspase 9 and caspase 3 are, however, lower when DMSO is added 24 h after isolation (Banic et al. 2011). Inhibition of caspase 3 maturation and apoptosis execution in cultured hepatocytes by DMSO is associated with downregulation of the apoptosis signal-regulating kinase 1/JNK-p38 pathway (Gilot et al. 2002). DMSO was therefore proposed to extend the survival of primary hepatocytes by modulating the preapoptotic cell stress response (Banic et al. 2011). DMSO also prevents cellular damage in cryopreserved hepatocytes by preventing the formation of ice crystals (Fu et al. 2001).
Phenobarbital
Phenobarbital, a prominent cytochrome P450 inducer, has a beneficial influence on the functionality of primary hepatocytes in vitro (Vinken et al. 2006). Exposure of cultured hepatocytes to phenobarbital is known to reduce cell detachment (Holzer and Maier 1987) and to positively affect the anti-apoptotic Bcl-2 and Bcl-xL proteins, a process involving the pregnane X receptor (Zucchini et al. 2005). However, it seems that phenobarbital is more effective in reducing experimentally induced apoptosis (Bohnenberger et al. 2001; Christensen et al. 1998; Schrenk et al. 2004) rather than spontaneous cell death in primary rat hepatocyte cultures (Bohnenberger et al. 2001).
Trichostatin A and 5-(4-dimethylaminobenzoyl)aminovaleric acid hydroxamide
Like in other organs, critical aspects of liver homoeostasis, including cell death, are associated with alterations in the chromatin structure and hence in epigenetic regulation of gene expression patterns. In this respect, induced apoptosis in primary hepatocyte cultures is accompanied by decreased levels of DNA methyltransferase 3a (Vinken et al. 2010) and opposing effects on ERK1/2 by histone deacetylases 1 and 2 (Lei et al. 2010). The latter, together with histone acetyltransferases, mediate reversible histone acetylation. Histone acetylation is typically associated with active gene expression, while the inverse holds true for histone deacetylation. Histone deacetylase inhibitors, with trichostatin A as a prototype, reduce spontaneous apoptosis in primary hepatocyte cultures as evidenced by decreased caspase 3 activity and lowered Bid steady-state protein levels (Vanhaecke et al. 2004). At the same time, trichostatin A effectively stabilises liver-specific functionality (Henkens et al. 2007). The trichostatin A structural analogue 5-(4-dimethylaminobenzoyl)aminovaleric acid hydroxamide also delays the onset of spontaneous apoptosis in primary hepatocyte cultures (Papeleu et al. 2007).
The restoration of intercellular contacts
Hepatocytes are in intimate contact with each other and also form heterotypic interactions with surrounding nonparenchymal cells within the liver, which is a prerequisite for maintaining homeostasis. Restoration of heterotypic cell interactions by cultivation of primary hepatocytes with another cell type, whether or not from hepatic origin, therefore was thought to be a way to counteract hepatocellular dedifferentiation in vitro (Vinken et al. 2006). A well-known co-culture system is the hepatocyte-rat liver epithelial cell model, in which the maintenance of the differentiated hepatocellular phenotype is kept over extended periods of time (Guguen-Guillouzo et al. 1983) and that displays less caspase 3 activity in comparison with pure hepatocyte cultures (Vanhaecke et al. 2004).
A complementary strategy to improve the differentiated status of primary cultured hepatocytes in vitro includes the boosting of homotypic hepatocyte interactions. This can be achieved by continuously rotating hepatocytes in suspension or by using cell-repelling substrates, such as porous alginate scaffolds. This results in the formation of so-called spheroids that maintain close cell–cell contacts (Vinken et al. 2006). Although these spheroids have been suggested to allow long-term cultivation of hepatocytes, considerable cell death is observed in the core of these entities. This cell death phenomenon is mainly of necrotic nature and is likely to result from the accumulation of bile acids in the centre of the spheroids (Dvir-Ginzberg et al. 2004). E-cadherin-mediated cell–cell adhesion was found to be a critical determinant for the reduction in caspase-independent cell death in hepatocyte spheroids (Luebke-Wheeler et al. 2009).
The re-establishment of an extracellular matrix scaffold
In liver, hepatocytes are supported by a broad set of ECM proteins, including collagens, proteoglycans and glycosaminoglycans, which is an indispensable condition to guarantee liver functionality (Vinken et al. 2006). Detachment of hepatocytes from the ECM backbone results in anoikis. Indeed, interaction between integrins and the ECM activates focal adhesion kinase, which triggers signalling molecules such as Akt and MAPK, ultimately resulting in the suppression of cell death (Hoshiba et al. 2007). Based on this knowledge, the re-introduction of an ECM scaffold in culture was considered an evident approach to improve cell survival in primary hepatocyte cultures.
Hepatocytes easily attach to plastic culture dishes, while they fail to do so in glass culture plates, resulting in low viability and a high number of apoptotic cells after 2 h of plating (Smets et al. 2002). When seeded on a layer of collagen type I or Matrigel®, a laminin-rich extract from Engelbreth-Holm-Swarm mouse tumour, expression of Bax, Bcl-2 and Fas is lower than in conventionally cultured hepatocytes (Qiao and Farrell 1999). In these matrices, hepatocytes switch from type II to type I Fas-mediated cell death, including activation of caspase 3 by Fas ligand independently of Bid cleavage, activation of Bax and Bad, and cytochrome C release (Walter et al. 2008). Upon addition of a second layer of ECM proteins on top of the cultured hepatocytes, the so-called sandwich culture system, a more pronounced beneficial effect on the differentiated phenotype is observed (Dunn et al. 1991, 1989; Vinken et al. 2006), which is associated with a reduction in the number of apoptotic cells (Tuschl et al. 2009; Vanhaecke et al. 2004). Godoy and colleagues showed that focal adhesion kinase is triggered in hepatocytes seeded on dried stiff collagen, leading to activation of Akt and ERK1/2. Akt causes resistance to transforming growth factor β1 (TGFβ1)-induced apoptosis by antagonising p38. By contrast, softer collagen gel does not activate focal adhesion kinase, keeping the hepatocytes in a state where they remain sensitive to TGFβ1-induced apoptosis. In this culture system, inhibition of p38 as well as overexpression of constitutively active Akt causes apoptosis resistance (Godoy et al. 2009). Other natural ECM matrices, such as fibronectin, also positively affect cell survival in primary hepatocyte cultures, whereas agarose is not a good cultivation substratum for hepatocytes. Furthermore, a number of synthetic scaffolds have been introduced in recent years, such as poly-N-p-vinylbenzyl-4-O-beta-d-galactopyranosyl-d-gluconamide and poly-l-lysine, which are efficient in suppressing cell death, in primary hepatocyte cultures, despite the absence of Akt signalling (Hoshiba et al. 2007).
Strategies to experimentally induce controlled apoptosis in hepatocyte cultures
Chemical induction of hepatocyte apoptosis
Fas-induced apoptosis
A frequently applied method to trigger apoptosis in primary hepatocyte cultures includes the use of monoclonal antibodies directed against the Fas receptor. However, unlike the Fas ligand, binding of the antibody to the Fas receptor does not result in the onset of a proapoptotic response per se (Fadeel et al. 1997; Legembre et al. 2003; Thilenius et al. 1997). In addition, Fas-mediated apoptosis induced by antibodies becomes typically manifested to a lesser extent compared to the in vivo situation (Nagata 1999; Ni et al. 1994). For this reason, Fas antibodies are frequently combined with inhibitors of gene expression or protein production, such as actinomycin D and cycloheximide, respectively (Nagata 1999; Ni et al. 1994; Rouquet et al. 1996). A more rationalised strategy that resembles the natural Fas pathway is the use of Fas ligand as such (Fu et al. 2004; Reinehr et al. 2002; Vinken et al. 2009). Fas ligand can also be presented to hepatocytes by cultivation partners. In this regard, a co-culture system consisting of primary mouse hepatocytes and 3T3 fibroblasts stably transfected with Fas ligand was found to be an effective in vitro model to study hepatocellular cell death, since the entire hepatocyte population undergoes apoptosis 24 h after its establishment (Schlosser et al. 2000).
Tumour necrosis factor α-induced apoptosis
In contrast to the Fas-mediated cascade, TNFα signalling has pleiotropic effects on hepatocytes, including induction of apoptosis, inflammatory responses and mitogenic activity (Cosgrove et al. 2008). Like for Fas antibodies and Fas ligand, the outcome of TNFα in primary hepatocyte cultures is often potentiated by simultaneous exposure to transcription inhibitors, such as d-galactosamine and α-amanitin (Hentze et al. 2004; Schlatter et al. 2011; Schmich et al. 2011). Cell death evoked by TNFα and d-galactosamine becomes even more exacerbated when hepatocytes are co-cultured with Kupffer cells (Abou-Elella et al. 2002). The cell killing effects of TNFα in primary hepatocyte cultures can also be reinforced by interleukins (Boer et al. 2003), LPS (Kudo et al. 2009), oxidative stress (Han et al. 2006) and hepatotoxic drugs (Cosgrove et al. 2009). TNFα-mediated apoptosis in cultured hepatocytes (Schwabe et al. 2004) relies on the JNK pathway.
Tumour necrosis factor α-related apoptosis-inducing ligand-mediated apoptosis
Of all cell death receptor ligands and corresponding signalling cascades, TRAIL has yet gained least attention in the context of in vitro modelling of hepatic apoptosis. This could be explained by the fact that TRAIL preferentially induces cell death in malignant cells and not in their normal counterparts (Corazza et al. 2009; Meurette et al. 2006). However, a number of reports described the occurrence of apoptosis in primary hepatocyte cultures upon TRAIL activation, a process boosted by free fatty acids (Malhi et al. 2007), glutathione depletion (Meurette et al. 2005) and chemotherapeutic drugs (Meurette et al. 2006).
Transforming growth factor β1-induced apoptosis
TGFβ1 is a multifunctional cytokine that is a potent inhibitor of hepatocellular cell growth both in vivo and in vitro (Michalopoulos and DeFrances 2005; Nguyen et al. 2007). It also induces apoptosis in primary mouse hepatocyte cultures (Lei et al. 2010; Oberhammer et al. 1996; Oberhammer et al. 1992), which depends on the involvement of reactive oxygen intermediates (Sanchez et al. 1996), the activation of p38 signalling (Yoo et al. 2003) and the upregulation of nicotinamide adenine dinucleotide phosphate oxidase Nox4 (Carmona-Cuenca et al. 2008). TGFβ1 equally evokes cell death in cultures of periportal and perivenous rat hepatocytes and is associated with a decrease in intracellular pH and lowered sodium/hydrogen exchanger activity (Benedetti et al. 1995). Activin, another member of the TGFβ family, also causes hepatocellular apoptosis in vivo and in vitro (Hully et al. 1994; Schwall et al. 1993).
Bile acid-induced apoptosis
Although bile salts are naturally secreted by hepatocytes, they inherently act as toxicants due to their detergent-like character. Hepatocellular accumulation of bile salts, as occurring during cholestasis, results in the onset of apoptosis (Hirschfield et al. 2010; Perez and Briz 2009). Specifically, hydrophobic bile salts, including taurolithocholic acid and glycochenodeoxycholic acid, induce apoptosis at low concentrations, while they cause necrosis at high concentrations. These cell death responses can be reduced by hydrophilic bile salts, such as ursodeoxycholic acid and tauroursodeoxycholic acid (Monte et al. 2009; Patel et al. 1994; Perez and Briz 2009). In primary hepatocyte cultures, the proapoptotic actions of hydrophobic bile salts involve NF-κβ (Wang et al. 2010), PI3K (Hohenester et al. 2010), nitric oxide (NO) (Wang et al. 2011), insulin-like growth factor 1 (Drudi Metalli et al. 2007) and protein kinase C (Wang et al. 2005). Moreover, their apoptotic outcome can be intensified in this in vitro setting by the presence of free fatty acids, such as palmitic acid (Pusl et al. 2008).
Microbiological induction of hepatocyte apoptosis
The role of hepatocellular apoptosis in hepatitis virus infection is controversial. Thus, hepatitis B virus (HBV) was reported to induce cell death in cultures of primary human hepatocytes (Arzberger et al. 2010), although this could not be reproduced by others (Schulze-Bergkamen et al. 2003). HBV X protein (HBX), which is critical for viral infection, triggers apoptosis in hepatocytes in vivo and in vitro independently of p53 (Terradillos et al. 1998). It also sensitises primary mouse hepatocytes to TNFα-induced apoptosis by a caspase 3-dependent mechanism (Kim et al. 2005). Again, however, HBX can act both proapoptotic and anti-apoptotic in primary rat hepatocyte cultures depending on the status of NF-κβ (Clippinger et al. 2009). Moreover, HBX can inhibit Fas-mediated cell death in primary human hepatocytes through upregulation of the stress-activated protein kinase (SAPK)/JNK pathway (Diao et al. 2001). In contrast, hepatitis C virus (HCV) infection induces apoptosis in cultured primary human hepatocytes through TRAIL signalling (Lan et al. 2008; Yang et al. 2011). The same outcome is achieved with HCV core protein, and this depends on the activation of protein kinase R (Realdon et al. 2004).
Miscellaneous induction of hepatocyte apoptosis
In addition to chemical and microbiological agents, a number of other conditions have been described to experimentally induce apoptosis in primary hepatocyte cultures. In this regard, apoptosis can be activated by cultivating hepatocytes in conditioned medium, collected over the first 3 h of cultivation from serum-free rat hepatocyte cultures (Bresgen et al. 2004). Omission of vital chemical factors from the hepatocyte culture medium is another way to evoke cell death, which has been demonstrated for magnesium (Martin et al. 2003) and glucose (Choi et al. 2003). Ischaemia/reperfusion, a condition known to strongly decrease cell survival of liver grafts in vivo, can be modelled in vitro through a hypoxia/reoxygenation approach (Laurens et al. 2005; Ozaki et al. 2003; Shimizu et al. 1996). Application of this approach to primary hepatocytes leads to caspase 3-mediated apoptosis and involves SAPK/JNK signalling (Crenesse et al. 2003). Similarly, irradiation of primary hepatocytes with ultraviolet light causes p53-dependent apoptosis (Bellamy et al. 1997; Prost et al. 1998; Worner and Schrenk 1996).
Detection and biomarkers of apoptosis in hepatocyte cultures
While studying apoptosis in vitro, in casu in primary hepatocyte cultures, a combined battery of general cytotoxicity assays and specific apoptosis detection methods is usually addressed. The former typically consists of classical techniques to assess cell viability. Thus, measurement of plasma membrane integrity can be achieved with dyes that only enter damaged cells, such as trypan blue (Tichy et al. 2010), or dyes that specifically stain vital cells, such as calcein acetoxymethylester (Alvarez et al. 2009). Plasma membrane damage can also be detected through monitoring of the leakage of cytosolic enzymes, such as lactate dehydrogenase, into the extracellular environment (Vinken et al. 2009). At the functional level, cytotoxicity is frequently studied by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, which becomes reduced by mitochondria of living cells to yield a purple formazan product (Hynes et al. 2006).
As regards apoptosis, morphological examination by light microscopy or transmission electron microscopy can reveal the typical features of this type of cell death, including blebbing, cell shrinkage, cytoplasmic and nuclear condensation, and the formation of apoptotic bodies (Kume et al. 2005; Vinken et al. 2009). An early event during the commitment of cells to apoptosis is the externalisation of phosphatidylserine, a negatively charged phospholipid that is normally restricted to the inner surface of the cell plasma membrane bilayer. This process can be monitored by using the phospholipid-binding protein Annexin V, for instance in a flow cytometry setup (Fu et al. 2001). Another crucial process in apoptosis involves the activation of caspases by proteolytic cleavage from their procaspase precursors. This can be studied at the protein level using techniques such as immunoblot analysis, and at the activity level by means of synthetic fluorescent caspase substrates (Vinken et al. 2009). In fact, caspase 3 activation was reported to be a reliable and sensitive marker of apoptosis in primary hepatocyte cultures (Gomez-Lechon et al. 2002). Given their central role in programmed cell death, mitochondria also deliver a number of apoptosis biomarkers. In this respect, subcellular fractionation and assessment of mitochondrial cytochrome C release are common techniques. Furthermore, changes in mitochondrial transmembrane potential can be traced by using lipophilic cationic dyes, such as 3,3-dihexyloxacarbocyanine iodide or rhodamine 123 (Hussain and Frazier 2003). A prototypical hallmark of apoptosis relates to changes in the nucleus. Indeed, DNA fragmentation can be visualised by gel electrophoresis and subsequent ethidium bromide staining (Ribeiro et al. 2010). However, a more popular assay is the terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labelling method, a histochemical technique that allows detection of DNA breaks (Fu et al. 2001).
Conclusions and perspectives
Since its first description in 1972 (Kerr et al. 1972), apoptosis has been observed in virtually all tissue types both as a mechanism to physiologically remove dysfunctional cells and as a protective response to pathological or toxic insults. Liver apoptosis, mainly occurring in hepatocytes, is particularly relevant in the latter scenario and relies on intrinsic and extrinsic signalling pathways (Gomez-Lechon et al. 2002; Guicciardi and Gores 2005; Malhi and Gores 2008; Malhi et al. 2006; Orrenius et al. 2011; Schattenberg et al. 2006; St-Pierre and Dufour 2012). It should be mentioned, though, that apoptosis is not the sole cell death mode that can be activated in the liver. Besides necrosis (Malhi et al. 2006), autophagy, a cellular self-digestion process which encompasses a highly conserved intracellular catabolic pathway that degrades proteins and organelles, also underlies a multitude of liver diseases (Rautou et al. 2010). Nevertheless, hepatic apoptosis still is a ubiquitous research topic, and this area frequently makes use of in vitro test systems. A number of such experimental settings have proven their utility in apoptosis research, such as precision-cut liver slices (Kasper et al. 2005), yet cultures of primary hepatocytes are considered as the gold standard for this purpose. However, they suffer from a number of fundamental disadvantages, which limit their applicability over the long term. Among those are the occurrence of spontaneous apoptosis, triggered during the hepatocyte isolation procedure and resulting in poor cell survival during subsequent cultivation (Elaut et al. 2006; Vinken et al. 2006, 2012b). Conventional strategies to counteract this process typically aim at mimicking the in vivo hepatocyte micro-environment in vitro (Table 1). It is expected that this classical methodological track will be largely pursued in near future. Sophisticated systems, such as tridimensional perfusion bioreactors in which primary hepatocytes are embedded in compartments that are continuously perfused, have been introduced in the last few years. Using this innovative device, it was found that the NO donor S-nitrosoglutathione reduces hepatocellular apoptotic cell death (Prince et al. 2010). Although promising, these classical strategies usually tackle the apoptotic response as such without affecting its actual triggers. In recent years, a number of novel approaches have been introduced in this respect. Based on the notion that levels of NO and inducible NO synthase strongly increase during hepatocyte isolation (Elaut et al. 2006), exposure of hepatocytes to the inducible NO synthase inhibitor l-omega-nitro-l-arginine methyl ester already started during the isolation procedure was found to strongly downregulate caspase activation and apoptosis (Donato et al. 2001). Likewise, addition of the epigenetic modifier trichostatin A to the perfusate used for hepatocyte isolation negatively affects caspase 3 processing as well as the expression of p53, Bid and Bax (Vanhaecke et al. 2006).
Table 1.
Strategies to counteract spontaneous apoptosis in primary hepatocyte cultures
| The addition of anti-apoptotic molecules to the cell culture medium |
| EGF/HGF |
| Insulin/glucagon |
| Dexamethasone |
| DMSO |
| Phenobarbital |
| Trichostatin A/5-(4-dimethylaminobenzoyl)aminovaleric acid hydroxamide |
| The restoration of intercellular contacts |
| Co-cultures with hepatic/non-hepatic partners Spheroid cultures |
| The re-establishment of an ECM scaffold |
| Single scaffold cultures on natural/synthetic/non-physiological substrata |
| Sandwich cultures between natural/non-physiological ECM layers |
DMSO dimethylsulphoxide, ECM extracellular matrix, EGF epidermal growth factor, HGF hepatocyte growth factor
Stabilised primary hepatocyte cultures, displaying only a minimal background of spontaneous apoptosis, are valuable in vitro tools for studying liver apoptosis at the mechanistic level, which is not possible in vivo. Experimental induction of apoptosis in primary hepatocyte cultures can be achieved in various ways, involving chemical, microbiological and a number of physical stimuli (Table 2). In turn, the apoptotic response triggered by these noxious factors can be monitored by addressing a number of cytomic markers, which preferentially cover a combined set of general cytotoxicity parameters and apoptosis-specific read-outs (Table 3). In the last decade, detection of apoptosis in primary hepatocyte cultures at a global scale by using transcriptomics (i.e. DNA micro-arrays) (Kume et al. 2005; Zucchini-Pascal et al. 2011) and proteomics (Rodriguez-Ariza et al. 2005; Rowe et al. 2010) technologies has generated a wealth of new sensitive mechanistic biomarkers. More recently, epigenetic hallmarks of hepatocellular cell death in vitro have also joined into this list (Lei et al. 2010; Vinken et al. 2010). Further exploration of such novel molecular signatures of hepatocellular apoptosis not only is of importance for its accurate and early detection in vitro, but is equally anticipated to yield new in vivo-relevant biomarkers that may be of potential clinical interest.
Table 2.
Strategies to experimentally induce controlled apoptosis in primary hepatocyte cultures
| Chemical induction of hepatocyte apoptosis |
| Fas antibodies/Fas ligand |
| TNFα |
| TRAIL |
| TGβ1/activin |
| Hydrophobic bile acids |
| Microbiological induction of hepatocyte apoptosis |
| HBV/HBX |
| HCV/HCV core protein |
| Miscellaneous induction of hepatocyte apoptosis |
| Conditioned medium |
| Omission of vital chemical factors |
| Hypoxia/reoxygenation |
| Ultraviolet light irradiation |
HBV hepatitis B virus, HBX hepatitis B virus X protein, HCV hepatitis C virus, TGFβ1 transforming growth factor β1, TNFα tumour necrosis factor α, TRAIL tumour necrosis factor α-related apoptosis-inducing ligand
Table 3.
Detection and biomarkers of apoptosis in primary hepatocyte cultures
| Biomarkers | Assays |
|---|---|
| General cytotoxicity read-outs | |
| Plasma membrane changes | LDH leakage assay/vital dye exclusion assay |
| Mitochondrial changes | MTT assay |
| Apoptosis-specific read-outs | |
| Morphological changes | Light/transmission electron microscopy |
| Plasma membrane changes | Annexin V staining/flow cytometry |
| Cytosolic changes | Caspase 3 activity assay/immunoblot analysis |
| Mitochondrial changes | Cytochrome C release/DiOC6 staining |
| Nuclear changes | Gel electrophoresis/TUNEL assay |
DiOC6 3,3-dihexyloxacarbocyanine iodide, LDH lactate dehydrogenase; MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium, TUNEL terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labelling
Acknowledgments
This work was financially supported by the grants of the University Hospital of the Vrije Universiteit Brussel (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research Flanders (FWO-Vlaanderen), the European Union (FP7/Cosmetics Europe projects HeMiBio and DETECTIVE) and the European Research Council (CONNECT project).
Abbreviations
- APAF-1
Apoptotic protease activating factor 1
- Bcl-2
B cell lymphoma 2
- Caspase(s)
Cysteinyl aspartate-specific proteinase(s)
- dATP
Deoxyadenosine triphosphate
- DiOC6
3,3-Dihexyloxacarbocyanine iodide
- DMSO
Dimethylsulphoxide
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- ERK
Extracellular signal-regulated kinase
- HBV
Hepatitis B virus
- HBX
Hepatitis B virus X protein
- HCV
Hepatitis C virus
- HGF
Hepatocyte growth factor
- IAP
Inhibitor of apoptosis
- JNK
c-Jun N-terminal kinase
- LDH
Lactate dehydrogenase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- NF-κβ
Nuclear factor-kappa beta
- NO
Nitric oxide
- PI3K
Phosphatidylinositol-3 kinase
- SAPK
Stress-activated protein kinase
- TGFβ1
Transforming growth factor β1
- TNFα
Tumour necrosis factor α
- TRAIL
Tumour necrosis factor α-related apoptosis-inducing ligand
- TUNEL
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labelling
Contributor Information
Mathieu Vinken, Department of Toxicology (FAFY), Faculty of Medicine and Pharmacy, Center for Pharmaceutical Research (CePhaR), Vrije Universiteit Brussel (VUB), Laarbeeklaan 103, 1090 Brussels, Belgium.
Michaël Maes, Department of Toxicology (FAFY), Faculty of Medicine and Pharmacy, Center for Pharmaceutical Research (CePhaR), Vrije Universiteit Brussel (VUB), Laarbeeklaan 103, 1090 Brussels, Belgium.
André G. Oliveira, Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Brazil
Bruno Cogliati, Department of Pathology, School of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil.
Pedro E. Marques, Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Brazil
Gustavo B. Menezes, Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Brazil
Maria Lúcia Zaidan Dagli, Department of Pathology, School of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil.
Tamara Vanhaecke, Department of Toxicology (FAFY), Faculty of Medicine and Pharmacy, Center for Pharmaceutical Research (CePhaR), Vrije Universiteit Brussel (VUB), Laarbeeklaan 103, 1090 Brussels, Belgium.
Vera Rogiers, Department of Toxicology (FAFY), Faculty of Medicine and Pharmacy, Center for Pharmaceutical Research (CePhaR), Vrije Universiteit Brussel (VUB), Laarbeeklaan 103, 1090 Brussels, Belgium.
References
- Abou-Elella AM, Siendones E, Padillo J, Montero JL, De la Mata M, Muntane Relat J. Tumour necrosis factor-alpha and nitric oxide mediate apoptosis by d-galactosamine in a primary culture of rat hepatocytes: exacerbation of cell death by cocultured Kupffer cells. Can J Gastroenterol. 2002;16:791–799. doi: 10.1155/2002/986305. [DOI] [PubMed] [Google Scholar]
- Alpini G, Phillips JO, Vroman B, LaRusso NF. Recent advances in the isolation of liver cells. Hepatology. 1994;20:494–514. [PubMed] [Google Scholar]
- Alvarez SD, Derfus AM, Schwartz MP, Bhatia SN, Sailor MJ. The compatibility of hepatocytes with chemically modified porous silicon with reference to in vitro biosensors. Biomaterials. 2009;30:26–34. doi: 10.1016/j.biomaterials.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterburn LM, Zurlo J, Yager JD, Overton RM, Heifetz AH. A morphological study of differentiated hepatocytes in vitro. Hepatology. 1995;22:175–187. [PubMed] [Google Scholar]
- Arzberger S, Hosel M, Protzer U. Apoptosis of hepatitis B virus-infected hepatocytes prevents release of infectious virus. J Virol. 2010;84:11994–12001. doi: 10.1128/JVI.00653-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier PK, Baumgartner U, Wolff-Vorbeck G, Hempel S, Hopt UT. Hepatocyte proliferation and apoptosis in rat liver after liver injury. Hepatogastroenterology. 2006;53:747–752. [PubMed] [Google Scholar]
- Bailly-Maitre B, de Sousa G, Boulukos K, Gugenheim J, Rahmani R. Dexamethasone inhibits spontaneous apoptosis in primary cultures of human and rat hepatocytes via Bcl-2 and Bcl-xL induction. Cell Death Differ. 2001;8:279–288. doi: 10.1038/sj.cdd.4400815. [DOI] [PubMed] [Google Scholar]
- Bailly-Maitre B, de Sousa G, Zucchini N, Gugenheim J, Boulukos KE, Rahmani R. Spontaneous apoptosis in primary cultures of human and rat hepatocytes: molecular mechanisms and regulation by dexamethasone. Cell Death Differ. 2002;9:945–955. doi: 10.1038/sj.cdd.4401043. [DOI] [PubMed] [Google Scholar]
- Banic B, Nipic D, Suput D, Milisav I. DMSO modulates the pathway of apoptosis triggering. Cell Mol Biol Lett. 2011;16:328–341. doi: 10.2478/s11658-011-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy CO, Prost S, Wyllie AH, Harrison DJ. UV but not gamma-irradiation induces specific transcriptional activity of p53 in primary hepatocytes. J Pathol. 1997;183:177–181. doi: 10.1002/(SICI)1096-9896(199710)183:2<177::AID-PATH909>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Di Sario A, Svegliati Baroni G, Jezequel AM. Transforming growth factor beta 1 increases the number of apoptotic bodies and decreases intracellular pH in isolated periportal and perivenular rat hepatocytes. Hepatology. 1995;22:1488–1498. [PubMed] [Google Scholar]
- Berry MN, Grivell AR, Grivell MB, Phillips JW. Isolated hepatocytes-past, present and future. Cell Biol Toxicol. 1997;13:223–233. doi: 10.1023/a:1007402505482. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Tousignant J, Ethier C, Rocheleau B, Raymond VA, Lapointe R. Anti-apoptotic effect of insulin on normal hepatocytes in vitro and in vivo. Apoptosis. 2004;9:609–617. doi: 10.1023/B:APPT.0000038040.54210.d1. [DOI] [PubMed] [Google Scholar]
- Boer U, Fennekohl A, Puschel GP. Sensitization by interleukin-6 of rat hepatocytes to tumor necrosis factor alpha-induced apoptosis. J Hepatol. 2003;38:728–735. doi: 10.1016/s0168-8278(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Bohnenberger S, Wagner B, Schmitz HJ, Schrenk D. Inhibition of apoptosis in rat hepatocytes treated with ‘non-dioxin-like’ polychlorinated biphenyls. Carcinogenesis. 2001;22:1601–1606. doi: 10.1093/carcin/22.10.1601. [DOI] [PubMed] [Google Scholar]
- Bresgen N, Ohlenschlager I, Wacht N, Afazel S, Ladurner G, Eckl PM. Ferritin and FasL (CD95L) mediate density dependent apoptosis in primary rat hepatocytes. J Cell Physiol. 2008;217:800–808. doi: 10.1002/jcp.21555. [DOI] [PubMed] [Google Scholar]
- Bresgen N, Rolinek R, Hochleitner E, Lottspeich F, Eckl PM. Induction of apoptosis by a hepatocyte conditioned medium. J Cell Physiol. 2004;198:452–460. doi: 10.1002/jcp.10439. [DOI] [PubMed] [Google Scholar]
- Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Choi BM, Pae HO, Kim YM, Chung HT. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003;37:810–823. doi: 10.1053/jhep.2003.50114. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Gonzales AJ, Cattley RC, Goldsworthy TL. Regulation of apoptosis in mouse hepatocytes and alteration of apoptosis by nongenotoxic carcinogens. Cell Growth Differ. 1998;9:815–825. [PubMed] [Google Scholar]
- Clippinger AJ, Gearhart TL, Bouchard MJ. Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-kappaB and the mitochondrial permeability transition pore. J Virol. 2009;83:4718–4731. doi: 10.1128/JVI.02590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazza N, Kassahn D, Jakob S, Badmann A, Brunner T. TRAIL-induced apoptosis: between tumor therapy and immunopathology. Ann N Y Acad Sci. 2009;1171:50–58. doi: 10.1111/j.1749-6632.2009.04905.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove BD, Cheng C, Pritchard JR, Stolz DB, Lauffenburger DA, Griffith LG. An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-alpha. Hepatology. 2008;48:276–288. doi: 10.1002/hep.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove BD, King BM, Hasan MA, Alexopoulos LG, Farazi PA, Hendriks BS, Griffith LG, Sorger PK, Tidor B, Xu JJ, Lauffenburger DA. Synergistic drug-cytokine induction of hepatocellular death as an in vitro approach for the study of inflammation-associated idiosyncratic drug hepatotoxicity. Toxicol Appl Pharmacol. 2009;237:317–330. doi: 10.1016/j.taap.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenesse D, Laurens M, Heurteaux C, Cursio R, Saint-Paul MC, Schmid-Alliana A, Gugenheim J. Rat liver ischemiareperfusion-induced apoptosis and necrosis are decreased by FK506 pretreatment. Eur J Pharmacol. 2003;473:177–184. doi: 10.1016/s0014-2999(03)01977-0. [DOI] [PubMed] [Google Scholar]
- Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- Diao J, Khine AA, Sarangi F, Hsu E, Iorio C, Tibbles LA, Woodgett JR, Penninger J, Richardson CD. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J Biol Chem. 2001;276:8328–8340. doi: 10.1074/jbc.M006026200. [DOI] [PubMed] [Google Scholar]
- Donato MT, Ponsoda X, O’Connor E, Castell JV, Gomez-Lechon MJ. Role of endogenous nitric oxide in liver-specific functions and survival of cultured rat hepatocytes. Xenobiotica. 2001;31:249–264. doi: 10.1080/00498250110052111. [DOI] [PubMed] [Google Scholar]
- Drudi Metalli V, Mancino MG, Mancino A, Torrice A, Gatto M, Attili AF, Alpini G, Alvaro D. Bile salts regulate proliferation and apoptosis of liver cells by modulating the IGF1 system. Dig Liver Dis. 2007;39:654–662. doi: 10.1016/j.dld.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. Faseb J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- Dvir-Ginzberg M, Elkayam T, Aflalo ED, Agbaria R, Cohen S. Ultrastructural and functional investigations of adult hepatocyte spheroids during in vitro cultivation. Tissue Eng. 2004;10:1806–1817. doi: 10.1089/ten.2004.10.1806. [DOI] [PubMed] [Google Scholar]
- Elaut G, Henkens T, Papeleu P, Snykers S, Vinken M, Vanhaecke T, Rogiers V. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 2006;7:629–660. doi: 10.2174/138920006778017759. [DOI] [PubMed] [Google Scholar]
- Elaut G, Vanhaecke T, Heyden YV, Rogiers V. Spontaneous apoptosis, necrosis, energy status, glutathione levels and biotransformation capacities of isolated rat hepatocytes in suspension: effect of the incubation medium. Biochem Pharmacol. 2005;69:1829–1838. doi: 10.1016/j.bcp.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Ethier C, Raymond VA, Musallam L, Houle R, Bilodeau M. Antiapoptotic effect of EGF on mouse hepatocytes associated with downregulation of proapoptotic Bid protein. Am J Physiol Gastrointest Liver Physiol. 2003;285:G298–G308. doi: 10.1152/ajpgi.00040.2003. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Thorpe CJ, Yonehara S, Chiodi F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;9:201–209. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- Feldmann G. Liver apoptosis. J Hepatol. 1997;26(Suppl 2):1–11. doi: 10.1016/s0168-8278(97)80491-6. [DOI] [PubMed] [Google Scholar]
- Fladmark KE, Gjertsen BT, Doskeland SO, Vintermyr OK. Fas/APO-1(CD95)-induced apoptosis of primary hepatocytes is inhibited by cAMP. Biochem Biophys Res Commun. 1997;232:20–25. doi: 10.1006/bbrc.1997.6214. [DOI] [PubMed] [Google Scholar]
- Fu T, Blei AT, Takamura N, Lin T, Guo D, Li H, O’Gorman MR, Soriano HE. Hypothermia inhibits Fas-mediated apoptosis of primary mouse hepatocytes in culture. Cell Transplant. 2004;13:667–676. doi: 10.3727/000000004783983495. [DOI] [PubMed] [Google Scholar]
- Fu T, Guo D, Huang X, O’Gorman MR, Huang L, Crawford SE, Soriano HE. Apoptosis occurs in isolated and banked primary mouse hepatocytes. Cell Transplant. 2001;10:59–66. [PubMed] [Google Scholar]
- Furukawa S, Usuda K, Fujieda Y, Tamura T, Miyamoto Y, Hayashi K, Ikeyama S, Goryo M, Okada K. Apoptosis and cell proliferation in rat hepatocytes induced by barbiturates. J Vet Med Sci. 2000;62:23–28. doi: 10.1292/jvms.62.23. [DOI] [PubMed] [Google Scholar]
- Gilot D, Loyer P, Corlu A, Glaise D, Lagadic-Gossmann D, Atfi A, Morel F, Ichijo H, Guguen-Guillouzo C. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J Biol Chem. 2002;277:49220–49229. doi: 10.1074/jbc.M207325200. [DOI] [PubMed] [Google Scholar]
- Gilot D, Serandour AL, Ilyin GP, Lagadic-Gossmann D, Loyer P, Corlu A, Coutant A, Baffet G, Peter ME, Fardel O, Guguen-Guillouzo C. A role for caspase-8 and c-FLIPL in proliferation and cell-cycle progression of primary hepatocytes. Carcinogenesis. 2005;26:2086–2094. doi: 10.1093/carcin/bgi187. [DOI] [PubMed] [Google Scholar]
- Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Müller A, Tuschl G, Mueller SO, Dooley S. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology. 2009;49:2031–2043. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, O’Connor E, Castell JV, Jover R. Sensitive markers used to identify compounds that trigger apoptosis in cultured hepatocytes. Toxicol Sci. 2002;65:299–308. doi: 10.1093/toxsci/65.2.299. [DOI] [PubMed] [Google Scholar]
- Gomez-Quiroz LE, Factor VM, Kaposi-Novak P, Coulouarn C, Conner EA, Thorgeirsson SS. Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis. J Biol Chem. 2008;283:14581–14589. doi: 10.1074/jbc.M707733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Clement B, Baffet G, Beaumont C, Morel-Chany E, Glaise D, Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983;143:47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GA, Jolley SL, Gilbert D, Coon DJ, Barros S, LeCluyse EL. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell–cell interactions. Cell Tissue Res. 2001;306:85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- Han D, Hanawa N, Saberi B, Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med. 2006;41:627–639. doi: 10.1016/j.freeradbiomed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Henkens T, Papeleu P, Elaut G, Vinken M, Rogiers V, Vanhaecke T. Trichostatin A, a critical factor in maintaining the functional differentiation of primary cultured rat hepatocytes. Toxicol Appl Pharmacol. 2007;218:64–71. doi: 10.1016/j.taap.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Hentze H, Latta M, Kunstle G, Dhakshinamoorthy S, Ng PY, Porter AG, Wendel A. Topoisomerase inhibitor camptothecin sensitizes mouse hepatocytes in vitro and in vivo to TNF-mediated apoptosis. Hepatology. 2004;39:1311–1320. doi: 10.1002/hep.20174. [DOI] [PubMed] [Google Scholar]
- Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Hohenester S, Gates A, Wimmer R, Beuers U, Anwer MS, Rust C, Webster CR. Phosphatidylinositol-3-kinase p110gamma contributes to bile salt-induced apoptosis in primary rat hepatocytes and human hepatoma cells. J Hepatol. 2010;53:918–926. doi: 10.1016/j.jhep.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer C, Maier P. DNA and protein contents of hepatocytes in primary cultures monitored by flow cytometry: effect of phenobarbital and dimethylsulphoxide. Toxicol In Vitro. 1987;1:203–213. doi: 10.1016/0887-2333(87)90022-1. [DOI] [PubMed] [Google Scholar]
- Hoshiba T, Nagahara H, Cho CS, Tagawa Y, Akaike T. Primary hepatocyte survival on non-integrin-recognizable matrices without the activation of Akt signaling. Biomaterials. 2007;28:1093–1104. doi: 10.1016/j.biomaterials.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hully JR, Chang L, Schwall RH, Widmer HR, Terrell TG, Gillett NA. Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology. 1994;20:854–862. doi: 10.1002/hep.1840200413. [DOI] [PubMed] [Google Scholar]
- Hussain SM, Frazier JM. Involvement of apoptosis in hydra-zine induced toxicity in rat primary hepatocytes. Toxicol In Vitro. 2003;17:343–355. doi: 10.1016/s0887-2333(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Hynes J, Hill R, Papkovsky DB. The use of a fluorescence-based oxygen uptake assay in the analysis of cytotoxicity. Toxicol In Vitro. 2006;20:785–792. doi: 10.1016/j.tiv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Isom HC, Secott T, Georgoff I, Woodworth C, Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci USA. 1985;82:3252–3256. doi: 10.1073/pnas.82.10.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Kasper HU, Dries V, Drebber U, Kern MA, Dienes HP, Schirmacher P. Precision cut tissue slices of the liver as morphological tool for investigation of apoptosis. In Vivo. 2005;19:423–431. [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WH, Hong F, Jaruga B, Zhang ZS, Fan SJ, Liang TJ, Gao B. Hepatitis B virus X protein sensitizes primary mouse hepatocytes to ethanol- and TNF-alpha-induced apoptosis by a caspase-3-dependent mechanism. Cell Mol Immunol. 2005;2:40–48. [PubMed] [Google Scholar]
- Kucera T, Canova NK, Farghali H, Martinek J. The morphological and immunocytochemical evaluation of primary rat hepatocytes undergoing spontaneous cell death: modulation by the nitric oxide donor S-nitroso-N-acetylpenicillamine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:75–82. doi: 10.5507/bp.2006.008. [DOI] [PubMed] [Google Scholar]
- Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168–175. doi: 10.1016/j.jhep.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Kume E, Aruga C, Takahashi K, Miwa S, Dekura E, Itoh M, Ishizuka Y, Fujimura H, Toriumi W, Doi K. Morphological and gene expression analysis in mouse primary cultured hepatocytes exposed to streptozotocin. Exp Toxicol Pathol. 2005;56:245–253. doi: 10.1016/j.etp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lan L, Gorke S, Rau SJ, Zeisel MB, Hildt E, Himmelsbach K, Carvajal-Yepes M, Huber R, Wakita T, Schmitt-Graeff A, Royer C, Blum HE, Fischer R, Baumert TF. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008;181:4926–4935. doi: 10.4049/jimmunol.181.7.4926. [DOI] [PubMed] [Google Scholar]
- Laurens M, Defamie V, Scozzari G, Schmid-Alliana A, Gugenheim J, Crenesse D. Hypoxia-reoxygenation-induced chemokine transcription is not prevented by preconditioning or intermittent hypoxia, in mice hepatocytes. Transpl Int. 2005;18:444–452. doi: 10.1111/j.1432-2277.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Legembre P, Beneteau M, Daburon S, Moreau JF, Taupin JL. Cutting edge: SDS-stable Fas microaggregates: an early event of Fas activation occurring with agonistic anti-Fas antibody but not with Fas ligand. J Immunol. 2003;171:5659–5662. doi: 10.4049/jimmunol.171.11.5659. [DOI] [PubMed] [Google Scholar]
- Lei WW, Zhang KH, Pan XC, Wang DM, Hu Y, Yang YN, Song JG. Histone deacetylase 1 and 2 differentially regulate apoptosis by opposing effects on extracellular signal-regulated kinase 1/2. Cell Death Dis. 2010;1:e44. doi: 10.1038/cddis.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke-Wheeler JL, Nedredal G, Yee L, Amiot BP, Nyberg SL. E-cadherin protects primary hepatocyte spheroids from cell death by a caspase-independent mechanism. Cell Transplant. 2009;18:1281–1287. doi: 10.3727/096368909X474258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kimura H, Koga N, Lin KH, Saito T. Cell density-dependent DNA fragmentation and its suppression by heparin in primary culture of adult rat hepatocytes. Biochem Biophys Res Commun. 1993;195:270–275. doi: 10.1006/bbrc.1993.2040. [DOI] [PubMed] [Google Scholar]
- Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- Martin H, Richert L, Berthelot A. Magnesium deficiency induces apoptosis in primary cultures of rat hepatocytes. J Nutr. 2003;133:2505–2511. doi: 10.1093/jn/133.8.2505. [DOI] [PubMed] [Google Scholar]
- Meurette O, Fontaine A, Rebillard A, Le Moigne G, Lamy T, Lagadic-Gossmann D, Dimanche-Boitrel MT. Cytotoxicity of TRAIL/anticancer drug combinations in human normal cells. Ann N Y Acad Sci. 2006;1090:209–216. doi: 10.1196/annals.1378.023. [DOI] [PubMed] [Google Scholar]
- Meurette O, Lefeuvre-Orfila L, Rebillard A, Lagadic-Gossmann D, Dimanche-Boitrel MT. Role of intracellular glutathione in cell sensitivity to the apoptosis induced by tumor necrosis factor {alpha}-related apoptosis-inducing ligand/anticancer drug combinations. Clin Cancer Res. 2005;11:3075–3083. doi: 10.1158/1078-0432.CCR-04-1764. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam L, Ethier C, Haddad PS, Bilodeau M. Role of EGF receptor tyrosine kinase activity in antiapoptotic effect of EGF on mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1360–G1369. doi: 10.1152/ajpgi.2001.280.6.G1360. [DOI] [PubMed] [Google Scholar]
- Muschen M, Warskulat U, Douillard P, Gilbert E, Haussinger D. Regulation of CD95 (APO-1/Fas) receptor and ligand expression by lipopolysaccharide and dexamethasone in parenchymal and nonparenchymal rat liver cells. Hepatology. 1998;27:200–208. doi: 10.1002/hep.510270131. [DOI] [PubMed] [Google Scholar]
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, Knight B, Yeoh GC, Fausto N, Parks WT. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology. 2007;45:31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- Ni R, Tomita Y, Matsuda K, Ichihara A, Ishimura K, Ogasawara J, Nagata S. Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- Nipic D, Pirc A, Banic B, Suput D, Milisav I. Preapoptotic cell stress response of primary hepatocytes. Hepatology. 2010;51:2140–2151. doi: 10.1002/hep.23598. [DOI] [PubMed] [Google Scholar]
- Oberhammer F, Froschl G, Tiefenbacher R, Inayat-Hussain SH, Cain K, Stopper H. Hepatocyte death following transforming growth factor-beta 1 addition. Microsc Res Tech. 1996;34:247–258. doi: 10.1002/(SICI)1097-0029(19960615)34:3<247::AID-JEMT7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA. 1992;89:5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Haga S, Zhang HQ, Irani K, Suzuki S. Inhibition of hypoxia/reoxygenation-induced oxidative stress in HGF-stimulated antiapoptotic signaling: role of PI3-K and Akt kinase upon rac1. Cell Death Differ. 2003;10:508–515. doi: 10.1038/sj.cdd.4401172. [DOI] [PubMed] [Google Scholar]
- Pagliassotti MJ, Wei Y, Wang D. Insulin protects liver cells from saturated fatty acid-induced apoptosis via inhibition of c-Jun NH2 terminal kinase activity. Endocrinology. 2007;148:3338–3345. doi: 10.1210/en.2006-1710. [DOI] [PubMed] [Google Scholar]
- Paine AJ, Andreakos E. Activation of signalling pathways during hepatocyte isolation: relevance to toxicology in vitro. Toxicol In Vitro. 2004;18:187–193. doi: 10.1016/s0887-2333(03)00146-2. [DOI] [PubMed] [Google Scholar]
- Papeleu P, Vanhaecke T, Henkens T, Elaut G, Vinken M, Snykers S, Rogiers V. Isolation of rat hepatocytes. Methods Mol Biol. 2006;320:229–237. doi: 10.1385/1-59259-998-2:229. [DOI] [PubMed] [Google Scholar]
- Papeleu P, Wullaert A, Elaut G, Henkens T, Vinken M, Laus G, Tourwe D, Beyaert R, Rogiers V, Vanhaecke T. Inhibition of NF-kappaB activation by the histone deacetylase inhibitor 4-Me2 N-BAVAH induces an early G1 cell cycle arrest in primary hepatocytes. Cell Prolif. 2007;40:640–655. doi: 10.1111/j.1365-2184.2007.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183–2192. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince JM, Vodovotz Y, Baun MJ, Monga SP, Billiar TR, Gerlach JC. The nitric oxide donor S-nitrosoglutathione reduces apoptotic primary liver cell loss in a three-dimensional perfusion bioreactor culture model developed for liver support. Tissue Eng Part A. 2010;16:861–866. doi: 10.1089/ten.tea.2009.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost S, Bellamy CO, Cunningham DS, Harrison DJ. Altered DNA repair and dysregulation of p53 in IRF-1 null hepatocytes. Faseb J. 1998;12:181–188. doi: 10.1096/fasebj.12.2.181. [DOI] [PubMed] [Google Scholar]
- Pusl T, Wild N, Vennegeerts T, Wimmer R, Goke B, Brand S, Rust C. Free fatty acids sensitize hepatocytes to bile acid-induced apoptosis. Biochem Biophys Res Commun. 2008;371:441–445. doi: 10.1016/j.bbrc.2008.04.113. [DOI] [PubMed] [Google Scholar]
- Qiao L, Farrell GC. The effects of cell density, attachment sub-stratum and dexamethasone on spontaneous apoptosis of rat hepatocytes in primary culture. In Vitro Cell Dev Biol Anim. 1999;35:417–424. doi: 10.1007/s11626-999-0117-2. [DOI] [PubMed] [Google Scholar]
- Raffray M, Cohen GM. Apoptosis and necrosis in toxicology: a continuum or distinct modes of cell death? Pharmacol Ther. 1997;75:153–177. doi: 10.1016/s0163-7258(97)00037-5. [DOI] [PubMed] [Google Scholar]
- Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Realdon S, Gerotto M, Dal Pero F, Marin O, Granato A, Basso G, Muraca M, Alberti A. Proapoptotic effect of hepatitis C virus CORE protein in transiently transfected cells is enhanced by nuclear localization and is dependent on PKR activation. J Hepatol. 2004;40:77–85. doi: 10.1016/j.jhep.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Graf D, Fischer R, Schliess F, Haussinger D. Hyperosmolarity triggers CD95 membrane trafficking and sensitizes rat hepatocytes toward CD95L-induced apoptosis. Hepatology. 2002;36:602–614. doi: 10.1053/jhep.2002.35447. [DOI] [PubMed] [Google Scholar]
- Ribeiro DH, Ferreira FL, da Silva VN, Aquino S, Correa B. Effects of aflatoxin b(1) and fumonisin b(1) on the viability and induction of apoptosis in rat primary hepatocytes. Int J Mol Sci. 2010;11:1944–1955. doi: 10.3390/ijms11041944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ariza A, Lopez-Sanchez LM, Gonzalez R, Corrales FJ, Lopez P, Bernardos A, Muntane J. Altered protein expression and protein nitration pattern during d-galactosamine-induced cell death in human hepatocytes: a proteomic analysis. Liver Int. 2005;25:1259–1269. doi: 10.1111/j.1478-3231.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- Rouquet N, Carlier K, Briand P, Wiels J, Joulin V. Multiple pathways of Fas-induced apoptosis in primary culture of hepatocytes. Biochem Biophys Res Commun. 1996;229:27–35. doi: 10.1006/bbrc.1996.1753. [DOI] [PubMed] [Google Scholar]
- Rowe C, Goldring CE, Kitteringham NR, Jenkins RE, Lane BS, Sanderson C, Elliott V, Platt V, Metcalfe P, Park BK. Network analysis of primary hepatocyte dedifferentiation using a shotgun proteomics approach. J Proteome Res. 2010;9:2658–2668. doi: 10.1021/pr1001687. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Alvarez AM, Benito M, Fabregat I. Apoptosis induced by transforming growth factor-beta in fetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J Biol Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liver Int. 2006;26:904–911. doi: 10.1111/j.1478-3231.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- Schlatter R, Schmich K, Lutz A, Trefzger J, Sawodny O, Ederer M, Merfort I. Modeling the TNFalpha-induced apoptosis pathway in hepatocytes. PLoS ONE. 2011;6:e18646. doi: 10.1371/journal.pone.0018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser SF, Azzaroli F, Dao T, Hingorani R, Nicholas Crispe I, Boyer JL. Induction of murine hepatocyte death by membrane-bound CD95 (Fas/APO-1)-ligand: characterization of an in vitro system. Hepatology. 2000;32:779–785. doi: 10.1053/jhep.2000.18422. [DOI] [PubMed] [Google Scholar]
- Schmich K, Schlatter R, Corazza N, Sa Ferreira K, Ederer M, Brunner T, Borner C, Merfort I. Tumor necrosis factor alpha sensitizes primary murine hepatocytes to Fas/CD95-induced apoptosis in a Bim- and Bid-dependent manner. Hepatology. 2011;53:282–292. doi: 10.1002/hep.23987. [DOI] [PubMed] [Google Scholar]
- Schrenk D, Schmitz HJ, Bohnenberger S, Wagner B, Worner W. Tumor promoters as inhibitors of apoptosis in rat hepatocytes. Toxicol Lett. 2004;149:43–50. doi: 10.1016/j.toxlet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Buchler P, Muller M, Krammer PH. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology. 2004;39:645–654. doi: 10.1002/hep.20138. [DOI] [PubMed] [Google Scholar]
- Schulze-Bergkamen H, Schuchmann M, Fleischer B, Galle PR. The role of apoptosis versus oncotic necrosis in liver injury: facts or faith? J Hepatol. 2006;44:984–993. doi: 10.1016/j.jhep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Schulze-Bergkamen H, Untergasser A, Dax A, Vogel H, Buchler P, Klar E, Lehnert T, Friess H, Buchler MW, Kirschfink M, Stremmel W, Krammer PH, Muller M, Protzer U. Primary human hepatocytes—a valuable tool for investigation of apoptosis and hepatitis B virus infection. J Hepatol. 2003;38:736–744. doi: 10.1016/s0168-8278(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFalpha- and Fas-mediated apoptosis in hepatocytes. Faseb J. 2004;18:720–722. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993;18:347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kamiike W, Akao Y, Kosaka H, Hasegawa J, Matsuda H, Tsujimoto Y. Involvement of ICE family proteases in apoptosis induced by reoxygenation of hypoxic hepatocytes. Am J Physiol. 1996;271:G949–G958. doi: 10.1152/ajpgi.1996.271.6.G949. [DOI] [PubMed] [Google Scholar]
- Shinzawa K, Watanabe Y, Akaike T. Primary cultured murine hepatocytes but not hepatoma cells regulate the cell number through density-dependent cell death. Cell Death Differ. 1995;2:133–140. [PubMed] [Google Scholar]
- Smets FN, Chen Y, Wang LJ, Soriano HE. Loss of cell anchorage triggers apoptosis (anoikis) in primary mouse hepatocytes. Mol Genet Metab. 2002;75:344–352. doi: 10.1016/S1096-7192(02)00004-5. [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Dufour JF. Biomarkers for hepatocellular apoptosis in the management of liver diseases. Curr Pharm Biotechnol. 2012;13:2221–2227. doi: 10.2174/138920112802502097. [DOI] [PubMed] [Google Scholar]
- Terradillos O, Pollicino T, Lecoeur H, Tripodi M, Gougeon ML, Tiollais P, Buendia MA. p53-independent apoptotic effects of the hepatitis B virus HBx protein in vivo and in vitro. Oncogene. 1998;17:2115–2123. doi: 10.1038/sj.onc.1202432. [DOI] [PubMed] [Google Scholar]
- Thilenius AR, Braun K, Russell JH. Agonist antibody and Fas ligand mediate different sensitivity to death in the signaling pathways of Fas and cytoplasmic mutants. Eur J Immunol. 1997;27:1108–1114. doi: 10.1002/eji.1830270510. [DOI] [PubMed] [Google Scholar]
- Tichy M, Pokorna A, Hanzlikova I, Nerudova J, Tumova J, Uzlova R. Primary rat hepatocytes in chemical testing and QSAR predictive applicability. Toxicol In Vitro. 2010;24:240–244. doi: 10.1016/j.tiv.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Hrach J, Walter Y, Hewitt PG, Mueller SO. Serum-free collagen sandwich cultures of adult rat hepatocytes maintain liver-like properties long term: a valuable model for in vitro toxicity and drug–drug interaction studies. Chem Biol Interact. 2009;181:124–137. doi: 10.1016/j.cbi.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Henkens T, Kass GE, Rogiers V. Effect of the histone deacetylase inhibitor trichostatin A on spontaneous apoptosis in various types of adult rat hepatocyte cultures. Biochem Pharmacol. 2004;68:753–760. doi: 10.1016/j.bcp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Henkens T, Snykers S, Elaut G, Papeleu P, Rogiers V. Effects of Trichostatin A on apoptosis-regulating proteins during hepatocyte isolation. ALTEX. 2006;23:441–444. [Google Scholar]
- Vinken M, Papeleu P, Snykers S, De Rop E, Henkens T, Chipman JK, Rogiers V, Vanhaecke T. Involvement of cell junctions in hepatocyte culture functionality. Crit Rev Toxicol. 2006;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, De Vuyst E, Leybaert L, Vanhaecke T, Rogiers V. Biochemical characterisation of an in vitro model of hepatocellular apoptotic cell death. Altern Lab Anim. 2009;37:209–218. doi: 10.1177/026119290903700210. [DOI] [PubMed] [Google Scholar]
- Vinken M, Snykers S, Fraczek J, Decrock E, Leybaert L, Rogiers V, Vanhaecke T. DNA methyltransferase 3a expression decreases during apoptosis in primary cultures of hepatocytes. Toxicol In Vitro. 2010;24:445–451. doi: 10.1016/j.tiv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Doktorova T, Ramboer E, De Vuyst E, Vanhaecke T, Leybaert L, Rogiers V. Characterization of spontaneous cell death in monolayer cultures of primary hepatocytes. Arch Toxicol. 2011;85:1589–1596. doi: 10.1007/s00204-011-0703-4. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Vanhaecke T, Leybaert L, Rogiers V. Connexin43 signaling contributes to spontaneous apoptosis in cultures of primary hepatocytes. Toxicol Sci. 2012a;125:175–186. doi: 10.1093/toxsci/kfr277. [DOI] [PubMed] [Google Scholar]
- Vinken M, Vanhaecke T, Rogiers V. Primary hepatocyte cultures as in vitro tools for toxicity testing: quo vadis? Toxicol In Vitro. 2012b;26:541–544. doi: 10.1016/j.tiv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Walter D, Schmich K, Vogel S, Pick R, Kaufmann T, Hochmuth FC, Haber A, Neubert K, McNelly S, von Weizsacker F, Merfort I, Maurer U, Strasser A, Borner C. Switch from type II to I Fas/CD95 death signaling on in vitro culturing of primary hepatocytes. Hepatology. 2008;48:1942–1953. doi: 10.1002/hep.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Wang H, Xu LN, Zou SQ. Hepatic injury in rats with obstructive jaundice: roles of the protein kinase C signal pathway and cytoprotection of fructose. Hepatobiliary Pancreat Dis Int. 2005;4:577–581. [PubMed] [Google Scholar]
- Wang K, Brems JJ, Gamelli RL, Holterman AX. iNOS/NO signaling regulates apoptosis induced by glycochenodeoxycho-late in hepatocytes. Cell Signal. 2011;23:1677–1685. doi: 10.1016/j.cellsig.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Wang K, Brems JJ, Gamelli RL, Holterman AX. Survivin signaling is regulated through nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim Biophys Acta. 2010;1803:1368–1375. doi: 10.1016/j.bbamcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Worner W, Schrenk D. Influence of liver tumor promoters on apoptosis in rat hepatocytes induced by 2-acetylaminofluorene, ultraviolet light, or transforming growth factor beta 1. Cancer Res. 1996;56:1272–1278. [PubMed] [Google Scholar]
- Yang D, Liu N, Zuo C, Lei S, Wu X, Zhou F, Liu C, Zhu H. Innate host response in primary human hepatocytes with hepatitis C virus infection. PLoS ONE. 2011;6:e27552. doi: 10.1371/journal.pone.0027552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zhang D, Luo Y, Zhang D, Huang A, Zhou W, Ren H. Fas ligand expression and apoptosis in primary rat hepatocytes induced by lipopolysaccharide. Zhonghua Gan Zang Bing Za Zhi. 2000;8:285–287. [PubMed] [Google Scholar]
- Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Jr, Liebermann DA, Bottinger EP, Roberts AB. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278:43001–43007. doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- Zucchini N, de Sousa G, Bailly-Maitre B, Gugenheim J, Bars R, Lemaire G, Rahmani R. Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes. Biochim Biophys Acta. 2005;1745:48–58. doi: 10.1016/j.bbamcr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Zucchini-Pascal N, de Sousa G, Pizzol J, Rahmani R. Molecular investigation of the effects of lindane in rat hepatocytes: microarray and mechanistic studies. Food Chem Toxicol. 2011;49:3128–3135. doi: 10.1016/j.fct.2011.09.033. [DOI] [PubMed] [Google Scholar]