Abstract
Solitary pulmonary nodule (SPN) is one of the most controversial clinical findings in patients. The aim of this study is to use 99mTc-ethylenediamine diacetic acid/hydrazine nicotinamide (HYNIC)-TATE scan technique to evaluate nodules. From 2008 to 2010, 21 patients with SPN underwent 99mTc-HYNIC-TATE scan after the initial assessment with high-resolution computed tomography and then accurate histopathologic diagnosis was established by trans-thoracic needle biopsy, Video Assisted Thoracic Surgery and thoracotomy. After demographic evaluations, specificity and sensitivity of this method was studied. A total of 21 patients were included in our study, of which 12 patients were male and 9 were female. Their mean age was 45 ± 14.3 years. About 43% of the patients were symptom-free and in patients with pulmonary complaints, the most prevalent symptom was cough. Final histopathology tests and clinical follow-up proved that 14 cases (67%) were benign and 7 (33%) were malignant. The diagnostic technique used in our study had no false negative and there were only 3 cases of false positive. Sensitivity and specificity of this method are 100% and 79%, respectively and the diagnostic accuracy is 86%. 99mTc-HYNIC-TATE scan can be helpful in evaluating patients with SPN and to reach a sensible decision on the method of treatment.
Keywords: Octreotate scan, sensitivity, solitary pulmonary nodule, specificity
Introduction
A solitary pulmonary nodule (SPN), also referred to as a coin lesion has been a challenging clinical problem for a long time[1,2] SPN is defined as a separate pulmonary lesion of 3 cm or less, surrounded by normal lung parenchyma free of adenopathy and atelectasis. Larger lesions are known as lung mass and must undergo tissue examination due to the high risk of malignancy. Based on available statistical data, SPNs are diagnosed in 1-2 of each 100 chest X-rays (CXRs). Most of the nodules are without clinical symptoms 90% of which are detected as an incidental finding.[3] Important issue, while encountering SPN is to determine the malignant or benign nature of the lesion and the need for surgery because unnecessary surgical interventions of a benign nodule may expose the patient to high morbidity and mortality. Even, risk factors associated with malignant SPN, including old age, smoking, larger nodules, and previous history of malignancies can help to estimate the probability of malignancy, but complementary diagnostic evaluations to refine the need for more invasive intervention versus follow-up are often advocated[1,2,4] The goal is to find a proper method to find suspicious nodules for malignancy as soon as possible, while sparing patients with benign lesions from unnecessary surgical interventions.[5] Radiolabeled somatostatin analogs single photon emission computed tomography (SPECT) method, such as radiolabeled depreotide, has been used to find these suspicious nodules with acceptable accuracy,[6,7,8] This study is primarily aimed at defining the accuracy of 99mTc-ethylenediamine diacetic acid (EDDA)/hydrazinonicotinamide (HYNIC)-TATE SPECT, which is cheaper and more available, in discrimination benign of malignant solitary lung nodule.
Materials and Methods
This is a retrospective study, which was done during the period between April 2008 and June 2010 in Imam-Reza Hospital thoracic surgery ward. Patients with single opacity on CXR with maximum 3 cm in diameters were included into the study. Inclusion criteria were: (1) Presence of SPN (2) voluntary participation in research (3) undergoing diagnostic surgeries or clinical follow-up. Exclusion criteria were as follows: (1) Non-SPN lesions (2) indefinite diagnosis. The lungs high-resolution computed tomography (HRCT) was done for all patients [Figures 1 and 2] showed CXR and computed tomograph scan of a patient with SPN. The patients with >1 nodule and nodules larger than 3 cm or with benign calcification were excluded. After giving informed consent, a total of 21 patients were enrolled for scintigraphy and tissue biopsy (gold standard) or clinical follow-up. The scintigraphy was done 2 h after injection of 20 mCi of the 99mTc-EDDA/HYNIC-TATE. The anterior and posterior planar scans were taken from head, chest, and abdomen by means of a two-headed Siemens E. Cam gamma camera using a high-resolution collimator for 5 min/scan [Figure 3]. After that chest SPECT was taken (128 steps, 128 × 128 matrix, and 25 s for each step). The images were evaluated by two nuclear medicine specialists qualitatively (any activity more than the contralateral lung tissue in the anatomical location of the SPN was considered positive) and semi-quantitatively using region of interest (ROI) (total count/total pixels) on the anatomical location of SPN and the mirrored ROI on the other side as the background. This means that the location of the tumor is defined and at the same slices the opposite site is considered as normal. This is recorded in all images and the tumor positive (T) to normal lung uptake (N) (T/N) is established as the basis of decision making [Figure 4]. Tissue biopsies were taken by open thoracotomy, thoracoscopy and trans-thoracic needle biopsy (TTNB) depending on patient general condition and location of lesion. Age, and gender, scintigraphy, and pathology result were evaluated. After demographic analysis, pathologic results were used to define sensitivity, specificity and accuracy of scintigraphy, with used validity or accuracy of diagnostic tests.
Figure 1.
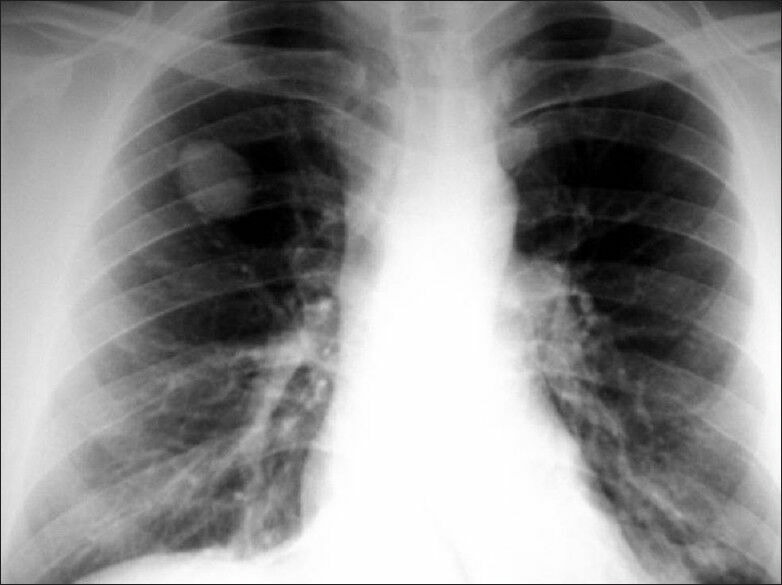
Chest X-ray of a patient with solitary pulmonary nodule
Figure 2.
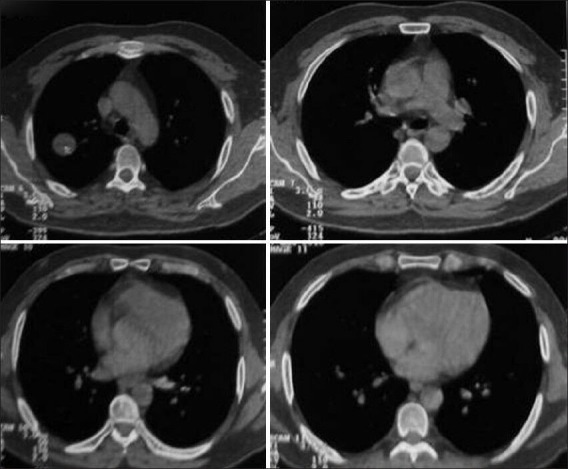
Computed tomography scan of the same patient
Figure 3.
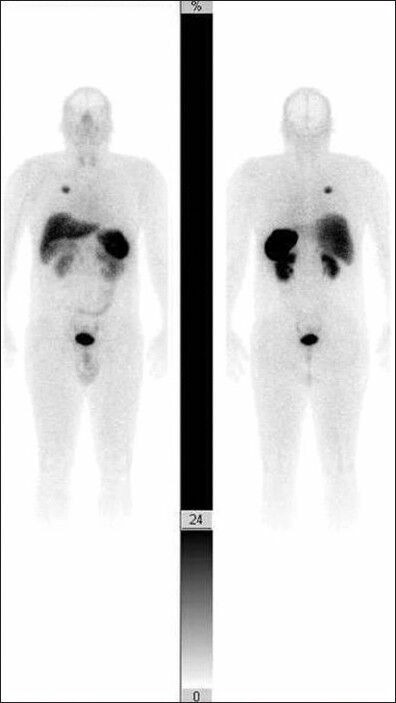
Patient's ant/post scan plans
Figure 4.
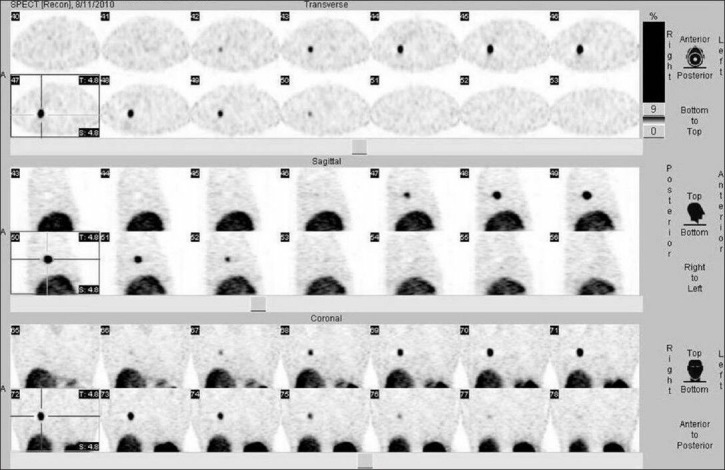
Normal lung scan compared to the involved area scan
This article approved in Ethic Committee of Mashhad University of Medical Sciences by Number: 89735.
Results
During 18 months of the study, 21 patients were included in the statistical population of which 12 were male and 9 were female. Their average age was 45 ± 14.3 years and their ages ranged from 22 to 77 years. SPN was an asymptomatic incidental finding in 9 patients (43%), but the others (57%) had referred because of pulmonary symptoms including chronic cough (n = 7; 33%), dyspnea (n = 3; 14%), and hemoptysis (n = 2; 10%). The pathology and scintigraphy results of patients' nodules are shown on Table 1. Tissue biopsies were taken by open thoracotomy, thoracoscopy and TTNB in 15, 4, and 2 cases, respectively. Considering the ratio of involved area to the normal area uptake (T/N), malignant patients ranged from 1.5 to 4.5 (mean: 3.65), and in nonmalignant group it ranged from 0.85 to 2.5 (mean: 1.33).
Table 1.
Pathology and scintigraphy results of patients
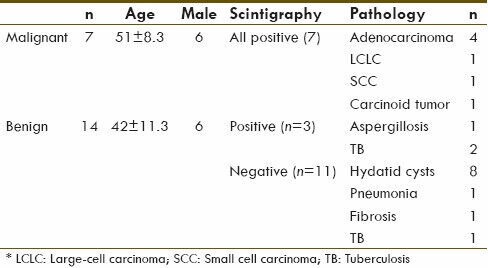
Pathologic evaluation reveals 7 malignant tumors (33%) and 14 benign lesions (67%) which according to that sensitivity of scintigraphy was 100% (no false negative) and its specificity was 79% (3 cases of false positive). Calculated positive predictive value and negative predictive value was 70% and 100%, respectively. The diagnosing accuracy of the test was 86%.
Discussion
The sophisticate management of SPNs is timely diagnosis and curative resection of all malignant nodules, while sparing benign nodules from any invasive intervention. Nevertheless, existing protocols do not fulfill this goal and most of them tend to result in frequent sampling or resection of benign nodules due to fear from poor outcome which may ensue when malignant nodule was missed,[1,3] Now-a-days, most protocols use standard HRCT and some sort of quantitative model for risk stratification of SPNs. Quantitative models usually determined by nodule size, patient age, smoking history, and overall prevalence of malignancy in the population. High- and low-risk SPNs for malignancy often manage with tissue biopsy and serial chest HRCT imaging, respectively[6,7] However, there is a wide controversy in management of intermediate risk SPNs which most unnecessary invasive interventions fall in this group. In such cases scintigraphy, as a less-aggressive and cost-effective diagnostic modality, is proved valuable for further refinement between malignant and benign nodules. Flourodeoxyglucose positron emission tomography (FDG-PET) scan with a sensitivity of 94% and a specificity 83% is now an accepted modality[8,9,10] but it is costly and not readily available. Tc-99m depretide is a synthetic somatostatin with a low molecular weight which bounds to somatostatin receptors.[6,11] The substance is used in the evaluation of SPN, carcinoid tumors, lung cancer, breast cancer, and renal cell carcinoma in which somatostatin marker is presented[12,13]T/N uptake ratio >2-2.22 is usually considered positive.[14]
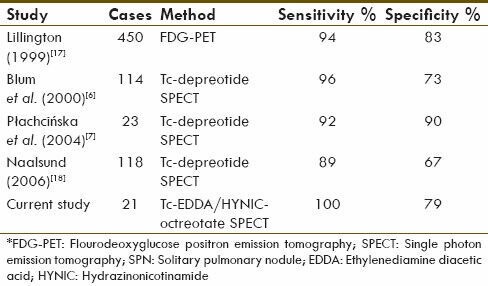
These drawbacks lead to introduce somatostatin analogs such as depreotide scan, which have comparable accuracy with FDG-PET scan [Table 2].[15,16,17] In our study, 99mTc-EDDA/HYNIC-TATE scan with a sensitivity of 100% and a specificity of 79% shows comparable result with depreotide and FDG-PET scan.
Table 2.
Sensitivity and specificity of scintigraphy to discrimination of malignant SPNs
Conclusion
Our study demonstrated that 99mTc-EDDA/HYNIC-TATE scintigraphy is a sensitive imaging modality with significant specificity and negative predictive value. We recommend this scan for the evaluation of SPNs, especially when PET is not available before biopsy because of its lower expenses and availability. Considering the small number of patients in the current study, further studies with wider samples are required to make a definite decision.
Acknowledgment
This paper is the result of a fellowship of pulmonary Medicine thesis Farshid Nattagh has been extracted, which is supported by deputy of research, Mashhad University of Medical Sciences. The authors wish to thank Vice Chancellor for Education and the Research Committee of University for their support. The authors also wish to thank Dr. Sadeghi Ramin, Akhlaghi Saeed, Naghavi Riyabi Fatemeh, Sheibani Shima and Adinepour Zohreh.
Footnotes
Source of Support: The authors would like to thank vice chancellery of Research of Mashhad University of Medical Sciences for financial support.
Conflict of Interest: None declared.
References
- 1.American College of Chest Physicians, Health and Science Policy Committee. Diagnosis and management of lung cancer: ACCP evidence-based guidelines. American College of Chest Physicians. Chest. 2003;123(1 Suppl: D-G):1S–337. [PubMed] [Google Scholar]
- 2.Aoki T, Tomoda Y, Watanabe H, Nakata H, Kasai T, Hashimoto H, et al. Peripheral lung adenocarcinoma: Correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220:803–9. doi: 10.1148/radiol.2203001701. [DOI] [PubMed] [Google Scholar]
- 3.Chang KJ, Wiersema MJ. Endoscopic ultrasound-guided fine-needle aspiration biopsy and interventional endoscopic ultrasonography. Emerging technologies. Gastrointest Endosc Clin N Am. 1997;7:221–35. [PubMed] [Google Scholar]
- 4.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 5.Cardillo G, Regal M, Sera F, Di Martino M, Carbone L, Facciolo F, et al. Videothoracoscopic management of the solitary pulmonary nodule: A single-institution study on 429 cases. Ann Thorac Surg. 2003;75:1607–11. doi: 10.1016/s0003-4975(02)04827-0. [DOI] [PubMed] [Google Scholar]
- 6.Blum J, Handmaker H, Lister-James J, Rinne N. A multicenter trial with a somatostatin analog (99m) Tc depreotide in the evaluation of solitary pulmonary nodules. Chest. 2000;117:1232–8. doi: 10.1378/chest.117.5.1232. [DOI] [PubMed] [Google Scholar]
- 7.Płachcińska A, Mikołajczak R, Maecke HR, Michalski A, Rzeszutek K, Kozak J, et al. 99mTc-EDDA/HYNIC-TOC scintigraphy in the differential diagnosis of solitary pulmonary nodules. Eur J Nucl Med Mol Imaging. 2004;31:1005–10. doi: 10.1007/s00259-004-1511-3. [DOI] [PubMed] [Google Scholar]
- 8.Shih WJ, Samayoa L. Tc-99m depreotide detecting malignant pulmonary nodules: Histopathologic correlation with semiquantitative tumor-to-normal lung ratio. Clin Nucl Med. 2004;29:171–6. doi: 10.1097/01.rlu.0000113855.93504.03. [DOI] [PubMed] [Google Scholar]
- 9.Henschke CI, Yankelevitz DF, Naidich DP, McCauley DI, McGuinness G, Libby DM, et al. CT screening for lung cancer: Suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231:164–8. doi: 10.1148/radiol.2311030634. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252–9. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 11.Morehead RS, Shih WJ. Tc-99m-labeled somatostatin receptor-binding peptide imaging for a pulmonary nodule. Clin Nucl Med. 2001;26:910–2. doi: 10.1097/00003072-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Miliziano JS, Bradley YC. Soft tissue metastases and lung cancer recurrence detected by Tc-99m depreotide scintigraphy. Clin Nucl Med. 2002;27:410–2. doi: 10.1097/00003072-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Montilla-Soler JL, Bridwell RS. Tc-99m depreotide scintigraphy of breast carcinoma. Clin Nucl Med. 2002;27:202–4. doi: 10.1097/00003072-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Montilla JL, Bridwell RS. Average vs maximal count region of interest quantitative analysis of lung nodules. Using Tc-99m depreotide. Is this nodule malignant? J Nucl Med. 2002;43:3–5. [Google Scholar]
- 15.Kalff V, Hicks RJ, MacManus MP, Binns DS, McKenzie AF, Ware RE, et al. Clinical impact of (18) F fluorodeoxyglucose positron emission tomography in patients with non-small-cell lung cancer: A prospective study. J Clin Oncol. 2001;19:111–8. doi: 10.1200/JCO.2001.19.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Libby DM, Smith JP, Altorki NK, Pasmantier MW, Yankelevitz D, Henschke CI. Managing the small pulmonary nodule discovered by CT. Chest. 2004;125:1522–9. doi: 10.1378/chest.125.4.1522. [DOI] [PubMed] [Google Scholar]
- 17.Lillington GA, Gould MK. Managing solitary pulmonary nodules: Accurate predictions and divergent conclusions. Mayo Clin Proc. 1999;74:435–6. doi: 10.4065/74.4.435. [DOI] [PubMed] [Google Scholar]
- 18.Naalsund A, Maublant J. The solitary pulmonary nodule - Is it malignant or benign? Diagnostic performance of 99mTc-depreotide SPECT. Respiration. 2006 Sep;Volume 73(Issue 5):634–41. doi: 10.1159/000093232. [DOI] [PubMed] [Google Scholar]


