Abstract
The increasing number of patients with coronary artery disease (CAD) undergoing major noncardiac surgery justifies guidelines concerning preoperative cardiac evaluation. This is compounded by increasing chances for a volatile perioperative period if the underlying cardiac problems are left uncorrected prior to major noncardiac surgeries. Preoperative cardiac evaluation requires the clinician to assess the patient's probability to have CAD, severity and stability of CAD, placing these in perspective regarding the likelihood of a perioperative cardiac complication based on the planned surgical procedure. Coronary events like new onset ischemia, infarction, or revascularization, induce a high-risk period of 6 weeks, and an intermediate-risk period of 3 months before performing noncardiac surgery. This delay is unwarranted in cases where surgery is the mainstay of treatment. The objective of this review is to offer a comprehensive algorithm in the preoperative assessment of patients undergoing noncardiac surgery and highlight the importance of myocardial perfusion imaging in risk stratifying these patients.
Keywords: Cardiac risk stratification, coronary artery disease, heart, noncardiac surgeries, stress myocardial perfusion imaging, surgery
Introduction
Of the 27 million patients who undergo surgeries in the U.S every year, approximately 8 million have coronary artery disease (CAD) or its risk factors.[1] Patients with a prior myocardial infarction (MI) have a high-risk of perioperative reinfarction compared with the normal population (5-8% vs. 0.1-0.7%). There is a 6% risk for reinfarction from surgery in patients with less than 3 months history of MI.[2] In patients undergoing general surgery procedures, the risk for perioperative MI is 0.8% in men over than 50 years[3] and varies with the cardiovascular status, comorbidities, and the extent of the procedure, reaching more than 20% among patients undergoing vascular surgery.[4] The risk of cardiac death is estimated to be 0.4%.[5] As patients become older, tend to have more comorbidities, minor surgical procedures even can get complicated with stormy postoperative period. To ensure a smooth uneventful recovery postoperatively, physicians must investigate the cardiac status of patients scheduled for major noncardiac surgeries.
Although procedural guidelines are in place for preoperative cardiac evaluations, there can be differences in the prevalence of CAD and its morbidity. Risk of surgery is also dependent on surgical skills, anesthetic care, and nursing quality. Each institution should therefore establish its own audit in order to take appropriate decisions when choices have to be made between different treatment modalities. In this article, we shall strive to emphasize on gated stress myocardial perfusion imaging (MPI), the most commonly used physiological imaging modality for cardiac risk stratification in preoperative setting. It is a noninvasive, cost-effective, and sensitivity investigation for detecting ischemic heart disease and also to assess physiological significance of known CAD.
Need for Preoperative Coronary Artery Disease Evaluation
Preoperative cardiac evaluation aims to lower the perioperative morbidity and mortality. It also helps in limiting the financial implications on the patient and to identify those high-risk patients with underlying CAD who will derive long-term benefit from a change in the perioperative management. However, nowadays advantages in anesthesia, use of beta blockers, statins, postoperative analgesia, and surgical techniques have contributed to a reduced rate of major cardiac complications. Low risk patients on the other hand need not undergo a preoperative cardiac assessment as it adds to their cost and also can lead to an undue delay in performing the relevant surgery. Thus, one need to understand an easy, cost-effective and robust methodology to risk stratify patients prior to noncardiac major surgeries.
Cardiac Risk Indices and Recommended Approach
Numerous risk factors have been set forth by various researchers and organizations to risk stratify patients planned for noncardiac surgeries. To estimate the cardiac risk, patients are risk stratified by the following factors:
Clinical predictors
Functional capacity predictors
Surgical risk predictors
Disease specific predictors.
Most of the centers worldwide follow the 2007 guidelines of American Heart Association and American College of Cardiology (AHA/ACC) to assess perioperative risk[6] .
Clinical predictors or risk factors
Patient related factors
High-risk factors include symptomatic CAD, advanced congestive cardiac failure (CCF), major valvular abnormalities, and arrhythmias.[7,8]
Cardiac related factors
Preoperative electrocardiogram (ECG) is recommended in all patients with history and physical findings suggesting heart disease. Men in the age group of 40-45 years, women over 55 years, patients with systemic conditions that may be associated with unrecognized cardiac abnormality, patients on cardiotoxic drugs and patients at risk for major electrolyte abnormalities are in need for further cardiac investigation.
Based on the presence or absence of cardiac diseases, patients can be divided into major, intermediate and minor predictors as described by Goldman[9]. This multifactorial index (used for cardiac risk assessment is known as Goldman index or “cardiac risk index.” It predicts the life-threatening cardiac complications or perioperative cardiac death based on the presence of preoperative risk factors. In this, probability of complications is shown on a logarithmic scale. The risks of major complications, defined as pulmonary edema, arrhythmic cardiac arrest, myocardial infarction, and death from cardiac causes were calculated by multiplying the prior odds of complications by the likelihood ratio for each class. The classes are defined as follows: Class I, 0-5 points on the index; Class II, 6-12 points; Class III, 13-25 points; and Class IV, 26 or more points (Table 1).
Table 1.
Goldman index or “cardiac risk index”
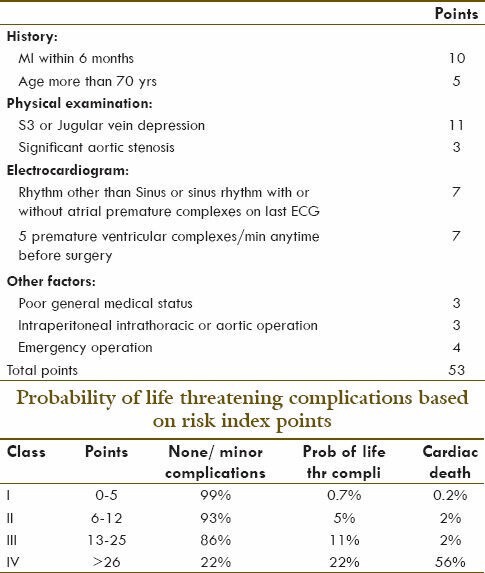
-
Major predictors or risk factors are markers of unstable CAD. There are five major risk factors:
- Recent MI (less than 6 weeks),
- Unstable angina (UA) (Class III-IV),
- Ischemia post MI,
- Ischemia and CCF,
- Malignant arrhythmias.
In patients with one or more of these major risk factors, the perioperative risk increases five-fold due to sympathetic stimulation and hypercoagulability.[3] These patients require an extensive preoperative cardiac evaluation with stress testing, echocardiography, nuclear testing to determine the severity and extent of CAD. Of these tests, MPI indicates a high negative predictive value for perioperative cardiac events when it is reported normal.
-
Intermediate predictors include:
- Previous MI,
- Stable angina (Class I-II),
- Renal insufficiency and diabetes mellitus,
- Low left ventricular (LV) ejection fraction (less than 35%),
- Compensated heart failure.
A few factors which are still considered to be controversial are age more than 70 years, hypertension and left ventricular hypertrophy (LVH). They either fall in intermediate or minor risk category. However, all patients in intermediate-risk category also need further evaluation by stress MPI or stress echocardiography before proceeding for surgery.[10]
-
Minor predictors are recognized markers of an increased probability of CAD and they include:
- Family history of CAD,
- Uncontrolled hypertension,
- Hypercholesterolemia,
- Smoking,
- Baseline ECG abnormalities (LVH, left bundle branch block [LBBB], arrhythmia),
- Post MI more than 3 months, asymptomatic without treatment,
- Post coronary artery bypass grafting (CABG)/percutaneous coronary angioplasty more than 3 months and less than 6 years, asymptomatic without treatment. In patients with minor predictors or low risk, further preoperative cardiac testing is not recommended as it may not alter the management of patients post major noncardiac surgeries.
Functional capacity predictors
Functional capacity of a patient can be estimated from treadmill exercise or from the ability to perform daily activities.[11] This measure is shown to be reliable for perioperative and long-term prediction of cardiac events. Patients with a low functional capacity of less than 4 metabolic equivalents (METS), marked stress-induced ST-segment changes or angina at low workloads, are markers for high-risk and need further investigations to evaluate cardiac risk.
Surgical risk predictors
The surgical risk factors are dependent on certain factors, like the type of surgery, timing of surgery and the degree of hemodynamic stress that can happen during the procedure. The reported rate of cardiac death or nonfatal myocardial infarction (MI) is more than 5 percent in high-risk procedures, between 1 and 5 percent in intermediate-risk procedures, and less than 1 percent in low-risk procedures. Institutional and/or individual surgeon experience with the procedure may increase or lower the risk. Emergency surgery is associated with particularly high risk, as cardiac complications are two to five times more likely than with elective procedures.[12] The timing of surgery also plays a role and affects the patient's risk of perioperative cardiac complications. Institutional and/or individual surgeon experience with the procedure may increase or lower the risk. Emergency surgery is associated with particularly high risk, as cardiac complications are two to five times more likely than with elective procedures.
The timing of surgery also plays a role and affects the patient's risk of perioperative cardiac complications. Institutional and/or individual surgeon experience with the procedure may increase or lower the risk. Emergency surgery is associated with particularly high risk, as cardiac complications are two to five times more likely than with elective procedures.
There can be significant hemodynamic abnormalities like variations in heart rate, blood pressure, vascular volume, pain, bleeding etc., intraoperatively. Thus, one need to take into account the type of procedure that is planned, the total time including the amount of blood loss that may occur during the surgery. High-risk procedures include major emergency surgery particularly in elderly patients, major and peripheral vascular surgery, and other prolonged procedures. Intermediate-risk procedures include carotid endarterectomy, head and neck procedures, intraperitoneal and intrathoracic, orthopedic, and prostate surgeries. Low-risk procedures include endoscopic, superficial procedures, cataract, and breast surgery.
Disease specific predictors
Associated cardiovascular diseases like CAD, hypertension, arrthymias, CCF, peripheral vascular disease, valvular heart diseases like aortic stenosis will need thorough evaluation and risk assessment. Aortic stenosis is the only valvular disease predictive of death, and it is included in the Cardiac Risk Index and modified Cardiac Risk Index.
Assessment of Coronary Artery Disease
Many algorithms have been proposed combining clinical risk indices, exercise treadmill testing, ECG, nuclear techniques such as radionuclide ventriculography, MPI, and coronary angiography. Myocardial perfusion abnormalities in MI or UA precede ECG and enzyme changes. which is explicitly described in the ‘ischemic cascade’.
Understanding ischemic cascade and perioperative myocardial infarction
Sequence of events in ischemic cascade is briefly described [Figure 1]. In 66% of patients, silent ischemic changes occur immediately after surgery, during recovery from anesthesia and coincide with postoperative physiological and anesthesia rebound phenomena such as tachycardia; hypertension, and raised sympathetic tone.[8] These changes happen when epicardial coronary flow is interrupted leading to an imbalance between myocardial oxygen supply and demand, myocardial hypoperfusion, regional ventricular dysfunction and ST-segment changes on the ECG and finally angina pectoris, if the ischemia reaches a clinical threshold. All these changes occur in quick succession; hence, the term - ischemic cascade has been described. Acute coronary syndrome (ACS) is typically initiated with acute plaque rupture and subsequent intracoronary thrombus development. These events lead to reduced blood flow, which, when severe, results in myocardial ischemia. If this condition is prolonged, myocardial necrosis occurs.
Figure 1.
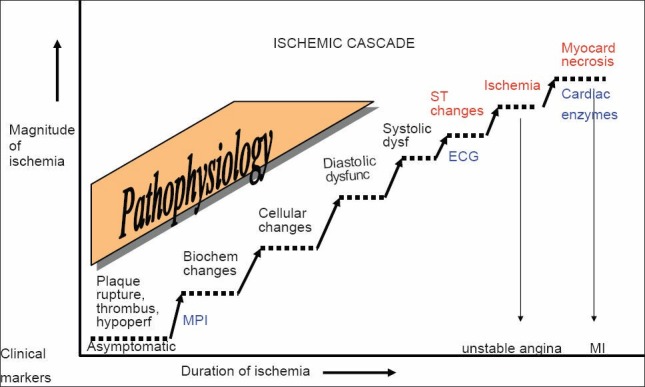
Depicts the myocardial ischemic cascade and stepwise changes, which occur at molecular and tissue level. Acute coronary syndrome is typically initiated with acute plaque rupture and subsequent intracoronary thrombus development. These changes happen when epicardial coronary flow is interrupted and leads to an imbalance between myocardial oxygen supply and demand, reduced myocardial perfusion, regional ventricular dysfunction, and ST-segment changes and finally angina pectoris, which when severe, results in myocardial ischemia. If this condition is prolonged, myocardial necrosis occurs. Therefore diagnostic techniques that can identify earlier components of this pathway, such as myocardial perfusion imaging have the potential for allowing earlier identification. The characteristic electrocardiogram changes in repolarization are noted 20-30 s after a coronary occlusion. Reduction in myocardial blood flow is the first detectable event in the ischemic cascade, while clinical anginal symptoms are the last in the temporal sequence of the ischemic cascade. Myocardial troponin usually rises by 8-24 h after surgery
Usually angina may be absent postoperatively due to raised catecholamine levels and hemodynamic instability making diagnosis more difficult unless there is a high degree of clinical suspicion.
Stress SPECT / PET MPI in Cardiac Assessment
Techniques
Cardiac nuclear medicine has evolved in the last 3 to 4 decades from 201Thallium (201Tl) chloride planar perfusion imaging to 99mTechnetium (99mTc) SestaMIBI/Tetrofosmin single photon emission computed tomography,[13] and positron emission tomography (PET) cardiac perfusion imaging using tracers such as 82Rubidium (82Rb), 13Ammonia adding sensitivity and image resolution.
Since most preoperative patients are either too sick or ill motivated to perform the desired level of exercise on a treadmill, the clinical experience of MPI is heavily leaned towards pharmacological stress with either vasodilators (adenosine, dipyridamole) or with dobutamine a beta agonist, as a method of stress during MPI. 99mTc based radiopharmaceuticals (SestaMIBI and Tetrofosmin) have largely replaced 201T1 chloride as the pharmaceutical of choice for MPI due to its favorable imaging characteristics, availability and possibility of gated images acquisition.
Myocardial perfusion imaging indications
Initial diagnosis or prognostic assessment of CAD in patients with intermediate pretest probability of disease, abnormal baseline ECG (e.g. LVH, LBBB) or inability to exercise.
Evaluation of patients following a change in clinical status (e.g. ACS) with abnormal baseline ECG or inability to exercise.
Prognostic assessment of LBBB patients undergoing initial evaluation for suspected or proven CAD.
Patients with intermediate/minor clinical risk predictors, poor functional capacity who require high-risk noncardiac surgery, when used in conjunction with pharmacologic stress.
Assessment of patients with intermediate clinical risk predictors, abnormal baseline ECGs, and moderate or excellent functional capacity (more than 4 METS) who require high-risk noncardiac surgery.
Patients with LV ejection fraction of less than 35%, severe diastolic dysfunction, with CCF, and in patients with dyspnea of unknown etiology.
Thus, MPI is indicated if at least two of the following conditions are met: The patient has intermediate clinical predictors, has poor functional capacity, or is undergoing a high-risk procedure. Dipyridamole[13] or adenosine vasodilator pharmacological stress MPI is the most preferred form of testing in this group of patients and is highly sensitive.
Patterns of myocardial insult in peri/postoperative period that can be identified by myocardial perfusion imaging
Unstable angina
Unstable angina is difficult to diagnose clinically as it produces no ST-segment change and there is no sufficient myocardial damage for release of cardiac biomarkers like troponins or CK-MB.
Non-ST-segment elevation myocardial infarction
There is no ST-segment change but there is myocardial necrosis producing an elevation of biomarkers but unreliable in peri- and post-operative setting.
ST-segment elevation myocardial infarction
This presentation of MI is the most classical. Here there is clear demonstration of ECG changes in the form of an ST segment elevation. Similarly as myocardial necrosis sets in, these patients reveal elevated toponin or CK -MB levels. However, if the patient had prior MI it may be difficult to interpret the fresh ECG changes in the background of pre existing ones to interpret fresh ECG changes.
MPI is useful across the entire spectrum of patients with ACS, from those with suspected ACS but without diagnostic initial ECG changes, to the now well-defined syndromes of UA/NSTEMI and STEMI. MPI techniques have a unique role, strongly supported by an evidence base, and supply simultaneous information on stress and rest perfusion as well as LV function.
Risk assessment by stress myocardial perfusion imaging single photon emission computed tomography
Of the various types of MPI, dipyridamole- 201T1 imaging has been studied most often. A negative scan predicts very low risk (likelihood ratio, 0.12; posttest probability, 1%), and a positive scan indicates increased risk (likelihood ratio, 3.02; posttest probability, 23%). There is a strong evidence that this imaging technique has a good predictive value for determining a low or high operative risk when applied to a selected population of clinical intermediate-risk, vascular patients. However, it has no real screening value, when applied to a large unselected vascular or nonvascular population, or among patients already classified clinically as low- or high-risk candidates for surgery.[14,15,16,17,18,19,20,21]
Independent scintigraphic predictors to be considered during interpretation of a positive study include; (a) number of reversible perfusion defects which act as a measure of ischemic extent, (b) magnitude of reversible perfusion defects which serves as a measure of ischemic severity, (c) heart rate achieved during stress (d) regional wall motion abnormalities. Evidence of residual ischemia after an MI is found to be a strong predictor of both fatal and nonfatal cardiac events. Patients with no scintigraphic evidence of ischemia have a very low cardiac events (less than 5%) while approximately 40-50% of patients with inducible ischemia develop subsequent cardiac events. MPI also adds incremental value to LV ejection fraction, LVEF. There is also a significant correlation of ischemic events with the magnitude of ischemia in perioperative period.[22]
Certain other scintigraphic variables that indicate underlying LV dysfunction include increased lung uptake of Thallium/ sestamibi or tetrofosmin, transient ischemic LV dilatation (TID) and marked ST-segment changes associated with angina. Magnitude of jeopardized myocardium (as shown by reversible perfusion defects and TID) has an exponential relationship to the likelihood of cardiac events. Patients with no stress perfusion defects are rated as low risk [Figure 2] while patients with or without infarcts with reversible ischemia [Figures 3 and 4] are categorized as intermediate to high-risk depending on the extent and size of perfusion defects. Quantification of their delayed redistribution of 201Tl chloride at 4-24 h is more predictive of cardiac death or MI than simple dichotomous interpretation in positive/negative results.
Figure 2.
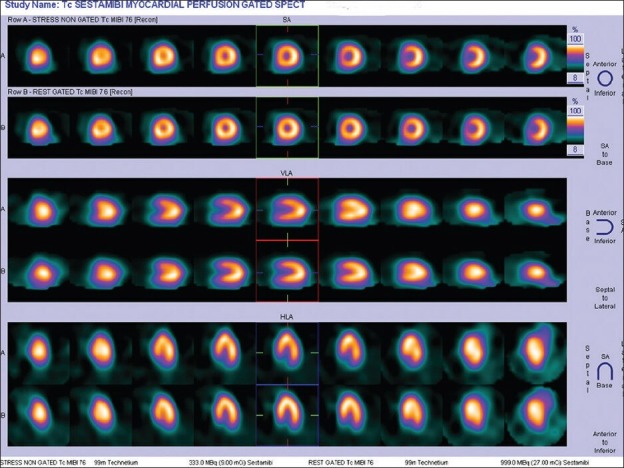
Normal stress myocardial perfusion scan in a 48-year-old lady planned for total hip replacement surgery – low risk for cardiac events
Figure 3.
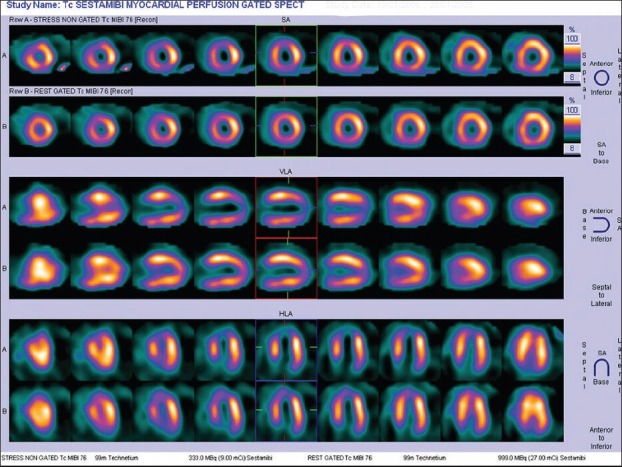
Pharmacological stress myocardial perfusion imaging in a 56-year-old male with peripheral vascular disease showing a small infarct involving apex with peri-infarct reversible ischemia. There is associated reversible ischemia in parts of anterior and inferior segments of left ventricular (left anterior descending and right coronary artery territory) -intermediate to high-risk for peri-and post-operative cardiac events if untreated prior to surgery
Figure 4.
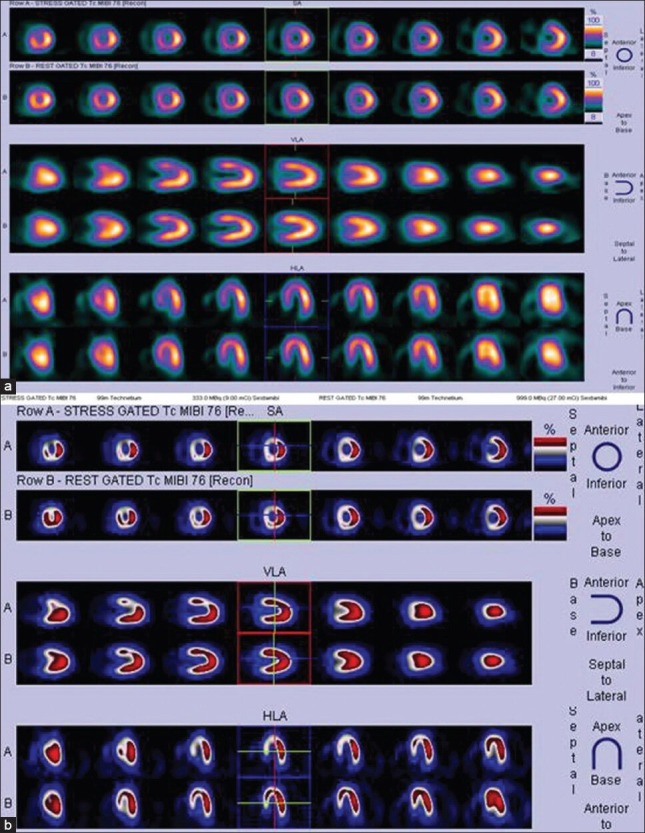
Stress and rest myocardial perfusion imaging images of a 32-year-old lady displayed in 2 different color scales (a and b). Patient presented with acute coronary syndrome. Images show reversible ischemia in anterior and septal segments of left ventricular myocardium (left anterior descending territory ischemia) -intermediate to high-risk for peri-and post-operative cardiac events if left untreated prior to surgery
Apart from the cardiac risk stratification, assessment of myocardial viability with 99mTc labeled SestaMIBI radiopharmaceutical is particularly important in patients with impaired LV function consequent to CAD, and the potential of revascularization preoperatively can be estimated. Studies have shown excellent correlation of 99mTc labeled SestaMIBI MPI with postoperative cardiac events, both perioperatively, within 30 days and at long-term follow-up.[5,22] Single photon emission computed tomography (SPECT) has a high sensitivity (90-94%) in multivessel coronary disease but limited sensitivity (60-76%) for detecting significant single-vessel disease and nonobstructive CAD.
On the literature review of studies conducted by multiple investigators, only 1.2% of normal MPI scan patients suffered coronary events postsurgery compared with 15.6% patients with reversible ischemia. Only 5% of surgical cases got cancelled due to highly abnormal MPI. Patients with normal MPI had a very low frequency of perioperative cardiac events (1.2%), while the event rate was 5% in patients with fixed defects and increased to 15% in patients showing reversible defects in MPI.[14,15,16,17,18,19,20,21]
Risk assessment by cardiac positron emission tomography imaging
With the increased clinical use of PET and PET/CT in stress MPI, data documenting its incremental prognostic value are beginning to emerge.
Positron emission tomography (like SPECT) uncovers the coronary territory supplied by the most severe stenosis. This is because the coronary vasodilator reserve is often abnormal in patients with CAD, even in territories supplied by noncritical anatomical stenosis[23,24] thereby reducing the heterogeneity of flow between “normal” and “abnormal” zones and limiting the ability to delineate the presence of multivessel CAD. An advantage of ECG-gated PET is its distinct ability to assess LV function at rest and during peak stress (as opposed to poststress with gated SPECT).
The prognostic value of dipyridamole stress 82Rb PET was investigated in 367 patients with follow-up for 3.1 + 0.9 years.[25] As has been previously described with SPECT, increased extent and severity of perfusion defects with stress PET was associated with increased frequency of adverse events. Importantly, the hard event rate (i.e. MI or cardiac death) in patients with normal stress PET was 0.4% per year.
However, this study was limited by the occurrence of only 17 hard events.
Preliminary data by Di Carli et al. in 1602 consecutive patients undergoing rest-stress 82Rb myocardial perfusion PET/CT also suggest that this technique provides incremental value to clinical variables in predicting overall survival.[26] Unlike previous studies, this study was adequately powered by the occurrence of 113 deaths (7% of the study cohort) during a median follow-up period of 511 days. In keeping with previous studies, increase in the extent and severity of stress perfusion defects translated into proportional increase in predicted mortality. In addition, preliminary data from a study be Dorbala et al. in 1274 consecutive patients also confirm the incremental prognostic value of LVEF over stress perfusion imaging.[27]
There is growing and consistent evidence that coronary hemodynamic quantitation by PET allows better definition of the extent of anatomic CAD. For example, Yoshinaga et al.[25] showed good agreement between SPECT defects and PET measures of coronary vasodilator reserve in only 16 of 58 (28%) territories supplied by vessels with 50% stenoses, as assessed by quantitative angiography. The remaining 42 of 58 (72%) territories with angiographic stenosis showed no regional perfusion defects by SPECT but an abnormal vasodilator reserve by PET. There is no head to head comparison until date of gated SPECT MPI and PET in intermediate cardiac risk patients undergoing noncardiac surgeries.
Correlative Imaging: Cardiac CT
Cardiac CT is rapidly evolving as a noninvasive imaging modality that allows the comprehensive assessment of cardiovascular anatomy, including the coronary arteries.
There are various indications of cardiac CT like assessing the calcium scoring, CABG graft and stent patency, identifying coronary anomalies and evaluation of cardiac masses.
Complimentary to MPI, cardiac CT uses coronary angiography for the detection of coronary artery stenosis in patients with known or suspected CAD. Evaluating coronary artery stenosis in patients with extensive coronary artery calcifications may be difficult and represents a major limiting factor. Reconstruction techniques in cardiac CT also have inherent disadvantages and needs care to avoid unwarranted artifacts. Calcified structures on CT tend to produce “blooming” and beam hardening artifacts that may lead to interpretational problems.
Salient key recommendations from American College of Cardiology/American Heart Association guidelines for cardiac risk assessment prior to noncardiac surgeries
The ACC/AHA 2007 perioperative guidelines[6] include an evidence-based algorithm for determining, which patients are candidates for cardiac testing as part of preoperative cardiac assessment. A stepwise approach takes into account the urgency of the surgery, the presence or absence of active cardiac conditions, the type of surgery and its risk level, and the patient's functional capacity and cardiac risk factors.
The following are among the algorithm's key recommendations
Patients requiring urgent noncardiac surgery should proceed to the operating room with perioperative surveillance (Class I, Level C).
Patients with active cardiac conditions who are undergoing non urgent surgery should be evaluated and treated per ACC/AHA guidelines before proceeding for surgery (Class I, Level B).
Patients scheduled for a low-risk procedure can proceed to surgery without testing (Class I, Level B).
Patients scheduled for intermediate-risk surgery or vascular surgery are to be assessed by functional capacity and clinical risk factors. Proceeding with planned surgery is appropriate in patients with good functional capacity (Class IIa, Level B). In patients with poor or unknown functional capacity undergoing vascular surgery who have three or more clinical risk factors, testing should be considered if the results would change management (Class IIa, Level B).
Patients with one or more clinical risk factors undergoing intermediate-risk surgery and those with fewer than three clinical risk factors undergoing vascular surgery may proceed to planned surgery with control of heart rate to diminish the stress response perioperatively (Class IIa, Level B), or they may undergo noninvasive testing, but only if the results would change management (Class IIb, Level B).
Patients undergoing intermediate-risk or vascular surgery who have poor or unknown functional capacity but no clinical risk factors may proceed to surgery without testing (Class I, Level B).
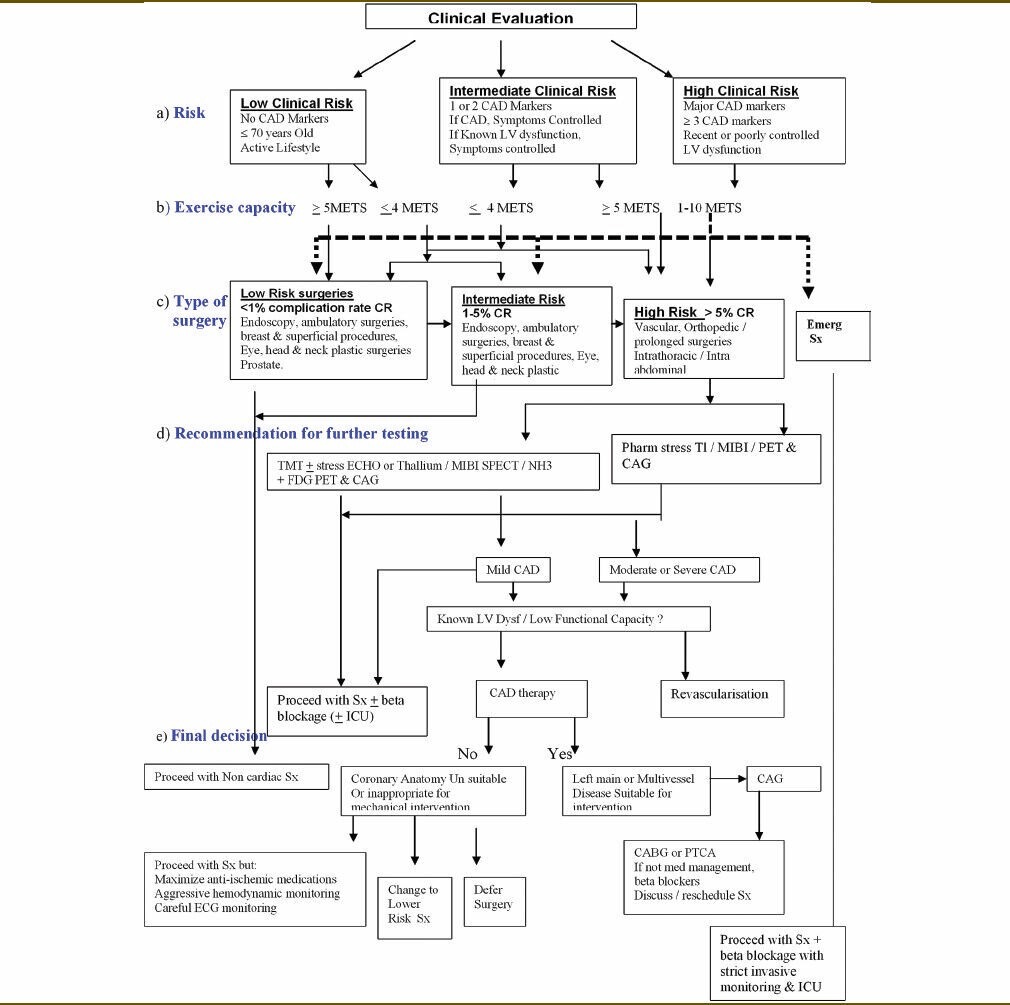
Flow chart [Table 2] depicting an easy effective preoperative cardiac risk assessment algorithm has been presented taking variables such as cardiac risk, functional capacity, type of surgery to recommend further cardiac testing in low-, intermediate-, and high-risk patients along with the final decision to proceed or not to proceed with surgery and precautions to be taken during surgery.
Table 2.
A flow chart depicting an easy effective preoperative cardiac risk assessment algorithm taking variables such as cardiac risk, functional capacity, type of surgery to recommend further cardiac testing in low-, intermediate-, and high-risk patients along with the final decision to proceed or not to proceed with surgery and precautions to be taken during surgery
Conclusion
Myocardial perfusion imaging has emerged as a key guide for major medical decisions involving patient with suspected and known CAD in preoperative situations. Presence of perfusion defects is a powerful long-term predictor of major ischemic events that enhances the prediction provided by clinical, exercise testing and coronary angiographic data. In view of its prognostic significance, extent of reversible perfusion defects might provide original information about improving prognosis by coronary revascularization. A normal preoperative MPI incurs both a low perioperative risk and a low long-term risk (2 years) even in groups with high clinical risk. Coronary revascularization prior to noncardiac surgery is generally indicated only in unstable patients and in patients with left main disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Maddox TM. Preoperative cardiovascular evaluation for noncardiac surgery. Mt Sinai J Med. 2005;72:185–92. [PubMed] [Google Scholar]
- 2.Rao TL, Jacobs KH, El-Etr AA. Reinfarction following anesthesia in patients with myocardial infarction. Anesthesiology. 1983;59:499–505. doi: 10.1097/00000542-198312000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Chassot PG, Delabays A, Spahn DR. Preoperative evaluation of patients with, or at risk of, coronary artery disease undergoing non-cardiac surgery. Br J Anaesth. 2002;89:747–59. [PubMed] [Google Scholar]
- 4.Ashton CM, Petersen NJ, Wray NP, Kiefe CI, Dunn JK, Wu L, et al. The incidence of perioperative myocardial infarction in men undergoing noncardiac surgery. Ann Intern Med. 1993;118:504–10. doi: 10.7326/0003-4819-118-7-199304010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Patel AD, Abo-Auda WS, Davis JM, Zoghbi GJ, Deierhoi MH, Heo J, et al. Prognostic value of myocardial perfusion imaging in predicting outcome after renal transplantation. Am J Cardiol. 2003;92:146–51. doi: 10.1016/s0002-9149(03)00529-0. [DOI] [PubMed] [Google Scholar]
- 6.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007;50:e159–241. doi: 10.1016/j.jacc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Palda VA, Detsky AS. Perioperative assessment and management of risk from coronary artery disease. Ann Intern Med. 1997;127:313–28. doi: 10.7326/0003-4819-127-4-199708150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Devereaux PJ, Goldman L, Yusuf S, Gilbert K, Leslie K, Guyatt GH. Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: A review. CMAJ. 2005;173:779–88. doi: 10.1503/cmaj.050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–50. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 10.Sutaria N, Mayet J. Preoperative screening for coronary disease: Who needs it and how do you do it. Heart? 2007;93:1497–9. doi: 10.1136/hrt.2006.108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single photon emission computed tomography: Report of3,400 patients from a single centre. J Am Coll Cardiol. 1997;30:641–8. doi: 10.1016/s0735-1097(97)00217-9. [DOI] [PubMed] [Google Scholar]
- 12.Mangano DT. Perioperative cardiac morbidity. Anesthesiology, 1990;72:153–84. doi: 10.1097/00000542-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Stratmann HG, Younis LT, Wittry MD. Dipyridamole techentium99m sestamibi myocardial tomography for preoperative cardiac risk stratification before major or minor non vascular surgery. Am Heart J. 1996;132:536. doi: 10.1016/s0002-8703(96)90235-5. [DOI] [PubMed] [Google Scholar]
- 14.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–8. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 15.Baron JF, Mundler O, Bertrand M, Vicaut E, Barré E, Godet G, et al. Dipyridamole-thallium scintigraphy and gated radionuclide angiography to assess cardiac risk before abdominal aortic surgery. N Engl J Med. 1994;330:663–9. doi: 10.1056/NEJM199403103301002. [DOI] [PubMed] [Google Scholar]
- 16.Eagle KA, Coley CM, Newell JB, Brewster DC, Darling RC, Strauss HW, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med. 1989;110:859–66. doi: 10.7326/0003-4819-110-11-859. [DOI] [PubMed] [Google Scholar]
- 17.Brown KA, Rowen M. Extent of jeopardized viable myocardium determined by myocardial perfusion imaging best predicts perioperative cardiac events in patients undergoing noncardiac surgery. J Am Coll Cardiol. 1993;21:325–30. doi: 10.1016/0735-1097(93)90670-v. [DOI] [PubMed] [Google Scholar]
- 18.Hendel RC, Whitfield SS, Villegas BJ, Cutler BS, Leppo JA. Prediction of late cardiac events by dipyridamole thallium imaging in patients undergoing elective vascular surgery. Am J Cardiol. 1992;70:1243–9. doi: 10.1016/0002-9149(92)90756-o. [DOI] [PubMed] [Google Scholar]
- 19.Lette J, Waters D, Cerino M, Picard M, Champagne P, Lapointe J. Preoperative coronary artery disease risk stratification based on dipyridamole imaging and a simple three-step, three-segment model for patients undergoing noncardiac vascular surgery or major general surgery. Am J Cardiol. 1992;69:1553–8. doi: 10.1016/0002-9149(92)90702-z. [DOI] [PubMed] [Google Scholar]
- 20.Bry JD, Belkin M, O'Donnell TF, Jr, Mackey WC, Udelson JE, Schmid CH, et al. An assessment of the positive predictive value and cost-effectiveness of dipyridamole myocardial scintigraphy in patients undergoing vascular surgery. J Vasc Surg. 1994;19:112–21. doi: 10.1016/s0741-5214(94)70126-1. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein H, Steingart R. Myocardial perfusion imaging for preoperative risk stratification. J Nucl Med. 2011;52:750–60. doi: 10.2967/jnumed.110.076158. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MC, Siewers AE, Dickens JD, Jr, Hill T, Muller JE. Perioperative and long-term prognostic value of dipyridamole Tc-99m sestamibi myocardial tomography in patients evaluated for elective vascular surgery. J Nucl Cardiol. 2003;10:464–72. doi: 10.1016/s1071-3581(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 23.Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331:222–7. doi: 10.1056/NEJM199407283310402. [DOI] [PubMed] [Google Scholar]
- 24.Yoshinaga K, Katoh C, Noriyasu K, Iwado Y, Furuyama H, Ito Y, et al. Reduction of coronary flow reserve in areas with and without ischemia on stress perfusion imaging in patients with coronary artery disease: A study using oxygen 15-labeled water PET. J Nucl Cardiol. 2003;10:275–83. doi: 10.1016/s1071-3581(02)43243-6. [DOI] [PubMed] [Google Scholar]
- 25.Yoshinaga K, Chow BJ, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029–39. doi: 10.1016/j.jacc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48:783–93. doi: 10.2967/jnumed.106.032789. [DOI] [PubMed] [Google Scholar]
- 27.Dorbala S, Hachamovitch R, Kwong R, Curillova Z, Di Carli MF. Incremental prognostic value of left ventricular ejection fraction assessment over myocardial perfusion imaging: A rubidium-82 PET/CT study. J Am Coll Cardiol. 2007;49:109A. [Google Scholar]


