Abstract
Objective
Reactive oxygen species (ROS) act as signaling molecules during angiogenesis, however, the mechanisms used for such signaling events remain unclear. Stromal cell-derived factor-1α (SDF-1α) is one of the most potent angiogenic chemokines. Here we examined the role of ROS in the regulation of SDF-1α-dependent angiogenesis.
Approach and results
Bovine aortic endothelial cells (BAECs) were treated with SDF-1α and intracellular ROS generation was monitored. SDF-1α treatment induced BAEC migration and ROS generation, with the majority of ROS generated by BAECs at the leading edge of the migratory cells. Antioxidants and NADPH oxidase (NOX) inhibitors blocked SDF-1α-induced endothelial migration. Furthermore, knockdown of either NOX5 or p22phox (a requisite subunit for NOX1/2/4 activation) significantly impaired endothelial motility and tube formation, suggesting that multiple NOXs regulate SDF-1α-dependent angiogenesis. Our previous study demonstrated that JNK3 activity is essential for SDF-1α-dependent angiogenesis. Here, we identified that NOX5 is the dominant NOX required for SDF-1α-induced JNK3 activation and that NOX5 and MKP7 (the JNK3 phosphatase) associate with one another but decrease this interaction upon SDF-1α treatment. Furthermore, MKP7 activity was inhibited by SDF-1α and this inhibition was relieved by NOX5 knockdown, indicating that NOX5 promotes JNK3 activation by blocking MKP7 activity.
Conclusions
We conclude that NOX is required for SDF-1α signaling and that intracellular redox balance is critical for SDF-1α-induced endothelial migration and angiogenesis.
Keywords: Reactive oxygen species, NADPH oxidase, SDF-1α, migration, angiogenesis
INTRODUCTION
Reactive oxygen species (ROS) are generated not only as by-products of mitochondrial metabolism, but also by a variety of cellular enzyme systems including NADPH oxidase (NOX), uncoupled endothelial nitric oxide synthase (eNOS), xanthine oxidase and arachidonic acid metabolizing enzymes. When cellular production of ROS exceeds the antioxidant capacity of cardiovascular cells, proteins, lipids and nucleic acids become damaged and may eventually contribute to the development of cardiovascular diseases such as atherosclerosis, hypertension, diabetic cardiovascular complications and ischemic-reperfusion injury. Conversely, low concentrations of ROS play a critical role in regulating cardiovascular functions such as angiogenesis and tissue repair.1–3 ROS are required for VEGF-induced endothelial migration, proliferation and tube formation.4, 5 During ischemia and reperfusion, ROS generation promotes capillary tube formation in human microvascular endothelial cells6 and the heart7, whereas inhibiting ROS through treatment with antioxidants or superoxide dismutases blocks vascularization and growth of tumors.8, 9 The precise molecular mechanisms, however, by which ROS mediate angiogenic responses are incompletely understood.
NOX is an important enzymatic source of ROS. There are seven Nox genes identified in mammalian organisms - Nox1-5 and Duox (Dual Oxidase) 1–2. NOXs are expressed in endothelial cells and other cardiovascular cells and regulate various functions such as cell survival, growth, apoptosis, differentiation, angiogenesis and contractility.10 The NOX enzymes are heteroprotein complexes (except NOX5) with different regulatory mechanisms, tissue distribution and subcellular localization and downstream targets. A membrane regulatory subunit, p22phox, is associated with NOXs 1, 2 and 4, and is required for their activity.10 NOXs 1 and 2 share a common overall structure with a very short cytoplasmic N-terminus, that is required for activation11–13, and six transmembrane domains.14, 15 In contrast, NOX4 is constitutively active and is regulated by gene expression.16 Interestingly, unlike other NOXs, NOX5 possesses a longer cytoplasmic N-terminus containing Ca2+-binding motifs, resulting in its activation by Ca2+ elevation.17 ROS generated by NOXs 1, 2 and 4 mediate angiogenic effects in response to angiogenic factors such as VEGF and angiotensin II.10 However, the exact function of these NOXs and the underlying mechanisms by which they mediate their actions remain unknown, partly due to inconsistent published observations and context-dependent efficacy.
Stromal cell-derived factor 1α (SDF-1α, also called CXCL12) is one of the most potent angiogenic CXC chemokines. Our previous studies have shown that SDF-1α requires MKP7 S-nitrosylation to activate JNK3 and promote endothelial migration and angiogenesis.18 MKP7 belongs to a subgroup of protein tyrosine phosphatases (PTPs), which are widely recognized as targets of ROS that can be oxidized at redox-sensitive cysteine residues resulting in the inhibition of phosphatase activity following growth factor treatment.19–21 MKP7 possesses a critical cysteine in its catalytic pocket that is highly sensitive to oxidation due to its low pKa.22 Therefore, we hypothesized that NOX-generated ROS may oxidize and inhibit MKP7, thereby regulating SDF-1α-dependent JNK3 activity and angiogenesis. By performing a series of biochemical and cell biological assays, we have developed strong evidence that suggests that NOXs, including NOX5, are novel positive regulators of SDF-1α-induced endothelial migration and angiogenesis.
MATERIAL AND METHODS
Materials and Methods are available in the online-only supplement.
RESULTS
SDF-1α induces transient generation of ROS in BAECs
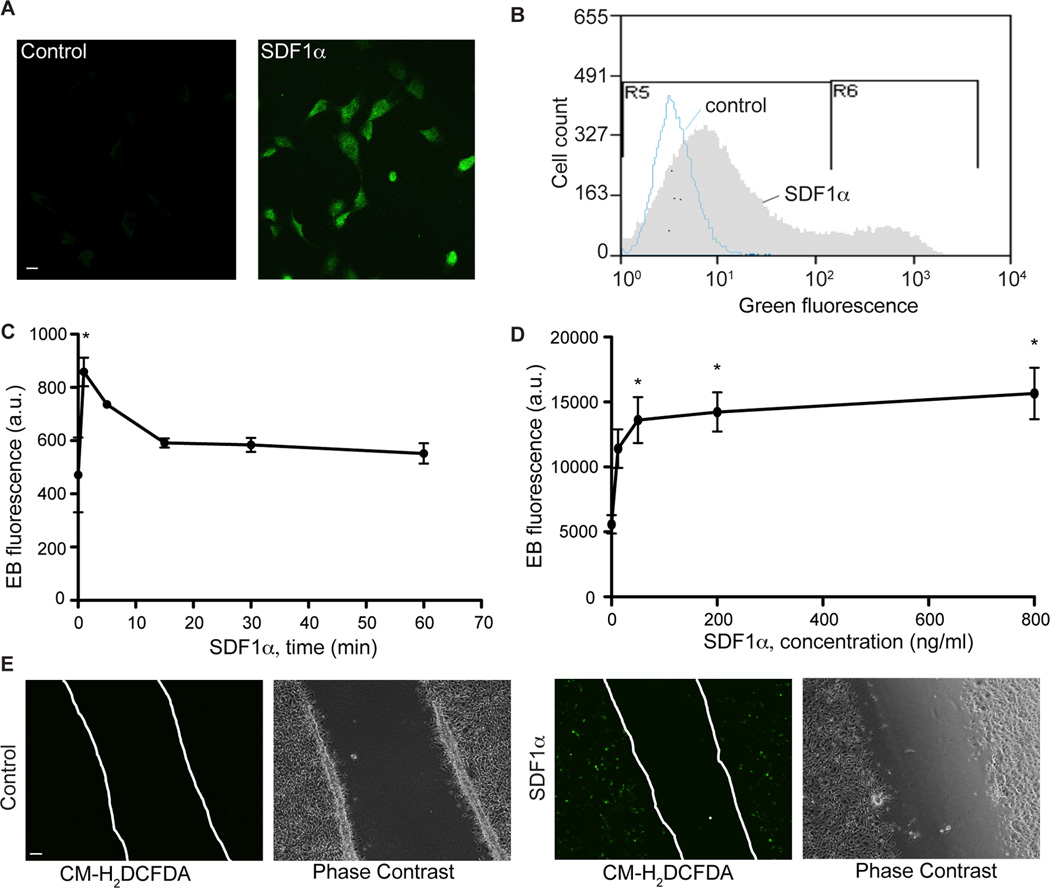
Our previous studies demonstrated that MKP7 S-nitrosylation and subsequent inhibition is required for SDF-1α-induced endothelial migration and angiogenesis.18 MKP7 activity can also be modified and inhibited through oxidation of a critical redox sensitive cysteine in its catalytic pocket.22 NOXs 1, 2 and 4 are the major source of ROS in endothelial cells.23. In addition, they regulate angiogenesis in response to growth factors such as VEGF and angiotensin II.10 However, the exact function of these NOXs remain elusive and whether NOX-generated ROS regulate SDF-1α-dependent signaling is unknown. We hypothesized that NOX-generated ROS may be another mechanism by which MKP7 activity is inhibited during SDF-1α-dependent angiogenesis. To investigate the role of NOX-generated ROS in SDF-1α-dependent angiogenesis, we first examined ROS generation in BAECs following SDF-1α treatment. The fluorescent dyes CM-H2DCFDA and dihydroethidium (DHE) were used to monitor and determine the level of hydrogen peroxide (H2O2) and superoxide (O2·−) production, respectively, in BAECs following treatment with SDF-1α. When cells were treated with SDF-1α for 5 minutes, an increase in fluorescent DCF signal was detected in BAECs by confocal microscopy (Figure 1A) and by flow cytometry (from 0.01% cells positive under control conditions to 15.45% cells in response to SDF-1α; Figure 1B & SIA). Moreover, this SDF-1α-induced DCF signal was inhibited by PEG-catalase, but not the inhibitors of nitric oxide synthase (NOS)- L-NAME and that of cyclooxygenase-diclofenac (Figure SIB, C, D and E), suggesting that this detected DCF signal is due to H2O2 generation, but not the formation of peroxynitrites or arachidonate metabolites. SDF-1α treatment of BAECs also resulted in O2·− generation, detected by the appearance of fluorescent ethidium generated from oxidation of DHE. SDF-1α-induced superoxide production rapidly increased ~2 fold, peaking at 5-minutes after the SDF-1α treatment began (Figure 1C). Moreover, the superoxide level was induced in a dose- dependent manner (Figure 1D). To determine whether ROS were generated during SDF-1α -induced endothelial cell migration, a wound scratch assay was performed to visualize the active migratory process of BAECs following SDF-1α treatment. Consistent with the observations above, SDF-1α treatment of BAECs resulted in generation of a fluorescent DCF signal that was most prominent at the leading edge of the BAECs where active migration takes place (Figure 1E). These data suggest that ROS are generated in response to SDF-1α treatment in BAECs and that this ROS generation correlates with the SDF-1α-dependent migratory response.
Figure 1.
ROS are generated following SDF-1α treatment. BAECs were treated with SDF-1α (100 ng/ml) for 5 minutes and ROS production was detected by CM-H2DCFDA (A-B) or DHE staining (C-D). A, Representative images of DCFDA-stained cells. Scale bar, 10 µm. B, Representative flow cytometry data obtained for H2O2 measurement that was detected as green fluorescence after CM-H2DCFDA staining. This is an overlay picture of two samples- the control (in blue) and 100 ng/ml SDF-1α-treated for 5 minutes (in gray). C–D, BAECs were treated with SDF-1α in 100 ng/ml for different time periods (C) or different concentrations for 5 minutes (D). O2·− production was quantified as ethidium bromide fluorescence. *, P<0.05, compared to control BAECs. n=3. E, Representative images of an endothelial cell monolayer with a scratch-wound. Cells were treated with SDF-1α (100 ng/ml) for 5 minutes and fixed for CM-H2DCFDA staining. Scale bar, 50 µm.
Antioxidants inhibit SDF-1α-induced JNK3 activation and endothelial migration
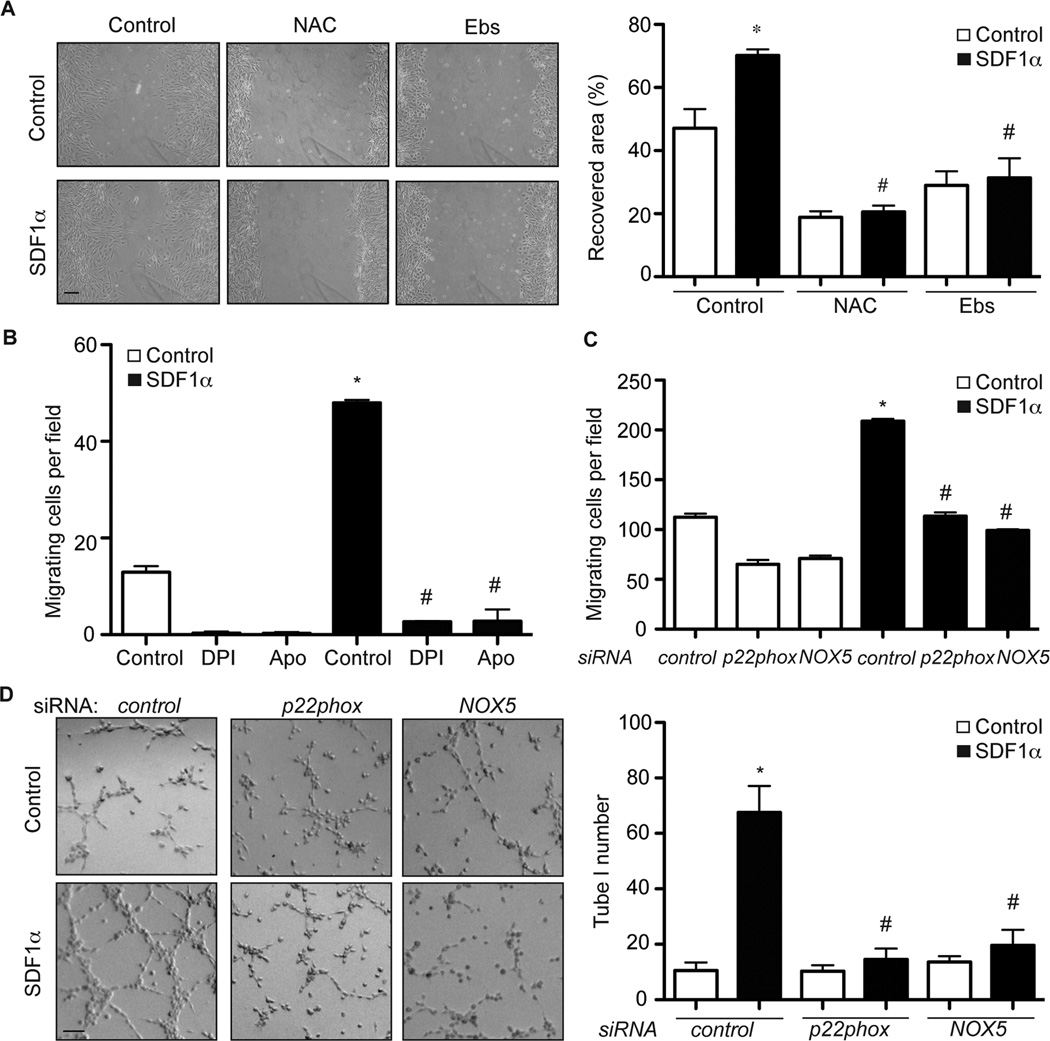
To determine whether the ROS generation seen at the leading edge of the migrating BAECs is necessary for SDF-1α-induced migration, the wound scratch assay was repeated in the presence of the two antioxidants NAC and ebselen. As shown in Figure 2A, SDF-1α-induced BAEC migration was significantly inhibited by pretreatment of cells with either NAC or ebselen, indicating that ROS generation is required for SDF-1α-induced cell migration.
Figure 2.
NOXs inhibit SDF-1α-induced migratory and angiogenic responses in BAECs. A, A wound healing assay was performed with BAECs. Cell monolayers were scratched, incubated with NAC (10 mM) or ebselen (Ebs, 40 µM) for 30 minutes and then treated with SDF-1α (100 ng/ml). 24 hours later, cells were fixed and images were taken. The level of cell migration into the scratch-wound was quantified as the recovered area. *, P<0.05, compared to control cells; #, P<0.01, compared to control cells that were treated with SDF-1α. n=3. B, Boyden chamber analysis of BAEC migration was performed using 100 ng/ml SDF-1α as the chemoattractant. *, P<0.001; compared to control cells without SDF-1α. #, P<0.001; compared to control cells with SDF-1α. n=3. C, Boyden chamber analysis of BAEC migration was performed using 100 ng/ml SDF-1α as the chemoattractant. BAECs were transfected with p22phox, NOX5 or control siRNA. *, P<0.01; compared to control cells without SDF-1α. #, P<0.01; compared to control cells with SDF-1α. n=3. D, In vitro Matrigel angiogenesis assays were performed with BAECs that were transfected with p22phox, NOX5 or control siRNAs. 100 ng/ml SDF-1α was used to induce tube formation. Images of formed tubes were taken and tube numbers were counted. *, P<0.001; compared to control cells without SDF-1α. #, P<0.05; compared to control cells with SDF-1α. Scale bar, 50 µm.
NOXs is required for SDF-1α-induced angiogenesis
Recent evidence indicates that NOXs are the major sources of ROS in endothelial cells.23 Given our observation that SDF-1α treatment of BAECs resulted in ROS generation that was associated with SDF-1α-induced cell migration, we next examined whether NOXs were also involved in these events. Using the Boyden chamber migration assay, we observed that SDF-1α treatment increased BAEC migration from 13±1 to 48±1 cells per field (Figure 2B), but that SDF-1α-induced migration could be completely inhibited by the two general NOX inhibitors DPI and apocynin (Figure 2B), indicating that NOX enzyme-dependent ROS generation is essential for SDF-1α-mediated BAEC migration.
Four different NOX enzymes (NOX1, 2, 4 and 5) are expressed in vascular endothelial cells such as BAECs (Figure SIIA).10 NOX 1, 2 and 4 have each been linked to angiogenic responses, however, the underlying molecular mechanisms behind these events are not completely understood. Furthermore, since previous experiments have been largely performed in rodent cells, which do not express NOX5, the role for NOX5 in angiogenesis has yet to be determined. Therefore, to determine which NOX was responsible for SDF-1α-induced angiogenesis, we first examined the effect of knockdown of NOX5 or p22phox, a crucial component of NOX1/2/4 active enzyme complexes10, on SDF-1α-induced endothelial migration in BAECs. p22phox or NOX5 siRNA specifically decreased the RNA and protein levels of p22phox or NOX5 respectively, but not other NOXs in BAECs (Figure SIIB, C). Similar to antioxidants or NOX inhibitors, knockdown of either NOX5 or p22phox decreased superoxide generation (Figure SIID). More excitingly, their knockdown blocked endothelial migration induced by SDF-1α (Figure 2C), suggesting that multiple NOXs, including NOX5, are involved in SDF-1α-induced endothelial migration. To assess the effect of NOX depletion on tube formation, BAECs were transfected with siRNA specific for either NOX5 or p22phox and the effect on SDF-1α-induced tube formation in Matrigel was analyzed. 72 hours following transfection with p22phox siRNA, NOX5 siRNA or control siRNA, BAECs cells were plated on Matrigel in medium containing 100 ng/ml SDF-1α. SDF-1α treatment significantly enhanced tube formation in BAECs transfected with control siRNA, with the tube number increasing from 12±3 to 68±10 tubes per field (Figure 2D). In contrast, SDF-1α-induced tube formation was significantly blocked in BAECs transfected with either p22phox siRNA or NOX5 siRNA, by 4.5- or 3.4-fold respectively (Figure 2D). To confirm this anti-angiogenic effect of p22phox and NOX5 siRNAs, we also performed a spheroid-sprouting angiogenesis assay using BAECs treated with SDF-1α. This assay measures the sprouting and network formation of gel-embedded aggregated endothelial cells. Endothelial spheroids were prepared with BAECs transfected with different siRNAs and then stimulated with SDF-1α. The total number of sprouts from each spheroid was counted and compared with control cells. As shown in Figure SIIE, SDF-1α-treated BAECs showed a 3.4-fold increase in sprouts/spheroids when compared with control BAECs. However, this increase in SDF-1α-induced sprout formation was inhibited in BAECs transfected with either p22phox or NOX5 siRNA. The data collected from the Matrigel and spheroid-sprouting angiogenesis assays strongly suggest that multiple NOXs, including NOX5 and probably NOX1/2/4, are critical mediators of SDF-1α-dependent angiogenesis in BAECs.
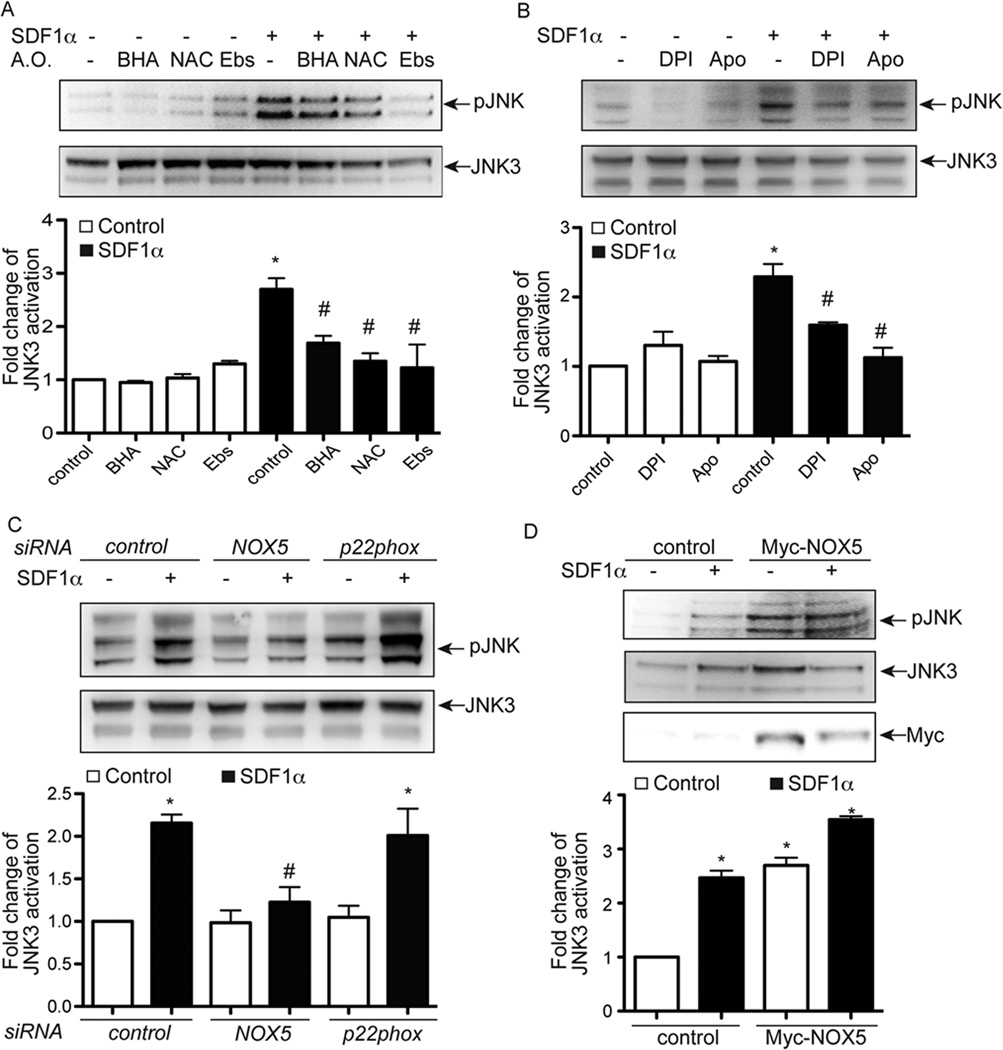
NOX5, but not NOX1/2/4, is required for JNK3 activation induced by SDF-1α
We have previously demonstrated that JNK3 is an important component of SDF-1α-mediated endothelial migration and angiogenesis18. Since the data above demonstrates that NOX-generated ROS also play an essential role in SDF-1α-dependent angiogenesis (Figure 2), we tested whether SDF-1α-induced JNK3 activation was influenced by NOX-dependent ROS generation. All three antioxidants- BHA, NAC and ebselen- inhibited SDF-1α-induced JNK phosphorylation in BAECs (Figure 3A and SIIIA). SDF-1α-induced JNK3 activation was also inhibited by the NOX inhibitors DPI and apocynin (Figure 3B). To test which NOX isoform was involved in SDF-1α-induced JNK3 activation, BAECs were transfected with p22phox siRNA, NOX5 siRNA or control siRNA. Activation of SDF- 1α-induced JNK3 activation was dramatically decreased by knockdown of NOX5, but not by knockdown of p22phox (Figure 3C). Furthermore, ectopic expression of NOX5 in BAECs was sufficient to activate JNK3 at baseline and augmented JNK3 activation following SDF-1α stimulation (Figure 3D). The inhibitory effect of NOX inhibitors and NOX5 siRNAs on SDF-1α-induced JNK3 activation was further confirmed in human vascular endothelial cells (HUVECs; Figure SIIIB, C and D). Moreover, we also observed that ERK activation in response to SDF-1α was also blocked by NOX inhibitors and NOX5 siRNAs, but not p22phox siRNAs in both BAECs and HUVECs (Figure SIIIC, E and F). In summary, these data suggest that NOX5, but not NOX1/2/4, is required and sufficient for SDF-1α-induced JNK3 and ERK activation. In addition to the effects seen by SDF-1α, we also observed other cytokines, including TNF1α and interleukin 1β (IL1β), but not bFGF, evoke an increase in superoxide generation in BAECs (Figure SIVA, B and C). Furthermore, the activation of JNK by TNF1α was inhibited by NOX5 siRNA, but not p22phox siRNA (Figure SIVD). The activation of NOX1, 2 and 4 and ROS generation regulate VEGF-induced angiogenesis24. However, the involvement of NOX5 has not been tested. To assess the effect of NOX5 depletion on VEGF-induced tube formation, HUVECs were transfected with NOX5 siRNA. VEGF increased the formed tube number in control cells. However, the knockdown of NOX5 inhibited tube formation in response to VEGF significantly (Figure SIVE, F). Taken together, we speculate that the NOX5-dependent signaling pathway may represent a relatively general mechanism for growth factors and cytokines.
Figure 3.
NOX5 is required for SDF-1α-induced JNK3 activation in BAECs. A, BAECs were incubated with BHA (200 µM), NAC (10 mM) or ebselen (Ebs, 40 µM) for 30 minutes and then treated with SDF-1α (100 ng/ml) for 10 minutes to activate JNK3. Cell lysates were used for Western blotting to detect the activation of JNK3 using the phospho-JNK antibody. The phosphorylation of JNK was interpreted as JNK3 phosphorylation since JNK3 is the major JNK moiety activated by SDF-1α- in endothelial cells in our system.18 B, BAECs were incubated with NOX inhibitors DPI (10 µM) or apocynin (Apo, 5 mM) for 30 minutes and then treated with SDF-1α (100 ng/ml) for 10 minutes to activate JNK3. Cell lysates were used for Western blotting to detect the activation of JNK3. C, BAECs were transfected with p22phox siRNA, NOX5 siRNA or control siRNA. Three days later, cells were treated with SDF-1α (100 ng/ml) for 10 minutes to activate JNK3. Cell lysates were used for Western blotting to detect the activation of JNK3. D, BAECs were transfected with Myc-tagged NOX5 or control vector. Two days later, cells were treated with SDF-1α (100 ng/ml) for 10 minutes to activate JNK3. Cell lysates were used for Western blotting to detect the activation of JNK3. *, P<0.05; compared to control cells without SDF-1α; #, P<0.05; compared to control cells with SDF-1α; n=3.
ROS induce the expression of numerous genes including those that code for antioxidant proteins. One such antioxidants, heme oxygenase-1 (HO-1) has previously been linked to SDF-1 induced angiogenesis25. Therefore, we were interested to test whether HO-1 expression in BAECs is also regulated by NOXs. Excitingly, when BAECs were treated with SDF-1α for 8 hours, the protein level of HO-1 increased dramatically, which is consistent with a previous report25. However, the induction in HO-1 protein level was inhibited in cells that were transfected with NOX5 siRNAs, but not p22phox siRNAs (Figure SV), indicating that HO-1 is likely one of the downstream mediators for NOX5’s angiogenic effect in BAECs.
Hyperglycemia disrupts SDF-1α-dependent ROS generation and JNK3 activation
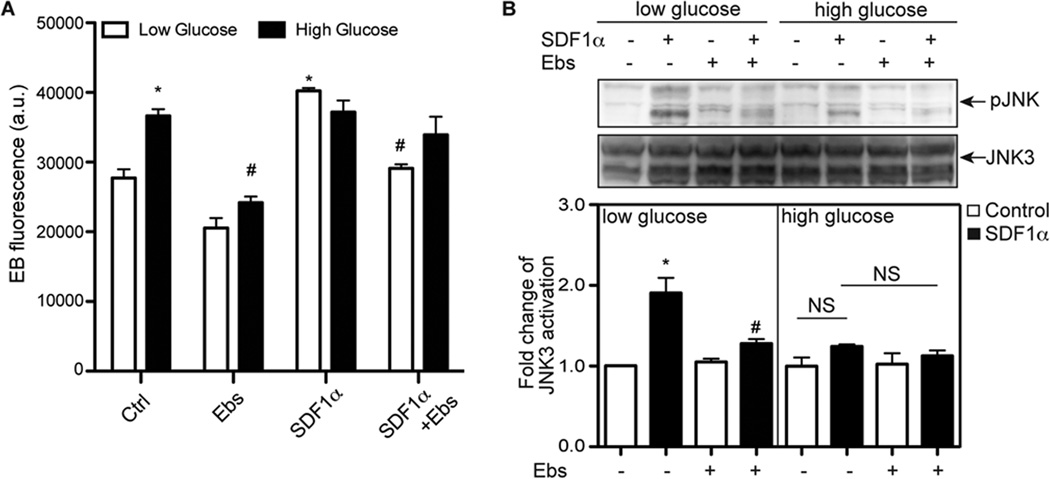
Increased oxidative stress is associated with many chronic diseases, including diabetes and related cardiovascular diseases26. SDF-1α is a dominant angiogenic factor in multiple pathological conditions such as diabetic retinopathy27. Therefore, we investigated the effect of elevated ROS level on SDF-1α-dependent signaling when BAECs were cultured under elevated glucose conditions. Comparing the cells cultured under normal glucose concentration (7 mM glucose), the incubation of cells with ~3.3-fold higher glucose level for three days significantly increased the intracellular ROS level (Figure 4A). Reports have demonstrated that NOX1/2/4, but not NOX5, are responsible for the elevation of ROS level in response to high glucose in vascular cells26, 28, 29. Interestingly, when cells were cultured under normal glucose concentrations, SDF-1α increased JNK3 activation and antioxidant-ebselen blocked it. However, when cells were cultured with a higher concentration of glucose, the response of JNK3 activation to SDF- 1α was blunted (Figure 4B). Together, these data suggest that SDF-1α-dependent NOX5 activation and its downstream signaling are inhibited under hyperglycemic conditions and that the regulatory roles of NOX1/2/4 and NOX5 are likely altered when cellular glucose homeostasis is disrupted.
Figure 4.
The response to SDF-1α is blunted when BAECs were cultured in the presence of high glucose concentrations. BAECs were cultured in media containing either normal glucose concentration (7 mM glucose + 23 mM mannitol) or high glucose concentration (30 mM glucose). Three days later, cells were incubated with ebselen (Ebs, 40 µM) for 30 minutes and then treated with SDF-1α (100 ng/ml) for 5 minutes to determine ROS generation and for 10 minutes to determine JNK3 activation. A, O2·− production was quantified as ethidium bromide fluorescence. B, JNK3 activation was determined by Western blotting. *, P<0.05, compared to control BAECs with low glucose and without SDF-1α. #, P<0.05; compared to control cells without ebselen; NS, not significant; n=3.
NOX5 is associated with MKP7 in BAECs and inhibits MKP7 activity
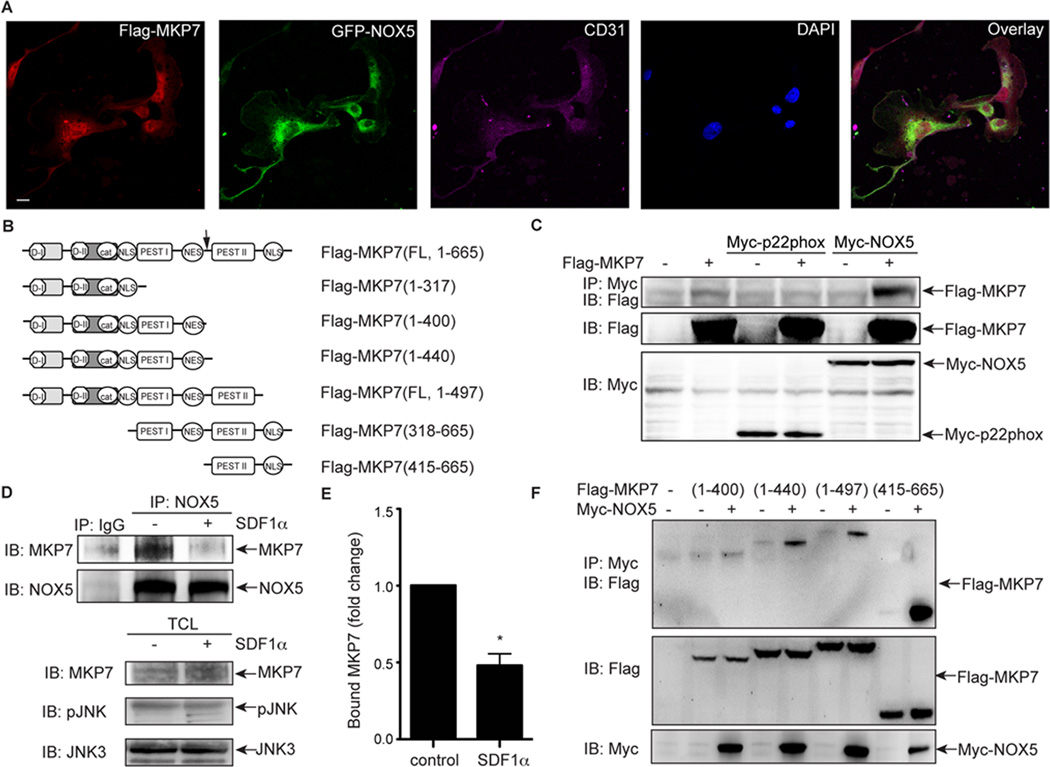
The preceding experiments indicate that NOX5 is the dominant NOX responsible for SDF-1α-induced JNK3 activation. However, the molecular mechanisms underlying this NOX5-dependent signaling event are not clear. S-nitrosylation of the catalytic cysteine of MKP7, which blocks its phosphatase activity, is required for the SDF-1α-induced activation of JNK3.18 Interestingly, the labile cysteine at the catalytic center of MKPs is also susceptible to oxidation.22 Indeed, oxidation of this cysteine by TNFα-generated ROS is critical for activation of JNK1 and the apoptotic effects of TNFα.22 This prompted us to examine whether ROS, generated by NOX5 could result in oxidation of MKP7. In order for NOX5-generated ROS to efficiently oxidize MKP7, NOX5 and MKP7 would need to be in close proximity30. To determine the relative localization of MKP7 and NOX5, Flag-tagged MKP7 and GFP-tagged NOX5 were transfected into BAECs and their localization determined using confocal microscopy. Both flag-tagged MKP7 (Figure 5B) and GFP-tagged NOX5 co-localized to the plasma membrane and cytoplasm of BAECs (Figure 5A), indicating a spatial proximity that would be favorable for NOX5 oxidation of MKP7. To determine whether these two proteins associate directly, immunoprecipitation assays were performed on transfected cells. As shown in Figure 5C, Flag-tagged MKP7 was detected in a protein complex immunoprecipitated with Myctagged NOX5 but not Myc-p22phox, suggesting that MKP7 was associated with NOX5 specifically in HEK 293 cells. The reverse co-immunoprecipitation experiment confirmed the association of MKP7 with NOX5 (Figure SVIA). Co-immunoprecipitation of endogenous MKP7 and NOX5 from BAECs confirmed that, under basal conditions, MKP7 and NOX5 were associated together (Figure 5D). However, following SDF-1α treatment, the proteins dissociated, suggesting a possible mechanism for reversible SDF-1α regulation of MKP7 (Figure 5D, E). To identify which region of MKP7 was responsible for NOX5 binding, a series of MKP7 deletion mutants (Figure 5B) were engineered. Using co-immunoprecipitation experiments once again, we determined that MKP7 (318–665) but not MKP7 (1–317) was associated with NOX5 (Figure SVIB). Further mutations of the 318–665 fragment revealed that MKP7 (415–440; indicated by black arrow in Figure 5B) was required for association with NOX5 (Figure 5F). Taken together, these data demonstrate that MKP7 and NOX5 associate in BAECs and that this association is dynamically regulated by SDF-1α.
Figure 5.
NOX5 is associated with MKP7. A, BAECs were transfected with Flag-MKP7 and GFP-NOX5. Two days later, cells were fixed and analyzed by confocal microscopy. Scale bar, 5µm. B, A schematic representation of full length Flag-MKP7(1–665) and its deletion mutant constructs. The black arrow indicates the fragment of MKP7 responsible for its minimal association with NOX5. C, HEK293 cells were transfected with Flag-MKP7, Myc-NOX5 or Myc-p22phox constructs. One day later, total cell lysates were used for immunoprecipitation for Myc-NOX5 and Myc-p22phox, and immunoblotting for Flag-MKP7. D, BAECs were treated with SDF-1α (100 ng/ml) for 5 minutes and harvested for immunoprecipitation for NOX5 and immunoblotting for MKP7. TCL, total cell lysates. E, The MKP7 proteins associated to NOX5 in BAECs were quantified as the fold change, compared to the control cells. *, P<0.05; compared to control cells without SDF-1α; n=3. F, HEK293 cells were transfected with different Flag-MKP7 mutants and Myc-NOX5 constructs. One day later, total cell lysates were used for immunoprecipitation for Myc-NOX5 and immunoblotting for Flag-MKP7.
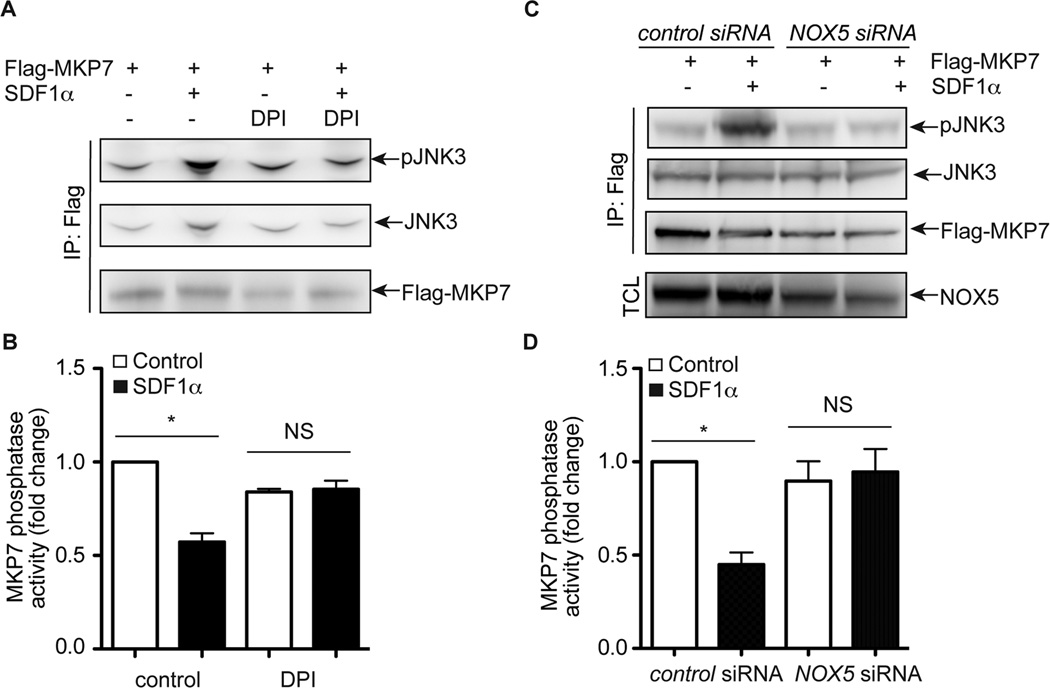
Having established that NOX5 and MKP7 do associate with one another in BAECs, we next sought to determine whether ROS generated from SDF-1α-induced activation of NOX5 oxidizes MKP7, resulting in the inhibition of MKP7 phosphatase activity and subsequent activation of JNK3. An in vitro MKP7 activity analysis was performed using active JNK3 protein and MKP7 concentrated from cell lysates following our established protocol18. At baseline, Flag-tagged MKP7 protein demonstrated strong phosphatase activity as shown by the decrease in JNK3 phosphorylation (Figure 6A). Similar to our previous report18, the treatment of SDF-1α blocked 42.8% of MKP7 phosphatase activity (Figure 6A and B). However, when cells were treated with the NOX inhibitor DPI, MKP7 retained high phosphatase activity at both baseline and following SDF-1α-treatment of cells (Figure 6A and B). Similarly, NOX5 knockdown via transfection of cells with NOX5 specific siRNAs relieved the inhibitory effect of SDF-1α on MKP7 phosphatase activity (Figure 6C and D). These data suggest that ROS generated by NOX5 inhibits MKP7 phosphatase activity, offering one plausible mechanism by which NOX5 promotes SDF-1α-induced JNK3 activation.
Figure 6.
MKP7 activity is regulated by NOX5. A, Results of an in vitro phosphatase assay using active JNK3 protein as the substrate. BAECs were transfected with Flag- MKP7 and then treated with DPI (10 µM) for 30 minutes and followed by SDF-1α (100 ng/ml) treatment for 5 minutes. The cell lysates concentrated for Flag-tagged MKP7 protein were used for in vitro phosphatase assays. B, Quantitative analysis of results from in vitro phosphatase assays of MKP7 in Figure 5A based on three independent experiments. *, P<0.05; compared to control cells. NS, not significant. C, Results of an in vitro phosphatase assay using active JNK3 protein as the substrate. BAECs were transfected with Flag-MKP7, and NOX5 or control siRNA and then treated with SDF-1α for 5 minutes. The cell lysates concentrated for Flag-tagged MKP7 protein were used for in vitro phosphatase assays. D, Quantitative analysis of results from in vitro phosphatase assays of MKP7 in Figure 5C based on three independent experiments. *, P<0.05; compared to control cells. NS, not significant.
Our previous study has demonstrated that MKP7 can be nitrosylated by nitric oxide generated by eNOS after eNOS is activated in response to SDF-1α treatment.18 Similar to eNOS activation leads to the inhibition of MKP7 phosphatase activity18, NOX5 activation blocked MKP7 activity and enhanced JNK3 activation. Therefore, we tested whether the blockage of both eNOS and NOX5 activities would result in synergistic inhibitory effects on JNK3 activation. BAECs were transfected with both siRNAs of eNOS and NOX5 and the changes of JNK3 activity were determined. Interestingly, the knockdown of NOX5 or eNOS blocked the activation of JNK3. However, the knockdown of both NOX5 and eNOS did not further significantly inhibit the activation of JNK3 (Figure SVII). It suggests that NOX5 and eNOS might work on the same pathway upstream of JNK3 activation. Reports have shown that NOX5 induces eNOS activity possibly by enhancing eNOS:hsp90 binding or increasing intracellular calcium level31. Another regulatory mechanism for their interaction could be that both eNOS and NOX5 regulate MKP7 activation.
Taken all together, our data identify NOX5 as a novel component of SDF-1α-dependent JNK3 activation and support the hypothesis that NOX-generated ROS are important mediators of SDF-1α-dependent angiogenesis. More importantly, both nitrous and oxidative stresses, and therefore the intracellular redox status, are important for SDF-1α- dependent signaling and endothelial activation in response to pathophysiologic conditions such as hyperglycemia.
DISCUSSION
NOXs are well accepted as being as novel players in the regulation of angiogenesis. However, the underlying mechanisms by which they exert their actions have not been completely elucidated. Using NOX inhibitors and NOX5-specific siRNA, we have demonstrated a requisite role for NOX5 in the regulation of SDF-1α-dependent JNK3 activation, and subsequent endothelial activation and angiogenesis. This is the first report demonstrating that NOX-generated ROS are required for the angiogenic effects of SDF-1α in endothelial cells. Moreover, we observed that multiple cytokines including SDF-1α, TNF1α and IL1β induced superoxide generation and the activation of JNK by TNF1α was inhibited by NOX5 siRNA (Figure SIV). This recapitulates previous evidence demonstrating that NOX-generated ROS play an important role in angiogenic signaling in response to growth factors and cytokines.10 Given the lack of NOX5 expression in rodents, the functional roles of NOX5 have not been well studied. However, our data hints at an unexpected role for NOX5 in the regulation of human pathophysiology that is associated with angiogenesis.
Increased oxidative stress is associated with many chronic diseases, including diabetes and related cardiovascular diseases26. Our observation that SDF-1α-dependent JNK3 activation was blunted under hyperglycemic conditions (Figure 4) indicates that NOX-dependent ROS generation normally required for angiogenesis is dysregulated in a microenvironment where significantly higher levels of oxidative stress are present. Therefore, both beneficial and detrimental roles of ROS, whose generation might be due to the activation of different enzyme and non-enzyme systems, on endothelial pathophysiology need further clarification to improve the efficacy and safety of therapeutic strategies involving use of antioxidants and NOX inhibitors. In addition, NOX5 is up-regulated in some cancers, such as prostate cancer, esophageal adenocarcinoma and hairy cell leukemia.32–34 DPI, a general NOX inhibitor, potently inhibits the migration, proliferation and invasion of prostate cancer cells by modulating the activity of growth signaling pathways including ERK1/2, p38 and causing cell cycle arrest.35 Based on our finding that NOX-generated ROS participate in angiogenic events, it is also possible that DPI blocks angiogenesis within the tumor, thereby increasing its usefulness as an anti-tumor therapy. Likewise, blockade of the SDF-1α/CXCR4 signaling axis, including NOX5, may represent a promising additional or alternative target for the treatment of some forms of cancer such as rectal carcinoma and colorectal cancer in which SDF-1α levels are seen to increase following reoccurrance of tumors after chemo- and anti- VEGF therapy36, 37. Targeting NOX5 in the SDF-1α pathway may also be useful in treatment against atherosclerosis. In the vascular system, NOX5 is predominantly expressed in endothelial cells, but is also detected in smooth muscle cells. In atherosclerotic aortas, the expression level of endothelial NOX5 is increased38. Given our data demonstrating that NOX5 is involved in angiogenic responses in endothelial cells, it is quite possible that the elevation in NOX5 expression in atherosclerotic lesions exacerbates plaque formation by promoting new vessel formation. Blockade of NOX5 may therefore be one mechanism by which the development of atherosclerotic plaques can be cured.
The data presented in this report is the first demonstration that NOXs are activated by the SDF-1α/CXCR4 signaling axis. All forms of NOX (NOX 1, 2, 4 and 5) are expressed in endothelial cells.10 Previous studies have demonstrated that p22phox is required for activity of NOX1, NOX2 and NOX4.10 Since knockdown of p22phox also blocked SDF- 1α-induced endothelial migration and angiogenesis, we conclude that NOX1, NOX2 and/or NOX4, in addition to NOX5, can be activated by SDF-1α in endothelial cells. That said, their activation and downstream effectors are very likely to be different based on their individual molecular structures. NOX5 possesses additional cytosolic N-terminal EF-hands for binding calcium, rendering it able to be activated by calcium, probably due to the fact that the binding of calcium to these EF-hands relieves the auto-inhibitory loop on the activity domain of NOX5.39 CXCR4, a G-protein coupled receptor, is ligated by SDF-1α and it activation initiates a series of signaling processes including an increase in calcium influx.40 Alternative calcium-independent mechanisms have also been reported to promote calcium sensitization and activation of NOX5.39 Whether calcium influx or other mechanisms of calcium sensitization is required for NOX5 activation by SDF-1α remains to be determined. In contrast to NOX5, the activation of NOX1, NOX2 and NOX4 are regulated differentially by small G proteins and multiple kinases. Rac GTPases are required for both NOX1 and NOX2 activity.41–44 PKC, PI3K and Src are kinases that regulate the assembly of the NOX2 oxidase complex by recruiting p47phox bound to p67phox and p40phox; Akt, PKC and PKA negatively modulate NOX1 activity10. Unlike NOX1 and NOX2, NOX4 exhibits constitutive activity. NOX4 agonists acutely increase its activity, probably through the fast induction of protein expression, increasing the available NADPH pool, or by promoting NOX4’s association with its receptors.10 The diverse range of activators and repressors of NOX 1, 2 and 4 activity suggest that numerous upstream mediators could be activated in response to SDF-1α. Therefore, further experiments are needed to characterize the detailed molecular mechanisms by which NOX1, NOX2 and NOX4 are activated by SDF-1α.
The downstream mediators of NOX1, NOX2, NOX4 and NOX5 responsible for promoting the effects of SDF-1α-induced endothelial migration and angiogenesis are unknown. By using NOX inhibitors and specific siRNAs of NOX5 and p22phox, we have demonstrated that JNK3 and ERK are specific mediators for NOX5 in the regulation of SDF-1α-induced angiogenesis (Figure 2, 3, SII, SIII). Moreover, we observed that the induction of HO-1, a known mediator of SDF-1α-induced angiogenesis, is mediated by NOX5 (Figure SV). However, whether JNK3 and ERK act as upstream mediators in response to SDF-1α to induce HO-1 expression still needs further investigation. Other than the downstream mediators that are mentioned above, multiple other pathways have been characterized as downstream mediators of NOX1/2/4 in the regulation of cell proliferation and migration. For example, Akt is reported to mediate NOX-promoted cell proliferation responses6, 45–47, whereas the PPARα pathways have been implicated as downstream effectors for NOX1’s effect on cell migration.48 The interaction of NOX2 and the scaffold protein IQGAP1 leads to the accumulation of NOX2 at the leading edge of migrating endothelial cells49 and NOX4 is reported to regulate eNOS expression in endothelial cells.50 Whether these pathways are involved in cell migration and proliferation associated with SDF-1α activation of these NOXs still needs further investigation.
The data presented in this report is a continuation of our recent observations that JNK3 is a major mediator of SDF-1α-dependent endothelial migration, and that MKP7, the JNK3 phosphatase, is nitrosylated by nitric oxide and this nitrosylation leads to inhibition of its phosphatase activity.18 Here, we present data demonstrating that MKP7 activity is also inhibited by NOX inhibitors or NOX5 siRNAs, suggesting that the intracellular redox status is critical for maintaining cellular functions for endothelial cells. Cysteine oxidation is one important mechanism by which ROS modulate protein function and the oxidation of the catalytic cysteine on MKP7 has been reported to be required for TNFα-induced JNK activation.22, 51 Therefore, we propose that MKP7 is oxidized by NOX5-generated ROS following SDF-1α administration, which in turn inhibits MKP7 phosphatase activity and sustains JNK3 activation. All MKPs share this cysteine-containing catalytic motif, suggesting they are similarly regulated by the intracellular redox status. In addition to cysteine oxidation, other amino acids such as lysine, proline, arginine and threonine can also be oxidized, and these modifications are recognized as being important for the pathogenesis of many degenerative diseases.52 Future studies aimed at further characterizing the role of protein oxidation may provide essential information that helps to explain the pathophysiologic mechanism of oxidative stress.
Supplementary Material
SIGNIFICANCE.
NOXs are well accepted as being novel players in the regulation of angiogenesis. However, the underlying mechanisms by which they exert their actions have not been completely elucidated. Using NOX inhibitors and NOX5-specific siRNA, our data identify NOX5 as a novel component of SDF-1α-dependent JNK3 activation and support the hypothesis that NOX-generated ROS are important mediators of SDF-1α-dependent angiogenesis. More importantly, both nitrous and oxidative stresses, and therefore the intracellular redox status, are important for SDF-1α-dependent signaling and endothelial activation in response to pathophysiologic conditions such as hyperglycemia.
ACKNOWLEDGEMENTS
We thank Robert Bagnell, Victoria J. Madden and Steven J. Ray in the UNC Microscopy Services Laboratories for help with immunofluorescence microscopy experiments.
SOURCES OF FUNDING
This work was supported in part by American Heart Association NCRP Science Development Grant 0930261N (to X.P.), NIH R01 HL112890 (to X.P.) and NIH R01 HL061656 (X.P.).
ABBREVIATIONS
- ROS
reactive oxygen species
- SDF-1α
stromal cell-derived factor-1α
- BAEC
bovine aortic endothelial cell
- NOX
NADPH oxidase
- eNOS
endothelial nitric oxide synthase
- Duox
dual Oxidase
- PTP
protein tyrosine phosphatase
- BHA
butylated hydroxyanisole
- NAC
N-acetyl-L-cysteine
- Ebs
ebselen
- DPI
diphenylene iodoniu
- Apo
apocynin
- DCF
dichlorofluorescein
- DHE
dihydroethidium
- HUVEC
human vascular endothelial cells
- HO-1
heme oxygenase-1
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 2.Skyschally A, Schulz R, Gres P, Korth HG, Heusch G. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals with ascorbic acid. Am J Physiol Heart Circ Physiol. 2003;284:H698–H703. doi: 10.1152/ajpheart.00693.2002. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JF, Jr, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97:1485–1490. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 5.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 6.Lelkes PI, Hahn KL, Sukovich DA, Karmiol S, Schmidt DH. On the possible role of reactive oxygen species in angiogenesis. Adv Exp Med Biol. 1998;454:295–310. doi: 10.1007/978-1-4615-4863-8_35. [DOI] [PubMed] [Google Scholar]
- 7.Maulik N. Redox regulation of vascular angiogenesis. Antioxid Redox Signal. 2002;4:783–784. doi: 10.1089/152308602760598927. [DOI] [PubMed] [Google Scholar]
- 8.Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, D'Agostini F, De Flora S, Noonan DM, Albini A. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab Invest. 1999;79:1151–1159. [PubMed] [Google Scholar]
- 9.Wheeler MD, Smutney OM, Samulski RJ. Secretion of extracellular superoxide dismutase from muscle transduced with recombinant adenovirus inhibits the growth of B16 melanomas in mice. Mol Cancer Res. 2003;1:871–881. [PubMed] [Google Scholar]
- 10.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 12.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 13.Ueno N, Takeya R, Miyano K, Kikuchi H, Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 14.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 15.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 16.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 18.Pi X, Wu Y, Ferguson JE, 3rd, Portbury AL, Patterson C. SDF-1alpha stimulates JNK3 activity via eNOS-dependent nitrosylation of MKP7 to enhance endothelial migration. Proc Natl Acad Sci U S A. 2009;106:5675–5680. doi: 10.1073/pnas.0809568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 20.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 22.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 24.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, Touyz RM. Oxidative stress, Nox isoforms and complications of diabetes--potential targets for novel therapies. J Cardiovasc Transl Res. 2012;5:509–518. doi: 10.1007/s12265-012-9387-2. [DOI] [PubMed] [Google Scholar]
- 27.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manea A, Fenyo IM, Florea IC, Raicu M. Distinct roles of NADPH oxidase isoforms in high glucose-induced reactive oxygen species formation in human aortic smooth muscle cells. Annals of RSCB. 2010;XV:26–31. [Google Scholar]
- 29.Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med. 2004;37:115–123. doi: 10.1016/j.freeradbiomed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Craige SE, Keaney JF., Jr Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal. 2009;11:2467–2480. doi: 10.1089/ars.2009.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 33.Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem. 2006;281:20368–20382. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- 34.Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH, Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- 35.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Duda DG, di Tomaso E, Ancukiewicz M, Chung DC, Lauwers GY, Samuel R, Shellito P, Czito BG, Lin PC, Poleski M, Bentley R, Clark JW, Willett CG, Jain RK. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 38.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedard K, Jaquet V, Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med. 2012;52:725–734. doi: 10.1016/j.freeradbiomed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Basu S, Broxmeyer HE. Transforming growth factor-{beta}1 modulates responses of CD34+ cord blood cells to stromal cell-derived factor-1/CXCL12. Blood. 2005;106:485–493. doi: 10.1182/blood-2004-10-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X, Carnevale KA, Cathcart MK. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J Biol Chem. 2003;278:40788–40792. doi: 10.1074/jbc.M302208200. [DOI] [PubMed] [Google Scholar]
- 44.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 45.Niu XL, Madamanchi NR, Vendrov AE, Tchivilev I, Rojas M, Madamanchi C, Brandes RP, Krause KH, Humphries J, Smith A, Burnand KG, Runge MS. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 47.Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- 48.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 50.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miki H, Funato Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J Biochem. 2012;151:255–261. doi: 10.1093/jb/mvs006. [DOI] [PubMed] [Google Scholar]
- 52.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.