Abstract
The elimination of large portions of axons is a widespread event in the developing and diseased nervous system. Subsets of axons are selectively destroyed to help fine tune neural circuit connectivity during development. Axonal degeneration is also an early feature of nearly all neurodegenerative diseases, occurs after most neural injuries, and is a primary driver of functional impairment in patients. In this review, we discuss the diversity of cellular mechanisms by which axons degenerate. Initial molecular characterization highlights some similarities in their execution, but also argues that unique genetic programs modulate each mode of degeneration. Defining these pathways rigorously will provide new targets for therapeutic intervention after neural injury or in neurodegenerative disease.
Keywords: Pruning, axon retraction, axosome shedding, axon degeneration, Wallerian degeneration, glia
Axon degeneration: natural versus pathological
Early in development neurons initially generate many more axonal connections with postsynaptic target cells than are necessary for the mature brain to function. At later developmental stages connectivity is fine-tuned such that the most appropriate axonal connections are retained and superfluous axonal connections are destroyed [1,2]. This over-wiring followed by the selective elimination of exuberant axons is referred to as axon pruning (Glossary), and represents an important mechanism to maximize the efficiency of mature neural circuits. There are a number of distinct modes of developmental axon pruning, and these can be characterized based on morphological changes that occur during axon disassembly. In some cases axons are clipped off from the parent neuron, undergo widespread granular fragmentation, and then are cleared by local phagocytes; in other situations axon simply retreat backwards and withdraw from an area while apparently remaining completely intact. Many axons are destroyed or remodeled without death of the parent neuron, which points to the existence of molecular pathways that can promote axonal disassembly in highly restricted cellular compartments.
Pathological axon loss occurs after nervous system injuries such as nerve crush or traumatic brain injury [3–5] and is an early pathological feature in many neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease [6–9]. The similarities in the morphology of axon disassembly are striking when one compares developmental axon pruning and degeneration after injury or in disease—but are these similar at the molecular level? Here we review three well-characterized cellular mechanisms by which axons degenerate during development and compare these with our current understanding of axonal degeneration after axotomy (i.e. Wallerian degeneration), a well characterized model for acute axonal degeneration. While it appears there are significant similarities in the morphology of axonal breakdown in these different modes of axon degeneration, emerging evidence suggests the underlying molecular pathways may be surprisingly distinct.
Pruning of axons during development
During the development of the nervous system, immature neurons are generated in excess, and their neurites grow out and establish an exuberant number of connections. Later in development, regressive events help refine connectivity of the mature neural circuit. Whole neurons are removed through activation of programmed cell death (PCD) in the cell body and corresponding degeneration of attached neurites [10–12]. At the same time, a more subtle and compartmentalized refinement of nervous system connectivity occurs through the selective, and often activity-dependent, pruning of axons, dendrites, or synapses. Below we describe three types of developmental axon pruning—simple axon retraction, shedding of axosomes and presynaptic debris, and local degeneration—which are discriminated by the morphology of axon breakdown, and more recently by the initial characterization of molecular pathways governing their execution. While we focus specifically on axonal pruning, similar mechanisms likely govern dendrite and synapse elimination [13,14].
Simple axon retraction
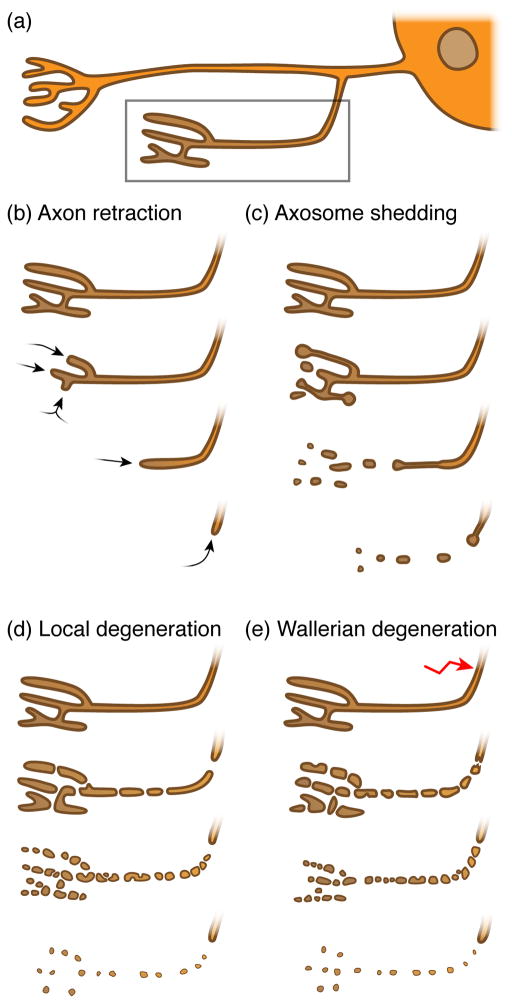
During axon retraction, axonal projections retract from the target area they have innervated in a distal to proximal manner (arrows, Figure 1a, b). In contrast to other types of axon degeneration, axons undergoing retraction remain morphologically intact and do not show signs of fragmentation [15]. Axonal tips involute their membrane distally, resulting in the formation of enlarged, vesicle-filled terminal axonal shafts, the bulbous tip of which is referred to as a retraction bulb. Internalized vesicles move in a retrograde manner up the axon, and, along with other intra-axonal contents in the retracting branch, are likely recycled to other parts of the neuron [16]. Retrograde involution of the terminal portion of the axon continues until it is completely resorbed into the parent arbor. This type of degenerative event is found primarily in axons undergoing local, short distance pruning.
Figure 1. Morphologically distinct modes of axon degeneration.
(a) Schematic neuron (soma including nucleus on the right) containing branched axons and two synapses (towards the left); dendrites are not shown. The synapse and its axon highlighted in the box will undergo various axon degenerative events over time, as shown in b – e. (b) During axon retraction, synapses & axons retract without the loss of axonal integrity. (c) While synapses and axons retract, they shed intact axoplasm-containing axosomes that are cleared by the surrounding glia in a process known as axosome shedding. (d) During local degeneration of axons, the synapse and its axon undergo catastrophic fragmentation, resulting in the removal of the axonal debris by surrounding glia. (e) During Wallerian degeneration, the distal axon separated from the soma undergoes catastrophic fragmentation. The surrounding glia then clears the resulting debris. Note that axon pruning and Wallerian degeneration share similar cellular qualities.
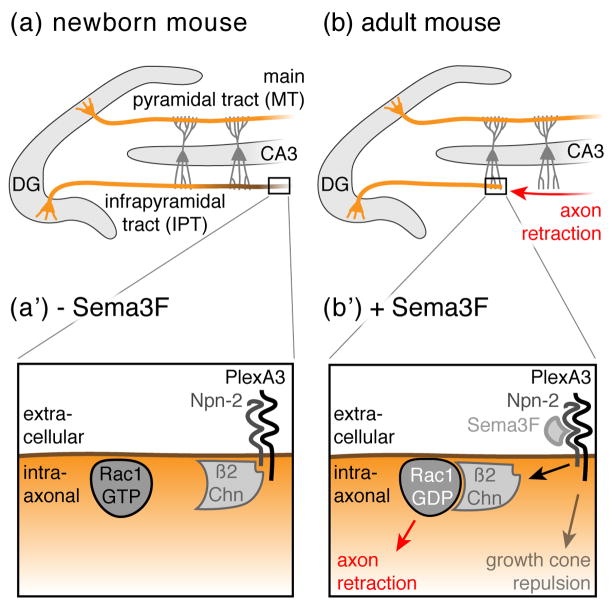
A limited number of signaling pathways modulating simple axonal retraction have been defined, some of which were first characterized for their repulsive effects on growth cones of growing axons [17]. Among these signaling proteins, Semaphorins and Plexins potently repel axon growth cones and act, at least in part, by modifying the activity of downstream small GTPases including Rac [18]. However, they also appear to drive axon retraction in the mammalian brain during neural circuit refinement. In the hippocampus of a neonatal mouse, Dentate Gyrus (DG) granule cells project their axons (mossy fibers) through both the main and the infrapyramidal tracts (MT and IPT, respectively) to terminate on CA3 pyramidal dendrites (Figure 2a). Within 1–2 postnatal months, the IPT is pruned by simple retraction rather than axonal fragmentation based on morphological studies [15] (Figure 2b). Plexin A3 (PlexA3) receptors cell-autonomously induce the retraction of IPT axons in response to Semaphorin 3F (Sema3F), which is likely supplied by local interneurons in the stratum oriens [15].
Figure 2. Axon retraction in the Dentate Gyrus.
(a) In neonatal mice, granule neurons (orange) located in the Dentate Gyrus (DG) project their axons (so called mossy fibers) into both the main and the infrapyramidal tracts (MT and IPT, respectively) to terminate on CA3 pyramidal dendrites (dark grey). (b) After two months, the IPT is remodeled through stereotyped retraction (red arrow) to generate adult structures. (a’ & b’) At the molecular level, Sema3F expression correlates with the regressive time period: in neonatal mice, Sema3F is not detectable (− Sema3F), however, shortly after birth, Sema3F expression is upregulated (+ Sema3F). Sema3F binds to the Neuropilin-2 (Npn-2) receptor, which in turn releases β2-Chimaerin (β2Chn) to axonal membranes where it triggers the hydrolysis of GTP to GTP + Pi in Rac1, thereby leading to axon retraction. By contrast, growth cone repulsion occurs independently of β2Chn.
The observed role for PlexA3/Sema3F in axon retraction might inspire one to draw parallels to the mechanisms of repulsive axon turning or dendritic spine reorganization, however the situation appears more complex with respect to molecular regulation. In the context of postnatal IPT axonal retraction, once Sema3F ligands reach critical threshold levels, they induce retraction through binding to Neuropilin-2 (Npn-2) receptors, which recruits the Rac-GAP β2-Chimaerin (β2Chn) to intracellular axonal membranes, thereby inhibiting Rac-dependent axoskeletal reorganization (Figure 2a’, inset, bottom). By contrast, during growth cone guidance, β2Chn is dispensable for signaling through Sema3F/PlexA3/Npn-2 (Figure 2b’, inset, bottom). Thus, different cytoplasmic effectors mediate PlexA3 signaling events to generate remarkably different outcomes (i.e. repulsion versus retraction) for the axon, despite significant overlap in key receptors and ligands.
Ephrins are cell-surface tethered guidance cues that bind to Eph receptor tyrosine kinases in trans on opposing cells, and this signaling module controls contact-mediated attraction or repulsion of axonal growth cones [19]. The Eph/Ephrin signaling cascade also mediates axon retraction in mouse hippocampal mossy fibers. Ephrin B signaling can promote axon retraction through a Grb4/Pak/Dock180-dependent signaling cascade which—like the PlexA3 signaling pathway described above—is ultimately upstream of Rac-mediated changes in cytoskeletal dynamics [20]. It remains unclear how Eph/Ephrin and Sema3F/Npn-2/β2Chn signaling are coordinated to promote axon retraction. However it has been proposed that Rac might spatially restrict endocytosis of repulsive axon guidance receptors at the axon terminal, thereby promoting a continuous collapse of the distal segment of the axon and ultimately retraction [21–23].
Shedding of axosomes and presynaptic debris at the neuromuscular junction
Muscle fibers in adult mammals are innervated at neuromuscular junctions (NMJs) by a single motorneuron (MN) axon, but this one-to-one relationship is the product of intense remodeling during NMJ formation. During development NMJs are initially innervated by axons from several MNs. These MNs compete for space on muscles, and eventually, “winner” MNs become stabilized whereas “loser” MNs are eliminated until a single MN remains. During elimination the loser MN axons recede from NMJs in a distal to proximal direction. Initially this regression was thought to be similar to simple axonal retraction since it showed no obvious signs of degenerative events: end bulb-like structure formed, and movement was in a proximal direction. However, ex vivo imaging of MN retraction at high resolution followed by correlative light-electron microscopy studies revealed something quite different – axon terminals in fact shed small vesicles (termed “axosomes”) that are internalized by surrounding Schwann cells [24] (Figure 1c). Axosomes shed by axons contain intact organelles and cytoskeletal elements similar to those seen in retraction bulbs, which are then internalized and degraded through lysosome-associated mechanisms [25].
The close association of Schwann cells with this regressing process and their highly dynamic nature during retraction implies that Schwann cells might be a driving force in retraction. Thus the cell biology of axosome shedding is quite different from simple axon retraction, where retreating axons internalize and reuse materials, and local axon degeneration where entire axon branches undergo wholesale granular degeneration (below). It may represent an example of axon retraction driven primarily by extrinsic mechanisms (i.e. the Schwann cell). To date the molecular signaling events underlying axosome shedding remain elusive, as does the precise relationship between the axon and Schwann cell.
A similar type of presynaptic shedding of neuronal debris has been described in both actively growing and retracting Drosophila NMJs [26]. NMJs undergo massive expansion during larval stages in Drosophila to maintain balance with the growing muscles of the larva. MNs constitutively generate new synaptic boutons, some of which become stabilized and form postsynaptic structures, while others (termed “ghost boutons”) fail to assemble postsynaptic elements, and are then shed from the parent arbor [26]. At the same time, significant amounts of presynaptic membrane is shed from the MN and appears as debris that remains reactive for neuronal epitopes [26]. Blocking engulfment activity in surrounding cells (e.g. in draper mutants) leads to the accumulation of large amounts of presynaptic material (shed debris and ghost boutons), indicating that glial or muscle cell engulfment is required for the clearance of presynaptic debris or ghost boutons, but not for the shedding by MNs [26]. Drosophila MNs also exhibit a pattern robust withdrawal from the NMJ when the larva transitions to the adult stage at metamorphosis. At metamorphosis larval muscles are destroyed, NMJs are dismantled, axon arbors retract, and retreating axons shed significant presynaptic material [27]. How this material is generated and cleared is not known, but this observation argues that the shedding of presynaptic material is a widespread event in MN remodeling.
Local axon degeneration
Unlike axonal retraction and axosome shedding, pruning through local axon degeneration is characterized by the locally restricted granular disintegration of axons and the degradation of their contents by other cells (Figure 1d). The entire portion of the axon destined for removal initially blebs, microtubule disassemble, axons swell locally, neurofilaments fragment (in mammals), most components of the axoplasm (e.g. mitochondria and vesicles) are destroyed, and then axon shaft degenerates in a synchronous manner. Once initiated this process takes typically only a few hours to complete, and surrounding glia then engulf and digest the remnants of these pruned axons [7]. This type of axon degeneration occurs more frequently during the regression of long axonal projections, while short projections are more likely to undergo simple retraction. This has been examined in detail in the mammalian neocortex where it is clear there is a correlation between the length of axonal projections and whether they undergo simple retraction or local degeneration – if a postnatal thalamocortical axon is less than 200μm it tends to retract, but if it is longer than 200μm it preferentially undergoes local degeneration and fragments [28]. Similar studies must be performed in other parts of the CNS to determine how widely this 200μm rule holds in other brain regions. However it seems reasonable to assume that the degeneration and subsequent clearance of a long axonal projection may be more efficient than expending the energy necessary to transport back all the axonal contents of a long axon to allow for simple retraction.
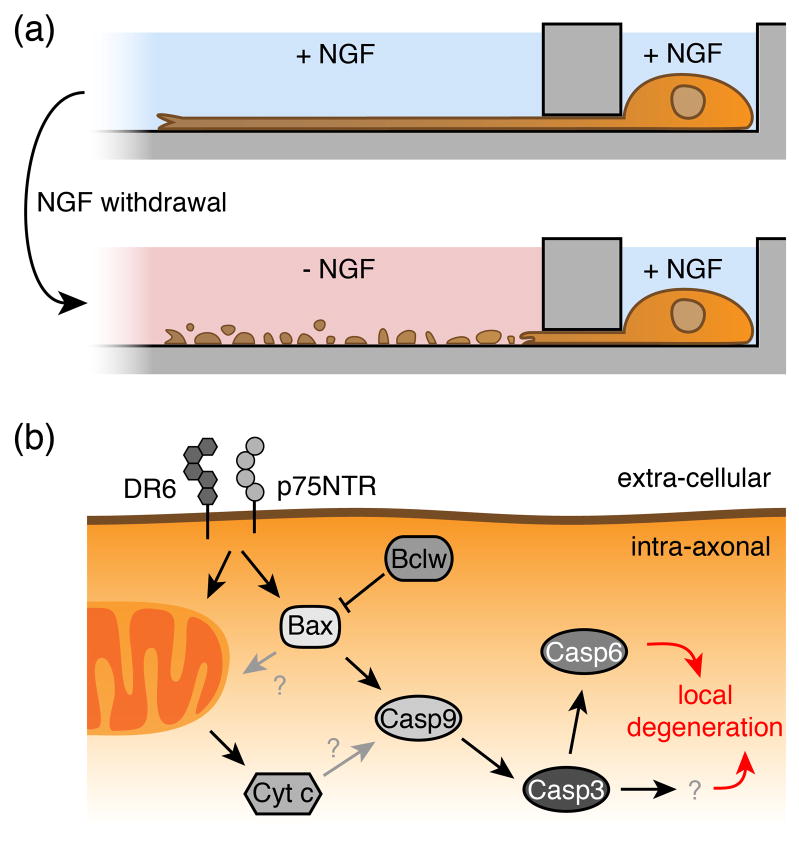
A simple and convenient experimental system in which to induce axon degeneration in neuronal cultures is through compartmentalized trophic factor deprivation of peripheral nervous system (PNS) ganglia in Campenot chambers [29], or the use of microfluidic chambers for neurons of the central nervous system (CNS) [30] (Figure 3a). In the presence of Nerve Growth Factor (NGF), neuronal cell bodies housed in one compartment extend their long axons to a second, isolated compartment. Depriving NGF solely from axonal compartments results in axon degeneration, whereas NGF withdrawal from compartments housing cell bodies leads to neuronal death and axon degeneration. NGF-induced axon degeneration has been used widely as a model for developmental axon pruning, and these approaches have revealed that components of the core apoptotic machinery are important for both the execution of neuronal cell death after trophic factor withdrawal, as well as axon degeneration itself.
Figure 3. NGF-deprived local axon degeneration.
(a) Nerve Growth Factor (NGF) withdrawal induces local degeneration of axons. Axon degeneration can be monitored in vitro upon local NGF withdrawal in neurons of the PNS using Campenot chambers [29], or in neurons of the CNS using microfluidic chambers [30]. (b) Molecular pathways mediating local axonal degeneration. Trophic factor deprivation leads to the activation of tumor necrosis factor (TNF) receptor family members such as death receptor 6 (DR6) or p75 neurotrophin receptor (p75NTR, also known as NGFR). They trigger the activation of specific members of the apoptotic signaling cascade, which results in pruning of axons. Receptor signaling leads to the upregulation and release of pro-apoptotic Bax from mitochondria, which triggers the activation of initiator caspase 9 and effector caspases 3 and 6. Bclw prevents pruning by inhibiting Bax. The role of Cytochrome c release from mitochondria in local axon degeneration remains unclear.
PCD in neurons is initiated by the upregulation of pro-apoptotic family members containing multiple Bcl-2 homology (BH) domains (e.g. Bax), which oligomerize and insert into the outer mitochondrial membrane to mediate mitochondrial damage and Cytochrome c (Cyt c) release. Cyt c, together with apoptotic protease-activating factor-1 (Apaf-1) and initiator Caspases (e.g. Casp9), subsequently lead to the oligomerization and activation of the effector Caspase 3 (Casp3) to initiate cell death (reviewed in [11]). Exciting recent work has demonstrated that pruning of axons requires Bax, the initiator Casp9, and two effector caspases (Casp3 and 6, respectively)(Figure 3b); however, Apaf-1 is dispensable for pruning [31–33]. Since Cyt c is crucial for cell survival, it remains technically challenging to discern whether Cyt c is involved in axonal pruning, nevertheless a core pathway resembling the apoptotic caspase cascade has been implicated.
Careful restriction of caspase activation to the compartment of the cell destined to be eliminated (i.e. the axon but not the cell body) is crucial for retention of the parent neuron. Somatic protection is ensured through upregulation of X-linked inhibitor of apoptosis protein (XIAP), which leads to the binding and inhibition of activated initiator and effector caspases in the soma [34]. A similar IAP-based mechanism of neurite protection has been shown to function during dendrite pruning in the dendritic arborizing (DA) sensory neuron ddaC in Drosophila, where caspase activity is activated throughout dendrites, and the soma is protected by the Drosophila XIAP homologue DIAP (Drosophila inhibitor of apoptosis protein) [35,36]. Could such local suppression of caspases be used as a mechanism to save specific neurites? Intriguingly, a recent study has shown in DRG cultures that mammalian XIAP is indeed present in axons, XIAP levels drop sharply in axons after NGF withdrawal prior to degeneration, and that overexpression of XIAP can suppress NGF withdrawal-induced degeneration [37]. It will be exciting to determine in the future whether this type of local caspase suppression is important for axon branch-specific protection in vivo.
Could there be additional checkpoints for caspase activation that are used in a local manner? While developing neurons are highly susceptible to PCD, circuit-integrated mature neurons are resistant to PCD and employ redundant strategies to prevent it, likely to ensure neuronal circuit stability over the life of the animal [12]. Blockade of PCD can certainly occur at several molecular checkpoints. Bax translocation to mitochondria can be inhibited by Bcl-2 family members [38] or by microRNA-29 (miR-29), which can modulate the translation of several BH3-only proteins required for Bax activation [39]. After Bax translocation to the mitochondrion, the blockade of PCD can still occur through transcriptional silencing of Apaf-1 and Casp3 in several neuronal tissues [40]. Whether these are also involved in the modulation of local axonal degeneration remains and exciting question for the future.
Extrinsic factors also appear capable of indirectly modulating caspase signaling in axons. For instance axonal transport of the messenger RNA (mRNA) for the anti-apoptotic gene bclw occurs in axons, where its local synthesis appears to enable axonal survival through suppression of caspase signaling. Specifically, Bclw interacts with Bax and suppresses Caspase-6 activity, which would otherwise result in axonal degeneration [41]. Lamin B2 (LB2) is normally associated with nuclear membranes, however, when triggered by guidance cues (mediated by the homeobox-containing gene Engrailed-1), lamin B2 mRNA is also transported into axons where it is locally translated. LB2 associates with axonal mitochondria, where it regulates mitochondrial function in order to meet the high metabolic demands of these axons [42]. While loss of LB2 does not affect axon guidance, it causes axon degeneration through mitochondrial dysfunction and defects in axonal transport. Thus, the translational control of axonal mRNAs seems an important mechanism for regulation axonal survival.
Drosophila has proven a valuable genetic model for identifying molecules that modulate local axon degeneration in vivo. During Drosophila metamorphosis, mushroom body (MB) γ neurons prune their axons and dendrites through local degeneration while the cell body and proximal axon shaft remain intact [43]. The first step in this process appears to be priming the neuron to prune through TFG-β signaling and the ecdysteroid receptor EcR (recently reviewed in [44]. The second step involves changes very similar to those in mammalian pruning: dramatic changes in axon ultrastructure including the disruption of the microtubule cytoskeleton, synaptic degeneration, and ultimately catastrophic axon fragmentation [45]. A limited number of signaling pathways have been described that act in the pruning event proper (rather than establishing competence to prune through EcR), including components of the ubiquitin-proteasome system (UPS) and cytoskeletal modulators. For example, overexpression of the yeast ubiquitin protease UBP2, which removes ubiquitin side chains from targets, potently suppresses MB γ neuron axon pruning, as does loss of ubiquitin activating enzyme 1 (Uba1) or the Drosophila proteasome regulatory particle subunits Mov34 or Rpn6 [45]. More recently Cullin-1, a core component of the RING E3 ligase SCF E3 ligase (Skp1-Cullin-F-box) was identified as a key E3 ligase required for axonal and dendritic pruning in Drosophila. The SCF E3 ligase has been proposed to function at least in part through inactivation of the InR/PI3K/TOR pathway [46]. However defining the precise role of the UPS pathway in driving axonal pruning remains an important unmet goal for the field.
In considering how axons are pruned we generally envision pathways that are activated at the right time to promote degeneration. However recent studies indicate that axon stability may in fact result from constitutive blockade of an endogenous degenerative program, and it appears this can be regulated very locally. RNAi-mediated knockdown of p190 RhoGAP (GTPase activating proteins) led to unexpected, early and robust retraction of the dorsal axonal branches of MB γ neurons [47]. In stable MB γ neuron axons, p190 activity is normally thought to drive RhoA to a GDP-bound (inactive) state, thereby suppressing downstream signaling. However inhibition of p190, led to the activation of RhoA through Drok signaling, enhanced phosphorylation of the myosin regulatory light chain, and ultimately axonal retraction of dorsal, but not medial axon branches [47]. These observations are intriguing for a number of reasons. First, they suggest that at least in some neurons a “silent” axon retraction signaling pathway is constitutively present, which is repressed during times of axonal stability, and de-repressed at the initiation of retraction. Second, the changes in axon retraction were specific to the dorsal branches of MB γ neuron axons and did not affect medial branches (which are normally pruned along with dorsal branches). These data therefore provide some of the first evidence that different axon branches attached to the same neuron may be differentially sensitive at the molecular level to signals activating degeneration [47].
Axon degeneration after injury: Wallerian degeneration
Axonal degeneration is also a widespread feature of nervous system injury or disease and is thought to be a primary cause of functional loss in patients. Axotomy, perhaps the most severe axonal insult, results in catastrophic fragmentation of the portion of the axon distal to the injury site [48,49]. A similar morphological fragmentation has been observed in Parkinson’s disease, Huntington’s disease, and Multiple Sclerosis [50]. In addition, many peripheral neuropathies present with an axonal “dying back” pathology, whereby the axon gradually degrades in a retrograde fashion from its tip over time [51]. Whether these types of pathological axon degeneration are molecularly similar to axon degeneration during development remains unclear, but is now a possibility that can be directly explored.
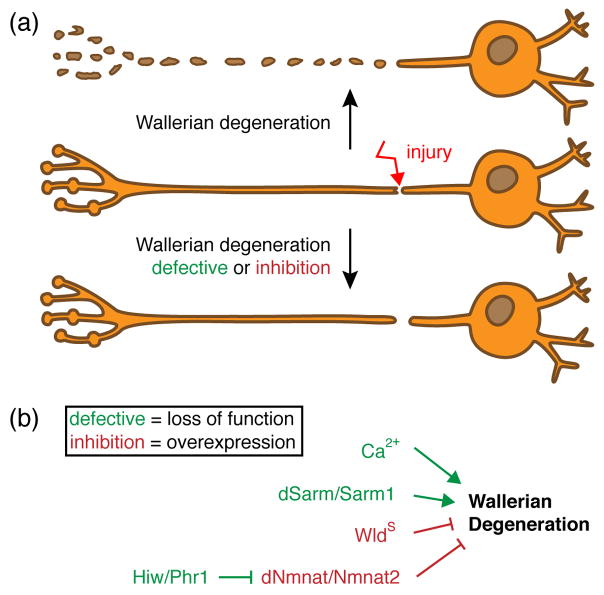
Axotomy serves as a simple model study axonal degeneration. After an axon is severed, the portion of the axon distal to the injury site exhibits (after a defined latent phase) widespread breakdown of the axonal cytoskeleton and destruction of internal organelles, and ultimately granular disintegration (Figure 4a). This degradative process, which is referred to as Wallerian degeneration (WD), was thought to result from the passive wasting away of axons due to lack of trophic support from the soma [52]. However the discovery of the spontaneous mouse mutant strain C57BL/WldS (Wallerian degeneration slow), where severed axons remain morphologically intact for weeks after axotomy, radically changed our views on the autonomy of the axonal compartment (Figure 4b).
Figure 4. Molecular pathways mediating Wallerian degeneration.
(a) Upon injury, the distal – from the soma separated – axon will undergo Wallerian degeneration (WD), which results in catastrophic axon self-destruction and clearance of resulting axonal debris by surrounding glial cells. WD can be attenuated, either by loss-of-function mutations of genes required for WD (green) or by over-expression of genes that inhibit it (red). (b) Factors that mediate WD are extracellular Ca2+ ions, dSarm/Sarm1 and the E3 ubiquitin ligase Highwire/Phr1. Loss of those candidates result in defective WD. In contrast, genes that antagonize WD are the neomorphic WldS and dNmnat/Nmnat2. Over-expression of those genes will lead to the inhibition of WD.
The WldS mutation, resulting in the production of a chimeric fusion protein consisting of the first 70 N-terminal amino acids of the E4 ubiquitin ligase Ube4b and the NAD+ scavenging enzyme Nmant1, functions cell-autonomously and protects severed axons in a dominant manner [53–55]. The Nmnat1 component in WldS appears to be the critical component and protects axons through gain-of- function mechanism (i.e. overexpression/mislocalization of Nmnat1 activity) which is clearly non-nuclear and likely axonal [56–58]. While the mechanistic action of WldS remains to be clearly defined, its protective effects are conserved across diverse species [59,60]. Interestingly, the ability of WldS to block axonal degeneration in vivo is specific to WD, as WldS does not block developmental axonal or dendritic pruning [61], and has no measureable effect on neuronal cell death [62]. Thus, WldS serves as a useful genetic tool with which to classify axon degenerative events—if the degenerative event is suppressed by WldS expression, it is characterized as “Wallerian-like”.
Once axons are severed, the earliest effector of WD is likely signaling via changes in axonal calcium (Ca2+) [63] (Figure 4b). Extracellular Ca2+ entry is both necessary and sufficient to induce WD, and ultimately leads to the activation of Ca2+-activated proteases of the calpain family [63]. These proteases execute the proteolysis of severed axons in WD, and their normal activation is constitutively inhibited by the calpain inhibitor calpastatin, which appears to be degraded in preparation for degenerative events [64,65]. Subsequent changes in mitochondria including the production of reactive oxygen species (ROS) and formation of the mitochondrial permeability transition pore (mPTP) ultimately precede and may drive axonal degeneration [66]. WldS seems to be upstream of all of these events since it can block immediate changes in Ca2+ signaling [67], mPTP and ROS production [68]. Bursts of Ca2+ signaling in dendrite branches is predictive of pruning in Drosophila [69], arguing for a role for dynamic Ca2+ changes in pruning, but whether ROS production or mPTP is important for driving dendritic pruning events is unknown.
Recent forward genetic approaches in Drosophila have begun to identify endogenous genes whose normal function is to promote Wallerian degeneration. Osterloh et al. recently identified the kinase scaffolding molecule dSarm (Drosophila sterile α/Armadillo/Toll-Interleukin receptor homology domain protein) as essential for axon degeneration. Remarkably, dsarm null alleles block axonal degeneration for the lifespan of the fly, and loss of the mammalian ortholog Sarm1 suppressed axon degeneration for up to two weeks after sciatic nerve lesion (while control axons degenerated within ~48 hrs). Sarm1 likely acts very early in the degeneration program since loss of Sarm1 led to robust preservation of the axonal cytoskeleton, and Sarm1 is required broadly in the nervous system to drive axon degeneration since Wallerian degeneration was delayed in multiple types of cultured mammalian CNS and PNS neurons in Sarm1−/− mutants [70] (Figure 4b). Pro-degenerative Sarm1 signaling in vitro requires the SAM and TIR domains of Sarm1 [71], but precisely how dSarm/Sarm1 promotes axonal degeneration remains to be determined. In C. elegans, TIR-1 (the worm homologue of Sarm1) is activated downstream of a Ca2+ signaling cascade [72], which suggests that dSarm/Sarm1 might be capable of directly responding to axotomy-induced increases in axonal Ca2+ to activate axon degeneration. dSarm provides further genetic evidence that local axon degeneration during pruning and Wallerian degeneration are molecularly distinct, since dSarm is dispensable for proper developmental axonal and dendritic pruning [70].
Flies lacking the E3 ubiquitin ligase Highwire (Hiw) have also recently been shown to exhibit robust morphological and functional preservation of severed axons [73]. Mouse mutants lacking the mammalian homologue Phr1 also exhibited a strong delay in axon degeneration after axotomy [74], again arguing for a strong evolutionary conservation of the axon death machinery. The proposed mechanism for Hiw/Phr1-mediated protection of axons provides an intriguing potential molecular link with WldS, as Hiw/Phr1 may normally act to degrade Nmant2, a cell body-derived axonal survival factor [75]. Briefly, several studies have clearly shown that the functional domain of WldS essential for axonal protective function is the NAD+ biosynthetic enzyme Nmnat1 [73,74,76,77]. It was proposed that the Nmnat activity of WldS substitutes for endogenous Nmnat2, which is a labile isoform of Nmnat that is actively transported down axons from the cell body, and whose elimination results in spontaneous axon degeneration in the absence of injury [75,78]. Hiw/Phr1 has been proposed to fine-tune Nmnat protein levels through degradation, and presumably the lack of Hiw/Phr1 leads to stabilization of axons due to perdurance of Nmant2 (Figure 4b). Consistent with this notion Nmnat levels are increased in severed mutant axons lacking Hiw/Phr1 [73,74].
How could injury lead to the activation of a Hiw/Phr1-dependent degradative event? Recent work found that axonal injury resulted in stabilization of DLK/MAP3K12, which was dependent on c-Jun N-terminal Kinase (JNK) and Hiw/Phr1 [79]. One possible explanation could be that the loss of Hiw/Phr1 blocks DLK stabilization. However, previous studies have argued that loss of DLK in Drosophila and mouse lead to stabilization of axons [80], which would seem to argue against this model. Future work to clarify the interaction between DLK, Hiw/Phr1, JNK, and Nmnat will be essential, as will be exploring the potential roles for Hiw/Phr1 in different types of axonal pruning.
Concluding remarks
Work over the past decade has led to a much clearer understanding of the cellular and molecular basis of axon degeneration during development and in some neurological conditions (most notably axotomy). A number of morphologically distinct modes of axon degeneration exist—those discussed here include simple retraction, axosome/debris shedding, pruning via local degeneration, and Wallerian degeneration—and that these are well-characterized makes them excellent models for rigorous genetic and molecular analysis. Initial molecular characterization seems to suggest that despite remarkable similarities in the cell biology of axonal self-destruction (e.g. local axon degeneration and Wallerian degeneration), the underlying molecular pathways are surprisingly dissimilar. That said, it must be pointed out that our understanding of these events are in their infancy, a broader analysis of many of these pathways in each mode of axon pruning and Wallerian degeneration remains to be performed, and as the field moves forward examples of molecular convergence might appear. The overlap between axon degeneration pathways, or their unique attributes, need to be considered and their delineation a key goal for the field (Box 1). Gaining a firm molecular grasp on how axons drive their own degeneration in these distinct contexts will be critically important to both understand why axons degenerate in neurological disease, and for the identification of new targets for therapeutic intervention in neurological conditions involving axon, dendrite, or synapse loss.
Box 1. outstanding questions.
Why do axons degenerate?
What molecular pathways dictate the necessity of axonal degeneration? Is axon degeneration activity-dependent, and if so is this true in all cases? What determines a “loser” versus a “winner” axon at the mammalian NMJ, and how is the retraction of the loser executed? How much is intrinsic to the axon versus driven by the Schwann cell? Could there be a non-autonomous trigger coming from the muscle cell? Synapses are susceptible to a wide array of neurodegenerative stimuli (physical trauma, infectious agents or in disease) [81]. Is synaptic degeneration an early event that inevitably triggers axonal “dying back”? How similar, or different, are the molecular pathways of axon degeneration during development versus disease?
How do axons degenerate?
Why are there distinct morphological types of axon degeneration? Can we tie specific programs of degeneration to specific types of retraction? Do we already know all existing morphological processes featuring “degeneration”, or are there additional degenerative events? Why do some axons undergo simple retraction, thereby keeping their axoplasm within the parent neuron through involution, whereas other axons gradually shed axosomes to surrounding glia? Why do some axons choose to undergo catastrophic fragmentation, where whole axons are lost, and resulting debris engulfed by surrounding glia? Is there in vivo cross talk between the recently identified modulators of axon degeneration? Do axon degenerative pathways converge on a common Nmnat-sensitive pathway as suggested previously [82]?
What are axon non-autonomous triggers for axon degeneration?
Glia, besides their role of providing myelin for rapid impulse propagation, are also required for the long-term survival of (long) axons [83]: surrounding glia support axons either by metabolites to increase survival [84,85], or by exosomes to increase stress-resistance [86]. These fundamental roles for glia add another level of complexity to our approach to understanding axonal survival and degeneration. Understanding the precise supportive roles for glia and their effect on axonal maintenance is a critical question for the field and carries tremendous therapeutic potential.
Acknowledgments
We would like to thank all Freeman lab members for comments and suggestions on the manuscript of this review. L.J.N. is a Charles A. King Trust Postdoctoral Fellow and is supported by the Harold Whitworth Pierce Charitable Trust. Work in the M.R.F. laboratory is supported from the NIH, The ALS Therapy Alliance, and M.R.F. is an Investigator of the Howard Hughes Medical Institute.
Glossary
- Pruning
an umbrella term of different cellular mechanisms used by neurons for the reduction of established synapses
- Axon retraction
axon branches retract in a distal to proximal manner, where axonal contents are recycled to other axonal/cellular compartments
- Axosome shedding
a cellular mechanism where retreating axons shed remnants, termed axosomes, containing the same organelles as observed in the bulb of the retreating axon
- Local axon degeneration
“catastrophic” self-destruction of whole axon branches
- Axotomy
applying mechanical injury to axons by cutting the shaft
- Wallerian degeneration (WD)
occurs upon injury of an axon shaft, where the distal axon, separated from the neuron’s cell body, degenerates
- Neuronal apoptosis or programmed cell death (PCD)
a genetic program driving the self-destruction of a whole neuron including its neurites
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raff MC, et al. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 2.Luo L, O’Leary DDM. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 3.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 4.Burke RE, O’Malley K. Axon degeneration in Parkinson’s disease. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson VE, et al. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunawardena S, Goldstein LSB. Polyglutamine diseases and transport problems: deadly traffic jams on neuronal highways. Arch Neurol. 2005;62:46–51. doi: 10.1001/archneur.62.1.46. [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.De Vos KJ, et al. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 9.Coleman MP. The challenges of axon survival: introduction to the special issue on axonal degeneration. Exp Neurol. 2013;246:1–5. doi: 10.1016/j.expneurol.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 12.Kole AJ, et al. Mature neurons: equipped for survival. Cell Death Dis. 2013;4:e689. doi: 10.1038/cddis.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao J, Rolls MM. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J Neurosci. 2011;31:5398–5405. doi: 10.1523/JNEUROSCI.3826-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajadi A, et al. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Current Biology. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 15.Bagri A, et al. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 16.Riley DA. Ultrastructural evidence for axon retraction during the spontaneous elimination of polyneuronal innervation of the rat soleus muscle. J Neurocytol. 1981;10:425–440. doi: 10.1007/BF01262414. [DOI] [PubMed] [Google Scholar]
- 17.Vanderhaeghen P, Cheng HJ. Guidance molecules in axon pruning and cell death. Cold Spring Harb Perspect Biol. 2010;2:a001859. doi: 10.1101/cshperspect.a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13:605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- 19.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends in Cell Biology. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurney WM, et al. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmer M, et al. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 23.Parker M, et al. Reverse endocytosis of transmembrane ephrin-B ligands via a clathrin-mediated pathway. Biochem Biophys Res Commun. 2004;323:17–23. doi: 10.1016/j.bbrc.2004.07.209. [DOI] [PubMed] [Google Scholar]
- 24.Bishop DL, et al. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Song JW, et al. Lysosomal activity associated with developmental axon pruning. J Neurosci. 2008;28:8993–9001. doi: 10.1523/JNEUROSCI.0720-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuentes-Medel Y, et al. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulanger A, et al. Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-β/BMP signaling and orphan nuclear receptors. PLoS ONE. 2012;7:e40255. doi: 10.1371/journal.pone.0040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portera-Cailliau C, et al. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005;3:e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor AM, et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Meth. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolaev A, et al. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Simon DJ, et al. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cusack CL, et al. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potts PR, et al. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163:789–799. doi: 10.1083/jcb.200307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams DW, et al. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- 36.Kuo CT, et al. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Unsain N, et al. XIAP regulates caspase activity in degenerating axons. Cell Rep. 2013;4:751–763. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Willis SN, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 39.Kole AJ, et al. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yakovlev AG, et al. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cosker KE, et al. Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. J Neurosci. 2013;33:5195–5207. doi: 10.1523/JNEUROSCI.3862-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon BC, et al. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148:752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Truman JW. Metamorphosis of the central nervous system of Drosophila. J Neurobiol. 1990;21:1072–1084. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- 44.Corty MM, Freeman MR. Cell biology in neuroscience: Architects in neural circuit design: Glia control neuron numbers and connectivity. J Cell Biol. 2013;203:395–405. doi: 10.1083/jcb.201306099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts RJ, et al. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 46.Wong JJL, et al. A Cullin1-based SCF E3 ubiquitin ligase targets the InR/PI3K/TOR pathway to regulate neuronal pruning. PLoS Biol. 2013;11:e1001657. doi: 10.1371/journal.pbio.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Billuart P, et al. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 48.Lee JC. Electron microscopy of Wallerian degeneration. J Comp Neurol. 1963;120:65–79. doi: 10.1002/cne.901200107. [DOI] [PubMed] [Google Scholar]
- 49.Thomas PK. Changes in the endoneurial sheaths of peripheral myelinated nerve fibres during Wallerian degeneration. J Anat. 1964;98:175–182. [PMC free article] [PubMed] [Google Scholar]
- 50.Adalbert R, Coleman MP. Axon pathology in age-related neurodegenerative disorders. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 51.Höke A. Neuroprotection in the peripheral nervous system: rationale for more effective therapies. Arch Neurol. 2006;63:1681–1685. doi: 10.1001/archneur.63.12.1681. [DOI] [PubMed] [Google Scholar]
- 52.Waller A. Experiments on the Section of the Glossopharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Philosophical Transactions of the Royal Society of London. 1850;140:423–429. [Google Scholar]
- 53.Lunn ER, et al. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 54.Perry VH, et al. Very Slow Retrograde and Wallerian Degeneration in the CNS of C57BL/Ola Mice. Eur J Neurosci. 1991;3:102–105. doi: 10.1111/j.1460-9568.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 55.Mack TG, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki Y, Milbrandt J. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem. 2010;285:41211–41215. doi: 10.1074/jbc.C110.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babetto E, et al. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci. 2010;30:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beirowski B, et al. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald JM, et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 60.Martin SM, et al. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137:3985–3994. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoopfer ED, et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Whitmore AV, et al. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–261. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- 63.George EB, et al. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, et al. Regulation of Axon Degeneration after Injury and in Development by the Endogenous Calpain Inhibitor Calpastatin. Neuron. 2013 doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 65.Ma M, et al. Calpains mediate axonal cytoskeleton disintegration during Wallerian degeneration. Neurobiol Dis. 2013;56:34–46. doi: 10.1016/j.nbd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrientos SA, et al. Axonal Degeneration Is Mediated by the Mitochondrial Permeability Transition Pore. J Neurosci. 2011;31:966–978. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avery MA, et al. Wld(S) Prevents Axon Degeneration through Increased Mitochondrial Flux and Enhanced Mitochondrial Ca(2+) Buffering. Curr Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Donnell KC, et al. Axon degeneration and PGC1α-mediated protection in a vertebrate model of α-synuclein toxicity. Dis Model Mech. 2014 doi: 10.1242/dmm.013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanamori T, et al. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013;340:1475–1478. doi: 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- 70.Osterloh JM, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou YJ, et al. SARM Is Required for Neuronal Injury and Cytokine Production in Response to Central Nervous System Viral Infection. J Immunol. 2013;191:875–883. doi: 10.4049/jimmunol.1300374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chuang CF, Bargmann CI. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 2005;19:270–281. doi: 10.1101/gad.1276505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiong X, et al. The highwire ubiquitin ligase promotes axonal degeneration by tuning levels of nmnat protein. PLoS Biol. 2012;10:e1001440. doi: 10.1371/journal.pbio.1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Babetto E, et al. The Phr1 Ubiquitin Ligase Promotes Injury-Induced Axon Self-Destruction. Cell Rep. 2013 doi: 10.1016/j.celrep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araki T, et al. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 77.Avery MA, et al. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilley J, et al. Rescue of Peripheral and CNS Axon Defects in Mice Lacking NMNAT2. J Neurosci. 2013;33:13410–13424. doi: 10.1523/JNEUROSCI.1534-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huntwork-Rodriguez S, et al. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol. 2013;202:747–763. doi: 10.1083/jcb.201303066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gillingwater TH, Wishart TM. Mechanisms underlying synaptic vulnerability and degeneration in neurodegenerative disease. Neuropathol Appl Neurobiol. 2013;39:320–334. doi: 10.1111/nan.12014. [DOI] [PubMed] [Google Scholar]
- 82.Vohra BPS, et al. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci. 2010;30:13729–13738. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 84.Fünfschilling U, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012 doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frühbeis C, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]