Summary
As humans age, they lose both muscle mass and strength (sarcopenia). Testosterone, a circulating hormone, progressively declines in aging and is associated with loss of muscle mass and strength. Joining of a young and old mouse (heterochronic parabiosis) activates Notch signaling and restores muscle regenerative potential in aged mice. We hypothesize that testosterone is at least one of the factor required for the improvement seen in muscles in old mice in heterochronic parabiosis with young mice. To test this hypothesis, we established the following heterochronic parabioses between young (Y; 5 months old) and old (O; 22–23 months old) C57BL6 male mice: 1) Y: O; 2) castrated Y: O (ØY: O); and 3) castrated + testosterone-treated Y: O (ØY+T: O). A group of normal young received empty implants and old mice were used as controls. Parabiotic pairings were maintained for 4 weeks prior to analysis. Serum testosterone levels were 3-fold higher in young in comparison with old mice. ØY+T: O pairing demonstrated significantly elevated levels of serum testosterone and improvement in gastrocnemius muscle weight, muscle ultrastructure, muscle fiber cross-sectional area, and Notch-1 expression in old mice. These changes were not present in aged mice in ØY: O pairing. Together, these data point to a critical role for testosterone in mediating improved muscle mass and ultrastructure seen in an experimental model of heterochronic parabiosis.
Keywords: Testosterone, parabiosis, muscle growth, aging, mouse
Introduction
Sarcopenia is defined as an age-related loss of skeletal muscle mass and strength. Multiple studies have demonstrated that it is both widespread and severely debilitating condition (Baumgartner et al. 1998; Roubenoff and Hughes 2000; Marzetti and Leeuwenburgh 2006). Such age-related loss of muscle mass has a profound effect on the elderly population, manifested by decreased healing after injury, impaired physical function, and increased risk of falls, fractures, dependency, and death (Glass and Roubenoff 2010). Despite its prevalence, mechanisms underlying this age-related loss of skeletal muscle mass remain poorly understood.
Adult skeletal muscles robustly regenerate throughout an organism life, but as the muscle ages, its ability to repair diminishes and eventually fails. This diminished regenerative potential of aged muscle is largely due to a decline in Notch signaling, which is essential for activation, proliferation, and myogenic progression of satellite cells (Conboy et al. 2003; Conboy et al. 2005; Shadrach and Wagers 2011). Heterochronic parabioses, where two mice of different ages are surgically joined such that they develop a shared blood circulation without immune rejection, is a powerful model to determine whether circulating factors can influence tissue function in aging and longevity (Conboy et al. 2005; Shadrach and Wagers 2011; Brack et al. 2007; Conboy et al. 2013). Heterochronic parabioses with young mice restore the regenerative capacity of aged satellite cells, through activation of Notch signaling, and promote successful muscle repair after injury (Conboy et al. 2005). Conversely, exposing a young mouse to an old systemic environment inhibits myogenesis (Brack et al. 2007). Importantly, these studies implicate a factor(s) in systemic circulation that regulates skeletal muscle stem cell niche and regeneration.
Testosterone level progressively declines in aging and is associated with loss of muscle mass strength (Sinha-Hikim et al. 2006; Cunningham and Toma 2011). Using a mouse model, we previously demonstrated that testosterone supplementation prevents loss of muscle mass in aging through stimulation of both Notch and Akt signaling in old mice (Kovacheva et al. 2010). Here, using a heterochronic parabiosis model, we provide preliminary evidence that testosterone is obligatory for restoring the systemic environment that supports muscle growth and improves muscle pathology in aging.
Materials and Methods
Heterochronic parabiosis
C57Bl-6J male mice were purchased from Harlan Laboratories (Indianapolis, IN) and aged in a standard animal facility at Charles R. Drew University of Medicine and Science under controlled temperature (22 °C) and photoperiod (12-h light, 12-h dark cycle) with food and water ad libitum. Young mice of 5 months age and aged mice of 22–23 months of age were utilized in this study. Young mice were treated as follows: i) control (sham operated), ii) castrated, and iii) castrated but received 1.0 cm testosterone implants. Orchiectomy and sub-dermal testosterone implantation were performed under anesthesia. Testosterone-filled implants were prepared from polydimethylsiloxane tubing (od, 1.96 mm; id, 1.47 mm; Dow-Corning). 1.0 cm testosterone implant size is based on the results of our earlier study (Kovacheva et al. 2010), which showed that this dose of testosterone fully reversed age-related decline in muscle mass and muscle fiber cross-sectional area (CSA) and suppressed age-specific increase in muscle cell apoptosis and oxidative stress. It also restored age-specific decreases in Akt and Notch signaling.
Heterochronic parabiotic pairings (N=5 pairs per group) were created between young and old mice as previously described (Wagers et al. 2002). In brief, the right side of the normal, castrated, or testosterone-supplemented castrated young mouse and the left side of the old mouse were shaved and sterilized. Matching skin incisions were made from the olecranon to the knee joint of each mouse. The subcutaneous tissue was next dissected to create a 0.5cm free skin flap, both dorsal and ventral. The elbows and knees of the parabiotic pair were attached by a single 5-0 coated vicryl suture and the dorsal and ventral skin flaps were each secured by a running 5-0 vicryl suture. These mice were maintained for 4 weeks following parabiosis. The following parabiotic pairings were established between young (Y) and old (O): 1) Y: O, 2) orchiectomized Y (ØY): O and 3) orchiectomized plus 1.0 cm testosterone-treated Y (ØY+T): O. A group of normal young received empty implants and old mice were used as controls. Animal handling and experimentation were in accordance with the recommendation of the American Veterinary Medical Association and were approved by the Charles R. Drew University School of Medicine and Science Animal Care and Use Review committee.
Blood collection and tissue preparation
All mice were euthanized with a lethal intraperitoneal injection of sodium pentobarbital (200 mg/kg body weight). Blood samples were collected from each animal by cardiac puncture immediately after death, and serum was separated and stored at −20°C for subsequent testosterone assay. The gastrocnemius muscles from each mouse were removed and weighed. Portions of the tissues were snap frozen in liquid N2 and stored frozen for subsequent analysis by Western blotting. Additional portions from each mouse were either fixed in 2.5% glutaraldehyde for electron microscopy or 4% formalin for histological and immunohistochemical studies. Portions of glutaraldehyde fixed muscles were further diced into small pieces, post-fixed into 1% osmium tetroxide and embedded in Epon 812 as described previously (Sinha-Hikim et al. 2007). Thin sections from selected tissue blocks were cut with an LKB ultramichrotome, stained with uranyl acetate and lead citrate, and examined with a Hitachi 600 electron microscope (Hitachi, Indianapolis, IN). The rationale for using gastrocnemius muscles was based on the results of several earlier studies, which show that this muscle exhibits a substantial decline in mass with age (Martin et al. 2007; Braga et al. 2008; Kovacheva et al. 2010; Sinha-Hikim et al. 2013).
Testosterone assay
Serum testosterone levels were measured by a previously reported radioimmunoassay (Sinha-Hikim et al. 2007; Kovacheva et al. 2010). The minimal detection limit in the assay was 0.6 ng/dl. The intra-assay and inter-assay coefficient of variations were 8.2% and 13.2%, respectively.
Muscle fiber CSA
Muscle fiber CSA was determined in 5 μm paraffin embedded, hematoxylin and eosin (H&E) stained sections of gastrocnemius muscles using the ImagePro Plus, version 5.1 software (Media Cybernetics, Silver Spring, MD) coupled to an Olympus BHS microscope equipped with a VCC video camera (Braga et al. 2008; Kovacheva et al. 2010). For each animal at least 50 fibers were measured.
Assessment of muscle pathology
Muscle pathology was evaluated using conventional histological analysis on H&E stained sections. Further evaluation of pathology was achieved by transmission electron microscopy (TEM). An observer who was unaware of the treatment assignment took and analyzed the electron micrographs. We also performed morphometric analysis to estimate the volume density (Vv), which is the volume of a given cellular component per unit myofibrillar volume. From each treatment group, 80 micrographs (20 micrographs/mouse) were selected for ultrastructural analysis. The point-counting method (Cruz-Orive and Weibel 1990; Mahapatra et al. 2005) was used to estimate the Vv of various myofibrillar components by superimposing a transparent overlay bearing a double-lattice grid on electron micrographs of muscles. The Vv was obtained by dividing the points on a given cellular organelle by the total number of points counted over the muscle fiber. Values were expressed as percentages of the myofibrillar volume (Vv %), obtained by multiplying volume densities by 100.
Western blotting
Western blotting was performed using muscle lysates as described previously (Braga et al. 2008; Kovacheva et al. 2010; Sinha-Hikim et al. 2013). In brief, proteins (50–80 μg) were separated on a 4–12% SDS-polyacrylamide gel with MES or MOPS buffer purchased from Invitrogen (Carlsbad, CA, USA) at 200V. Gel was transferred on an Immuno-blot PVDF Membrane (Bio-Rad, Hercules, CA) overnight at 4°C. Membranes were blocked in blocking solution (0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk) for 1 h at room temperature then probed using rabbit polyclonal Notch 1 (1:200) and proliferating cell nuclear antigen (PCNA; 1:200) for 1 h at room temperature or overnight at 4°C with constant shaking. All antibodies were obtained from Santa Cruz Biotechnology Inc., Santa Cruz, CA. Following 3 X 10-min washes in TBS-T buffer, membranes were then incubated in anti-rabbit (Amersham Biosciences, Piscataway, NJ, USA) IgG-HRP secondary antibody at a 1:2000 dilution. All antibodies were diluted in blocking buffer. For immunodetection, membranes were washed three times in TBS-T wash buffer, incubated with ECL solutions per the manufacturer’s specifications (Amersham Biosciences), and exposed to Hyper film ECL. The membranes were stripped and re-probed with a rabbit polyclonal GAPDH (1:2000) for normalization of the loading. Band intensities were determined using Quantity One software from Bio-Rad (Hercules, CA, USA).
Statistical analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Corporation, San Rafael, CA). Data are presented as mean ± SEM. We used one-way ANOVA to compare group differences. If overall ANOVA revealed significant differences, post hoc (pairwise) comparisons were performed using Student-Newman-Keuls method. Differences were considered significant if P<0.05. Pearson product moment correlation coefficients were computed to relationship between variables.
Results
Testosterone levels, muscle weight, and muscle fiber CSA
Serum testosterone levels were 3-fold higher in young mice as compared to their aged counterparts (Fig. 1a). Compared with ØY: O pairing, old mice in ØY: O+T joining had significantly (P<0.01) elevated levels of serum testosterone (Fig. 1a). Gastrocnemius muscle mass was significantly lower by 15.4% in old mice in comparison to young mice (P<0.05). Notably, gastrocnemius muscle mass in old parabiont was increased in ØY+T: O pairing (153 ± 8 mg) but not with ØY: O (127 ± 2 mg) pairing (Fig. 1b). Changes in gastrocnemius muscle mass were significantly (r = 0.92; P <0.02) and positively correlated with changes in testosterone levels.
Fig. 1.
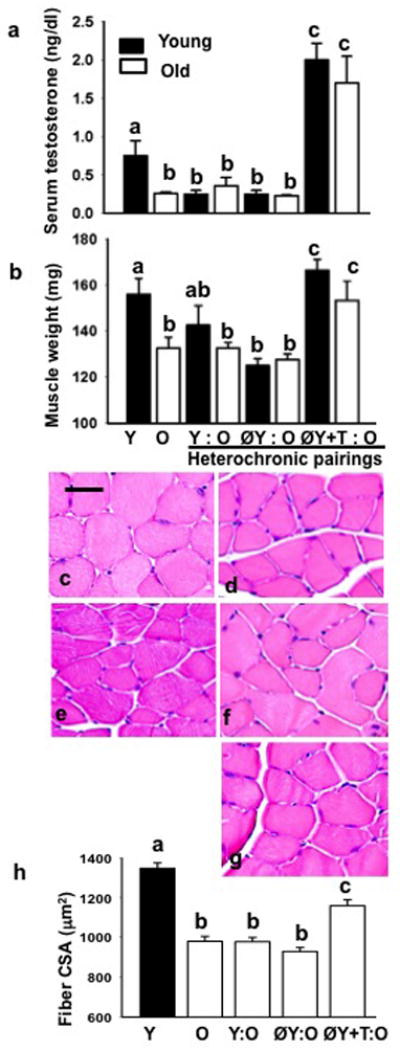
Testosterone levels, muscle weights, muscle histology, and muscle fiber CSA. a, Serum testosterone levels were 3-fold higher in young mice as compared to their aged counterparts. Compared with Y: O or ØY: O pairing, old mice in ØY+T: O pairing had significantly elevated levels of serum testosterone. Values are given as mean ± SEM (n=5). Means with unlike superscripts are significantly different. b, The weight of the gastrocnemius muscles was decreased significantly in the old animals compared to young mice. Notably, ØY+T: O but not ØY: O pairing fully restored gastrocnemius muscles weight in aged mice to levels seen in young controls. Values are given as mean ± SEM (n=5). Means with unlike superscripts are significantly different. Representative H&E stained gastrocnemius muscle sections from Y (c), O (d), and old mice in Y: O (e), ØY: O (f), or ØY+T: O (g)pairing reveals that only ØY+T: O pairing results in an apparent increase in muscle fiber size in old mice (g)similar to the phenotype observed in young mice (c). Scale bar = 25 μm. h, Quantitative analysis of muscle fiber CSA in various parabionts. Values are given as mean ± SEM (n=5). Means with unlike superscripts are significantly different.
H&E stained muscle section revealed smaller muscle fibers in old mice (Fig. 1d) compared to those of young males (Fig. 1c). Muscle fiber histology in old mice in Y: O (Fig. 1e) or ØY: O pairing (Fig. 1f) was indistinguishable from that of old controls (Fig. 1d). Notably, ØY+T: O pairing resulted in an increase in muscle fiber size in old mice (Fig. 1g) similar to phenotype observed in young controls (Fig. 1c). As summarized in Fig. 1h, there was a significant (P<0.05) reduction in mean fiber CSA in old mice (980 ± 25 μm2) compared to that of young males (1346 ± 27 μm2). No significant differences in muscle fiber CSA were noted between old mice (980 ± 25 μm2) and old mice in Y: O (978 ± 21 μm2) or ØY:O (929 ± 20 μm2) pairing. However, ØY+T: O pairing resulted in a significant (P<0.05) increase in muscle fiber CSA (1158 ± 30 μm2) in aged mice.
ØY+T: O joining improves myofibrillar ultrastructure in old mice
We next performed TEM to evaluate myofibrillar architecture in various parabiotic pairings (Fig. 2). Gastrocnemius muscle from young mice exhibited normal architecture (Fig. 2a) with abundant normal looking mitochondria, no intramyofibrillar lipid (IML) accumulation, and no tubular aggregation (TA). In contrast, varying degrees of abnormalities were noted in aged muscle, including mitochondrial swelling with broken cristae, mitochondrial vacuolization, increased IML accumulation, and presence of TA (Fig. 2b). Y: O pairing reversed some of these changes in old mice, as the old parabionts, exhibited decreased IML accumulation, less mitochondrial abnormalities, and smaller areas of TA (Fig. 2c). In contrast, muscles from old partners in ØY: O pairing failed to reverse these age-associated changes (Fig. 2d–f). Instead, typical of old muscle, muscle from these mice demonstrated larger areas of TA (Fig. 2d), vacuolated (Fig. 2e) and swollen mitochondria with broken cristae (Fig. 2f), and increased IML accumulation. ØY+T: O pairing, however, showed remarkable improvement in muscle ultrastructure (Fig. 2g). The ultrastructural appearance of muscle in these old parabionts was similar to that seen in young mice (Fig. 2a).
Fig. 2.
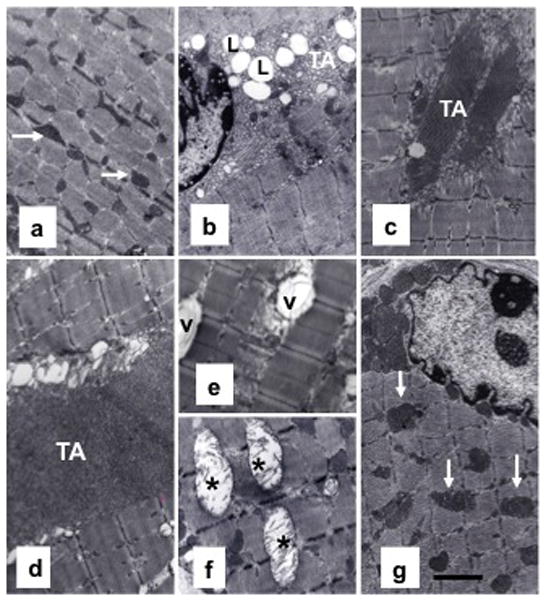
ØY+T: O joining improves myofibrillar ultrastructure in old mice (a–g). Representative TEM image of a portion of a gastrocnemius muscle from a young mouse exhibits normal myofibrillar cytoarchitecture and sarcomere organization (a) with abundance of mitochondria (arrow). In striking contrast, muscle from an old mouse exhibits perturbed muscle ultrastructure (b), including IML accumulation (L) and formation of large areas of tubular aggregation (TA). Muscle from an aged parabiont in Y: O pairing shows no IML accumulation and relatively smaller areas of TA formation (c). In contrast, muscles from aged partners in ØY: O pairing (d–f) show larger areas of TA formation and vacuolated (V) and swollen mitochondria with broken cristae (asterisk). Notably, ØY+T: O pairing restores normal cytoarchitecture and sarcomere organization with abundant hypertrophied mitochondria in aged mice (g). Scale bar = 2.5 μm.
Ultrastructural analysis of volumetric composition of myofibrillar organelles is summarized in Table 1. Compared with muscle from young mice, a significant (P<0.05) decrease in the Vv% of normal-looking mitochondria together with increases in the Vv% of vacuolated mitochondria, IML accumulation, and TA were noted in muscle from old mice. Y: O pairing fully attenuated age-related decline in Vv% of normal looking mitochondria. There were also significant (P<0.05) reductions in the Vv% of vacuolated mitochondria (by 39.5%) and IML (47%) in aged parabionts in comparison with aged controls. Intriguingly, ØY+T: O pairing was even more effective than Y: O pairing in reversing such age-related changes in muscle ultrastructure. The Vv% of normal looking mitochondria was increased to 627% and 195% over the values measured in young and old controls, respectively. There were also significant (P<0.05) reductions in the Vv% of vacuolated mitochondria (51.6%), IML (70.3%), and TA (61%) in these parabionts compared to that of old controls. In contrast, muscle in ØY: O pairing failed to reverse these age-associated changes.
Table 1.
Morphometric data on volumetric composition (Vv%) of normal looking mitochondria (NMIT), vacuolated mitochondria (VMIT), intramyofibrillar lipid (IML) accumulation, and tubular aggregation (TA) in skeletal muscles of young (Y), old (O), and O mice in various parabiotic pairings with untreated (Y:O), castrated (ØY:O), and castrated + 1cm testosterone-treated young males (ØY+T:O).
| Treatment | NMIT | VMIT | IML | TA |
|---|---|---|---|---|
| Y | 5.55 ± 1.17A | 0.25 ± 0.05A | 0.17 ± 0.05A | 0A |
| O | 1.73 ± 0.33B | 2.23 ± 0.33B | 2.32 ± 0.01B | 9.30 ± 2.14B |
| Y: O | 4.40 ± 0.55A | 1.35 ± 0.29C | 1.23 ± 0.21C | 5.93 ± 0.55B |
| ØY: O | 1.92 ± 0.16B | 2.81 ± 0.91B | 3.02 ± 0.85B | 18.45 ± 4.63C |
| ØY+T: O | 10.86 ± 2.24C | 1.08 ± 0.07C | 0.69 ± 0.09D | 3.63 ± 0.44D |
Vv%, volume density expressed as a percentage of the myofibrillar volume. Values are given as mean ± SEM. In each column, means with unlike superscripts are significantly (P<0.05) different.
Muscle hypertrophy of old mice in ØY+T: O pairing is associated with stimulation of Notch signaling
As shown in Fig. 3a and b, there was a significant (P<0.01) decrease in Notch-1 levels in gastrocnemius muscles from old mice when compared with young animals. Following Y: O or ØY+T: O pairing muscle from old parabionts exhibited increased Notch-1 expression in comparison to old controls. There was no upregulation of Notch-1 expression in old mice in ØY: O pairing. Notch-1 expression was associated with cell proliferation as demonstrated by increased expression of PCNA in muscle lysates (P<0.05, Fig. 3a and c). Notch-1 expression was significantly and positively correlated with changes in serum testosterone levels (r = 0.93; P <0.02) throughout groups.
Fig. 3.
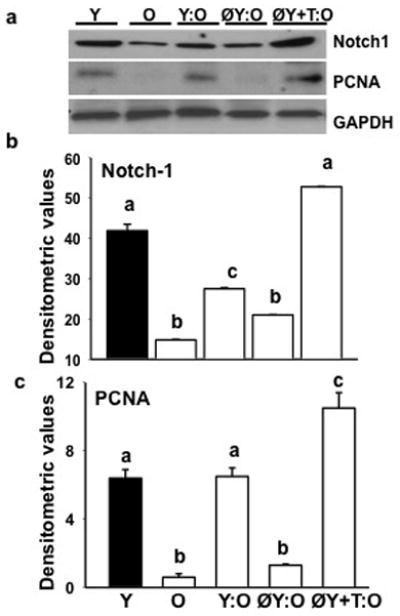
Muscle hypertrophy in old mice in ØY+T: O pairing is associated with stimulation of notch signaling. a, Western blots of muscle lysates show ØY+T: O but not ØY: O pairing fully restores Notch-1 expression to levels seen in young (Y) controls. Activation of Notch signaling in these heterochronic old parabionts is associated with increased expression of PCNA in muscle lysates. b and c, Quantification of band intensities. Values are mean ± SEM. Means with unlike superscripts are significantly (P<0.05) different.
Discussion
The central hypothesis of this study is that testosterone is obligatory for restoring the systemic environment that supports muscle growth and improves muscle pathology in aging. We tested this hypothesis using a heterochronic parabiosis model between young and old mice with additional manipulation. The results of the present study confirm and extends earlier findings by demonstrating that ØY+T: O pairing attenuated age-related decrease in muscle mass, induced muscle fiber hypertrophy, improved skeletal muscle ultrastructure, and fully restored Notch-1 expression in aged mice. Notably, these improvements in skeletal muscles were not present in aged mice in ØY: O pairing. Importantly, we further show that such reversal of aging muscle phenotype could be achieved with normal levels of testosterone (1.7 ± 0.3 ng/ml) in aged heterochronic parabionts as opposed to supra-physiological levels (8.0 ± 3.1 ng/ml) of testosterone required for muscle hypertrophy in old control (non-parabiosed) mice as noted in our previous study (Kovacheva et al. 2010). Thus, we speculate that the observed muscle hypertrophy in old partner with normal levels of testosterone, as opposed to supra-physiological levels (Kovacheva et al. 2010), in ØY+T: O pairing may arise from activation of old muscle stem cell niche. Given the adverse effects associated with testosterone administration in elderly (Cunningham and Toma 2011; Spitzer et al. 2013), our study has important clinical implication.
The diverse ultrastructural changes seen in aged muscles, including mitochondrial swelling with broken cristae, mitochondrial vacuolization, increased IML accumulation and presence of TA were consistent with those described in the earlier literature in humans as well as in rodents (Kaminska et al. 1998; Agbulut et al. 2000; Corsetti et al. 2008; Crane et al. 2010). It is worth noting here that TA formation can also be seen several human muscle disorders, including myopathies with exercise-induced cramps and muscle pain, myoasthenic disorders and in some familial myopathies (Niakan et al. 1985; Rosenberg et al. 1985; Mahjneh et al. 2007; Jain et al. 2008). We do not know how TA formation and myopathies are connected. The pathophysiology of TA formation in aging also remains unknown but may be a manifestation of perturbed muscle structure and function in aging. The amount of oxidative stress increases as an organism ages and is postulated to be a major causal factor for loss of muscle mass and function in aging (Braga et al. 2008; Kovacheva et al. 2010; Crane et al. 2010; Sinha-Hikim et al. 2013). Increased oxidative stress has also been implicated in mitochondrial damage and IML accumulation in aged muscles (Martin et al. 2007; Bonnard et al. 2008). Furthermore, Bonard and colleagues (2008) demonstrated that increased generation of oxidative stress, triggered by high-fat and high sucrose diet or streptozotocin treatment, led to a reduction in mitochondrial density and marked alterations in mitochondrial structure. These effects were blocked by attenuation of oxidative stress either through normalization of glycemia or by antioxidant treatment. It is pertinent to note here that testosterone is able to reduce oxidative stress in aged muscles (Kovacheva et al. 2010). Collectively, these data suggest that testosterone restores muscle architecture in aged parabionts possibly through suppression of oxidative stress.
Notch signaling is essential for activation, proliferation, and myogenic progression of satellite cells (Conboy et al. 2003; Conboy et al. 2005; Shadrach and Wagers 2011). Our previous studies on both elderly men (Sinha-Hikim et al. 2006) and aged mice (Kovacheva et al. 2010) indicate the involvement of Notch signaling in testosterone-mediated muscle growth in aging. Consistent with a role of Notch signaling in muscle growth, we found increased Notch-1 expression in aged muscle in Y: O pairing. Interestingly, no upregulation of Notch-1 expression was detected in muscle from old parabionts in ØY: O pairings. In contrast, ØY+Y: O pairing resulted in a marked upregulation of Notch-1 expression in aged muscle to levels even higher than that seen in young controls. Muscle Notch-1 expression was positively correlated to the changes in circulating testosterone levels (r = 0.93; P<0.02). It is worth noting here that previous work examining the effects of heterochronic parabiosis between young and old mice showed activation of Notch signaling as well as the proliferation and rejuvenation of aged satellite cells (Conboy et al. 2005). Together, it is tempting to speculate that in the present model testosterone may restore the aged systemic milieu to its youthful state, through stimulation of Notch signaling and induce muscle hypertrophy in aging.
Although our study has important implications in the rapidly expanding filed of modulating the local environment that promotes muscle growth in aging, it has some limitations. One limitation is that we do not use this model of in vivo or in vitro heterochronic parabiosis to characterize the stimulatory effects of testosterone on aged muscle progenitor cells. An additional short fall of our study is that the functionality of testosterone-induced muscle hypertrophy was not determined. Clearly, this merits further investigation.
In summary, our results indicate that testosterone may be one of the serum factor(s) necessary for muscle growth seen in aged mice in an experimental of heterochronic parabiosis model. A deeper understanding of the mechanism by which testosterone improves the local environment that supports muscle regeneration and growth in aging may provide new entry points for therapeutic strategies in clinical settings.
Acknowledgments
This work was supported by National Institutes of Aging Grant F32 AG034703 (IS) and NIH 1 RO1 AG033053 and 1 P30 AG031679 (AJW). We thank the oxidative core laboratories of the National Institutes of Health Accelerating Excellence in Translational Science grant (U54 MD007598) at Charles R. Drew University of Medicine and Science for performing Western blot and hormone assays.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- Agbulut O, Destombes J, Thiesson D, Butler-Browne G. Age-related appearance of tubular aggregates in the skeletal muscle of almost all male inbred mice. Histochem Cell Biol. 2000;114:477–481. doi: 10.1007/s004180000211. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bonnard c, Durund A, Peyrol S, Chanseaume E, Chauvin M-A, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Braga M, Sinha Hikim AP, Datta S, Ferrini M, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13:822–832. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:60–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetti G, Pasini E, D’Antona G, Nisoli E, Flati V, Assanelli D, Dioguardi FS, Bianchi R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am J Cardiol. 2008;101 (suppl):26E–34E. doi: 10.1016/j.amjcard.2008.02.078. [DOI] [PubMed] [Google Scholar]
- Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65A:119–128. doi: 10.1093/gerona/glp179. [DOI] [PubMed] [Google Scholar]
- Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: a brief survey. Am J Physiol. 1990;258:L148–156. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- Cunningham GR, Toma SM. Why is androgen replacement in males controversial? J Clin Endocrinol Metab. 2011;96:38–52. doi: 10.1210/jc.2010-0266. [DOI] [PubMed] [Google Scholar]
- Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci. 2010;1211:25–36. doi: 10.1111/j.1749-6632.2010.05809.x. [DOI] [PubMed] [Google Scholar]
- Kaminska AM, Fidzianska A, Schulze G, Coper H, Ossowska K, Wolfarth S, Hausmanowa-Petrusewicz I. Ultrastructural changes in the skeletal muscle of senile rats with significant age-dependent motor deficits. Basic Appl Myol. 1998;8:185–190. [Google Scholar]
- Kovacheva EL, Sinha Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone Supplementation Reverses Sarcopenia in Aging through Regulation of Myostatin, c-Jun NH2-Terminal Kinase, Notch, and Akt Signaling Pathways. Endocrinology. 2010;151:628–638. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra N, O’Connor DV, Vainganker SM, Sinha Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy B, Ziegler M, Ross J, Mahata S. Targeted ablation of chromogranin A gene: elevated blood pressure rescued by human homolog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Dubouchaud H, Mosoni L, Chardigny J-M, Oudot A, Fontaine E, Vergely C, Keriel C, Rochette L, Leverve X, Demaison L. Abnormalities of mitochondrial function can partly explain the metabolic disorders encountered in sarcopenic gastrocnemius. Aging Cell. 2007;1:1–13. doi: 10.1111/j.1474-9726.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerentol. 2006;41:234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerentol A Biol Sci Med Sci. 2000;55:716–724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- Shadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Phil Trans R Soc B. 2011;366:2297–2306. doi: 10.1098/rstb.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community dwelling, older men. J Clin Endocrinol Metab. 2006;91:3024–3033. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Braga M, Shen R, Sinha Hikim AP. Involvement of c-Jun NH2-terminal kinase and nitric oxide-mediated mitochondria-dependent intrinsic pathway signaling in cardiotoxin-induced muscle cell death: role of testosterone. Apoptosis. 2007;12:965–1978. doi: 10.1007/s10495-007-0120-6. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Sinha-Hikim AP, Parveen M, Shen R, Goswami R, Tran P, Crum A, Norris KC. Long-term supplementation with a cystine-based antioxidant delays loss of muscle mass in aging. J Gerontol A Biol Sci Med Sci. 2013;68:749–759. doi: 10.1093/gerona/gls334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risk and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9:414–424. doi: 10.1038/nrendo.2013.73. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood R, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]


