Abstract
Background
The clinical significance of antibodies directed against antigens other than MHC antigens is poorly understood and there are few large animal models in which such antibodies can be examined. We studied, both retrospectively and prospectively, the development of antibodies to non-MHC antigens in tolerant miniature swine.
Methods
Our database was assessed for cases of anti-donor antibody formation in tolerant animals over the last 20 years. Flow cytometry, absorption assays and familial analyses for inheritance pattern of the gene(s) potentially responsible for the antibody reactivities were carried out and an animal determined to be negative for this reactivity was immunized by a skin graft and subcutaneous injections of PBMCs from an antigen-positive donor.
Results
Sixteen of 469 tolerant animals tested were found to have developed anti-donor antibodies. These antibodies were found to be specific for the same, presumably single, non-MHC antigen. Familial analyses indicated that the gene encoding this antigen was expressed in an autosomal dominant manner in approximately 95% of the herd. In a prospective study, anti-donor antibodies with the same specificity as those observed retrospectively were successfully induced in an antigen-negative animal after immunization with PBMCs.
Conclusions
To our knowledge, this is the first report of the development of antibodies to a highly prevalent, non-MHC antigen present on peripheral blood mononuclear cells and developing in tolerant animals without signs of graft dysfunction. Considering the concern often raised by the appearance of anti-donor antibodies in transplant recipients, these data could have important implications for clinical transplantation.
Keywords: Antibody, MHC, antigen specificity, tolerance, MGH swine
INTRODUCTION
Our laboratory has previously demonstrated that class I mismatched renal transplantation, followed by 12 days of high-dose Cyclosporine A (CyA) in euthymic miniature swine uniformly induces tolerance of the kidney graft (1-3). These animals have thereby provided a reproducible preclinical model for studies of transplantation tolerance. Tolerant animals exhibit donor-specific unresponsiveness in-vitro, and following removal of the tolerated graft, uniformly accept second donor MHC matched kidneys, without rejection (1). During experiments carried out over the last 20 years using this model, we have observed that a small percentage (<5%) of tolerant animals nevertheless developed antibody reactive to donor peripheral blood mononuclear cells (PBMCs), while the tolerated graft was in place and its function unperturbed.
Recent speculations on the importance of anti-donor antibodies on clinical outcome of transplanted allografts (4-6), especially with regard to monitoring of drug minimization protocols (7,8) and attempts toward induction of transplantation tolerance (9), have led us examine the potential importance of anti-donor antibody responses in our class I tolerance model. We have performed a retrospective analysis of known cases of antibody development in animals tolerant of renal allografts, and a prospective study on the intentional induction of such antibody. Our results suggest that when anti-donor antibodies are produced in this model, they appear to be directed toward one predominant, non-MHC allelic antigen, segregating in the herd. We have termed these antibodies ANSDA, or anti-non-SLA (Swine Leukocyte Antigen) Donor Antibodies. Our laboratory has previously reported the existence of a non-MHC allelic antigen detected serologically with a monoclonal antibody, known as PAA (10). We have therefore given the new antigen detected by ANSDA the tentative name of “Pig Allelic Antigen 2”, or PAA-2 (see discussion). Both antigens are expressed on the cell surface of peripheral blood mononuclear cells and neither appears to cause graft rejection. These findings may have implications for the importance of pre- and post-transplant donor-specific, non-HLA antibodies in human transplant patients.
RESULTS
Detection of anti-donor antibodies in tolerant animals
From among 469 class-I mismatched kidney transplants performed in miniature swine since 1992 using our standard tolerance-induction protocol (bilateral native nephrectomy and class-I disparate renal transplantation, followed by 12 days of high-dose CyA (1-3)), we identified 16 tolerant recipients (3.4%) that produced anti-donor antibodies while maintaining stable renal function (Table 1). All 16 animals were considered long-term tolerant (LTT), as defined by stable renal function and donor-specific cellular unresponsiveness in vitro for >100 days.
Table 1.
Table summarizing the 16 animals which developed ANSDA: Graft function was not affected, and all animals were tolerant in-vivo and in-vitro (data not shown). Animals developed antibodies against PAA-2 after transplantation of minor mismatched or class I mismatched kidneys (see column 5).
| No. | Animal | Sex | Protocol | SLA leading to antibody | Tolerant |
|---|---|---|---|---|---|
| 1 | 2016 | F | Tolerance induction | Class-I Disparate | Yes |
| 2 | 2107 | F | Tolerance induction1 | Class-I Disparate | Yes |
| 3 | 2816 | M | Tolerance induction1 | Class-I Disparate | Yes |
| 4 | 3013 | F | Tolerance induction1 | Class-I Disparate | Yes |
| 5 | 10233 | M | Tolerance induction1 | Class-I Disparate | Yes |
| 6 | 10310 | M | Tolerance induction1 | Class-I Disparate | Yes |
| 7 | 10349 | M | Tolerance induction1 | Class-I Disparate | Yes |
| 8 | 10511 | M | Tolerance induction1 | Class-I Disparate | Yes |
| 9 | 10719 | F | Tolerance induction1 | Class-I Disparate | Yes |
| 10 | 10817 | M | Acceptance of Retransplanted Donor MHC graft2 | Class-I Disparate | Yes |
| 11 | 10906 | M | Acceptance of Retransplanted Donor MHC graft2 | Class-I Disparate | Yes |
| 12 | 18349 | F | Abrogate stable tolerance3 | MHC matched | Yes |
| 13 | 18350 | M | Abrogate stable tolerance3 | MHC matched | Yes |
| 14 | 18351 | M | Abrogate stable tolerance3 | MHC matched | Yes |
| 15 | 18354 | M | Abrogate stable tolerance3 | MHC matched | Yes |
| 16 | 19312 | F | Abrogate stable tolerance3 | Class-I Disparate | Yes |
class I mismatched KTx + 12-day course of CyA;
class I mismatched KTx + 12-day course of CyA, graftectomy and retransplant of a donor-matched kidney without immunosuppression >100 days after the first Tx;
class I mismatched KTx + 12-day course of CyA, graftectomy and retransplant of a kidney (donor-matched or recipient-matched) without immunosuppression >100 days after the first Tx;
Antibody Specificity
To determine whether the antibodies produced by these 16 animals were specific for MHC antigens of the kidney donor, their sera were tested against PBMCs of animals bearing a variety of different MHC haplotypes. Both positive and negative reactivities were observed on target cells from both donor and recipient MHC-matched animals (Table 2), implying that the antibodies were ANSDA, directed toward an antigen (or antigens) determined by a non-MHC linked gene (or genes). We have described in the past a different, non-MHC allelic antigen known as “pig allelic antigen” (PAA) detected by a monoclonal antibody (10). Because of the different allelic distribution, we have given the new putative antigen the name of “Pig Allelic Antigen 2”, or PAA-2 (see Discussion).
Table 2.
Antibody was directed at a common antigen, or set of antigens: Sera from three animals (18439, 19312, and 20392) which developed antibodies to PAA2 were tested against target cells from different SLA sublines. Animals were originally in experiments performed several years apart. None of the tested sera reacted to cells from the other antibody-producing animals. Positive and negative reactions were observed on target cells from different MHC sublines (SLAdd, SLAgg, SLAhh). Plus “+” signs indicate antibody binding to target PBMCs (Median Fluorescence Intensity > 65), whereas minus “-” signs indicate no binding (Median Fluorescence Intensity < 40).
| Sera from antibody-producing animals | |||
|---|---|---|---|
| Target cells (haplotype) | 18349 | 19312 | 20392 |
| 18349 (DD) | - | - | - |
| 19312 (DD) | - | - | - |
| 20392 (DD) | - | - | - |
| 20394 (HH) | - | - | - |
| 21406 (DD) | + | + | + |
| 19886 (GG) | + | + | + |
| 20077 (HH) | + | + | + |
Number of antigens detected
Since these experiments took place over >20 years, we reasoned that ANSDA from early experiments may have been directed at antigens different from those observed more recently. To assess this possibility, we tested sera form antibody-producing animals on cells from other animals that had produced antibodies. Despite the fact that these animals were from experiments separated by several years, none of the reactions were positive. This result suggested that the antibodies produced were directed toward an antigen or a set of antigens that was absent in all antibody-producing animals (Table 2).
In order to determine the number of antigens detected by the sera from antibody-producing (PAA-2 negative) animals, we performed a series of serum absorption studies. Sera from PAA-2 negative animals were absorbed on cells from PAA-2 positive animals. The supernatants from the absorbed sera were then tested back on cells from positive animals bearing different MHC haplotypes. In every case tested, cells from PAA-2 positive animals were capable of removing all reactivity to all other PAA-2 positive animals, regardless of SLA haplotype (Fig 1A and 1B), suggesting that a single antigen (or a set of antigens that segregate together) was being detected (see Discussion).
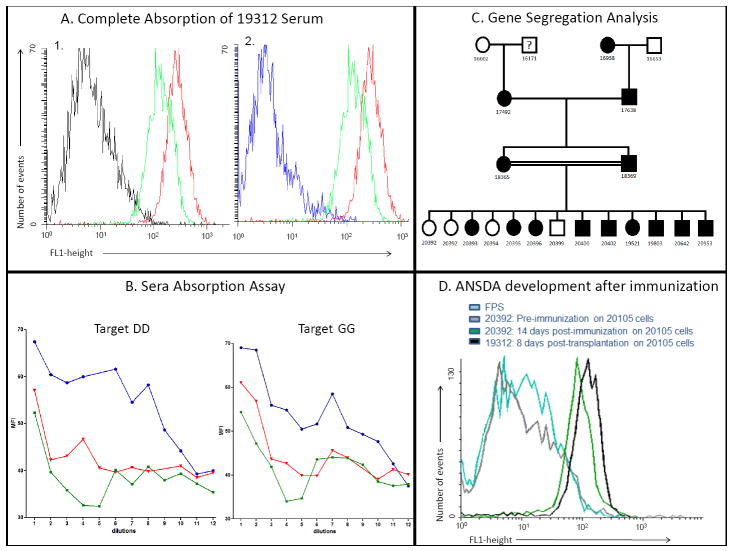
Figure 1.
A: Determining the number of antigens involved and gene segregation: In Figure 1A-1, PBMCs from a SLAdd PAA-2 positive animal were incubated for 30’ with serum from an antibody-producing SLAdd animal (19312, green curve), or fetal pig serum (FPS, black curve, negative control) or anti-SLAdd pig serum (red curve, positive control). After a wash, FITC labeled goat-anti swine IgG was added and incubated for 30’. After two more washes, the cells were analyzed by flow cytometry. Serum from 19312 showed presence of antibodies directed to an antigen present on the surface of the MHC-matched (SLAdd) target cells. Fig 1A-2: The same curves as shown in A-1, except that serum from 19312 was absorbed on PAA-2 positive cells prior to incubation with the target SLAdd PAA-2 positive cells (blue curve) and the negative control curve of fetal pig serum is not shown (for clarity, since it was essentially identical to that of the absorbed serum). Figure 1B: Representative data of the serum absorption assays. Serum from 20392, a PAA-2 negative animal that developed anti-PAA-2 antibodies after immunization, was absorbed at serial dilutions on cells from several PAA-2 positive animals bearing different MHCs (SLAdd, red curves, and SLAgg, green curves). The absorbed serum was then tested back on target cells from animals from the SLAdd (left panel) and SLAgg (right panel) lines. Essentially identical serum absorption curves were observed for absorption with either SLAdd and SLAgg cells, indicating that PAA-2 is a single antigen, or a set of antigens that segregate together. The blue lines are positive controls of serial dilutions of unabsorbed sera tested on the same target cells. Figure 1C: Pedigree of a family of animals analyzed in this study. By convention, circles indicate female animals and squares male animals. The segregation of PAA-2 positive (filled) vs. negative (open) animals is most consistent with Mendelian inheritance of the gene for PAA-2 in an autosomal dominant manner. Cells from animal 16171 were unfortunately not available for testing. Figure 1D: Antibody production in a naïve PAA-2 negative animal after immunization with a PAA-2 positive skin graft and subcutaneous PBMCs injections: Prior to immunization (grey curve) 20392 had no detectable anti-PAA-2 antibodies to 20105 (an animal known to be PAA-2 positive). Animal 20392 developed antibodies to PAA-2 after immunization with a skin graft and subcutaneous PBMCs injection (green curve). Animal 19312 had known anti-PAA-2 antibodies and was used as a positive control (black curve). The turquoise curve is a negative control in which the target cells were incubated with FPS before staining with the secondary goat-anti swine antibody.
Dominance and segregation of PAA-2
By analyzing the pedigree of one of the antibody-producing animals, a family inheritance analysis could be constructed (Fig. 1C). PAA-2 positive animals were present in every generation. Also, in a large litter with PAA-2 positive and negative siblings, the frequency of PAA-2 positive animals was about 70% (9 of 13). These observations suggested that PAA-2 was likely to be inherited in an autosomal dominant manner. No significant correlation was observed between PAA-2 and swine leucocyte antigens (SLA) or PAA-1 (data not shown), suggesting that the gene(s) encoding PAA-2 does not appear to be linked to the MHC nor to the gene encoding PAA-1.
Response to PAA-2 positive skin grafts
Of 55 young animals in the herd screened prospectively, 3 were found to be negative for PAA-2 (5.5%), consistent with expectations from the retrospective data (see above). The difference between the incidence of PAA-2 negative animals identified prospectively by screening of the current herd (5.5%) versus retrospectively (3.4%) was not significant (p=0.44). In order to study antibody development prospectively, one PAA-2 negative animal (20392) underwent skin grafting from an MHC-matched, PAA-2 positive donor (20105). The animal developed low titers of antibody to PAA-2 by 8 days after skin grafting. Subsequent booster immunizations with donor PBMCs injected subcutaneously, led to high and sustained levels of anti-PAA-2 activity (Fig. 1D).
DISCUSSION
In 1999, our laboratory reported a monoclonal mouse anti-pig antibody detecting a non-MHC-linked cell surface antigen, not associated with rejection, which we previously named PAA (10). All animals in our herd are routinely screened for the presence of this antigen, which is frequently used to detect chimerism following hematopoietic cell transplants between SLA-matched animals (10). Unlike PAA, which was detected following xenogeneic immunization (i.e. mouse anti-pig) the ANSDA antibodies described in the present study were produced by alloimmunization and the antigen detected showed a segregation pattern unrelated to that of PAA. Thus, the specificity detected in the present study represents a second, non-SLA, pig cell-surface, allelic antigen. We therefore now propose redefinition of the previously described PAA antigen as PAA-1 and definition of this newly detected alloantigen as PAA-2.
Most of the data for animals in this study were collected retrospectively from experiments which took place over approximately 20 years. To determine if antibody-producing animals over this entire period all developed ANSDA to the same antigen (or antigens), we tested antibody-positive sera obtained (and stored frozen) from historically antibody-positive animals on PBMCs taken from a recent antibody-producing animal. We found that the historical sera failed to react on PBMCs from current producers of anti-PAA-2 antibodies. Conversely, these sera did react with current animals that were PAA-2 positive, suggesting that same antigen was responsible for ANSDA development in all of the cases tested (Table 2).
In order to determine whether ANSDA were produced against a single antigen or multiple antigens, we performed a series of serum absorption assays (Fig. 1B). Sera from animals that developed antibodies to PAA-2 were absorbed on cells from PAA-2 positive animals of different haplotypes (SLAdd and SLAgg). When the absorbed sera were tested back on cells from both the SLAdd and SLAgg lines, comparably complete absorption was observed in all cases tested. The absence of residual antibody binding to other PAA-2 positive cells after such absorptions strongly suggests that these antibodies were produced against the same antigen (or a set of antigens segregating together).
The familial analysis presented in Fig. 1C suggests that PAA-2 is segregating in a simple Mendelian autosomal dominant pattern in our herd. The dominance of PAA-2 expression is consistent with the high frequency of the PAA-2 positive animals observed in our prospective screening (approximately 95% were positive).
As the majority of our data was obtained in a retrospective manner, we sought to prospectively demonstrate the antigenicity of PAA-2 by inducing antibody development through immunization with skin and cells obtained from an animal known to express the antigen. This required the identification of an animal that did not express PAA-2. In order to do so, we prospectively screened a subset of animals in the herd. Of 55 animal tested, we identified 3 animals that did not express PAA-2. Swine 20392, a SLAdd PAA-2 negative animal, was immunized with skin and PBMCs from a SLAdd PAA-2 positive animal. This immunization led to antibody development despite the use of a donor that was MHC-matched to the recipient (i.e. SLAdd to SLAdd), thus confirming that antibody development was to non-MHC antigens. This experiment differed somewhat from our retrospective experience insofar as the skin recipient was not already tolerant of a renal allograft. It also calls attention to the potential implications of our observation in those previous tolerant animals, that antibody to PAA-2 was able to develop despite the tolerant state. We have previously demonstrated that tolerance across a class-I mismatch in MGH miniature swine is mediated by regulatory T cells (Tregs) (11,12) and that this tolerance is apparent at both the level of T cells and B cells (i.e. antibodies). We have also demonstrated that PBMCs from tolerant animals exhibit linked suppression of responses to other MHC antigens on the same antigen presenting cells (APC) when tolerant animals are exposed to F1 grafts (13). Thus, the fact that anti-PAA-2 antibodies developed in animals tolerant of a renal allograft, suggests that linked suppression does not extend to B cell responses against these non-MHC antigens, despite the fact that they are present on the same APCs. The mechanism responsible for this disparity in the control of B cell responses is not clear, but could involve the absence of antigenic determinants for T cell responses on the same cell-surface molecules.
While we have observed expression of PAA-2 on all peripheral blood mononuclear cells by flow cytometry, we have not yet studied expression on other tissues. Thus, it remains possible that PAA may be expressed only on cells of hematopoietic lineage and not on renal parenchymal cells. Such expression could explain the apparent inability of anti-PAA-2 antibodies, once formed, to negatively impact renal allograft survival. However, since the relevant APC are presumably of hematopoietic origin, it would still not explain why peripheral tolerance of MHC antigens on these APC did not prevent B cell responses to the PAA-2 antigens on these same cells. Further studies on the tissue distribution of PAA-2 could shed further light on this issue and are currently in progress.
In addition to these theoretical considerations, our results may also have implications for clinical transplantation. Previous studies have suggested an association between the presence of antinon-MHC antibodies and chronic allograft rejection (14-16). Standard crossmatch techniques generally utilize peripheral blood mononuclear cells and may not distinguish between anti-non- MHC antibodies that are or are not deleterious to allograft function. It seems possible that this difference may be dependent on the nature of the antigen toward which those antibodies are directed. Clearly, our data suggest that anti-donor antibodies directed toward PAA-2 are not harmful to the transplanted graft. Perhaps the absence of linked suppression of antibody responses to antigens like PAA-2 may correlate with the fact that antibodies to these antigens are innocuous. In that case, if this distinction could be made on the basis of an in vitro assay, no intervention would be required for appearance of such antibodies in transplant recipients.
MATERIALS AND METHODS
Animals
Animals were cared for according to the guidelines of the Massachusetts General Hospital Institutional Animal Care and Use Committee. The immunogenetic characteristics of this herd and of the intra-MHC recombinant haplotypes available have been described previously (17-19).
Kidney Transplantation and Re-transplantation
The surgical procedures for primary and kidney re-transplantation have been previously described in detail (1,3,20).
Immunosuppression and Rejection Monitoring
CyA (Sandimmune) was generously provided by Novartis Pharmaceutical Corp. (Hanover, NJ). It was administered as an intravenous suspension daily at a dose of 10 to 13 mg/kg (adjusted to maintain a trough blood level of 400–800 ng/ml) for 12 days, starting on the day of the primary renal transplantation and whole blood trough levels were determined by a monoclonal radioimmunoassay (20,21).
Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
For separation of PBMCs, freshly heparinized whole blood was diluted 1:2 with Hank’s balanced salt solution (HBSS; GIBCO BRL, Gaithersburg, MD) and the mononuclear cells were obtained by gradient centrifugation using lymphocyte separation medium (Organon Teknika, Durham, NC) as previously described (20).
Antibodies and Flow Cytometry
The presence of anti-donor immunoglobulin (IgM and IgG) in the serum of experimental swine was examined by indirect flow cytometry using a Becton Dickinson FACScalibur (Sunnyvale, CA). FITC-labeled goat anti-swine IgM or IgG polyclonal antibodies were used as secondary reagents (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD). For staining, 1 × 10ˆ6 cells per tube of donor-type PBMCs were resuspended in HBSS (GIBCO BRL, Gaithersburg, MD) and incubated for 30 minutes at 4°C with decomplemented test sera. After two washes, a saturating concentration of FITC-labeled goat anti-swine IgM or IgG was added and incubated for 30 minutes at 4°C. After a final wash, cells were analyzed by means of flow cytometry with propidium iodide gating to exclude dead cells. Fetal pig serum (FPS) was used as a control for specific binding.
Skin Grafts and Cellular Immunizations
Skin grafting in swine has been described (21,22). Briefly, split-thickness skin grafts (3×4 cm) were harvested from donors with a Zimmer dermatome and placed on graft beds on the dorsum of the recipient. Grafts were assessed daily by visualization and palpation and were considered rejected when less than 10% of the graft appeared to be viable. PAA-2 negative animal 20392 was immunized by rejection of a skin graft followed by subcutaneous injection with 2.5×10ˆ7 PBMCs from PAA-2 positive animal 20105 in 5 ml of saline.
Cellular Absorptions
Doubling dilutions of 100 μl of serum from animal 20392 (a PAA-2 negative animal that produced antibodies after immunization) were made in Hank’s solution using a V-bottom 96-well plate (Costar, Cambridge, MA), starting at a dilution of 1:16. Equal volumes of cell suspensions (at a concentration of 2×10ˆ7 cells/mL) were then added for absorption. The contents of the plates were mixed, incubated for 30 min and then centrifuged at 1600 rpm for 10 minutes. The supernatants of the cell suspensions were carefully withdrawn with a 25 μl pipette and delivered into the rows below, where 100 μl of target cells suspensions (at a concentration of 1×10ˆ7 cells/mL) were then added. The plates were mixed and incubated at 4 °C for 30 min. The plates were then washed, and a secondary, FITC labeled goat anti-swine IgG antibody was added. After a 30’ min incubation at 4 °C the plates were washed twice and the content of each well was transferred into test tubes and analyzed by flow cytometry.
Statistics
The potential significance of the change in the incidence of the PAA-2 phenotype with time was calculated using the standard formula for significance of proportions (23).
Acknowledgments
The authors would like to acknowledge Rebecca Brophy for her expert editorial assistance and Dr. Rolf Barth for his critical review of these data. This work was sponsored by a grant from the American Society of Transplantation (awarded to J. Scalea), and NIAID 5U19AI102405, RO1AI084903 and 5P01AI45897. The authors also gratefully acknowledge support from CO6RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine.
Abbreviations
- APC
antigen presenting cells
- CDC
complement-dependent cytotoxicity
- CyA
Cyclosporine A
- FPS
Fetal pig serum
- LTT
long-term tolerant
- PAA
pig allelic antigen
- PBMC
peripheral blood mononuclear cells
- SLA
Swine Leukocyte Antigen
Footnotes
No conflict of interest for each author
Reference List
- 1.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Gianello P, Fishbein JM, Sachs DH. Tolerance to primarily vascularized allografts in miniature swine. Immunol Rev. 1993;133:19–44. doi: 10.1111/j.1600-065x.1993.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosengard BR, Fishbein JM, Gianello PR, et al. Retransplantation in miniature swine: lack of a requirement for graft adaptation for maintenance of specific renal allograft tolerance. Transplantation. 1994;57:794–799. [PubMed] [Google Scholar]
- 4.Tambur AR, Bray RA, Takemoto SK, et al. Flow cytometric detection of HLA-specific antibodies as a predictor of heart allograft rejection. Transplantation. 2000;70:1055–1059. doi: 10.1097/00007890-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Lee PC, Ozawa M. Reappraisal of HLA antibody analysis and crossmatching in kidney transplantation. Clin Transpl. 2007:219–226. [PubMed] [Google Scholar]
- 6.Billen EV, Christiaans MH, van den Berg-Loonen EM. Clinical relevance of Luminex donor-specific crossmatches: data from 165 renal transplants. Tissue Antigens. 2009;74:205–212. doi: 10.1111/j.1399-0039.2009.01283.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Fructuoso AI, Santiago JL, Perez-Flores I, Calvo RN, Valero R. De novo anti-HLA antibodies in renal allograft recipients: a cross-section study. Transplant Proc. 2010;42:2874–2876. doi: 10.1016/j.transproceed.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 8.Butani L, Perez RV, Gallay BJ. Success of a steroid-minimization immunosuppression protocol for renal transplantation in the presence of donor-specific antibodies. Pediatr Transplant. 2009;13:624–627. doi: 10.1111/j.1399-3046.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchimoto Y, Huang CA, Shimizu A, Seebach J, Arn JS, Sachs DH. An allelic non-histocompatibility antigen with wide tissue distribution as a marker for chimerism in pigs. Tissue Antigens. 1999;54:43–52. doi: 10.1034/j.1399-0039.1999.540105.x. [DOI] [PubMed] [Google Scholar]
- 11.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550–559. [PubMed] [Google Scholar]
- 12.Ierino FL, Yamada K, Hatch T, Sachs DH. Preliminary in vitro evidence for regulatory cells in a miniature swine renal allograft model. Transplant Proc. 1997;29:1165. doi: 10.1016/s0041-1345(96)00515-5. [DOI] [PubMed] [Google Scholar]
- 13.Griesemer AD, LaMattina JC, Okumi M, et al. Linked suppression across an MHC-mismatched barrier in a miniature swine kidney transplantation model. J Immunol. 2008;181:4027–4036. doi: 10.4049/jimmunol.181.6.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu GD, Jin YS, Salazar R, et al. Vascular endothelial cell apoptosis induced by anti-donor non-MHC antibodies: a possible injury pathway contributing to chronic allograft rejection. J Heart Lung Transplant. 2002;21:1174–1187. doi: 10.1016/s1053-2498(02)00457-6. [DOI] [PubMed] [Google Scholar]
- 15.Carter V, Shenton BK, Jaques B, et al. Vimentin antibodies: a non-HLA antibody as a potential risk factor in renal transplantation. Transplant Proc. 2005;37:654–657. doi: 10.1016/j.transproceed.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 16.Karpinski M, Rush D, Jeffery J, et al. Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol. 2001;12:2807–2814. doi: 10.1681/ASN.V12122807. [DOI] [PubMed] [Google Scholar]
- 17.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII. Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66–71. [PubMed] [Google Scholar]
- 19.Pescovitz MD, Thistlethwaite JR, Jr, Auchincloss H, Jr, et al. Effect of class II antigen matching on renal allograft survival in miniature swine. J Exp Med. 1984;160:1495–1508. doi: 10.1084/jem.160.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497–506. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okumi M, Scalea JR, Gillon BC, et al. The induction of tolerance of renal allografts by adoptive transfer in miniature swine. Am J Transplant. 2013;13:1193–1202. doi: 10.1111/ajt.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianello PR, Lorf T, Yamada K, et al. Induction of tolerance to renal allografts across single- haplotype MHC disparities in miniature swine. Transplantation. 1995;59:884–890. [PubMed] [Google Scholar]
- 23.Milton JS. Statistical methods in the Biological and Health Sciences. McGraw Hill. 1998 [Google Scholar]