Abstract
There is strong evidence to indicate that a positively charged nitrogen of endogenous and exogenous opioid ligands forms a salt bridge with the Asp residue in the third transmembrane helix of opioid receptors. To further examine the role of this electrostatic interaction in opioid receptor binding and activation, we synthesized ‘carba’-analogues of the highly potent μ opioid analgesic carfentanil (3), in which the piperidine nitrogen was replaced with a carbon. The resulting trans isomer (8b) showed reduced, but still significant MOR binding affinity (Kiμ = 95.2 nM) with no MOR versus DOR binding selectivity and was a MOR partial agonist. The cis isomer (8a) was essentially inactive. A MOR docking study indicated that 8b bound to the same binding pocket as parent 3, but its binding mode was somewhat different. A reevaluation of the uncharged morphine derivative N-formylnormorphine (9) indicated that it was a weak MOR antagonist showing no preference for MOR over KOR. Taken together, the results indicate that deletion of the positively charged nitrogen in μ opioid analgesics reduces MOR binding affinity by 2–3 orders of magnitude and may have pronounced effects on the intrinsic efficacy and on the opioid receptor selectivity profile.
Keywords: carfentanil, ‘carba’-carfentanil, opioid activity profiles, μ opioid receptor binding mode
1. Introduction
There is strong evidence to indicate that formation of a salt bridge between the Asp residue in the third transmembrane helix ((TMH3) of opioid receptors and a positively charged nitrogen present in most non-peptide opiates and most opioid peptides plays an important role in receptor binding and activation. The recent determinations of the crystal structures of the μ (MOR), δ (DOR) and κ (KOR) opioid receptors in complex with an antagonist confirmed the existence of a salt bridge between one or two charged nitrogens of the ligand with the TMH3 Asp residue in each case.1–3 Two studies on mutations of the TMH3 Asp residue of the DOR4,5 and a subsequent re-interpretation of the results6 indicated that the protonated amine of opioid ligands indeed interacts with Asp128 in the wild-type receptor. An elegant two-dimensional mutagenesis study revealed that the carboxylate group of Asp147 (TMH3) in the MOR also engages in an electrostatic interaction with the protonated nitrogens of naltrexone and morphine.7
In opioid peptides the positively charged amino group of the N-terminal tyrosine residue is thought to play the same role as the protonated nitrogen of opiates in the interaction with the TMH3 Asp residue of opioid receptors. In cyclic opioid peptide analogues containing a 2′,6,-dimethyltyrosine (Dmt) residue in place of Tyr1, elimination of the positive charge through removal of the N-terminal amino group or its replacement with a methyl group resulted in selective antagonists with very high binding affinity8,9 and in a DOR agonist with moderate potency.10
The positively charged nitrogen of the piperidine ring in fentanyl (1) (Fig. 1) is thought to form a salt bridge with the MOR TMH3 Asp147 residue. In a fentanyl analogue containing a 3-(guanidinomethyl)-benzyl group in place of the phenyl moiety attached to the ethylamide group, the piperidine nitrogen was replaced with a carbon (2).11 The resulting cis and trans isomers (2a,2b) showed deceased MOR binding affinities but retained full MOR agonist activity, indicating that an electrostatic interaction of the protonated nitrogen in the piperidine ring of fentanil analogues with the THM3 Asp147 residue is not a requirement for receptor activation. However, these ‘carba’-analogues still carry a positive charge on the guanidine group which, according to a performed receptor docking study, interacts with Asp216 of the MOR.11 This interaction compensates to some extent for the loss of the salt bridge with Asp147.
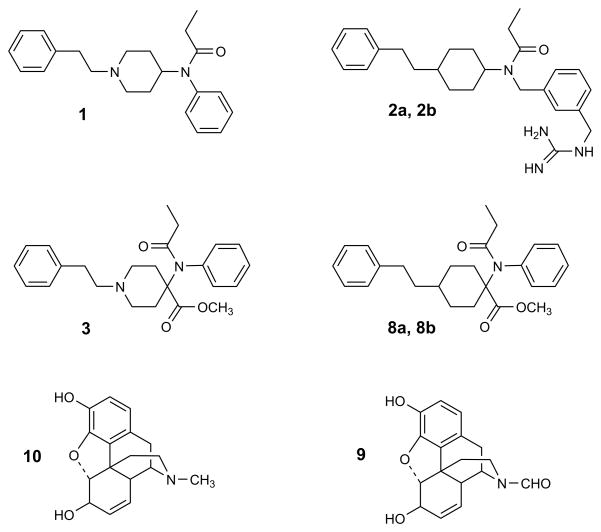
Figure 1.
Structural formulas of fentanyl (1), fentanyl analogues (2a, 2b), carfentanil (3), ‘carba’-carfentanils (8a, 8b), N-formylnormorphine (9) and morphine (10).
Carfentanil (3) is one of the most potent μ opioid analgesics known.12 In the present study we describe the synthesis and in vitro opioid activity profiles of the two isomers (trans and cis) of carfentanil in which the piperidine nitrogen was replaced with a carbon. The resulting ‘carba’-carfentanils (c-carfentanils) 8a (cis) and 8b (trans) lack a positive charge and are unable to engage in salt bridge formation with the receptor. Because of the extraordinary potency of carfentanil it was thought that a ‘carba’-analogue might still retain significant opioid receptor binding affinity despite the loss of an electrostatic interaction with the receptor. Furthermore, it was of interest to determine the effect of the carbon substitution on the intrinsic efficacy and on opioid receptor selectivity.
2. Results and discussion
2.1. Chemistry
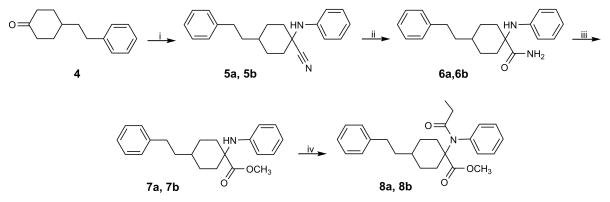
The ‘carba’-analogues of carfentanil were synthesized according to Scheme 1. Phenylethylcyclohexanone 4 was prepared as described.11 An anhydrous modification of the Strecker reaction using 4, aniline and trimethylsilyl cyanide (TMSCN) in glacial acetic acid13 yielded isomers 5a and 5b (isomer ratio 1:3) in 90% yield. The conversion of nitriles 5a and 5b to amides 6a and 6b was performed with basic H2O2 in DMSO.14 Methyl esters 7a and 7b were obtained by direct methanolysis of the amides in the presence of p-toluenesulfonic acid in a sealed tube.15 Acylation of 7a and 7b with propionic anhydride gave target compounds 8a and 8b. NMR resonance assignments based on 1H-1H and 1H-13C correlations permitted the stereochemical assignment. 8a and 8b were identified as the cis and trans isomer, respectively.
Scheme 1.
Synthetic route to compounds 8a and 8b. Reagents and conditions: (i) aniline, TMSCN, AcOH, rt, 16 h, 90%; (ii) 30% H2O2, DMSO, K2CO3 × H2O, rt, 10 – 40 h, 90 – 94%; (iii) p-TSOH, MeOH, 105 – 110° C, 4 days, 30 – 48%; (iv) (CH3CH2CO)2O, reflux, 3.5 – 72 h, 34 – 93%.
2.2. In vitro opioid activity determinations
The trans isomer of c-carfentanil (8b) showed marked binding affinity for the MOR in the nanomolar range (Kiμ = 95.2 nM) (Table 1). In comparison with the carfentanil parent (3), its MOR binding affinity is about 4000-fold decreased. Interestingly, the DOR binding affinity of 8b (Kiδ = 110 nM) is comparable to that at the MOR. This lack of selectivity is in contrast to the preference of the carfentanil parent for MOR over DOR (Kiδ/Kiμ = 138). The compound showed no significant KOR binding affinity (Kiκ > 5 μM). In the guinea pig ileum (GPI) assay, 8b turned out to be a MOR partial agonist (maximal inhibition of electrically evoked contractions = 50%) with an IC25 of 116 nM. The effect was completely reversible with naloxone (50 nM), indicating that it was mediated by opioid receptors. In the DOR-representative mouse vas deferens (MVD) assay, 8b was a weak partial agonist (70% inhibition of contractions) with an IC35 of 3890 nM. The relatively weak DOR partial agonist activity of 8b as compared to its DOR binding affinity may be due to differences between central and peripheral DORs or to somewhat impeded DOR access in the vas preparation.
Table 1.
Opioid receptor binding affinities of ‘carba’-carfentanil and N-formylnormorphine
| No. | Compound | Kiμ (nM)a | Kiδ (nM)a | Kiκ (nM)a | potency ratio
|
|---|---|---|---|---|---|
| μ/δ/κ | |||||
| 8a | c-carfentanil (cis) | > 5000 | > 5000 | > 5000 | - |
| 8b | c-carfentanil (trans) | 95.2 ± 16.3 | 110 ± 5 | > 5000 | 1/1/> 53 |
| 3 | carfentanilb | 0.024 ± 0.04 | 3.30 ± 0.04 | 43 ± 4 | 1/138/1790 |
| 9 | N-formylnormorphine | 420 ± 30 | 17700 ± 1200 | 363 ± 32 | 1/49/1 |
| 10 | morphine | 1.45 ± 0.16 | 82.5 ± 9.2 | 28.1 ± 2.8 | 1/57/19 |
Mean of 3–4 determinations ± SEM.
Data taken from ref.32.
The cis isomer of c-carfentanil (8a) showed no significant MOR-, DOR- or KOR-binding affinity at concentrations up to 5 μM. In the GPI assay, it was a very weak MOR and KOR antagonist (Keμ = 4050 nM; Keκ = 2980 nM) and it was inactive in the MVD assay. The lack of significant opioid activity of 8a can be explained with the different orientation of its phenylethyl substituent (axial position) as compared to 3 and 8b (equatorial position) (see section 2.3).
For comparative purposes, the in vitro opioid activity profile of N-formylnormorphine (9), a derivative of morphine (10) lacking a positive charge, was determined. This compound had previously been synthesized and characterized in a MOR binding assay and in a MOR [35S]GTPγS] binding assay.7 It had been found to be unable to activate the MOR, but antagonist activity had not been determined. In the opioid receptor binding assays, N-formylnormorphine (9) showed MOR affinity (Kiμ = 420 nM) 290-fold lower as compared to morphine (10) (Kiμ = 1.45 nM) and MOR versus DOR selectivity (Kiδ/Kiμ = 49) similar to that of its parent (10) (Kiδ/Kiμ = 57) (Table 1). Its binding affinity for the KOR (Kiκ = 363 nM) was similar to that to that for the MOR. Thus, unlike morphine (Kiκ/Kiμ = 19), it showed no preference for the MOR over the KOR. In the GPI assay, N-formylnormorphine (9) was a weak antagonist at the MOR (Keμ = 1240 nM) and at the KOR (Kiκ = 1890 nM). In agreement with its very low DOR binding affinity, it was inactive in the MVD assay.
2.3. Molecular modeling studies
A molecular mechanics study was performed to determine the lowest energy conformations of carfentanil and its ‘carba’-analogues. The lowest-energy conformers of all compounds have the piperidine or cyclohexane ring in the chair conformation. In the lowest-energy conformers of carfentanil (3) and the trans-’carba’-analogue (8b) the phenylethyl- and N-phenylpropionamido substituents are equatorial and the methyl carboxylate is axial. The lowest-energy conformer of the cis ‘carba’ analogue (8a) has the N-phenylpropionamido substituent in the equatorial position and the phenylethyl and methyl carboxylate groups in the axial positions.
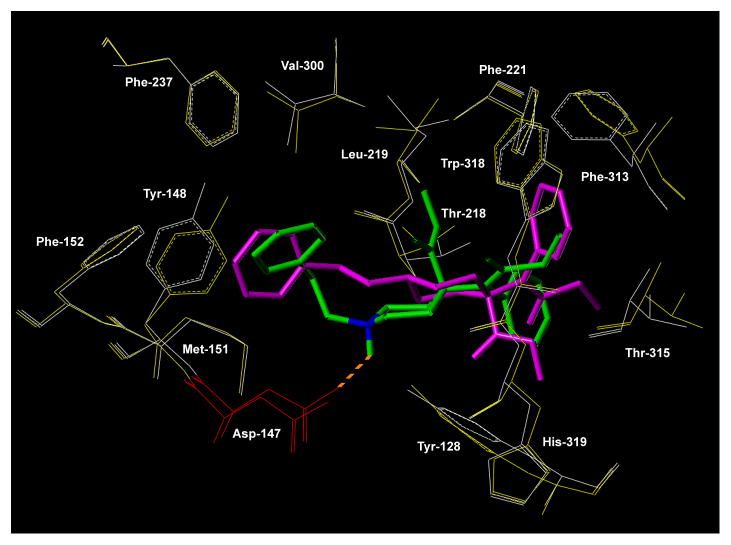
A study of flexible docking of carfentanil (3) and of the trans isomer of c-carfentanil (8b) in their lowest-energy conformations was performed using Mosberg’s model of the MOR in the activated form16 (Fig. 2). Carfentanil (3) fits into a binding pocket formed by helices 2, 3, 5, 6 and 7. Its positively charged piperidine nitrogen forms a salt bridge with Asp147 in TMH3 with a distance of 2.8 Å between the nitrogen and the Asp147 carboxylate group. The phenylethyl substituent is in an extended conformation with the phenyl ring interacting with Tyr148, Val300, Phe237, Met151 and Phe152. The phenyl ring of the N-phenylpropionamido substituent interacts with Tyr128 and Thr218, and the methyl carboxylate group is in close proximity to Trp318 and Leu219.
Figure 2.
Docking study. Carfentanil (3) in green bound to the MOR in the activated state (key residues depicted in white) and ‘carba’-carfentanil (trans) (8b) in magenta bound to the MOR in the activated state (key residues depicted in yellow). Asp147 depicted in red.
The trans ‘carba’-carfentanil analogue (8b) is located in the same binding pocket as carfentanil, but is rotated by about 180° relative to the orientation of its parent. The centroid of the cyclohexane ring of 8b is shifted by 1.8 Å relative to the centroid of the piperidine ring of 3. Obviously, there is no salt bridge formation due to the replacement of the piperidine nitrogen with a carbon which is located at an increased distance (6.7 Å) from the Asp147 -COOH group as compared to the distance between that residue and the piperidine nitrogen in carfentanil (2.8 Å). The phenylethyl substituent is in an extended conformation and its aromatic ring interacts with the same five residues with which the phenyl moiety of the phenylethyl substituent of carfentanil interacts. Due to the rotated orientation of the of MOR-bound 8b relative to 3, the two other substituents on the cyclohexane ring have different orientations as compared to the corresponding substituents in 3. The phenyl group of the N-phenylpropionamido substituent interacts with Trp318 and Phe313, and the methylcarboxylate group is located close to Thr315 and Tyr128.
3. Conclusions
The trans isomer of ‘carba’-carfentanil (8b) has about 4000-fold lower MOR binding affinity than the carfentanil parent (3), corresponding to a loss in binding energy (ΔG) of 5 kcal/mol. This reduction in binding energy is due to the inability of 8b to engage in an electrostatic interaction with Asp147. Such electrostatic interactions typically contribute about 3 – 5 kcal/mol to the binding energy in ligand-protein interactions.17 The receptor docking study revealed that while the phenylethyl substituent in 3 and 8b interacts with the same receptor residues, the MOR binding mode of 8b differs somewhat from that of 3, with its cyclohexane ring rotated and shifted relative to the to the position of the piperidine ring of 8b and with the N-phenylpropionamido- and methylcarboxylate substituent assuming a different orientation and interacting with different receptor residues. The different binding mode of 8b may induce a distinct receptor conformation, resulting in the MOR partial agonist behaviour of this compound. Interestingly, the carbon substitution also had an effect on receptor selectivity, as 8b showed no preference for MOR over DOR, in contrast to the high MOR versus DOR selectivity of 3.
In contrast to the MOR partial agonist behaviour of 8b, the ‘carba’ analogues containing a guanidinomethyl group in the meta position of the phenyl moiety attached to the ethylamido group (2a, 2b) were full agonists. In these compounds the guanidino group forms a salt bridge with Asp216. N-formylnormorphine (9) showed 290-fold lower MOR binding affinity than morphine (10), corresponding to a ΔG reduction of ~ 3.5 kcal/mol due to the loss of salt bridge formation. As described above, 9 turned out to be an antagonist with an altered MOR versus KOR binding selectivity profile. Taken together, the results obtained with 2a, 2b, 8b and 9 indicate that deletion of the positively charged nitrogen can have diverse effects on the intrinsic efficacy (agonism, partial agonism, antagonism). As formation of a salt bridge with the TMH3 Asp147 residue is no longer possible, ligand moieties other than the positively charged nitrogen present in the parent compounds may play an important role in inducing or recognizing distinct receptor conformations, leading to altered opioid activity profiles.
A number of ‘non-classical’ opioid receptor ligands lacking a positively charged nitrogen have been described in the literature. The neoclerodane diterpene salvinorin A (KOR agonist)18 and its analogue herkinorin (MOR agonist)19 are nonnitrogenous opioid receptor ligands with high binding affinities. However, there is evidence to indicate that these compounds have receptor binding modes totally different from the binding modes of all ‘classical’ nitrogen-containing agonists.20 A neutral cyclic pentapeptide, c[YpwFG] (MOR agonist)21 and an uncharged cyclic tetrapeptide, c[FpFW] (CJ-15,208) (KOR agonist),22,23 are opioid receptor ligands with quite high binding affinities that likely have receptor binding modes quite different from that of ‘classical’ opioid peptides.
4. Experimental section
4.1. Chemistry: general
Melting points were recorded on an Electrothermal melting point apparatus and are uncorrected. Reactions were monitored by ascending TLC using precoated plates (silica gel 60F254, 250 μm, Merck Darmstadt). Flash chromatography was performed with silica gel columns (particles size 230–400 μm). Preparative reversed-phase HPLC was performed using a Vydac 218-TP1022 column (22×250mm) with a linear gradient of 55–85% MeOH in 0.1% TFA/H2O over 30 min at a flow rate of 12 ml/min. Purity of compounds was determined using an analytical Vydac 218-TP54 column (5×250mm) with a linear gradient of 45–75% MeOH in 0.1% TFA/H2O over 30 min at a flow rate of 1 ml/min. High-resolution mass spectra were obtained by electrospray mass spectrometry on a hybrid Q-Tof mass spectrometer interfaced to a Mass Lynx 4.0 data system. 1H and 13C NMR spectra were recorded on Varian INOVA 500 MHz and Bruker 700 MHz AVANCEII spectrometers, and are referenced with respect to the residual signals of the solvent. Singlet, broad singlet, doublet, triplet and multiplet are abbreviated as s, br s, d, t, and m, respectively. J stands for the coupling constant measured in hertz (Hz). IR spectra were recorded on an ABB-Bomem MR Series spectrophotometer.
4.1.1. Phenethyl-1-(phenylamino)cyclohexanecarbonitrile 5a, 5b
To a stirred solution of 4-phenethylcyclohexanone 4 (3.5 g, 17.3 mmol) and aniline (1.88 g, 21 mmol) in 30 ml of glacial acetic acid, trimethylsilyl cyanide (2.48 ml, 17.3 mmol) was added dropwise over 5 min. The solution was stirred at room temperature overnight and then poured into 50 ml of NH4OH conc. and 50 ml of ice. For adjustment of the pH to 10 an additional portion of NH4OH was added. The reaction mixture was extracted with CHCl3 (3 × 50ml) and the combined organic phase was washed with water and dried over MgSO4. Evaporation of the organic layer afforded 5 (4.8 g, 90%) as a white solid of the mixture of cis and trans isomers. The isomer ratio (5a/5b = 1:3) was determined from the 1H NMR spectrum by integration of the peaks at 2.33 and 2.47 ppm, respectively. Purification by flash chromatography on silica gel with hexane/ethyl acetate (9:1) afforded 5a (0.5 g) and 5b (2.8 g).
5a (isomer 1)
yield 10%; mp 102–103° C; IR (KBr) 2234 cm−1 ∪ (CN); 1H NMR (500 MHz, CDCl3) δ 7.17–7.35 (m, 7H), 6.91–6.96 (m, 3H), 3.60 (br s, 1H), 2.62 (t, 2H, J = 8 Hz), 2.33 (d, 2H, J =14 Hz), 1.94 (t, 2H, J = 14 Hz), 1.74–1.78 (m, 2H), 1.49–1.62 (m, 1H), 1.35–1.43 (m, 2H); 13C NMR (125MHz, CDCl3) δ 143.6, 142.5, 129.5, 128.6, 128.5, 126.0, 122.4, 120.7, 117.4, 45.0, 37.7, 35.5, 34.2, 33.4, 26.6; HRMS (EI) m/e calcd for C21H25N2 [M+H]+ 305.2096, obsd 305.2019.
5b (isomer 2)
yield 50%; mp 136–138°C; IR (KBr) 2237 cm−1 ∪ (CN); 1H NMR (500 MHz, CDCl3) δ 7.19–7.32 (m, 7H), 6.92–6.95 (m, 3H), 3.82 (br s, 1H), 2.66 (t, 2H, J = 8 Hz), 2.48 (d, 2H, J =12 Hz), 1.92 (d, 2H, J = 15 Hz), 1.63 (dd, 2H, J = 6 Hz, J = 10 Hz), 1.43–1.57 (m, 4H), 1.32–1.45 (m, 1H); 13C NMR (125MHz, CDCl3) δ 143.8, 142.5, 129.5, 128.6, 125.5, 126.0, 121.2, 120.9, 118.2, 45.0, 38.2, 37.0, 36.3, 33.4, 29.3; HRMS (EI) m/e calcd for C21H25N2 [M+H]+ 305.2096, obsd 305.2016.
4.1.2. 4-Phenethyl-1-(phenylamino)cyclohexanecarboxamide (6a, 6b)
To a solution of nitrile 5a (0.3 g, 1 mmol) or 5b (0.9 g, 3 mmol) in DMSO (10 or 20 ml) at room temperature was added K2CO3 × 1.5 H2O (0.2 g or 0.6 g, respectively) and dropwise 30% H2O2 (1 or 2 ml). After 10 h for isomer 5a and 40 h for 5b no more substrate was observed (monitoring on TLC [hexane/ AcOEt =1:1]. The reaction mixture was then poured into cool water and the obtained solid precipitate was filtered and washed with cool water to give 6a (304 mg, 94%) or 6b (870 mg, 90%) respectively.
6a (isomer 1)
yield 94%; mp 230–230°C; IR (KBr) 1669 cm−1 ∪ (CO); 1H NMR (500 MHz), CDCl3) δ 6.67–7.35 (m, 12H), 4.04 (br s, 1H), 2.61 (t, 2H, J = 8Hz), 1.98–2.12 (m, 4H), 1.72 (d, 2H, J = 12 Hz), 1.53 (dd, 2H, J = 7 Hz, J = 9 Hz), 1.39–1.42 (m, 1H), 0.99–1.08 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 179.5, 144.1, 142.7, 129.3, 128.5, 128.3, 125.9, 119.3, 116.4, 60.3, 39.1, 36.3, 33.3, 31.0, 27.8; HRMS (EI) m/e calcd for C21H27N2O [M+H]+ 323.2118, obsd 323.2123.
6b (isomer 2)
yield 90%; mp 142–144°C; IR (KBr) 1668 cm−1 ∪ (CO); 1H NMR (500 MHz, CDCl3) δ 7.30–7.77 (m, 9H), 6.91 (t, 1H, J = 14 Hz), 6.77 (d, 2H, J = 7.8 Hz), 4.0 (br s, 1H), 2.61 (t, 2H, J = 8 Hz), 2.34 (m, 2H) 1.59–1.69 (m, 6H), 1.50 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 179.0, 144.5, 142.8, 129.3, 128.6, 128.5, 125.3, 119.3, 116.2, 60.0, 35.8, 34.3, 33.9, 31.9, 27.3; HRMS (EI) m/e calcd for C21H27N2O [M+H]+ 323.2118, obsd 323.2119.
4.1.3. Methyl 4-phenethyl-1-(phenylamino)cyclohexanecarboxylate (7a, 7b)
Amide 6a (250 mg, 0.77 mmol) or 6b (337 mg, 1 mmol), p-toluenesulfonic acid monohydrate (670 mg, 3.5 mmol) and 10 ml MeOH was sealed in a glass pressure vessel. The reaction vessel was kept in an oil bath at 105–110°C for 4 days. After cooling the vessel was opened and was vented. The mixture was filtered and the solvent evaporated. A concentrated aqueous solution of NH4OH was added to the residue until a pH of 8 was reached and the solution was then extracted with AcOEt (3 × 25ml). The combined organic extracts were dried (MgSO4) and evaporated. Purification by flash chromatography on silica gel with hexane/AcOEt (7:3) afforded 7a (80 mg) or 7b (100 mg) in the form of a colorless oil in both cases.
7a (isomer 1)
yield 48%;1H NMR (500 MHz, CDCl3) δ 7.15–7.3 (m, 7H), 6.75 (t, 1H, J =7 Hz), 6.57 (d, 2H, J =8 Hz), 4.03 (br s, 1H), 3.70 (s, 3H), 2.62 (t, 2H, J = 8 Hz), 2.22 (d, 2H, J =13 Hz), 1.82 (t, 2H, J =13 Hz), 1.70 (d, 2H, J = 13 Hz), 1.56 (dd, 2H, J =6 Hz, J =10 Hz), 1.26–1.40 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 177.1, 144.9, 142.8, 129.3, 128.5, 125.9, 118.7, 115.3, 60.0, 52.5, 38.7, 36.4, 33.3, 32.3, 27.5; HRMS (EI) m/e calcd for C22H28NO2 [M+H]+ 338.2115, obsd 338.2112.
7b (isomer 2)
yield 30%; 1H NMR (500 MHz, CDCl3) δ 7.12–7.35 (m, 10H), 4.0 (br s, 1H), 3.77 (s, 3H), 2.59 (t, 2H, J =8 Hz), 2.40 (d, 2H, J = 12 Hz), 1.78–1.85 (m, 4H), 1.50 (dd, 2H, J = 7 Hz, J = 9Hz), 1.37 (m, 1H), 1.09–1.17 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 173.5, 142.8, 129.4, 128.5, 128.4, 125.9, 122.1, 118.8, 63.6, 52.4, 38.1, 35.9, 33.8, 33.6, 29.1; HRMS (EI) m/e calcd for C22H28NO2 [M+H]+ 338.2115 obsd 338.2116.
4.1.4. Methyl 4-phenethyl-1-(N-phenylpropionamido)cyclohexanecarboxylate (8a, 8b)
A solution of 7a or 7b (60 mg, 0.2 nmol) in propionic anhydride (2 ml) was stirred and refluxed for 72 h or 3.5 h, respectively (completion of the reaction was monitored by TLC [hexane/AcOEt = 4:1]). After cooling the mixture was poured into ice and basified with conc. NH4OH. The separated oil was extracted into CHCl3. The organic layer was washed with H2O, dried (MgSO4) and the solvent was evaporated. Purification of the crude products by preparative HPLC afforded 8a (20 mg) or 8b (55 mg). Compound 8a was identified as the cis isomer and 8b as the trans isomer (see Supplementary data).
8a (cis isomer)
yield 34%; 1H NMR (500 MHz, CDCl3) δ 7.12–7.50 (m, 10H), 3.79 (s, 3H), 2.52 (t, 2H, J = 8 Hz), 2.17 (d, 2H, J = 4 Hz), 1.86–1.92 (m, 4H), 1.56–1.59 (m, 2H), 1.43–1.47 (m, 2H), 1.36 (m, 1H), 0.93–1.0 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 175.8, 175.0, 142.8, 140.4, 131.4. 129.2, 128.6, 128.5, 128.4, 125.9, 62.2, 52.5, 38.0, 35.0, 33.4, 31.5, 29.2, 27.5, 9.4; HRMS (EI) m/e calcd for C25H32NO3 [M+H]+ 394.2377 obsd 394.2385.
8b (trans isomer)
yield 93%; 1H NMR (500 MHz, CDCl3) δ 7.13–7.45 (m, 10H), 3.80 (s, 3H), 2.55 (t, 2H, J = 8 Hz), 2.34 (d, 2H, J = 13 Hz), 1.86 (dd, 2H, J = 7 Hz, J = 8 Hz), 1.64 (br d, 2H), 1.39–1.53 (m, 4H), 1.12–1.22 (m, 3H), 0.96 (t, 3H, J = 7 Hz); 13C NMR (125 MHz, CDCl3) δ 174.3, 174.2, 142.9, 139.7, 130.6, 129.5, 128.8, 128.5, 125.8, 64.9, 52.2, 38.6, 36.4, 34.2, 33.4, 29.3, 9.5; HRMS (EI) m/e calcd for C25H32NO3 [M+H]+ 394.2377 obsd 394.2385.
4.2. Theoretical conformational analyses and receptor docking studies
All calculations were performed using the SYBYL software version 7.0 (Tripos Associates, St. Louis, MO). The Tripos force field was used for energy calculations. A dielectric constant of 78 was used for the conformational analysis of carfentanil and ‘carba’-carfentanil. A stepwise approach was used to determine the low-energy conformations of carfentanil (3) and analogues 8a and 8b.24 First, the piperidine or cyclohexane ring was retrieved from the fragment library. The nitrogen of the piperidine ring was in the charged (protonated) form. Functional groups were attached to the six-membered ring in each of the four different arrangements: equatorial-equatorial, equatorial-axial, axial-equatorial and axial-axial, resulting in four different starting structures for each compound studied. For each structure, a systematic conformational grid search was performed to identify low-energy structures. Each exocyclic rotatable bond was rotated in 30° increments over all space. Energies were calculated and the obtained conformers were grouped into low-energy families. The lowest-energy member of each family was minimized and the resulting conformations were ranked according to energy.
The software program GLIDE (Schrödinger LLC) was used in the studies of flexible docking of compounds 3 and 8b to a model of the MOR in the activated state.16 A dielectric constant of one was used in the docking studies and in all subsequent calculations involving ligand-receptor complexes. Each of the resulting ligand-receptor complexes was minimized using the conjugate gradient approach.25 Molecular dynamics simulations of 100 ps at 300 K were performed to assess the stability of each complex. In each case, no significant change in the complex structure was observed during the simulation.
4.3. In vitro bioassays and receptor binding assays
Opioid receptor binding studies were performed as described in detail elsewhere.26 Binding affinities for the MOR and DOR were determined by displacing, respectively, [3H]DAMGO (Multiple Peptide Systems, San Diego, CA) and [3H]DSLET (Multiple Peptide Systems) from rat brain membrane binding sites and KOR binding affinities were measured by displacement of [3H]U69,593 (Amersham) from guinea pig brain membrane binding sites. Incubations were performed for 2 h at 0° C with [3H]DAMGO, [3H]DSLET and [3H]U69,593 at respective concentrations of 0.72, 0.78 and 0.80 nM. IC50 values were determined from log-dose response curves, and Ki values were calculated from the IC50 values by means of the equation of Cheng and Prusoff,27 using values of 1.3, 2.6 and 2.9 nM for the dissociation constants of [3H]DAMGO, [3H]DSLET and [3H]U69,593, respectively.
The GPI28 and MVD29 bioassays were carried out as described elsewhere.26,30 A dose-response curve was determined with [Leu5]enkephalin as standard for each ileum or vas preparation, and IC50 values of the compounds being tested were normalized according to a published procedure.31
Supplementary Material
Table 2.
GPI and MVD assay of ‘carba’-carfentanil and N-formylnormorphine
Acknowledgments
The work was supported by grants from the U.S. National Institutes of Health (DA004443) and the Canadian Institutes of Health Research (MOP-89716).
Abbreviations
- c-carfentanil
‘carba’-carfentanil
- DAMGO
H-Tyr-D-Ala-Gly-Phe(NMe)-Gly-ol
- Dmt
2′,6′-dimethyltyrosine
- DSLET
H-Tyr-D-Ser-Gly-Phe-Leu-Thr-OH
- GPI
guinea pig ileum
- HPLC
high performance liquid chromatography
- MVD
mouse vas deferens
- TFA
trifluoroacetic acid
- TLC
thin layer chromatography
- TMH3
transmembrane helix 3
- TMSCN
timethylsilyl cyanide
- U69,593
(5α,7α,8β)-(—)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Nature. 2012;485:321. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Nature. 2012;485:400. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Nature. 2012;485:327. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Befort K, Tabbara L, Bausch S, Chavkin C, Evans C, Kieffer BL. Mol Pharmacol. 1996;49:216. [PubMed] [Google Scholar]
- 5.Cavalli A, Babey AM, Loh HH. Neuroscience. 1999;93:1025. doi: 10.1016/s0306-4522(99)00280-8. [DOI] [PubMed] [Google Scholar]
- 6.Décaillot FM, Kieffer BL. In: The Delta Receptor. Chang K-J, Porreca F, Woods JH, editors. Dekker; New York: 2004. pp. 41–60. [Google Scholar]
- 7.Li J-G, Chen C, Yin J, Rice K, Zhang Y, Matecka D, de Riel JK, DesJarlais RL, Liu-Chen L-Y. Life Sci. 1999;65:175. doi: 10.1016/s0024-3205(99)00234-9. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Nguyen TMD, Weltrowska G, Berezowska I, Lemieux C, Chung NN, Schiller PW. J Med Chem. 2001;44:3048. doi: 10.1021/jm0101186. [DOI] [PubMed] [Google Scholar]
- 9.Schiller PW, Weltrowska G, Nguyen TMD, Lemieux C, Chung NN, Lu Y. Life Sci. 2003;73:691. doi: 10.1016/s0024-3205(03)00389-8. [DOI] [PubMed] [Google Scholar]
- 10.Berezowska I, Lemieux C, Chung NN, Wilkes BC, Schiller PW. Chem Biol Drug Design. 2009;74:329. doi: 10.1111/j.1747-0285.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weltrowska G, Chung NN, Lemieux C, Guo J, Lu Y, Wilkes BC, Schiller PW. J Med Chem. 2010;53:2875. doi: 10.1021/jm9019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casy AF, Parfitt T. Opioid Analgesics. Plenum; New York: 1986. pp. 287–301. [Google Scholar]
- 13.Feldman PL, Brackeen MF. J Org Chem. 1990;55:4207. [Google Scholar]
- 14.Katritzky AR, Pilarski B, Urogdi I. Synthesis. 1989:949. [Google Scholar]
- 15.Traber DF, Rahimizadeh M. J Org Chem. 1992;57:4037. [Google Scholar]
- 16.Anand JP, Purington LC, Pogozheva ID, Traynor JR, Mosberg HI. Chem Biol Drug Des. 2012;80:763. doi: 10.1111/cbdd.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burley SK, Petsko GA. Adv Protein Chem. 1988;39:125. doi: 10.1016/s0065-3233(08)60376-9. [DOI] [PubMed] [Google Scholar]
- 18.Roth BL, Bauer K, Westkaemper R, Siebel D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Proc Natl Acad Sci USA. 2002;99:11934. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J Med Chem. 2005;48:4765. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 20.Kane BE, McCurdy CR, Ferguson DM. J Med Chem. 2008;51:1824. doi: 10.1021/jm701040v. [DOI] [PubMed] [Google Scholar]
- 21.Cardillo G, Centilucci L, Tolomelli A, Spinosa R, Calienni M, Qasem AR, Spampinato S. J Med Chem. 2004;47:5198. doi: 10.1021/jm0498811. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Hiria H, Kim YJ, Kojima Y, Matsunaga Y, Nishida H, Sakakibara T, Suga O, Sujaku T, Kojima N. J Antibiot. 2002;55:847. doi: 10.7164/antibiotics.55.847. [DOI] [PubMed] [Google Scholar]
- 23.Ross NC, Kulkarni SS, McLaughlin JP, Aldrich JV. Tetrahedron. 2010;51:5020. doi: 10.1016/j.tetlet.2010.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkes BC, Schiller PW. Biopolymers. 1990;29:89. doi: 10.1002/bip.360290113. [DOI] [PubMed] [Google Scholar]
- 25.Powell MJD. Mathematical Programming. 1977;12:241. [Google Scholar]
- 26.Schiller PW, Lipton A, Horrobin DF, Bodanszky M. Biochem Biophys Res Commun. 1978;85:1332. doi: 10.1016/0006-291x(78)91149-x. [DOI] [PubMed] [Google Scholar]
- 27.Cheng YC, Prusoff WH. Biochem Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 28.Paton WDM. Br J Pharmacol Chemother. 1957;12:119. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson G, Hughes J, Kosterlitz HW. Br J Pharmacol. 1972;46:764. doi: 10.1111/j.1476-5381.1972.tb06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiMaio J, Nguyen TMD, Lemieux C, Schiller PW. J Med Chem. 1982;25:1432. doi: 10.1021/jm00354a008. [DOI] [PubMed] [Google Scholar]
- 31.Waterfield AA, Leslie FM, Lord JAH, Ling N, Kosterlitz HW. Eur J Pharmacol. 1979;58:11. doi: 10.1016/0014-2999(79)90334-0. [DOI] [PubMed] [Google Scholar]
- 32.Maguire P, Tsai N, Kamal J, Cometta-Morini C, Upton C, Loew G. Eur J Pharmacol. 1992;213:219. doi: 10.1016/0014-2999(92)90685-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.