Abstract
Tissue-nonspecific alkaline phosphatase (TNAP) is an enzyme present on the surface of mineralizing cells and their derived matrix vesicles that promotes hydroxyapatite crystal growth. Hypophosphatasia (HPP) is an inborn-error-of-metabolism that, dependent upon age of onset, features rickets or osteomalacia due to loss-of function mutations in the gene (Alpl) encoding TNAP. Craniosynostosis is prevalent in infants with HPP and other forms of rachitic disease but how craniosynostosis develops in these disorders is unknown. Objectives: Because craniosynostosis carries high morbidity, we are investigating craniofacial skeletal abnormalities in Alpl−/− mice to establish these mice as a model of HPP-associated craniosynostosis and determine mechanisms by which TNAP influences craniofacial skeletal development. Methods: Cranial bone, cranial suture and cranial base abnormalities were analyzed by micro-CT and histology. Craniofacial shape abnormalities were quantified using digital calipers. TNAP expression was suppressed in MC3T3E1(C4) calvarial cells by TNAP-specific shRNA. Cells were analyzed for changes in mineralization, gene expression, proliferation, apoptosis, matrix deposition and cell adhesion. Results: Alpl−/− mice feature craniofacial shape abnormalities suggestive of limited anterior-posterior growth. Craniosynostosis in the form of bony coronal suture fusion is present by three weeks after birth. Alpl−/− mice also exhibit marked histologic abnormalities of calvarial bones and the cranial base involving growth plates, cortical and trabecular bone within two weeks of birth. Analysis of calvarial cells in which TNAP expression was suppressed by shRNA indicates that TNAP deficiency promotes aberrant osteoblastic gene expression, diminished matrix deposition, diminished proliferation, increased apoptosis and increased cell adhesion. Conclusions: These findings demonstrate that Alpl−/− mice exhibit a craniofacial skeletal phenotype similar to that seen in infants with HPP, including true bony craniosynostosis in the context of severely diminished bone mineralization. Future studies will be required to determine if TNAP deficiency and other forms of rickets promote craniosynostosis directly through abnormal calvarial cell behavior, or indirectly due to deficient growth of the cranial base.
Keywords: bone, disorders of phosphate, animal models, craniofacial, micro-CT (μCT)
1. 1 Introduction
Hypophosphatasia (HPP) is an inborn-error-of-metabolism that, dependent upon age of onset, features rickets or osteomalacia due to loss-of function mutations in the gene (Alpl) encoding the tissue-nonspecific alkaline phosphatase isozyme (TNAP or TNSALP) [1-4]. Clinical manifestations of HPP are broad-ranging both in terms of severity and age of onset, with more severe forms of the disorder presenting in utero or infancy [5]. The HPP phenotype is primarily characterized by defective bone mineralization as a result of inadequate TNAP activity [5-8]. In the more severe forms of HPP, epilepsy may also occur due to diminished hydrolysis of pyridoxal-5’-phosphate (PLP) leading to vitamin B6 deficiency in neurons [1,2,9]. Young patients with severe HPP who survive also show an increased incidence of craniosynostosis (the premature fusion of cranial bones) [5]. Craniosynostosis was most recently reported to occur in approximately 40% of patients with infantile or childhood hypophosphatasia [9]. It is unknown how a deficiency in TNAP activity leads to this craniofacial abnormality.
Previous reports demonstrated that Alpl−/− mice phenocopy the metabolic, long bone and tooth abnormalities seen in infantile HPP [6,10,11-12], and these mice were previously used to demonstrate that a mineral-targeted recombinant form of TNAP showed biologic efficacy in a pre-clinical setting, for correcting these aspects of the disorder [13-16]. Similar to the presentation of infantile HPP in humans, Alpl−/− mice are born with a normally mineralized skeleton but develop rickets within a week after birth [6]. Alpl−/− mice die within 20 days of birth due to severe skeletal hypomineralization, respiratory insufficiency from chest deformity and seizures [10]. Significantly, treatment of Alpl−/− mice with a mineral-targeted recombinant form of human TNAP by injection initiating at birth prevents the skeletal and tooth mineralization defects seen in these mice [13-16].
Success of this treatment in mice supported rationale for testing this recombinant protein for enzyme replacement studies in human infants with life-threatening HPP. Initial results from these studies indicate that TNAP enzyme replacement therapy normalizes plasma levels of the TNAP substrates inorganic pyrophosphate and pyridoxal 5′-phosphate, improves skeletal mineralization and pulmonary function, and prevents seizures [17]. Significantly, while TNAP enzyme replacement dramatically improved skeletal mineralization, motor and cognitive function; the incidence of craniosynostosis remained at approximately 40% in treated patients, suggesting that the treatment regimen might not alter the incidence of this craniofacial abnormality [17]. Craniosynostosis can occur in utero or during early post-natal development, dependent upon genotypic abnormality and phenotypic severity [18,19]. In some infants, obvious clinical manifestations of HPP can first appear and worsen after birth [20], suggesting that the onset of craniosynostosis and therefore a therapeutic window for prevention may be postnatal in some patients. Craniosynostosis cannot be reversed after it has occurred therefore prevention is dependent upon initiating treatment prior to the onset of craniosynostosis. In utero delivery may therefore be required to prevent craniosynostosis in patients with prenatal onset. Because treatment with recombinant TNAP was initiated weeks to years after birth in the study, the presence of craniosyostosis in treated patients indicates either that treatment was initiated at too advanced a developmental time point (after the onset of craniosynostosis) or that the treatment is not efficacious for the prevention of craniosynostosis and associated craniofacial abnormalities.
Cranial sutures are fibrous joints between cranial bones. Cranial sutures are primary sites of osteogenesis, providing osteoprogenitor cells and allowing for new bone formation along the outer edge of each cranial bone. In craniosynostosis, the cranial suture is lost, cranial bones become fused and no more skull growth in that area can occur [21]. Craniosynostosis can be a debilitating condition. Severity increases with earlier age of onset and greater number of affected cranial bones [22]. While milder forms of craniosynostosis may only involve abnormal skull and/or facial shapes, more severe forms can lead to high intracranial pressure, airway impairments, brain abnormalities, blindness, deafness, seizures and death [23-32]. The only current treatment option for craniosynostosis is surgery [23].
Prompted by the recognition that craniosynostosis occurs at high prevalence in infants with HPP [5,9] and that investigational TNAP enzyme replacement therapy does not appear to alter the incidence of craniosynostosis in these patients [17], we are undertaking studies of bony craniosynostosis and craniofacial skeletal abnormalities in the Alpl−/− mouse model of infantile HPP. Our goal is to establish Alpl−/− mice as a model for the craniofacial components of infantile HPP to investigate the mechanisms by which lack of TNAP affects craniofacial skeletal development. Ultimately we hope to determine if earlier intervention with the mineral-targeted recombinant TNAP enzyme can prevent craniosynostosis and associated craniofacial abnormalities in severely affected TNAP-deficient mice.
1.2 Materials and Methods
1.2.1 Animals
Preparation and genotyping of Alpl+/− mice were previously reported [7,10]. Alpl−/− and wild type (Alpl+/+) mice were obtained by heterozygous breeding. All animals (breeders, nursing mothers and their pups, and weanlings) in this study were given free access to modified laboratory rodent diet 5001 containing increased levels (325 ppm) of pyridoxine. The Alpl−/− mice are maintained in a 12.5% C57Bl/6–87.5% 129J background. This mixed genetic background has been maintained intentionally as it results in Alpl−/− mice with variable phenotype severity reminiscent of the variability observed in human infantile HPP. Homozygous mice are identified at birth (day 0) by the lack of enzyme activity, compared to about half of the WT activity in heterozygote Alpl+/− mice. We used 0.5 μl whole blood obtained at the time of toe clipping and measured serum ALP activity in a total reaction volume of 25 μL, velocity of 30 min at OD 405, with 10 mM p- nitrophenolphosphate (pNPP) [13]. The genotype of the animals was confirmed by PCR using tail DNA obtained at the time of tissue collection as previously described [14]. All animal procedures were approved by the Sanford-Burnham Animal Care and Use Committee (La Jolla, CA, USA).
1.2.2 Linear Craniofacial Measurements
The craniofacial bones of Alpl−/− mice are present but severely under-mineralized, making visualization of some skeletal landmarks difficult on micro-computed tomographic images. Therefore, digital calipers were utilized to conduct craniofacial linear measurements. P15 (postpartum day 15) Alpl−/− (n=24), P15 Alpl+/+ (n=24), P20 Alpl−/− (n=37) and P20 Alpl+/+ (n=39) mouse skulls were carefully dissected and fixed. Linear measurements were made using previously reported skeletal landmarks [33,34], including five standard measurements currently in use by the Craniofacial Mutant Mouse Resource of the Jackson Laboratory (Bar Harbor, ME) which are skull length (nasale to paro), skull width (measured between right and left intersections between the squamosal body to the zygomatic process of the squamous portion of the temporal bone), inner canthal distance (measured between right and left intersections of frontal process of maxilla with frontal and lacrimal bones), nose length (nasale to bregma) and nasal bone length (measured from nasale to nasion). The Jackson Laboratory skull height measurement was substituted with a cranial height measurement taken between pari and the inferior portion of the spheno-occipital synchondrosis, due to the omission of the mandible in our study. Linear measurements were also calculated for frontal bone length (nasion to bregma) and parietal bone length (bregma to pari). Linear distances of bilateral structures were averaged from right and left measurements for each mouse. Measurements were performed twice and an average of the two measurements was utilized for statistical comparison of genotypes. Because the overall size of Alpl−/− mice is smaller than Alpl+/+ mice (all craniofacial linear measurements were smaller in TNAP−/−mice), linear measurements were normalized to total skull length (measured from nasale to opisthion).
1.2.3 Micro-computed tomography
Whole dissected calvaria from P15 Alpl−/− (n=24), P15 Alpl+/+ (n=24), P20 Alpl−/− (n=37) and P20 Alpl+/+ (n=39) were fixed then scanned in water at an 18 μm isotropic voxel resolution using the eXplore Locus SP micro-computed tomography imaging system (GE Healthcare Pre-Clinical Imaging, London, ON, Canada). Measurements were taken at an operating voltage of 80 kV and 80 mA of current, with an exposure time of 1600 ms using the Parker method scan technique [35], which rotates the sample 180 degrees plus a fan angle of 20 degrees. Scans were calibrated to a hydroxyapatite phantom and 3D images were reconstructed at an effective voxel size of 18 μm3. A fixed threshold of 1400 Hounsfield Units was used to discriminate mineralized tissue. Regions of interest (ROI's) for parietal and frontal bones were established as 1 mm in length, 1 mm in width, depth equivalent to thickness of bone and position starting at a 0.75 mm distance from sagittal and coronal sutures, using the Advanced ROI tool [36]. The ROI tool was also utilized to isolate bones of the cranial base for analysis. Bone volume fraction, bone mineral density, bone mineral content, tissue mineral density and tissue mineral content were measured using Microview version 2.2 software (GE Healthcare Pre-Clinical Imaging, London, ON) and established algorithms [37,38]. Micro-CT bone data was analyzed and is reported in accordance with the recommendations of Bouxsein et al. 2010 [39].
1.2.4 Craniosynostosis Assessment
Fusion of the coronal suture (fusion between frontal and parietal bones) and sagittal suture (fusion between the right and left parietal bones) was assessed using the micro-CT scans of Alpl−/− and Alpl+/+ dissected calvaria. Cranial sutures were viewed using the two-dimensional micro-CT slices in an orthogonal view across the entire length of the coronal suture, as previously described [33,34]. Due to the severity of hypomineralization seen in some calvarial bones of Alpl−/− mice, craniosynostosis was only documented by micro-CT between bones that were mineralized in these mice. Bone fusion assessments from micro-CT images were verified by visualization of all specimens under a dissecting microscope (Leica M60 TL5000; Leica Microsystems Inc., Buffalo Groves, IL).
1.2.5 Histology
Cranial bones form and grow by intramembraneous ossification while bones of the cranial base form and grow by endochondral ossification. Histologic sections were therefore taken to examine bones and sutures of the cranium, and bones and synchondroses of the cranial base. Dissected and fixed skulls were serially dehydrated, washed in isopropanol, incubated in xylene and embedded in methyl methacrylate. This method does not remove mineral and therefore allows for assessment of bone fusion. Methacrylate blocks were trimmed in the sagittal plane to within 2 mm of the sagittal suture. 4 μM sections perpendicular to the coronal suture were prepared with a Leica RM2255 microtome equipped with a tungsten carbide blade (Leica Microsystems Inc., Buffalo Groves, IL). Sections were transferred to slides and dried at 42°C in a slide press overnight. Sections were stained by Von Kossa with toluidine blue by incubation in 5% AgNO3 followed by staining in a 1% toludine blue 1% sodium borate solution.
1.2.6 Establishment of TNAP deficient cells
MC3T3E1(C4) pre-osteoblastic cranial cells were transduced with lentiviral particles expressing a puromycin resistance gene and TNAP specific shRNA (Sigma Mission) or non-target shRNA (Sigma Mission, SHC002V) in the presence of 8 ug/ml hexadimethrine bromide. Puromycin resistant colonies were expanded and tested for expression of TNAP.
1.2.7 Cell Culture and Assay
Cells were passaged in custom formulated αMEM containing no ascorbate, supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (P/S). Cells were differentiated by media supplementation with 50 μg/ml ascorbate. To induce mineralization, cells were grown for five days in αMEM containing 50 μg/ml ascorbate followed by supplementation with 2.5 mM NaPO4 or 5 mM β-glycerophosphate. For Von Kossa staining, cells were fixed with ethanol and rehydrated in a graded ethanol series. Cells were then rinsed with dH2O, incubated in 5% AgNO3, rinsed again with dH2O, and exposed to light. Alkaline phosphatase enzyme activity was assayed using the colorimetric substrate, NBT/ BCIP (Sigma). For collagenous matrix staining, cells were fixed in Bouin's fixative, washed, air-dried and then incubated with Sirius Red followed by rinsing with .01 N HCl. For quantification of mineral, alkaline phosphatase enzyme activity and Sirius Red staining; wells were scanned and densitometry was performed using NIH Image software. To assay cellular apoptosis, a Cell Death Detection kit (Roche) was utilized according to the manufacturer's instructions. This assay uses antibodies directed against DNA and histones, to quantify mono- and oligonucleosomes that are released into the cytoplasm of cells that die from apoptosis. Briefly, 10,000 cells were seeded into 96-well plates and grown in media containing 10% or 0.5% FBS for 48 hours. Cell lysate was utilized to quantify apoptosis by a colorimetric reaction and absorbance was measured at 405 nm (reference wavelength of 490 nm). To assay cellular proliferation, cells were seeded and grown in media containing 10% FBS. Cells were stained with trypan blue and counted for each time point. Type I collagen coated plates utilized in cell adhesion and mineralization assays (to determine if TNAP-deficient cell abnormalities in adhesion and/or mineralization could be rescued by supplying an exogenous collagenous matrix), were commercially obtained (Greiner Bio-One).
Real-time PCR was utilized to measure mRNA levels in TNAP deficient cells. RNA was isolated using Trizol reagent (Invitrogen) following manufacturer protocols. Real time PCR was performed utilizing the murine GAPDH primer/probe set Mm9999915_g1, the murine osteocalcin (OCN) Mm03413826_mH primer/probe set, the murine bone sialoprotein (BSP) primer/probe set Mm00492555_m1, the murine tissue non-specific alkaline phosphatase (TNAP) primer/probe set Mm00475834_m1, the murine osteopontin (OPN) primer/probe set Mm01204014_m1, the murine collagen type 1, alpha 1 (Col1a1) primer/probe set Mm00801666_g1, the murine Runx2 primer/probe set Mm00501578_m1 and Taqman Universal PCR Master Mix (Applied Biosystems). Real-time PCR was performed on a ViiA7 thermocyler (Life Technologies) and quantified by comparison to a standard curve.
1.2.8 Statistical Analysis
No significant difference between genders was found therefore genders were combined for comparison of genotypes. Student's t-tests were used to compare quantitative results between genotypes. Fisher's exact test based upon the number of fused versus patent sutures was performed to establish statistical significance between genotypes for the craniosynostosis assessment.
1.3 Results
1.3.1 Qualitative Assessment of Overall Craniofacial Phenotype in Alpl−/− Mice
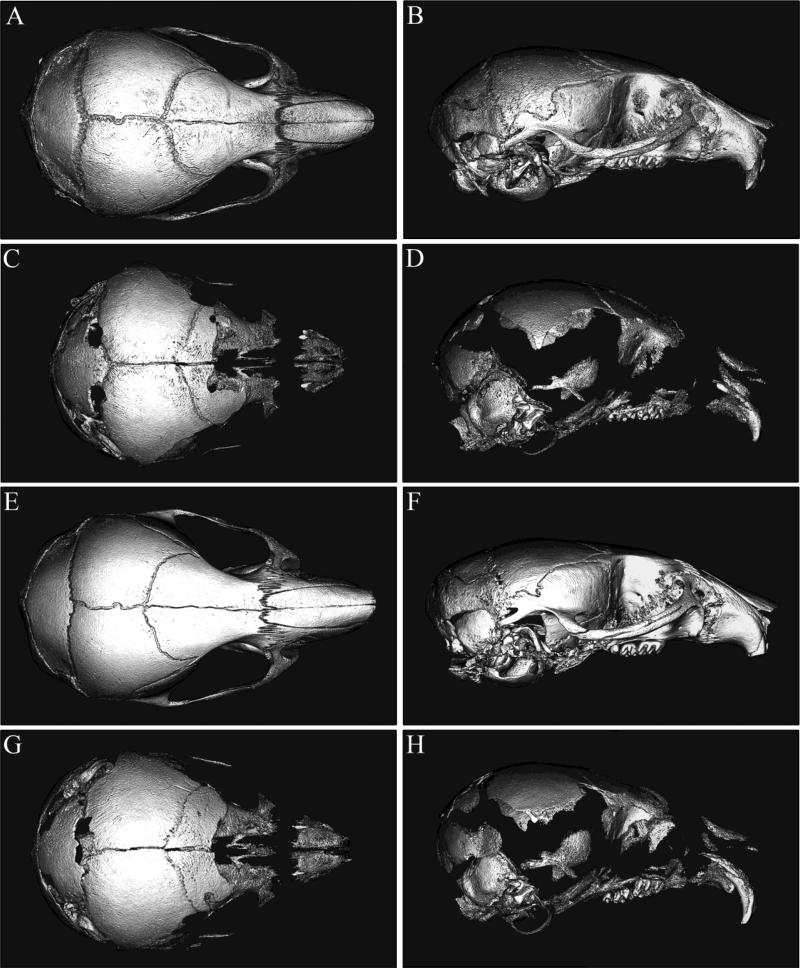
Micro-CT isosurface images of P15 and P20 Alpl−/− mouse skulls (Fig. 1C,D,G,H) revealed an obvious craniofacial phenotype involving hypomineralization and an abnormal craniofacial shape (Fig. 1A,B,E,F). Multiple bones of the cranial vault and face including the squamosal, temporal, frontal, maxillary and zygomatic bones were so severely under-mineralized in Alpl−/− mice that they in part or whole did not appear on micro-CT scans that were calibrated to a hydroxyapatite phantom and constrained to a bone tissue threshold. Parietal, interparietal, occipital, sphenoid and posterior frontal bones in contrast, appeared relatively less affected in the Alpl−/− skull. These findings suggested the presence of severe osteomalacia that is individual bone specific. Notably, while there did appear to be some increase in mineralization of parietal and frontal bones in the P20 Alpl−/− skull as compared to the P15 Alpl−/− skull, bones that were not mineralized in the P15 Alpl−/− skull were also not mineralized in the P20 Alpl−/− skull. In addition to the mineralization abnormalities, the overall shape of the Alpl−/− skull appeared different than that of the Alpl+/+ skull. The skulls of Alpl−/− mice appeared acrocephalic (taller) and brachycephalic (wider) relative to anterior-posterior length, leading to an overall dome shaped appearance when compared to Alpl+/+ skulls. These shape abnormalities were apparent in both P15 and P20 Alpl−/− mice.
Figure 1. Alpl−/− mice exhibit craniofacial shape and mineralization abnormalities.
Micro CT isosurface images of P15 Alpl+/+ (A,B), P15 Alpl−/− (C,D), and P20 Alpl+/+ (E,F) and P20 Alpl−/− (G,H) mouse skulls are shown. Multiple cranial vault and facial bones are so severely hypomineralized in P15 and P20 Alpl−/− mice that they do not appear on isosurface images calibrated to a bone threshold. The Alpl−/− skull appears decreased in anterior-posterior length but increased in height when compared to the Alpl+/+ skull, and is more dome-shaped in overall appearance. Notably, P15 and P20 Alpl−/− skulls are quite similar in overall appearance.
1.3.2 Craniofacial Skeletal Linear Analysis
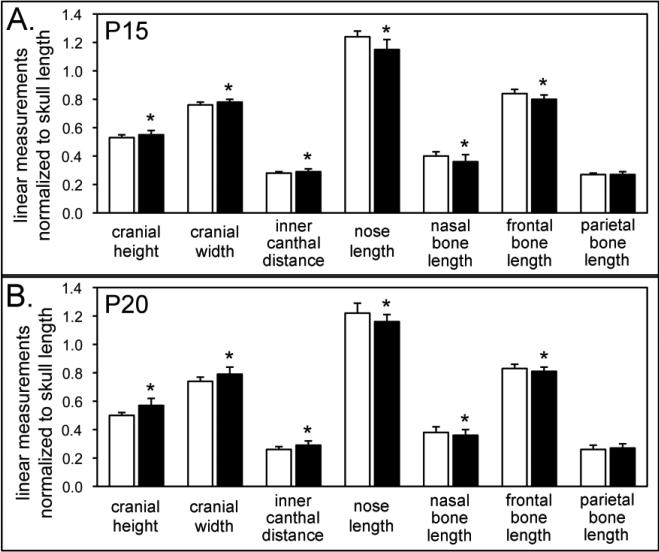
To quantify form abnormalities (form includes differences in size and shape) in the craniofacial skeleton of Alpl−/− mice, we utilized linear data generated from digital caliper measurements of Alpl−/− and Alpl+/+ skulls. These measurements demonstrated that the craniofacial form of Alpl−/− mice was consistently different than that of Alpl+/+ mice as early as two weeks after birth, in that multiple linear measurements were statistically different between P15 Alpl−/− and Alpl+/+ mice (data not shown). Similar differences were seen between P20 Alpl−/− and Alpl+/+ mice (data not shown). When normalized to skull length, cranial height, cranial width, and inner canthal distance were longer; while nose length, nasal bone and frontal bone length were shorter in P15 Alpl−/− mice when compared to Alpl+/+ mice (Fig. 2A). Parietal bones were not different in Alpl−/− and Alpl+/+ mice. Similar results were seen for P20 Alpl−/− skulls (Fig. 2B). Together these results supported our subjective assessment of the Alpl−/− skull as being different in form from that of Alpl+/+ mice. Because we report measurements that are normalized for skull size, these results also indicated that Alpl−/− P15 and P20 skulls were different in shape from those of Alpl+/+ mice. Overall the skulls of Alpl−/− mice are acrocephalic and brachycephalic compared to Alpl+/+ mice, similar to that which has been reported in infants with severe HPP [5,9].
Figure 2. Linear analysis of craniofacial forms demonstrates craniofacial shape abnormalities in Alpl−/− mice.
The craniofacial form of Alpl−/− mice is different than that of Alpl+/+ mice as early as two weeks after birth. Linear measurements demonstrate significantly increased cranial height, cranial width and inner canthal distance, with significantly diminished nose, nasal bone and frontal bone lengths in both P15 (A) and P20 (B) Alpl−/− mice, as compared to Alpl+/+ mice. Parietal bone length is not different between Alpl−/− and Alpl+/+ mice at either age. Together, these differences cause the skulls of Alpl−/− mice to be shorter in anterior-posterior length, but taller and wider compared to Alpl+/+ mice. *p < .05 vs. Alpl+/+. White = Alpl+/+, black = Alpl−/−.
1.3.3 Micro-CT Based Assessments of Craniofacial Bone Volume, Density and Mineral Content
To determine the extent to which mineralization of the craniofacial skeleton was affected in Alpl−/− mice, we performed micro-CT based analyses of bone volume, density and mineral content on calvarial and cranial base bones of Alpl−/− and Alpl+/+ mice. Because isosurface images based upon the micro-CT scans (Fig. 1) showed apparently diminished craniofacial bone mineralization that appeared more severe in frontal than parietal bones, and because we suspected based upon the overall skull shape that these two bones could be involved in fusions, we specifically quantified bone parameters for these two bones. Results (Table 1) show that normalized micro-CT based parameters of P15 Alpl−/− frontal and parietal bones (bone volume fraction, bone mineral density and tissue mineral density) were not significantly different from those of P15 Alpl+/+ frontal and parietal bones, indicating that mineralization of these bones was not significantly affected by a lack of TNAP enzyme by two weeks post-birth. In contrast, bone volume fraction, bone mineral density and tissue mineral density of P20 Alpl−/− frontal and parietal bones were significantly lower than that seen in P20 Alpl+/+ frontal and parietal bones, indicating that TNAP deficiency leads to significant hypomineralization of these calvarial bones by approximately three weeks after birth. Bone volume fraction, bone mineral density and tissue mineral density were not significantly greater in frontal bones of P20 Alpl−/− mice than those of P15 Alpl−/− mice, indicating that bone mineralization did not progress in this bone as the mice continued to slowly grow. Bone volume fraction, bone mineral density and tissue mineral density were significantly greater in parietal bones of P20 Alpl−/− mice than those of P15 Alpl−/− mice, indicating that bone mineralization did occur in this bone as the mice continued to grow, albeit to a lesser extent as that seen in wild type mice.
Table I.
Frontal Bone Volume, Density and Mineral Content in Alpl−/− and Alpl+/+ Mice.
| Bone Volume (mm3) | Bone Volume Fraction | Bone Mineral Content (mg) | Bone Mineral Density (mg/cc) | Tissue Mineral Content (mg) | Tissue Mineral Density (mg/cc) | |
|---|---|---|---|---|---|---|
| P15 TNAP−/− frontal | .008 +/− .003* | 0.54 +/− 0.14 | .007 +/− .002* | 478 +/− 43 | .005 +/− .002* | 563 +/− 23 |
| P15 TNAP+/+ frontal | .006 +/− .003 | 0.47 +/− 0.18 | .006 +/− .001 | 468 +/− 51 | .004 +/− .002 | 557 +/− 30 |
| P20 TNAP−/− frontal | .008 +/− .005* | 0.54 +/− 0.28 | .007 +/− .003* | 478 +/− 111* | .005 +/− .003* | 588 +/− 34* |
| P20 TNAP+/+ frontal | .010 +/− .004 | 0.65 +/− 0.25 | .009 +/− .002 | 538 +/− 77 | .006 +/− .003 | 610 +/− 34 |
| P15 TNAP−/− parietal | .007 +/− .002 | 0.58 +/− 0.13 | .006 +/− .001 | 484 +/− 33 | .004 +/− .001 | 558 +/− 27 |
| P15 TNAP+/+ parietal | .007 +/− .002 | 0.61 +/− 0.12 | .006 +/− .001 | 501 +/− 30 | .004 +/− .001 | 568 +/− 19 |
| P20 TNAP−/− parietal | .011 +/− .002* | 0.68 +/− 0.01* | .009 +/− .001* | 539 +/− 32* | .007 +/− .001* | 612 +/− 21* |
| P20 TNAP+/+ parietal | .015 +/− .003 | 0.74 +/− 0.08 | .011 +/− .002 | 574 +/− 30 | .009 +/− .002 | 637 +/− 19 |
Indicates statistical significance between genotypes.
Micro-CT analysis of P15 of Alpl−/− cranial base bones showed significantly diminished tissue mineral density in P15 Alpl−/−mice, indicating that, in contrast to the calvarial bones, mineralization of cranial base bones was significantly affected by a lack of TNAP enzyme by approximately two weeks post-birth. Bone volume fraction, bone mineral density and tissue mineral density were significantly lower in P20 Alpl−/− cranial base bones than in P20 Alpl+/+ cranial base bones, indicating that osteomalacia of these bones was also present at three weeks after birth. It is also worth noting that bone volume fraction and bone mineral density decreased significantly from fifteen to twenty days after birth in Alpl−/− cranial base bones. Finally, tissue mineral density (a volume normalized bone parameter) was significantly diminished in Alpl−/− cranial base bone but not in Alpl−/− frontal or parietal bones at P15, indicating that hypomineralization of the cranial base occurs earlier during development and/or is more severe than that of calvarial bones.
1.3.4 Craniosynostosis Assessment
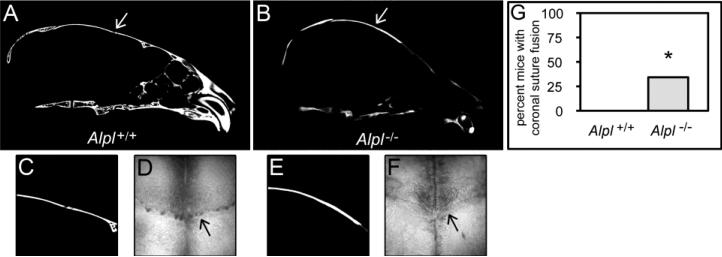
Any degree of craniosynostosis will prevent growth of involved cranial bones along the entire length of the fused suture, because separation of cranial bones is necessary for new bone growth to occur in that area [21]. The overall craniofacial shape of Alpl−/− mice is similar to that seen in patients and mice with coronal suture fusion [35,40], which suggested fusion between frontal and parietal bones of Alpl−/− mice. We therefore next assessed fusion vs. patency of the cranial sutures in P20 Alpl−/− and Alpl+/+ mice using the two-dimensional micro-CT slices in an orthogonal view across the entire length of the coronal suture [33,34]. The coronal suture was patent in all Alpl+/+ mice and partially fused in 34% of TNAP−/− mice (Fig. 3). The sagittal suture was patent in all mice, regardless of genotype. Please note that craniosynostosis was not evident in P15 mice, regardless of genotype. Presence or lack of craniosynostosis was also documented by histology (Figs. 4, 5).
Figure 3. Craniosynostosis in Alpl−/− mice.
Coronal suture fusion assessment in 20-day-old mice. Representative micro CT images show fusion of the coronal suture in Alpl−/− (B) but not in Alpl+/+ (A) skulls (white arrows point to open coronal suture in wild type and fused coronal suture in mutant). Magnification of coronal suture and bone surrounding the coronal suture inAlpl+/+ (C) and Alpl−/− (E) mice. 32× Magnification of dissected skulls also demonstrates loss of patency of the coronal future near the sagittal midline in Alpl−/− (F) mice but not Alpl+/+ (D) mice (black arrows point to patent coronal suture in Alpl+/+ mice and fused coronal suture in Alpl−/− mice). Statistical comparison of the incidence of coronal suture fusion demonstrates that a significant number of Alpl−/− mice exhibit craniosynostosis in the form of coronal suture fusion when compared to Alpl+/+ mice (G). No P20 mice exhibited fusion of the sagittal or lambdoid sutures, regardless of genotype. No P15 mice exhibited fusion of the coronal, sagittal or lambdoid sutures, regardless of genotype. Fisher's exact test, *p<.05 vs. Alpl+/+.
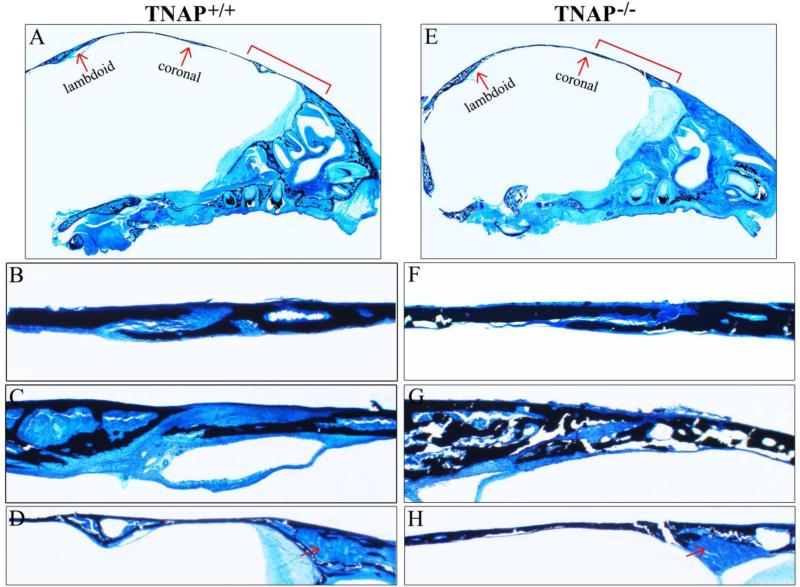
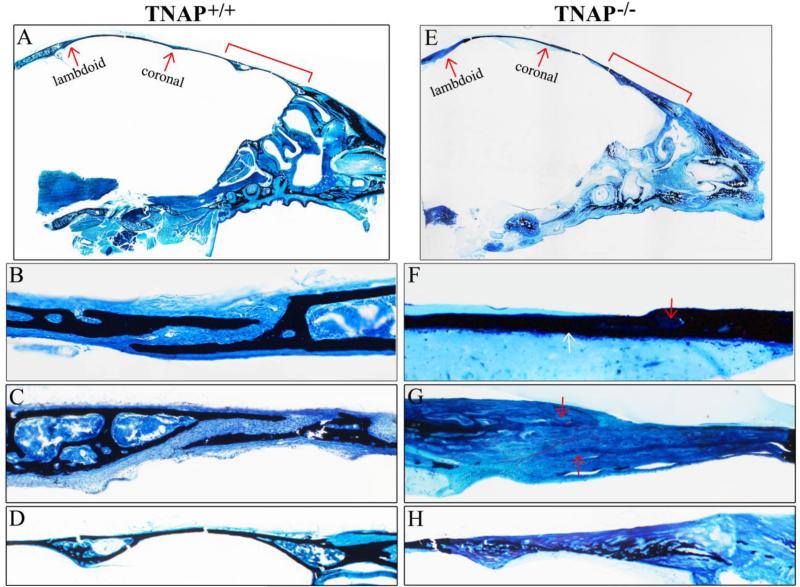
Figure 4. Histology of P15 calvarial bones and sutures.
Histologic staining of non-decalcified sagittal sections of P15 Alpl+/+ (A) and Alpl−/− (E) mouse skulls are shown (black stains mineral). Red arrows point to lamdoid and coronal sutures. (B,F) 10x magnification of Alpl+/+ (B) and Alpl−/− (F) coronal suture and surrounding tissues. (C,G) 10× magnification of Alpl+/+ (C) and Alpl−/− (G) lambdoid suture and surrounding tissues. (D,H) 10x magnification of Alpl+/+ (D) and Alpl−/− (H) anterior frontal bone (red bracket shown in A,E). In addition to diminished mineralization, the Alpl−/− bone exhibit diminished marrow space. Red arrow points to marrow in Alpl+/+ and bone matrix in Alpl−/− mice.
Figure 5. Histology of P20 calvarial bones and sutures.
Histologic staining of non-decalcified sagittal sections of P20 Alpl+/+ (A) and Alpl−/− (E) mouse skulls are shown. Red arrows point to lamdoid and coronal sutures. (B,F) 10x magnification of Alpl+/+ (B) and Alpl−/− (F) coronal suture and surrounding tissues. White arrow points to bony fusion between Alpl−/− parietal and frontal bones along the inferior aspect of the coronal suture. Red arrow points to remaining Alpl−/− coronal suture area. (C,G) 10x magnification of Alpl+/+ (C) and Alpl−/− (G) lamdoid suture and surrounding tissues. Note clearly demarcated bone surrounding cellular suture area in the Alpl+/+ skull and lack of clearly demarcated bone surrounding cellular suture area in the Alpl−/− skull. Red arrows point to thin layers of hypo-mineralization bone projecting towards the lamdoid suture in the Alpl−/− skull. Red outline highlights edges of non-mineralized bone matrix. While the lamdoid suture is patent, it appears diminished in width in the Alpl−/− skull. (D,H) 10x magnification of Alpl+/+ (D) and Alpl−/− (H) anterior frontal bone (red bracket shown in A,D). Again, note the diminished mineralization plus lack of distinct cortex, trabeculae and marrow space the Alpl−/− skull .
1.3.5 Histologic Assessment of the Craniofacial Skeleton
To further investigate fusion between calvarial bones and to assess the overall effect of TNAP deficiency on craniofacial skeletal development, we next performed histologic staining of non-decalcified tissue sections. Von Kossa with toluidine blue staining of P15 and P20 Alpl−/− mice confirmed that all craniofacial bones were present and that many bones had large regions lacking mineralization (Figs. 4, 5). Additionally, while calvarial and facial bones of Alpl+/+ mice appeared well-defined with clearly established cortices, trabeculae and marrow; many Alpl−/− calvarial and facial bones appeared thickened, with disorganized bone morphology including no clear distinction between cortical and trabecular bone, and diminished or absent marrow spaces. The coronal suture appeared normally patent in both P15 and P20 Alpl+/+mice. The coronal suture was also patent in P15 Alpl−/− mice, although diminished in width when compared to that of P15 Alpl+/+mice (Fig. 4B, F). In P20 Alpl−/− mice, while some suture tissue was present, parietal and frontal bones were fused along the inferior aspect of the coronal suture (Fig. 5 B,F). The lambdoid suture was patent in both P15 and P20 Alpl−/− and Alpl+/+mice although lamdoid suture width was diminished in both P15 and P20 Alpl−/− mice (Fig. 4C,G; Fig. 5C,G). Thickened and hypo-mineralized bone matrix was present in bones surrounding the lambdoid suture in P20 Alpl−/− mice. The anterior frontal bone also exhibited thickening and hypomineralization with diminished marrow space that was worse in P20 than P15 in Alpl−/− mice (Fig. 4D,H; Fig. 5D,H). These results demonstrate that bony craniosynostosis does not appear in Alpl−/− mice until approximately three weeks after birth.
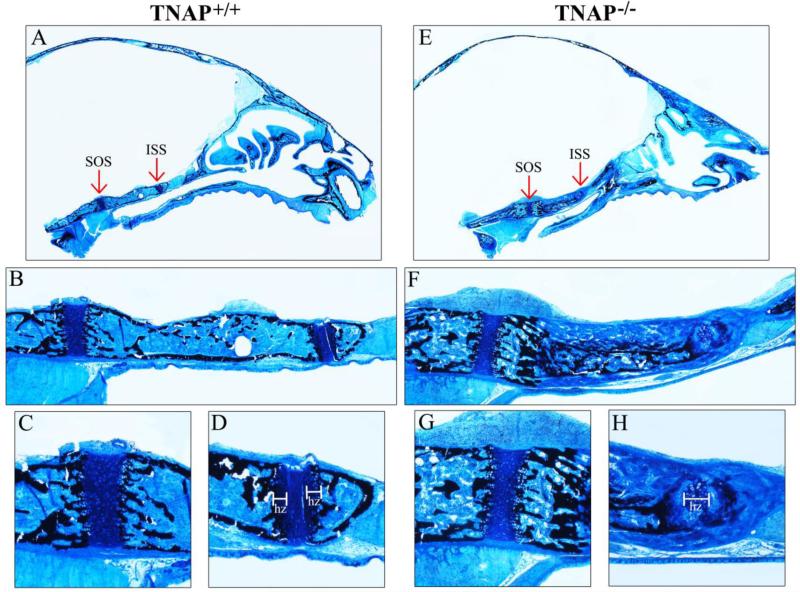
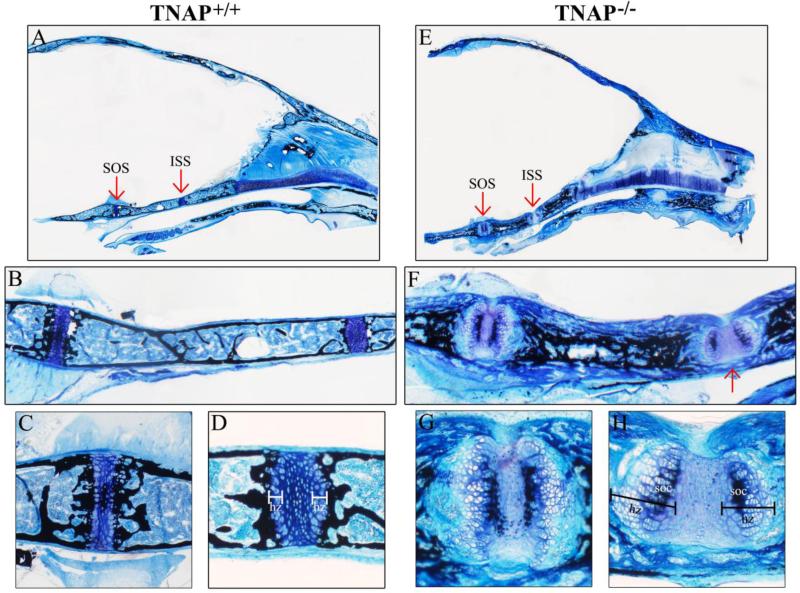
We also performed histologic staining of the cranial base. Abnormalities were evident in both cartilaginous and bony aspects of the P15 and P20 Alpl−/− cranial base. In P15 Alpl−/− skulls, the sphenoid exhibited a thick layer of hypo-mineralized bone matrix present external to the mineralized portion of the physis (Fig. 6F). This tissue appeared disorganized; lacking in cortex, trabeculae and marrow spaces. Additionally, while the spheno-occipital synchondrosis (SOS) of P15 Alpl−/− mice appeared fairly normal relative to that of P15 Alpl+/+ mice (Fig. 6C,G), the intersphenoidal synchondrosis (ISS) was abnormal, exhibiting a widely expanded single hypertrophic zone (Fig. 6D,H). Mineral was also absent from hypertrophic cell columns in the ISS of P15 Alpl−/− mice. More striking abnormalities were seen in cartilaginous and bony aspects of the P20 Alpl−/− cranial base (Fig. 7). The bone of both the sphenoid and occipital bones appeared thickened and disorganized with no clear delineation between cortices and trabeculae (Fig. 7F). Mineral was present within the inner third of bone but did not extend to the outer surface. Marrow space appeared diminished. Notably, there was also evidence of nonmineralized bone matrix bridging (fusion) between anterior and posterior aspects of the sphenoid along the inferior aspects of the intersphenoidal synchondrosis (ISS). In addition, the synchondroses themselves were quite abnormal. In contrast to the single secondary ossification center present in the P20 Alpl+/+ SOS and no secondary ossification center present in the P20 Alpl+/+ ISS, both Alpl−/− synchondroses contained two secondary ossification centers and exhibited marked expansion of hypertrophic zones (Fig. 7C,D,G,H). Mineral was absent from the lateral hypertrophic zones in both the SOS and ISS of Alpl−/− mice (does not insert between hypertrophic cell columns). These findings confirm that TNAP is essential for normal endochondral bone growth, including the cranial base. Together, these results also demonstrate that cranial base abnormalities are apparent within two weeks after birth, and get worse with continued growth in Alpl−/− mice.
Figure 6. Histology of P15 cranial base bones and synchondroses.
Histologic staining of nondecalcified mid-sagittal sections of P15 Alpl+/+ (A) and Alpl−/− (E) mouse skulls are shown (black stains mineral). Red arrows point to spheno-occipital (SOS) and inter-sphenoidal (ISS) synchondroses. (B,F) 10× magnification of Alpl+/+ (B) and Alpl−/− (F) the cranial base including SOS and ISS. Note clearly demarcated cranial base cortical bone surrounding marrow and trabeculae in all Alpl+/+ cranial base bones. In contrast, the Alpl−/− posterior sphenoid bone exhibits a less well defined cortex and diminished marrow space compared that that of Alpl+/+ mice. Hypo-mineralized bone matrix is also present external to the mineralized aspect of the Alpl−/− posterior sphenoid bone. Additionally, the ISS appears markedly abnormal in the Alpl−/− skull, as compared to that seen in the Alpl+/+ skull. (C,D,G,H) 40× magnification of Alpl+/+ SOS (C), Alpl+/+ ISS (D), Alpl−/− SOS (G) and Alpl−/− ISS (H). Note presence of two laterally positioned hypertrophic zones in the Alpl+/+ ISS but one widened and medially positioned hypertrophic zone in the Alpl−/− ISS. Bone matrix is well mineralized adjacent to the ISS hypertrophic zones in Alpl+/+ but not in Alpl−/− mice. hz= hypertrophic zone.
Figure 7. Histology of P20 cranial base bones and synchondroses.
Histologic staining of non-decalcified mid-sagittal sections of P20 Alpl+/+ (A) and Alpl−/− (E) mouse skulls are shown. Red arrows point to spheno-occipital (SOS) and inter-sphenoidal (ISS) synchondroses. (B,F) 10× magnification of Alpl+/+ (B) and Alpl−/− (F) the cranial base including SOS and ISS. Note clearly demarcated cranial base cortical bone surrounding marrow and trabeculae in the Alpl+/+ skull. In contrast, the Alpl−/− cranial base bones lack distinct cortex, trabeculae and marrow. Well mineralized bone is present internal to hypo-mineralized bone matrix in the Alpl−/− cranial base bones. Red arrow points to non-mineralized bone matrix extending across the inferior aspect of the ISS, connecting the anterior to the posterior sphenoid bone. Additionally, both the SOS and ISS appear abnormal in the Alpl−/− skull, as compared to those in the Alpl+/+ skull. (C,D,G,H) 40× magnification of Alpl+/+ SOS (C), Alpl+/+ ISS (D), Alpl−/− SOS (G) and Alpl−/− ISS (H). Note presence of two secondary ossification centers plus dramatically increased width of hypertrophic zones in both the SOS and ISS of Alpl−/− mice. Bone matrix is well mineralized adjacent to the hypertrophic zones in Alpl+/+ but not in Alpl−/− mice. hz= hypertrophic zone, soc = secondary ossification center.
1.3.6 TNAP Deficient Calvarial Cells Exhibit Aberrant Osteoblastic Cell Behavior
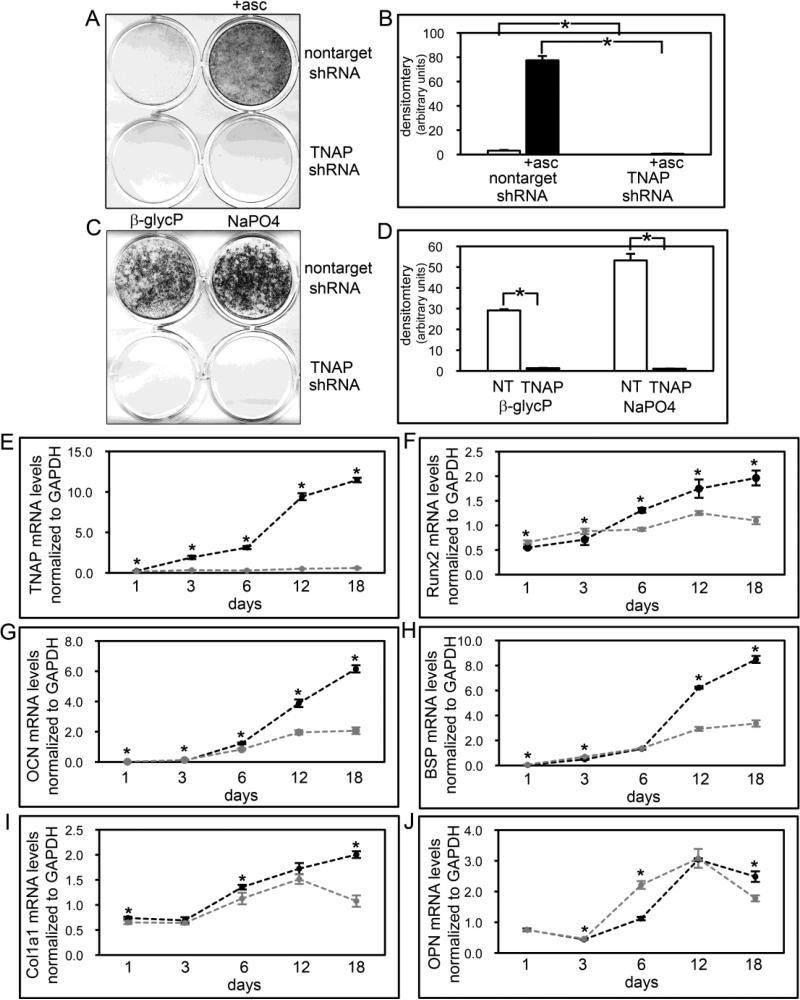
Histologic staining of Alpl−/− calvarial bones revealed apparent mineralization and bone morphologic abnormalities (bones lacked clearly defined cortical bone, trabecular bone and marrow), suggesting that the influence of TNAP on calvarial cells may extend beyond that of promoting hydroxyapatite crystal growth. Therefore, we next sought to establish how TNAP deficiency promotes abnormal calvarial bone development by stably suppressing TNAP expression via shRNA in calvarial MC3T3E1(C4) cells. Results of control experiments confirmed that TNAP shRNA expressing cells have extremely low levels of alkaline phosphatase enzyme activity and TNAP mRNA (Fig. 8A,B,E), and that these cells do not form mineralized nodules when provided with either TNAP dependent (β-glycerophosphate) or TNAP independent (NaPO4) sources of phosphate for mineralization (Fig. 8C,D). A time course of gene expression during osteoblast differentiation in culture showed a different overall pattern of mRNA expression in TNAP shRNA expressing, than non-target shRNA expressing cells (Fig. 8F-J). Runx2 mRNA expression increased upon differentiation in control cells, and this increased expression was maintained for up to 18 days of culture in differentiation media. In contrast, while Runx2 mRNA expression was also increased upon differentiation of TNAP shRNA expressing cells, it did so to a lesser extent. Additionally, the higher expression level of Runx2 was not maintained and actually decreased from 12 to 18 days of differentiation in the TNAP shRNA expressing cells. Similarly, while osteocalcin (OCN), bone sialoprotein (BSP) and col1a1 mRNA expression increased in both non-target and TNAP shRNA expressing cells at early differentiation time points, expression increased to a significantly greater extent in non-target than TNAP shRNA expressing cells at later time points of differentiation (col1a1 mRNA expression actually decreases at later time points). Osteopontin (OPN, Spp1) mRNA expression was higher in TNAP shRNA expressing cells at intermediate differentiation time points and was lower in TNAP shRNA expressing cells at later stages of differentiation. Overall the results showed that TNAP shRNA expressing cells expressed significantly higher levels of Runx2, BSP and OCN mRNA at days 1 and 3 of differentiation and OPN at days 3 and 6 of differentiation. Overall it appears that TNAP deficiency alters osteoblastic gene expression, and that the expression of osteoblastic genes may be normal or even enhanced in early and intermediately differentiated TNAP deficient calvarial cells, but this expression becomes diminished with continued differentiation, when compared to control cells.
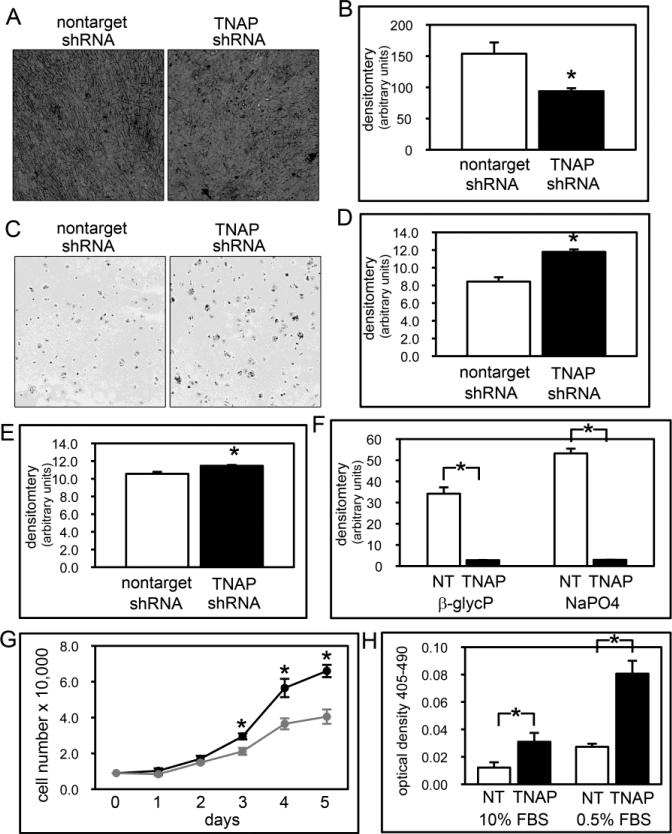
Figure 8. TNAP deficient calvarial cell mineralization and osteoblastic gene expression.
(A,B) MC3T3E1(C4) calvarial cells stably expressing TNAP specific or non-target shRNA were cultured with or without ascorbate to induce osteoblast differentiation. TNAP enzyme activity was visualized by incubation of cells with a colorimetric substrate and quantified by densitometry. Results are shown as means +/− standard deviations of triplicate experiments. (C,D) Cells were cultured with ascorbate and β-glycerophosphate or NaPO4 to induce mineralized nodule formation. Mineralized nodules were stained by Von Kossa and quantified by densitometry. (E-J) Cells were cultured with ascorbate to induce osteoblast differentiation. RNA was isolated at indicated time points. Tissue non-specific alkaline phosphatase (TNAP), osteocalcin (OCN), bone sialoprotein (BSP), col1a1 and osteopontin (OPN) mRNA levels were measured by real time PCR. Results are presented as normalized to GAPDH. Black lines = non-target shRNA, grey lines = TNAP shRNA. *p<.05 vs. non-target shRNA cells.
Because col1a1 mRNA expression was diminished in TNAP shRNA expressing cells and because histology of Alpl−/− calvarial bones showed evidence of abnormal matrix deposition (thickening, with a lack of clearly demarcated cortices or marrow spaces), we next stained the TNAP and non-target shRNA cells for collagen deposition. Sirius red staining showed apparently ample collagen deposition by both experimental and control cells, yet quantification of the stain revealed diminished collagen deposition by the TNAP shRNA expressing cells (Fig. 9B). Staining also revealed that while the non-target shRNA expressing cells exhibited a well-aligned cellular pattern that is typical of differentiating osteoblasts in culture, the TNAP shRNA expressing cells exhibited a more disorganized cellular pattern (Fig. 9A). This latter finding suggested that TNAP shRNA expressing cells were potentially interacting with their matrix in a different manner than control cells. Cell adhesion experiments subsequently showed significantly greater cell adhesion by TNAP shRNA than control cells (Fig. 9C,D). Because TNAP shRNA cells produced less collagen than control cells, we next assayed for cell adhesion on collagen-coated plates. Cell adhesion increased for control cells but did not change for the TNAP shRNA expressing cells on collagen-coated plates, diminishing the likelihood that lower collagen deposition by these cells contributed significantly to their phenotype (Fig. 9E). We also assayed for mineralization on collagen-coated plates but found no increase in mineral nodule formation by TNAP shRNA expressing cells on these plates (Fig. 9F).
Figure 9. TNAP deficient calvarial cell matrix deposition, adhesion, proliferation and apoptosis.
(A,B) Cells were cultured with ascorbate to induce osteoblast differentiation and collagen was stained with Sirius Red. Staining was quantified by densitometry. Results are shown as means +/− standard deviations of triplicate experiments. Note lack of cell alignment in in TNAP shRNA expressing, as compared to control cells. (C,D) Photograph and quantification of cell adhesion assay. Cell adhesion (E) and mineralization (F) assays were performed on collagen pre-coated plates and quantified. (G) Cells were stained with trypan blue and counted at indicated time points after plating to assay for proliferation. (H) Cells were cultured with 10% FBS (fetal bovine serum) or 0.5% FBS to induce apoptosis. Generation of apoptotic changes in DNA was assayed by a colorimetric reaction. Results are shown as means +/− standard deviations of triplicate experiments.
TNAP shRNA expressing cells also appeared to grow slower than non-target shRNA expressing cells. When quantified, results clearly showed diminished proliferation by the TNAP deficient cells (Fig. 9G). Finally, TNAP shRNA expressing cells were tested for their tendency to undergo apoptosis. Results of these experiments demonstrated that a greater percentage of TNAP shRNA expressing cells underwent apoptosis than control cells under normal and lower serum conditions (Fig. 9H). Together these results show that TNAP deficiency leads to multiple changes in calvarial osteoblastic cell behavior in vitro, and that some of these changes occur prior to osteoblast differentiation, matrix deposition and matrix mineralization.
1.4 Discussion
Alpl−/− mice exhibit significantly decreased craniofacial bone volume, density and mineral content as assessed by micro-CT. These findings are consistent with previous reports showing diminished mineralization of the skull vault in juvenile Alpl−/− mice by alizarin staining [10,43]. TNAP has long been known to be essential for long bone and tooth mineralization in both mice and humans [1-8,10-12], therefore it is not surprising that TNAP deficient mice exhibit craniofacial bone hypomineralization. It is notable that the severity of craniofacial bone hypomineralization appears to be skeletal site-specific. Alpl−/− bones of the face, for example, are present and measurable with digital calipers, but are so hypo-mineralized that they do not appear on micro-CT scans constrained to a bone tissue threshold. Future studies are required to determine why TNAP is more essential to the mineralization of some craniofacial bones over others.
A subset of Alpl−/− mice also develop craniosynostosis in the form of bony coronal suture fusion, within three weeks of birth. Craniosynostosis in HPP patients is also variable [5]. While much of this variability is likely genetic mutation dependent [44], phenotype severity can also vary in patients with the same genetic mutation [45], indicating that environmental and/or genetic modifiers likely also exist. Indeed, Alpl−/− mice, maintained in a hybrid genetic background, exhibit phenotypic variability reminiscent of the human disease, though they all carry the same Alpl null mutation. Future studies will focus on determining if craniosynostosis location and severity in inbred Alpl−/− strains are genetic background dependent [8,10].
The craniofacial shape of Alpl−/− mice is taller and wider, but shorter in anterior-posterior length. This shape is associated with coronal and/or lambdoid suture fusion in patients (Fig. 10), and is presumably due to limited anterior-posterior skull growth with compensating vertical and transverse growth [33,46], although deficient growth of the cranial base can also limit anterior-posterior skull growth [47,48]. We find that Alpl−/− mice exhibit abnormalities in both calvarial bones and the cranial base. It is worth noting here that craniofacial shape abnormalities and craniosynostosis are also seen at high incidence in other forms of rachitic disease including X-linked hypophosphatemic rickets and Vitamin D dependent rickets (19,49,50), suggesting that rickets may predispose to craniosynostosis and associated craniofacial shape abnormalities. Future studies comparing mice with a conditional ablation of Alpl only in osteoblasts to mice with a conditional ablation of Alpl in both chondrocytes and osteoblasts will be conducted to determine if TNAP deficiency in calvarial osteoblasts leads directly to craniosynostosis, or if craniosynostosis in HPP results indirectly from cranial base abnormalities.
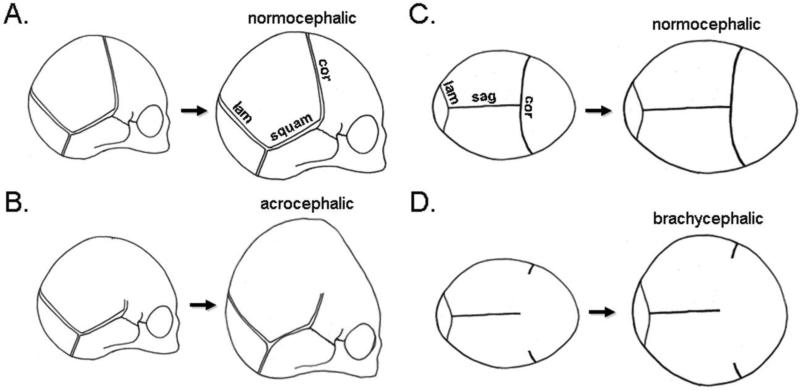
Figure 10. Schematic of coronal suture fusion influence on overall skull growth and shape.
A newborn skull is comprised of separate cranial bones separated by fibrous cranial sutures. During normal development, the skull increases in size via bony apposition along the outer edge of each cranial bone. With normal growth the skull increases in size and maintains a normocephalic shape (A,C). Upon coronal suture fusion, growth can no longer occur along the anterior aspect of the parietal bones and the posterior aspect of the frontal bones. This leads to an acrocephalic (taller relative to anterior-posterior length) (C) and/or brachycephalic (wider relative to anterior-posterior length) shape (D) due to limited anterior-posterior growth along the coronal suture with compensating vertical and transverse growth along remaining patent sutures such as the sagittal suture, the lambdoid suture and the squamosal suture. cor = coronal suture, lam = lambdoid suture, squam = squamosal suture, sag = sagittal suture.
Histologic staining revealed morphologic abnormalities in intramembraneous calvarial bones including hypomineralization, lack of clear cortices, excess osteoid and diminished marrow space. These findings are typical of rachitic disease [51,52] and demonstrate that TNAP is essential for normal bone mineralization and morphology by calvarial osteoblasts. TNAP deficiency leads to hypomineralized matrix, which can itself alter cell behavior. To determine if TNAP deficiency has more direct and/or additional influences on cell behavior, we suppressed TNAP expression by shRNA in MC3T3E1(C4) calvarial osteoblasts for in vitro assay. We found that TNAP deficient calvarial cells exhibit altered osteoblastic gene expression. Expression of Runx2, bone sialoprotein, osteocalcin, col1a1 and osteopontin were lower in TNAP shRNA than control cells at later stages of differentiation. Hypomineralization of the matrix could account for the diminished osteoblastic gene expression seen at later differentiation time points in TNAP deficient cells. In contrast, osteopontin mRNA levels were higher at six days of differentiation in TNAP shRNA than control cells, suggesting that this difference may not be due to a matrix abnormality. Early studies of primary calvarial cells isolated from Alpl−/−mice suggested no differences in osteoblastic gene expression as compared to cells isolated from Alpl +/− or Alpl+/+ mice [40] but these studies utilized semi-quantitative reverse transcription polymerase chain at one time point of differentiation, which may be less accurate and sensitive than current methods. For example, our results are consistent with more recent studies that showed that OPN levels are high in Alpl−/− cells and mice [41,42,53]. Because inorganic phosphate and pyrophosphate were previously shown to regulate OPN gene expression [54-56], altered local levels of inorganic phosphate and/or pyrophosphate due to TNAP deficiency could account for the high OPN gene expression seen in TNAP shRNA expressing cells at earlier differentiation time points. The idea that TNAP deficiency leads to changes in cell behavior independent of matrix hypomineralization is also supported by our in vitro studies showing that pre-differentiated TNAP deficient calvarial cells exhibit altered cell adhesion, proliferation and apoptosis. While previous studies demonstrated no difference in proliferation, DNA or protein content in fibroblasts isolated from HPP and control patients [57], osteoblasts from HPP patients have not yet been studied. Future studies utilizing primary osteoblastic and chondrocytic cells and tissues from Alpl−/− mice and HPP patients are needed to confirm these results.
Histologic staining also revealed marked abnormalities in the cranial base synchondroses of Alpl−/− mice including marked expansion of hypertrophic zones, lack of mineral at hypertrophic zone/bone interfaces, and duplication of the secondary zones of ossification. These results are not entirely surprising because TNAP is expressed in pre-hypertrophic and hypertrophic chondrocytes [6], and abnormal metaphyseal growth plates are seen in patients with HPP and in Alpl−/− mice [6,10,58,59]. It is worth noting here that hypertrophic zone expansion in long bones is characteristic of multiple forms of rickets [60-62]. Previous reports have also shown that low systemic inorganic phosphate levels promote premature chondrocyte differentiation and diminished hypertrophic chondrocyte apoptosis leading to hypertrophic zone expansion [63-65]. While patients and mice with HPP do not exhibit systemic hypophosphatemia [2,20,59], TNAP is an enzymatic generator of local inorganic phosphate, via its ability to hydrolyze ATP as well as PPi [7,66-68]. Our results therefore suggest the possibility that a similar mechanism involving locally reduced phosphate and accumulation of PPi, thus an altered Pi/PPi ratio, may be at work in the cranial base synchondroses of Alpl−/− mice.
Here we report that the Alpl−/− mouse model of infantile HPP exhibits craniosynostosis and associated craniofacial shape abnormalities similar to that which has been reported in life-threatening HPP [1,2,5,9]. Results presented here suggest that craniofacial skeletal abnormalities involving calvarial bones and the cranial base are present in Alpl−/− mice by two weeks after birth, that these abnormalities worsen with continued growth and that craniosynostosis occurs by three weeks after birth. This mouse model can now be utilized to determine if prenatal or early postnatal TNAP enzyme replacement therapy will significantly diminish craniosynostosis and/or cranial base abnormalities in Alpl−/− mice. Because bone structure, turnover and growth are different in rodents than humans, a primate model of HPP would also be informative.
Highlights.
Alpl−/− mice phenocopy the craniofacial skeletal abnormalities seen in infantile hypophosphtasia
Craniosynostosis is evident in a subset of Alpl−/− mice by three weeks after birth.
Alpl−/− mice show marked histologic abnormalities of calvarial bones and the cranial base within two weeks of birth.
Pre-differentiated and differentiated TNAP deficient calvarial cells exhibit abnormal cell behavior in vitro.
Table II.
Comparison of Cranial Base Bone Volume, Density and Mineral Content in Alpl−/− vs. Alpl+/+ Mice.
| Bone Volume (mm3) | Bone Volume Fraction | Bone Mineral Content (mg) | Bone Mineral Density (mg/cc) | Tissue Mineral Content (mg) | Tissue Mineral Density (mg/cc) | |
|---|---|---|---|---|---|---|
| P15 TNAP−/− | 2.0 +/− 0.8* | 0.06 +/− 0.02 | 4.1 +/−1.3* | 119 +/− 17 | 1.2 +/− 0.5* | 599 +/− 19* |
| P15 TNAP+/+ | 2.5 +/− 0.6 | 0.06 +/− 0.02 | 5.1 +/− 0.7 | 125 +/− 21 | 1.6 +/− 0.4 | 642 +/− 28 |
| P20 TNAP−/− | 1.5 +/− 0.9* | 0.04 +/− 0.02* | 3.1 +/− 1.3* | 94 +/− 26* | 0.9 +/− 0.6* | 610 +/− 25* |
| P20 TNAP+/+ | 3.4 +/− 1.1 | 0.08 +/− 0.02 | 5.7 +/− 1.4 | 127 +/− 21 | 2.3 +/− 0.8 | 667 +/− 24 |
Indicates statistical significance between genotypes.
Acknowledgements
This work was supported by National Institute of Health collaborative supplement to grant DE012889 (to J.L.M.), an Individual Biomedical Research Award from The Hartwell Foundation (to N.E.H.) and by Alexion Pharma International Sarl (Lausanne, Switzerland).
Abbreviations used are
- TNAP
tissue-nonspecific alkaline phosphatase
- HPP
hypophosphatasia
- FGFR
fibroblast growth factor receptor
- micro-CT
micro-computed tomography
- OPN
osteopontin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have no conflicts of interest.
References
- 1.Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2:40. doi: 10.1186/1750-1172-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci. 2010;1192:190–200. doi: 10.1111/j.1749-6632.2010.05387.x. [DOI] [PubMed] [Google Scholar]
- 3.OnlineMendelian Inheritance in Man and OMIM. Johns Hopkins University; Baltimore, Md, USA: 2009. MIM Number 171760 http://omim.org/entry/171760. [Google Scholar]
- 4.Millan JL. Mammalian Alkaline Phosphatases: From Biology to Applications in Medicine and Biotechnology. Wiley-VCH; 2006. [Google Scholar]
- 5.Fraser D. Hypophosphatasia. Am J Med. 1957;22(5):730–46. doi: 10.1016/0002-9343(57)90124-9. [DOI] [PubMed] [Google Scholar]
- 6.Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14(12):2015–26. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, MacGregor GR. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet. 1995;11(1):45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- 9.Collmann H, Mornet E, Gattenlöhner S, Beck C, Girschick H. Neurosurgical aspects of childhood hypophosphatasia. Childs Nerv Syst. 2009;25(2):217–23. doi: 10.1007/s00381-008-0708-3. [DOI] [PubMed] [Google Scholar]
- 10.Narisawa S, Frohlander N, Millan JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Beertsen W, VandenBos T, Everts V. Root development in mice lacking functional tissue non-specific alkaline phosphatase gene: inhibition of acellular cementum formation. J Dent Res. 1999;78(6):1221–9. doi: 10.1177/00220345990780060501. [DOI] [PubMed] [Google Scholar]
- 12.Foster BL, Nagatomo KJ, Tso HW, Tran AB, Nociti FH, Jr, Narisawa S, et al. Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J Bone Miner Res. 2013;28(2):271–82. doi: 10.1002/jbmr.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millan JL, Narisawa S, Lemire I, Loisel TP, Boileau G, Leonard P, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–87. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav MC, Lemire I, Leonard P, Boileau G, Blond L, Beliveau M, et al. Dose response of bone-targeted enzyme replacement for murine hypophosphatasia. Bone. 2011;49(2):250–6. doi: 10.1016/j.bone.2011.03.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee MD, Nakano Y, Masica DL, Gray JJ, Lemire I, Heft R. Enzyme replacement prevents dental defects in a mouse model of hypophosphatasia. J Dent Res. 2011;90:470–6. doi: 10.1177/0022034510393517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav MC, de Oliveira RC, Foster BL, Fong H, Cory E, Narisawa S, et al. Enzyme replacement prevents enamel defects in hypophosphatasia mice. J Bone Miner Res. 2012;27(8):1722–34. doi: 10.1002/jbmr.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366(10):904–13. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 18.Connolly JP, Gruss J, Seto ML, Whelan MF, Ellenbogen R, Weiss A, Buchman SR, Cunningham ML. Progressive postnatal craniosynostosis and increased intracranial pressure. Plast Reconstr Surg. 2004;113(5):1313–23. doi: 10.1097/01.prs.0000111593.96440.30. [DOI] [PubMed] [Google Scholar]
- 19.Seruya M, Oh AK, Boyajian MJ, Myseros JS, Yaun AL, Keating RF, Rogers GF. Age at initial consultation for craniosynostosis: comparison across different patient characteristics. J Craniofac Surg. 2013;24(1):96–8. doi: 10.1097/SCS.0b013e318270fb83. [DOI] [PubMed] [Google Scholar]
- 20.Whyte MP. Hypophosphatasia. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson K, Mitchell G, editors. OMMBID - The Online Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill; New York, NY: 2013. [May 16, 2014]. http://ommbid.mhmedical.com/content.aspx?bookid=474&Sectionid=45374222. [Google Scholar]
- 21.Rice DP. Developmental Anatomy of Craniofacial Sutures. In: Rice DP, editor. Craniofacial Sutures Development, Disease and Treatment. Karger, Switzerland: 2008. pp. 1–21. [Google Scholar]
- 22.Cohen MM., Jr Sutural biology and the correlates of craniosynostosis. Am J Med Genet. 1993;47:581–616. doi: 10.1002/ajmg.1320470507. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen SA, Yazdy MM, Frías JL, Honein MA. Priorities for public health research on craniosynostosis: summary and recommendations from a Centers for Disease Control and Prevention-sponsored meeting. Am J Med Genet A. 2008;146A(2):149–58. doi: 10.1002/ajmg.a.32106. [DOI] [PubMed] [Google Scholar]
- 24.Renier D, Lajeunie E, Arnaud E, Marchac D. Management of craniosynostoses. Childs Nerv Syst. 2000;16(10-11):645–58. doi: 10.1007/s003810000320. [DOI] [PubMed] [Google Scholar]
- 25.Seruya M, Oh AK, Boyajian MJ, Posnick JC, Keating RF. Treatment for delayed presentation of sagittal synostosis: challenges pertaining to occult intracranial hypertension. J Neurosurg Pediatr. 2011;8(1):40–8. doi: 10.3171/2011.4.PEDS1160. [DOI] [PubMed] [Google Scholar]
- 26.Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207(5):637–53. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreiborg S. Craniofacial growth in plagiocephaly and Crouzon syndrome. Scand J Plast Reconstr Surg. 1981;15(3):187–97. doi: 10.3109/02844318109103433. [DOI] [PubMed] [Google Scholar]
- 28.Cohen MM, Kreiborg S. Upper and lower airway compromise in the Apert syndrome. Am J Med Gene. 1992;44(1):90–3. doi: 10.1002/ajmg.1320440121. [DOI] [PubMed] [Google Scholar]
- 29.Okajima K, Robinson LK, Hart MA, Abuelo DN, Cowan LS, Hasegawa T, et al. Ocular anterior chamber dysgenesis in craniosynostosis syndromes with a fibroblast growth factor receptor 2 mutation. Am J Med Genet. 1999;85(2):160–70. doi: 10.1002/(sici)1096-8628(19990716)85:2<160::aid-ajmg11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.Shah PS, Siriwardena K, Taylor G, Steele L, Ray P, Blaser S, et al. Sudden infant death in a patient with FGFR3 P250R mutation. Am J Med Genet A. 2006;140(24):2794–6. doi: 10.1002/ajmg.a.31517. [DOI] [PubMed] [Google Scholar]
- 31.Flapper WJ, Anderson PJ, Roberts RM, David DJ. Intellectual outcomes following protocol management in Crouzon, Pfeiffer, and Muenke syndromes. J Craniofac Surg. 2009;20(4):1252–5. doi: 10.1097/SCS.0b013e3181acdf9a. [DOI] [PubMed] [Google Scholar]
- 32.Baird LC, Gonda D, Cohen SR, Evers LH, Lefloch N, Levy ML, Meltzer HS. Craniofacial reconstruction as a treatment for elevated intracranial pressure. Childs Nerv Syst. 2011;28(3):411–8. doi: 10.1007/s00381-011-1615-6. [DOI] [PubMed] [Google Scholar]
- 33.Perlyn CA, DeLeon VB, Babbs C, Govier D, Burell L, Darvann T, et al. The craniofacial phenotype of the Crouzon mouse: analysis of a model for syndromic craniosynostosis using three-dimensional MicroCT. Cleft Palate Craniofac J. 2006;43(6):740–8. doi: 10.1597/05-212. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Nam HK, Wang E, Hatch NE. Further Analysis of the Crouzon Mouse, Effects of the FGFR2C342Y Mutation are Cranial Bone Dependent. Calc Tissue Int. 2013;92(5):451–466. doi: 10.1007/s00223-013-9701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker DL. Optimal short scan convolution reconstruction for fanbeam CT. Med Phys. 1982;9(2):254–7. doi: 10.1118/1.595078. [DOI] [PubMed] [Google Scholar]
- 36.Du L Y, Umoh J, Nikolov H N, Pollmann S I, Lee TY, Holdsworth DW. A quality assurance phantom for the performance evaluation of volumetric micro-CT systems. Phys Med Biol. 2007;52:7087–108. doi: 10.1088/0031-9155/52/23/021. [DOI] [PubMed] [Google Scholar]
- 37.Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone. 2009;45(6):1104–1116. doi: 10.1016/j.bone.2009.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umoh JU, Sampaio AV, Welch I, Pitelka V, Goldberg HA, Underhill TM, et al. In vivo micro-CT analysis of bone remodeling in a rat calvarial defect model. Phys Med Biol. 2009;54(7):2147–61. doi: 10.1088/0031-9155/54/7/020. [DOI] [PubMed] [Google Scholar]
- 39.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 40.Wennberg C, Hessle L, Lundberg P, Mauro S, Narisawa S, Lerner UH, Millán JL. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J Bone Miner Res. 2000;15(10):1879–88. doi: 10.1359/jbmr.2000.15.10.1879. [DOI] [PubMed] [Google Scholar]
- 41.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millán JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164(4):1199–209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M, Terkeltaub R, Millán JL. Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(−/−) mice. J Bone Miner Res. 2006;21(9):1377–86. doi: 10.1359/jbmr.060619. [DOI] [PubMed] [Google Scholar]
- 43.Anderson HC, Harmey D, Camacho NP, Garimella R, Sipe JB, Tague S, et al. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am J Pathol. 2005;166:1711–1720. doi: 10.1016/S0002-9440(10)62481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mornet E. Hypophosphatasia: the mutations in the tissue-nonspecific alkaline phosphatase gene. Hum Mutat. 2000;15(4):309–15. doi: 10.1002/(SICI)1098-1004(200004)15:4<309::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann C, Girschick H, Mornet E, Schneider D, Jakob F, Mentrup B. Unexpected high intrafamilial phenotypic variability observed in hypophosphatasia. Eur J Hum Genet. 2014:26. doi: 10.1038/ejhg.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen MM., Jr Perspectives on craniosynostosis: sutural biology, some well-known syndromes, and some unusual syndromes. J Craniofac Surg. 2009;20(Suppl 1):646–51. doi: 10.1097/SCS.0b013e318193d48d. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg P, Arlis HR, Haworth RD, Heier L, Hoffman L, LaTrenta G. The role of the cranial base in facial growth: experimental craniofacial synostosis in the rabbit. Plast Reconstr Surg. 1997;99(5):1396–407. doi: 10.1097/00006534-199704001-00030. [DOI] [PubMed] [Google Scholar]
- 48.Stewart RE, Dixon G, Cohen A. The pathogenesis of premature craniosynostosis in acrocephalosyndactyly (Apert's syndrome). A reconsideration. Plast Reconstr Surg. 1977;59(5):699–707. doi: 10.1097/00006534-197705000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Reilly BJ, Leeming JM, Fraser D. Craniosynostosis in the Rachitic Spectrum. J Pediatr. 1964;64:396–405. doi: 10.1016/s0022-3476(64)80192-x. [DOI] [PubMed] [Google Scholar]
- 50.Gjørup H, Kjaer I, Sonnesen L, Haubek D, Beck-Nielsen SS, Hintze H, Poulsen S. Craniofacial morphology in patients with hypophosphatemic rickets: a cephalometric study focusing on differences between bone of cartilaginous and intramembranous origin. Am J Med Genet A. 2011;155A(11):2654–60. doi: 10.1002/ajmg.a.34242. [DOI] [PubMed] [Google Scholar]
- 51.Elder CJ, Bishop NJ. Rickets. Lancet. May 10. 2014;383(9929):1665–76. doi: 10.1016/S0140-6736(13)61650-5. [DOI] [PubMed] [Google Scholar]
- 52.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. British Journal of Haematology. 2007;139:351–362. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- 53.Narisawa S, Yadav MC, Millán JL. In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin. J Bone Miner Res. 2013;28(7):1587–98. doi: 10.1002/jbmr.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck GR, Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97(15):8352–7. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282(21):15872–83. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 56.Nam HK, Liu J, Li Y, Kragor A, Hatch NE. Ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) protein regulates osteoblast differentiation. J Biol Chem. 2011;286(45):39059–71. doi: 10.1074/jbc.M111.221689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whyte MP, Vrabel LA, Schwartz TD. Adult hypophosphatasia: generalized deficiency of alkaline phosphatase activity demonstrated with cultured skin fibroblasts. Trans Assoc Am Physicians. 1982;95:253–63. [PubMed] [Google Scholar]
- 58.Ornoy A, Adomian GE, Rimoin DL. Histologic and ultrastructural studies on the mineralization process in hypophosphatasia. Am J Med Genet. 1985;22(4):743–58. doi: 10.1002/ajmg.1320220410. [DOI] [PubMed] [Google Scholar]
- 59.Caswell AM, Whyte MP, Russell RG. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: clinical and laboratory aspects. Crit Rev Clin Lab Sci. 1991;28(3):175–232. doi: 10.3109/10408369109106862. [DOI] [PubMed] [Google Scholar]
- 60.Eicher EM, Southard JL, Scriver CR, Glorieux FH. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976;73(12):4667–71. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139(10):4391–6. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 63.Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci U S A. 2005;102(27):9637–42. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miedlich SU, Zalutskaya A, Zhu ED, Demay MB. Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J Biol Chem. 2010;285(24):18270–5. doi: 10.1074/jbc.M109.098616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong M, Carney DH, Jo H, Boyan BD, Schwartz Z. Inorganic phosphate induces mammalian growth plate chondrocyte apoptosis in a mitochondrial pathway involving nitric oxide and JNK MAP kinase. Calcif Tissue Int. 2011;88(2):96–108. doi: 10.1007/s00223-010-9433-5. [DOI] [PubMed] [Google Scholar]
- 66.Johnson KA, Hessle L, Vaingankar S, Wennberg C, Mauro S, et al. Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1365–1377. doi: 10.1152/ajpregu.2000.279.4.R1365. [DOI] [PubMed] [Google Scholar]
- 67.Ciancaglini P, Yadav MC, Simão AM, Narisawa S, Pizauro JM, et al. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res. 2010;25(4):716–23. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millán JL. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int. 2013;93(4):299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]