Abstract
Facial branchiomotor neurons (FBMs) of vertebrates typically develop in rhombomere 4 (r4) and in mammals and several other vertebrate taxa, migrate caudally into r6 and subsequently laterally and ventrally to the pial surface. How similar or dissimilar these migratory processes between species are at a molecular level remains unclear. In zebrafish and mouse, mutations in certain PCP genes disrupt normal caudal migration of FBMs. Zebrafish prickle1a (prickle-like 1a) and prickle1b, two orthologs of Prickle1, act non-cell autonomously and cell-autonomously, respectively, to regulate FBM migration. Here we show that in Prickle1C251X/C251X mice, which have reduced Prickle1 expression, the caudal migration of FBMs is affected. Most FBM neurons do not migrate caudally along the floor plate. However, some neurons perform limited caudal migration such that the neurons eventually lie near the pial surface from r4 to anterior r6. FBMs in Prickle1C251X/C251X mice survive until P0 and form an ectopic nucleus dorsal to the olivo-cochlear efferents of r4. Ror2, which modifies the PCP pathway in other systems, is expressed by the migrating mouse FBMs, but is not required for FBM caudal migration. Our results suggest that in mice, Prickle1 is part of a molecular mechanism that regulates FBM caudal migration and separates the FBM and the olivo-cochlear efferents. This defective caudal migration of FBMs in Prickle1C251X mutants resembles Vangl2 mutant defects. In contrast to other developing systems that show similar defects in Prickle1, Wnt5a and Ror2, Wnt5a and Ror2 only have limited or no effect on FBM caudal migration.
Keywords: Prickle1, Facial branchiomotor neurons, PCP, motoneuron migration, Ror2
Introduction
The Prickle1 (Prickle like 1) gene is important for the nervous system development: 1) mutation in PRICKLE genes leads to epilepsy in humans, mice, zebrafish and Drosophila (Bassuk, et al., 2008, Mei, et al., 2013, Tao, et al., 2011); 2) in human and zebrafish, the mutant protein fails to interact normally with REST, which is an transcriptional repressor that represses neuronal genes in non-neuronal tissues (Bassuk, et al., 2008, Mapp, et al., 2011); 3) the neurite outgrowth is affected in cell cultures or Prickle mutants (Mapp, et al., 2010, Mei, et al., 2013, Okuda, et al., 2007, Tao, et al., 2011); and 4) the caudal migration of facial branchiomotor neurons (FBMs) is impaired in zebrafish (Carreira-Barbosa, et al., 2003, Mapp, et al., 2011, Mapp, et al., 2010, Rohrschneider, et al., 2007). These results together suggest conserved function of PRICKLE from flies to humans, but whether and how Prickle1 mutation causes similar neurite outgrowth and/or neuronal migration defects in mammals has not yet been explored.
Prickle1 is believed to be an integral part of the planar cell polarity (PCP) pathway. In flies, it is recruited by the protein Vangl2 (Van Gogh like 2) to the cell membrane to establish cell polarity (Bastock, et al., 2003, Carreira-Barbosa, et al., 2003, Strutt, et al., 2013, Takeuchi, et al., 2003, Tao, et al., 2009, Tree, et al., 2002). Current data support the notion that interaction between Prickle and Vangl is conserved across phyla: vangl2 and prickle1a/1b mutants in zebrafish have similar FBM caudal migration defects (Carreira-Barbosa, et al., 2003, Mapp, et al., 2011, Mapp, et al., 2010, Rohrschneider, et al., 2007); Prickle1C251X and Vangl2lp mouse mutants have similar limb growth defects (Gao, et al., 2011, Walsh, et al., 2011, Yang, et al., 2013a); Vangl2 is critical to establish hair cell polarity in the inner ear, and the asymmetric Prickle1 protein localization is disrupted in cochlea of Smurf1 (SMAD specific E3 ubiquitin protein ligase 1) mutants suggesting that Prickle1 may play a role in establishing hair cell polarity like Vangl2 (Murdoch, et al., 2001, Narimatsu, et al., 2009, Torban, et al., 2004). These data suggest that the function of Prickle1 in the nervous and sensory system is tied to the function of Vangl2.
An example of this conserved interaction in the nervous system is the zebrafish FBM caudal migration. In hindbrain development, there is a transient phase of rhombomere formation to subdivide the rostro-caudal axis. FBMs become postmitotic in rhombomere 4 (r4) in all jawed vertebrates with a variable addition of r5 (Fritzsch, 1998, Murakami, et al., 2004, Szekely and Matesz, 1993). Their axons combine together as the facial nerve to exit at r4 on the ipsilateral side while the soma of r4 derived FBMs migrate in some vertebrates caudally to r6 and ventrolaterally to the pial surface (Fritzsch, 1998, Fritzsch and Nichols, 1993, Szekely and Matesz, 1993, Wanner, et al., 2013), where they form various subnuclei that innervate the different muscles of the face and hyoid (Komiyama, et al., 1984, Matsuda, et al., 1979, Nieuwenhuys, et al., 1998).
In zebrafish, vangl2 functions in the floor plate cells and non-cell autonomously regulates migration of the FBMs (Jessen, et al., 2002, Sittaramane, et al., 2013). Prickle1a and prickle1b are essential for FBM caudal migration in zebrafish, acting non-cell autonomously and cell-autonomously, respectively (Carreira-Barbosa, et al., 2003, Mapp, et al., 2011, Mapp, et al., 2010, Rohrschneider, et al., 2007).
The PCP pathway includes not only prickle and vangl, but also dvl, fzd and wnt. Among these genes, fzd3 is critical for FBM caudal migration in zebrafish (Jessen, et al., 2002), but neither wnt5a nor dvl has a role in zebrafish (Jessen, et al., 2002). These results suggest either a redundant or non-essential role of wnt and dvl genes in zebrafish FBM migration. These possibilities raise the question as to whether FBM caudal migration is a PCP process since only certain PCP genes are required for FBM caudal migration.
Similarly, in mice, the function of PCP in FBM caudal migration is controversial. Vangl2 protein is required for FBM caudal migration. However, Vangl2 is expressed broadly in the hindbrain, including the FBMs, before and during FBM caudal migration, and thus whether Vangl2 functions cell autonomously or non-cell autonomously is still unclear. Fzd3 is critical (Jessen, et al., 2002), while Wnt5a has only a minor role in FBM caudal migration (Vivancos, et al., 2009). Dvl1/2 are not required whereas the role of Dvl3 has not been examined (Glasco, et al., 2012, Jessen, et al., 2002). It is therefore possible that Prickle1 is required for FBM caudal migration in mice comparable to the essential role of prickle1a and/or prickle1b in zebrafish (Carreira-Barbosa, et al., 2003, Mapp, et al., 2011, Mapp, et al., 2010, Rohrschneider, et al., 2007). Here we show that Prickle1 mutation affects FBM migration in mice
Another gene associated with PCP is Ror2, which is expressed in the post-migration FBMs at E14.5 (http://www.eurexpress.org). Ror2, when bound to Wnt5a, can modulate Vangl2 activity and thus PCP signaling (Gao, et al., 2011, Wang, et al., 2011). Consistent with this, Ror2 mutants have limb phenotype similar to Vangl2, Wnt5a and Prickle1C251X mutants (Gao, et al., 2011, Raz, et al., 2008, Schwabe, et al., 2004, Wang, et al., 2011, Witte, et al., 2010, Yang, et al., 2013a). In addition, Ror2 mutants have cleft palate much like Wnt5a and Prickle1C251X mutant mice (He, et al., 2008, Yang, et al., 2013b). Since Vangl2 plays a critical role in FBM caudal migration and is expressed in FBMs, it is possible that Ror2 is also required for this process. As with Prickle1, the role of Ror2 has not been explored in mice FBM migration. We show that Ror2 is expressed in pre-migratory and migrating neurons. However, while Prickle1 is essential for FBM caudal migration, Ror2 is not essential. Our data suggests overlapping expression but a strikingly different function of Ror2 and Prickle1 in caudal migration of FBMs which contrasts sharply with their apparently similar function in the limb and palate development. These data support notions of context dependent signaling of these PCP related proteins.
Materials and Methods
Mice
All the animal treatment was approved by University of Iowa IACUC (ACURF 0804066) and (ACURF1109204). We used the Prickle Cys251X mutant mice previously described (Tao, et al., 2011, Yang, et al., 2013a, Yang, et al., 2013b). Given apparent similarity in phenotypes in at least two developing systems we also used Ror2W749X and Ror2−/− mice (Raz, et al., 2008, Takeuchi, et al., 2000). Noon on the day of vaginal plug visualization was designated as E0.5. Embryos from timed breeding were fixed in 4% paraformaldehyde (PFA). Tails were collected for PCR and sequencing for genotyping. Genotyping was conducted as previously described (Raz, et al., 2008, Takeuchi, et al., 2000, Yang, et al., 2013a).
In situ hybridization
The probes for in situ hybridization were generated by in vitro transcription from the plasmid and then labeled with digoxigenin. Tbx20, Wnt5a, Ror2, Nkx6.1 and Prickle1 probes were previously described (Glasco, et al., 2012, Müller, et al., 2003, Okuda, et al., 2007, Schwabe, et al., 2004, Song, et al., 2006). Mutant and wild-type littermate embryos were reacted in the same tube for the same probe to minimize the reaction variability. Samples were digested with 20µg/ml Proteinase K for half an hour and proceed to in situ hybridization following the protocol described previously (Duncan, et al., 2011). Samples were then mounted in glycerol and viewed in a Leica M205 FA microscope. Images were captured with Leica application suite V3. Unless indicated otherwise, at least two animals were prepared for a given stage.
Lipophilic dye tracing
FBMs were labeled with lipophilic dyes (NeuroVue; Molecular Targeting Technologies; MTTI) (Fritzsch, et al., 2005). The E12.5 and the E13.5 brains were labeled from both sides. Dye was placed into the left ear and the right orbit to label the vestibulo-cochlear efferent and facial motor neurons of the left side, and the right abducens motor neurons, respectively. This dye was false colored as green during imaging. Another dye labelling with a different color was placed into the right ear to label the vestibulo-cochlear efferent and facial motor neurons on the right side, which was false colored as red (Fritzsch, et al., 2005, Maklad and Fritzsch, 2003). The E12.5 and the E13.5 hindbrain was incubated in 4% PFA at 60°C for two days, dissected out, and mounted in open-book configuration in glycerol.
To label the VIIa nuclei, one dye was place between the eye and the ear to label the trigeminal nerve in E13.5 embryos. A second dye was place anterio-ventrally to the ear to label the facial nerve and the accessory facial nerve as they exit the stylo-mastoid foramen.
The head of E18.5 embryo was separated into halves. A dye was placed into the cochlea to specifically label the vestibulo-cochlear efferent ipsilaterally. A second dye application with a different color was placed into the tympanic segment of the facial nerve just beneath the lateral semicircular canal. After incubating the sample in 4% PFA at 60°C for seven days, the brain was dissected out and sectioned coronally into 100µm sections with a Vibratome. Sections or whole mounted brains were imaged with a Leica SP5 confocal microscope.
Plastic sectioning of the facial nerve
Previous work had indicated that FBM developmental failures can be associated with loss of facial nerve axons. Facial nerve exiting the inner ear was dissected and embedded in plastic and sectioned into 2µm thick sections as previously described (Fritzsch, et al., 1997). Sections were imaged and their area compared in the nerves: an ellipse was draw to best fit the nerve section and the area of the ellipse was calculated. Since no apparent differences in areas were found no further statistics was employed.
Results
Prickle1 is expressed by FBMs
The factors that affect neuron migration can be divided into two groups: the non-cell autonomous environment and the autonomous signals intrinsic to the neurons themselves. The former provides the directional cues to the neurons and the latter translates the signals into action (Wanner, et al., 2013). Therefore, we asked where Prickle1 was expressed in mouse hindbrain, which potentially provided clues as to whether it affected FBM migration cell-autonomously or non-cell autonomously, thus acting either similarly to prickle1a or prickle1b in zebrafish (Carreira-Barbosa, et al., 2003, Mapp, et al., 2011, Mapp, et al., 2010).
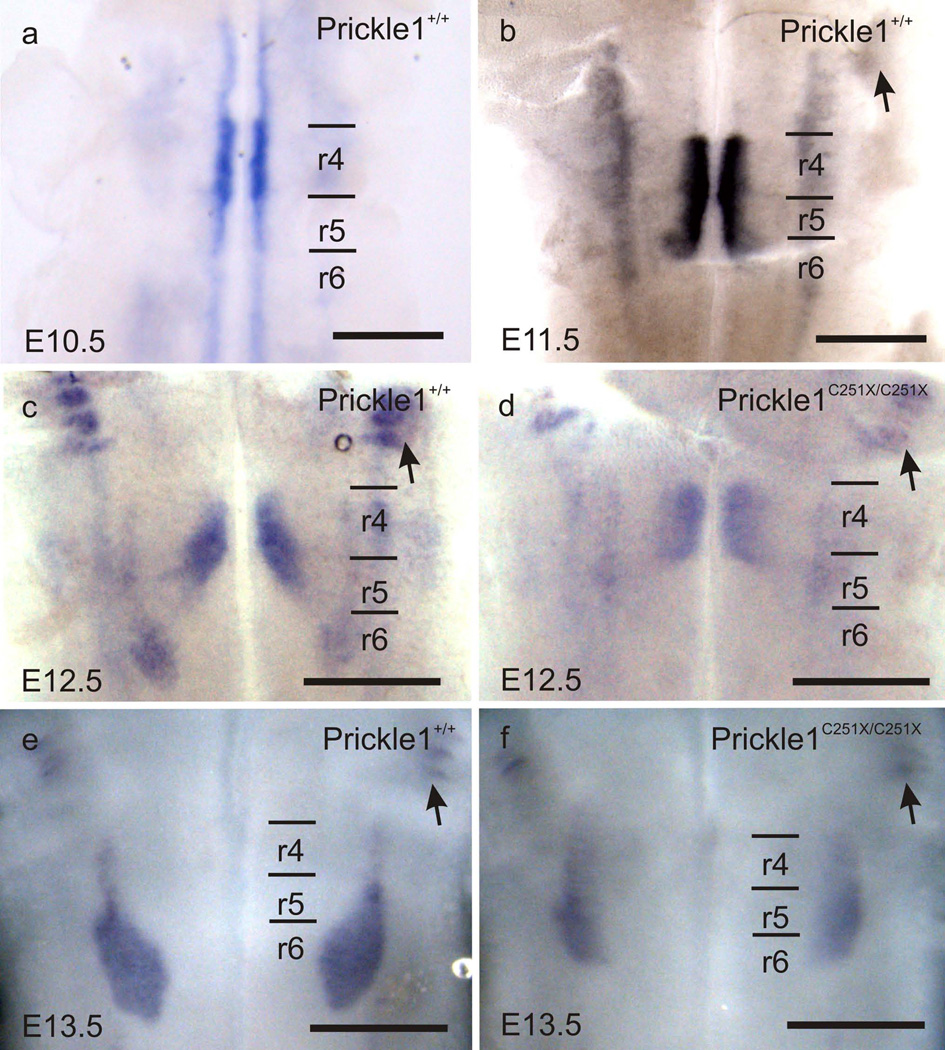
We examined Prickle1 mRNA expression in E10.5 to E13.5 mice by whole-mount in situ hybridization. At E10.5 (Fig. 1a), Prickle1 was highly expressed in the FBMs in r4. In addition, Prickle1 expression was detected in other motor neurons along the floor plate, similar to the expression pattern of Isl1 (Vivancos, et al., 2009). From E11.5 to E13.5, Prickle1 was expressed by the migrating FBMs (Fig. 1b, c and e) and the trigeminal motor neurons (arrowhead in Fig. 1b – f). The prominent Prickle1 expression in the neurons supports a cell autonomous role for Prickle1 in FBM caudal migration, presumably acting like prickle1b in zebrafish (Wanner, et al., 2013).
Fig. 1.
Prickle1 is expressed by the migrating FBMs as revealed by mRNA in situ hybridization. a: Prickle1 is highly expressed by the pre-migration FBMs at E10.5. In addition, Prickle1 expression is also detected in other motor neurons. b–f: Prickle1 is expressed by the FBMs from E11.5 to E13.5, and trigeminal neurons (arrows). d and f: The expression level in Prickle1C251X/C251X is reduced and the facial nucleus is barely visible. The FBM nucleus forms in r6 in the wild-type, but spans from r4 to r6 in the homozygotic mutant. Arrow: trigeminal neurons; r4–r6: rhombomere 4–6.The scale bar is 500µm
Prickle1 knockout mice die around E6.5 (Tao, et al., 2009), which excludes the possibility of analyzing FBM migration at later stages. Therefore, we analyzed the FBM migration in Prickle1C251X mice, which has a nonsense mutation in the third LIM domain (Tao, et al., 2011, Yang, et al., 2013a, Yang, et al., 2013b). It has been suggested that Prickle1 mutant protein with LIM and C-terminal protein domain deleted acts dominant-negatively to inhibit the function of normal Prickle1 protein (Liu, et al., 2013). However, the fact that mice of this mutant line survive longer than the knockout line suggests there is limited Prickle1 function in Prickle1C251X mutant mice and the truncated protein, if generated at all, has only a limited function.
As previously reported in other developing systems (Yang, et al., 2013a, Yang, et al., 2013b), the expression in Prickle1C251X mutant was markedly reduced (Fig. 1 d and f) . Nevertheless, the limited Prickle1 expression in the mutant FBMs showed that FBMs failed to migrate caudally to r6. These results support that Prickle1 is required for FBM caudal migration from r4 to r6, and may act cell-autonomously.
Prickle1 is required for FBM caudal migration
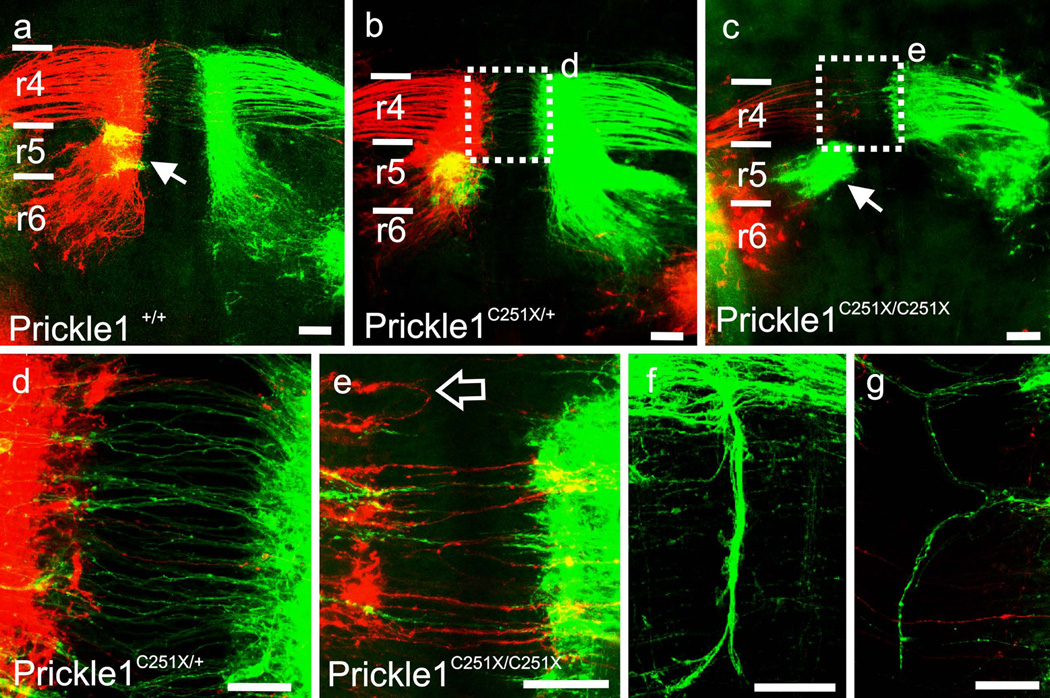
Since there is limited expression of Prickle1 in the Prickle1C251X mutants, it is possible that the mutant FBMs unlabeled by in situ hybridization in the mutant still migrate normally. Therefore, we examined the neuronal migration with dye tracing. We examined FBM migration in E13.5 mouse embryos by backfilling the neurons from the peripheral nerves with differently colored lipophilic dyes as previously described (Fritzsch and Nichols, 1993, Studer, et al., 1996). We also labeled abducens motor neurons from the eye, which served as landmarks for r5 (Fig. 2 a–c, green labeling on the right half of the brain, arrow). The brainstem was prepared as openbook, viewed from the ventral side. In the wild-type mouse, FBMs translocated caudally past the abducens that located in r5, into r6; they began also lateral migration in r6 (Fig. 2 a). In Prickle1C251X/+ mutants, the majority of the FBM neurons started lateral migration when they reached r5 with fewer neurons migrating along the floor plate to r6 (Fig. 2 b). In Prickle1C251X/C251X mutants, the majority of the neurons stayed within r4 (Fig. 2c). Only few FBMs migrated caudally into r5 and anterior r6 (Fig 2c). The results suggest that Prickle1 is required for coordinated FBM caudal migration in a dosage-dependent way. This dosage effect is consistent with previous study which showed that Prickle1C251X/+ have reduced threshold to seizures (Tao, et al., 2011).
Fig. 2.
Prickle1 is required for normal migration of motor neurons in a dosage dependent manner. a–c: FBM caudal migration is impaired in proportion to available Prickle1. The facial branchiomotor neurons are labeled from the ear using different colored dyes on the left and right side (which are false colored as green and red, respectively). The abducens neurons (Fig 2 a–c, arrows), are labeled ipsilaterally from the eye using a different color of dye (colored green, or yellow when merged with red in a and b). In wild type (a), most neurons migrate pass r5 (abducens neurons, triangle) to r6. In heterozygous mutant (b), most neurons start lateral migration at or in r5 although some migrate to r6. In homozygous mutant (c), most neurons fail to migrate to r6 but stay within r4 and r5 (c). d–g: Prickle1 also affects vestibule-cochlear efferent axon outgrowth across the floor plate. d: The efferents of the inner ear project their axons to the contralateral side in both wild-type and heterozygotic mutants. e–g: However, some efferents in the heterozygous Prickle1 C251X mutants fail to cross the midline in the homozygotic mutants (empty arrow in e). Some efferent axons grow along the mid-line (f and g). Scale bar is 50um in d, f and g, and 100um in a–c and e. Arrow, abducens neurons; empty arrow, vestibule-cochlear efferents that failed to cross the midline; r4–r6, rhombomere 4–6
We quantified the distance that the FBMs migrated (Fig 2 a, dashed bar). The FBMs in the controls migrated 786.7±103.6 µm while the FBMs in Prickle1C251X/C251X embryos only migrated 492.5±83.4µm (E12.5, n=5, t-test, p<0.001).
In addition to the failure of caudal migration of FBMs, we also noticed variability in how the vestibulo-cochlear efferent (Fritzsch and Nichols, 1993, Simmons, et al., 2011) crossed the floor plate in Prickle1C251X/C251X mutants (Fig. 2 d–f). In wild-type or Prickle1C251X/+ mice, the vestibulo-cochlear efferent axons crossed the midline without any caudal extension (Fig. 2 d). However, in the homozygotic Prickle1 mutants, fewer vestibulo-cochlear efferent axons crossed the floor plate (Fig. 2e arrow) and some crossing fibers extended along the midline (Fig. 2f–g) as previously described in EphB2 mutants (Cowan, et al., 2000).
The differences in migration within r4 of the Prickle1C251X/C251X mutants and control littermates was even more obvious in a lateral view of hemisected brains with afferent fiber tracts and nerve roots as reference points (Fig. 3a and b). In this preparation, trigeminal nuclei were labeled in red and facial nuclei were labeled in green. Therefore, the VIIa appears as yellow. Our data show that the VIIa remained in rostral r4 and caudal r3 in our Prickle1C251X/C251X embryos (Fig. 3 a and b, right brackets) overlapping with the caudal trigeminal motoneurons like in their littermate controls (Fig. 3).
Fig. 3.
Prickle1C251X mutation affects the FBM neuronal polarity but does not affect the position of the VIIa nucleus shown by dye tracing. a–b: The trigeminal neurons (red), the FBMs (green) and the VIIa neurons (yellow) are back filled from the peripheral applications (see material and methods). In Prickle1+/+ embryos, the FBMs migrate from r4 to r6. The VIIa neurons remainin caudal r3 and rostral r4 (right brackets). Note: the split of FBMs in r4 is due to split of the brains when mounting (triangle in A). A single plane of image from the region in dashed square is shown in higher power as c and d. c: At the neuron migrating front at r6 in Prickle1+/+, the neurons are oriented rostral-caudally (arrow). d: In Prickle1C251X/C251X embryos, the FBMs within r4 are oriented and migrat only medio-laterally (arrow). The scale bar is 200µm in a–b and 50µm in c-d. Right brackets, VIIa nucleus; triangle, split of the brain during preparation; dashed square, where the higher power images of c and d are taken from; r3–r6, rhombomere 3–6
Individual neurons and their processes could occasionally be visualized. We found that in control embryos, the FBMs that are within r6 were oriented caudal-laterally (Fig. 3c, arrow). In contrast, in the Prickle1C251X/C251X mutants, neurons were oriented within r4 medio-laterally (Fig. 3d, arrow), much like accessory neurons in r3. These results suggested that the cell polarity was affected in some FBMs in the Prickle1C251X/C251X embryos, which migrated laterally within r4.
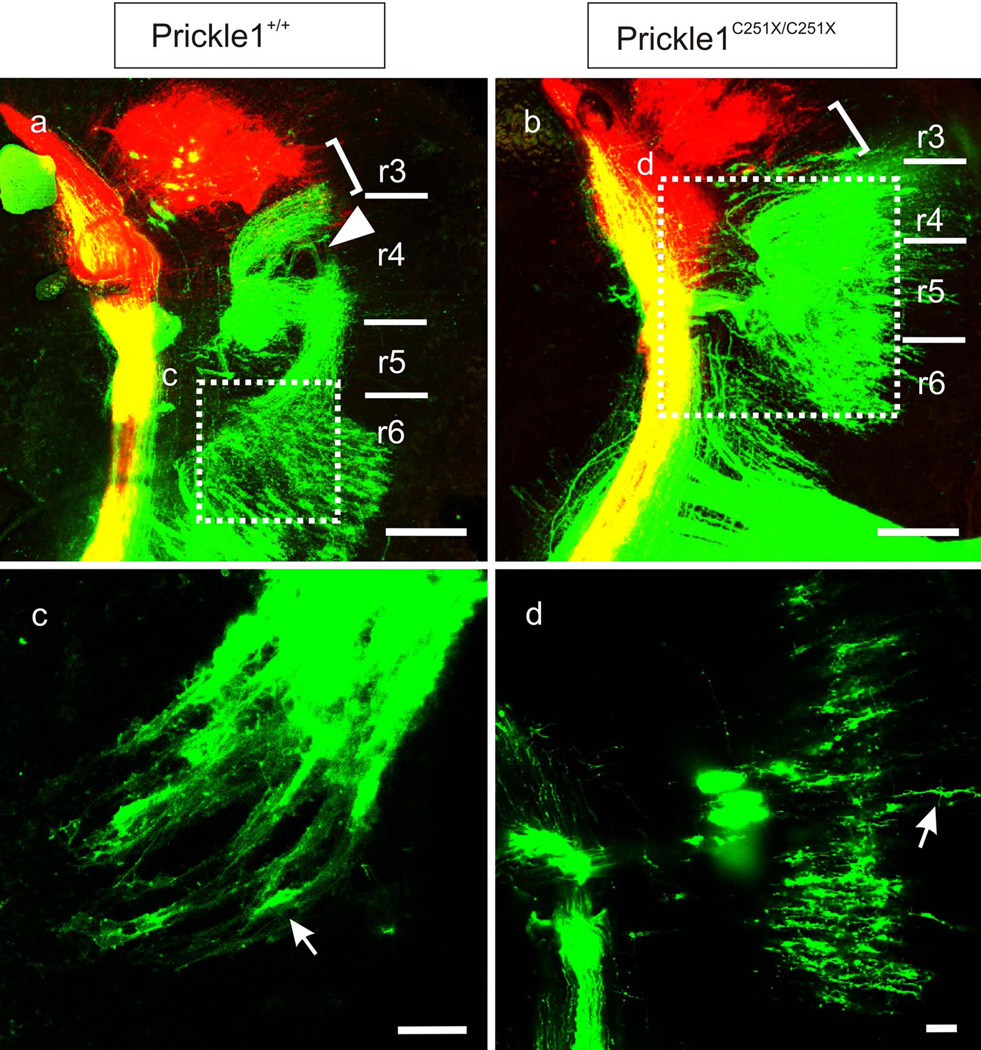
The ectopic FBM nucleus in Prickle1 mutants lies dorsal to the olivo-cochlear efferent nucleus
Since the olivo-cochlear efferents form the superior olivary complex near the pial surface of r4–r5 (Simmons, et al., 2011), we asked whether the mutant FBMs that migrated to r5 could still formed a nucleus near the pial surface and/or became dispersed among the olivo-cochlear efferents situated in the superior olive complex. We back-filled the FBM and inner ear efferent neurons from the facial nerve and the ear, respectively, using differently colored lipophilic dyes at E18.5. Brains were removed and coronal sections were taken to examine the position of FBMs. In the wild-type, FBMs migrated to a position caudal to the olivo-cochlear efferent nucleus, and formed a nucleus adjacent to the ventral pial surface (Fig. 4 a and d). Therefore, the nerve and the nucleus were present in different coronal section (about four 100µm-thick sections between the nerve root and the nucleus). In homozygous Prickle1C251X mutants, FBMs neurons did not reach the pial surface. Instead they formed an ectopic nucleus in r4 and r5, superior to, but segregated from the olivo-cochlear efferents (Fig. 4 b–c). In addition, the nucleus could be found in the same coronal section with the axons entering at r4 (Fig. 4 c) demonstrating that in Prickle1C251X/C251X mutants many FBMs remain within r4 with a migration roughly comparable to olivo-cochlear efferents (Karis, et al., 2001). Heterozygous mutants had intermediate phenotypes between wild-type and homozygous mutants: many FBM neurons formed an ectopic FBM nucleus dorsal to olivo-cochlear efferents, but others had migrated into r6 and formed a small FBM nucleus in the normal position. Strikingly, FBMs and olivo-cochlear efferents of Prickle1 mutants did not mix despite overall similarities in migration (Fig. 4). This suggests a mechanism separating the two motor neuron populations that is independent of the caudal migration of FBMs.
Fig. 4.
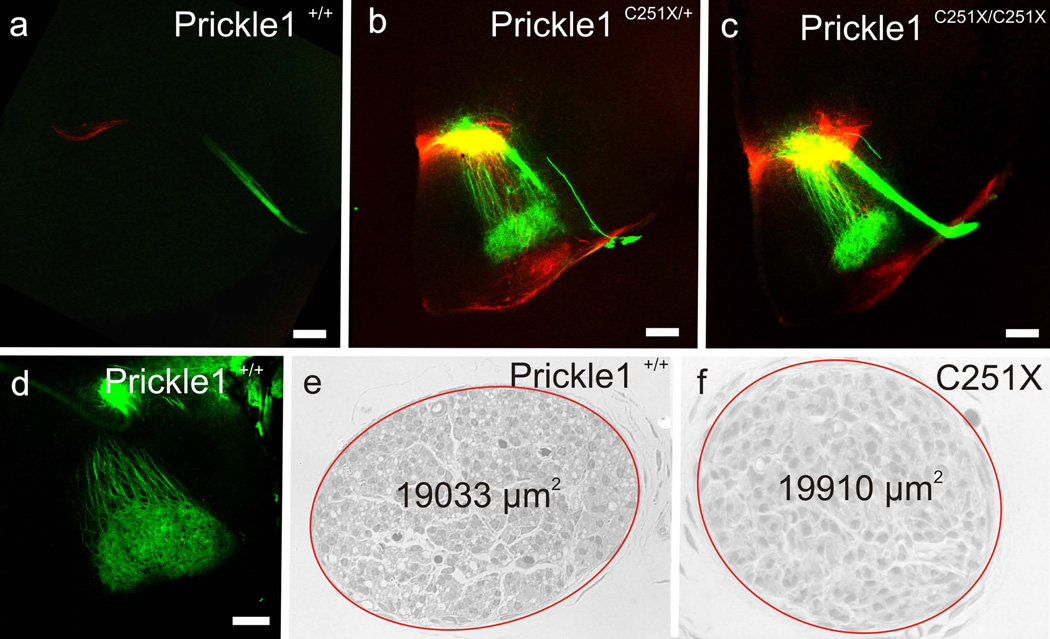
The mutant FBMs form an ectopic nucleus dorsal to olivo-cochlear efferents. The facial neurons are labeled from the facial nerve and shown in green, whereas the olivo-cochlear efferents are labeled from the ear and are shown in red. a and d: In wild-type, the facial nerve and the FBM nucleus are not in the same section and the nucleus lies near the pial surface (d). b: In heterozygotic mutants, some FBM neurons are found in the same section of the facial nerve and the FBM nucleus lies dorsally to the olivo-cochlear efferents (red). c: In homozygotic mutants, most FBM neurons are in the same section of the facial nerve, and the FBM neurons are dorsal to the olivo-cochlear efferents of the superior olivary complex.e-f: cell survival is not affected by Prickle1C251X mutation as the territory of the cross section of the nerve is similar in wild-type (e) and homozygotic mutant (f). Due to space limitation in f, Prickle1C251X/C251X is written as C251X. Red ellipse marks the facial nerve. The scale bar in a–d is 200 µm
The survival of FBMs is not affected in Prickle1 C251X mutants
Previous work showed that FBM survival is affected by Hoxb1 and Vangl2lp mutants by E12.5 (Glasco, et al., 2012, Studer, et al., 1996). Therefore, we asked whether the FBMs could survive in newborn Prickle1C251X mutants, the latest stage we could obtain Prickle1C251X/C251X mice. We isolated the facial nerve at birth near the stylo-mastoid foramen where it is composed only of FBM axons (Fritzsch, et al., 1997). There was no obvious difference in the cross-sectional nerve territory (Fig. 4 e and f). This suggests that forming the nucleus at the wrong position affects neither neuronal survival up to P0 nor projection of FBM axons within the facial nerve. Due to the early lethality of the mutants, we could not test long-term viability and function of the unusually positioned FBM neurons.
Prickle1C251X mutation does not affect expression of several genes implicated in migration
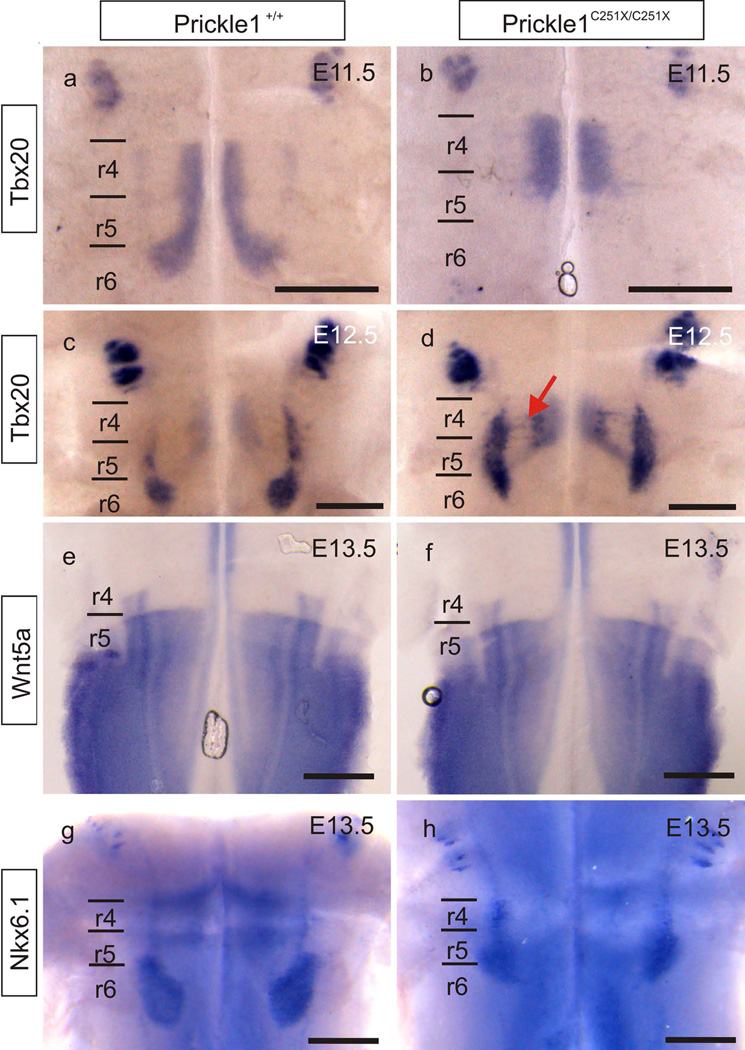
Tbx20 is a transcription factor necessary for FBM migration, and its down-regulation impairs the expression of genes in the PCP family, including Prickle1, Fzd7, Wnt11, Vangl1 and Vangl2 (Song, et al., 2006). We investigated whether Prickle1C251X mutation could, in turn, affect expression of its upstream transcription factor Tbx20 in FBMs. Tbx20 expression in FBMs was unchanged in the Prickle1C251X/C251X mutants (Fig. 5 a–d). This suggests that the migratory defect of FBMs in Prickle1 mutant mice is not mediated by down-regulation of Tbx20. The Tbx20 expression again illustrated the defective caudal migration in Prickle1C251X mutants.
Fig. 5.
Prickle1C251X mutation does not affect expression of crucial genes in the hindbrain. a–d: Tbx20 is expressed by the migrating FBMs in both wild-type and the Prickle1C251X/C251X mutants at both E11.5 and E12.5. In Prickle1C251X/C251X mutants, FBM neurons do not migrate caudally past r5 at E11.5 (b), but migrate lateral within r4 (arrow in d) at E12.5. e–f: Wnt5a is only expressed in r5–8 but not in r4. The expression is not affected by Prickle1C251X mutation. g–h: Nkx6.1 is expressed by the migrating FBMs in the wild-type. Nkx6.1 expression is not affected by Prickle1C251X mutation. The expression Nkx6.1 shows the wide distribution of mutant FBMs from r4 to r6. Black line: boundary of rhombomeres; r4–r6: rhombomere 4–6; red arrow: the laterally migrating FBMs. The scale bar is 500µm
Previously we showed the expression pattern of Wnt5a, a typical ligand of the PCP pathway, was affected in Prickle1C251X/C251X limbs (Yang, et al., 2013a) and in Vangl2lp/lp hindbrain (Glasco, et al., 2012). Although the affected Wnt5a expression in Vangl2lp mutants is probably not associated with defective FBM caudal migration in Vangl2lp mutants (Glasco, et al., 2012), the change in expression suggests a feedback mechanism from Vangl2 to Wnt5a through an unknown mechanism. We therefore examined the expression of Wnt5a to see whether a similar feedback mechanism exists from Prickle1 to Wnt5a in the hindbrain as in the limb. Wnt5a was expressed by the ventricular zones posterior to r4 (Fig. 5 e). However, we did not detect any obvious change in expression pattern in the Pricke1 mutant hindbrain (Fig. 5 f). This difference between the effect of Vangl2 mutation and Prickle1 mutation on Wnt5a expression in the hindbrain implies different molecular roles of PCP pathway in different developing systems. It should be noted that Wnt5a plays only a limited role in FBM migration (Vivancos, et al., 2009), suggesting either redundant or non-essential role of this Wnt signaling ligand. Similarly, there was no obvious change in expression of Nkx6.1, another transcription factor that is necessary for FBM migration (Müller, et al., 2003), in the PrickleC251X mutant hindbrain (Fig. 5 g–h).
Ror2 is not necessary for FBM caudal migration
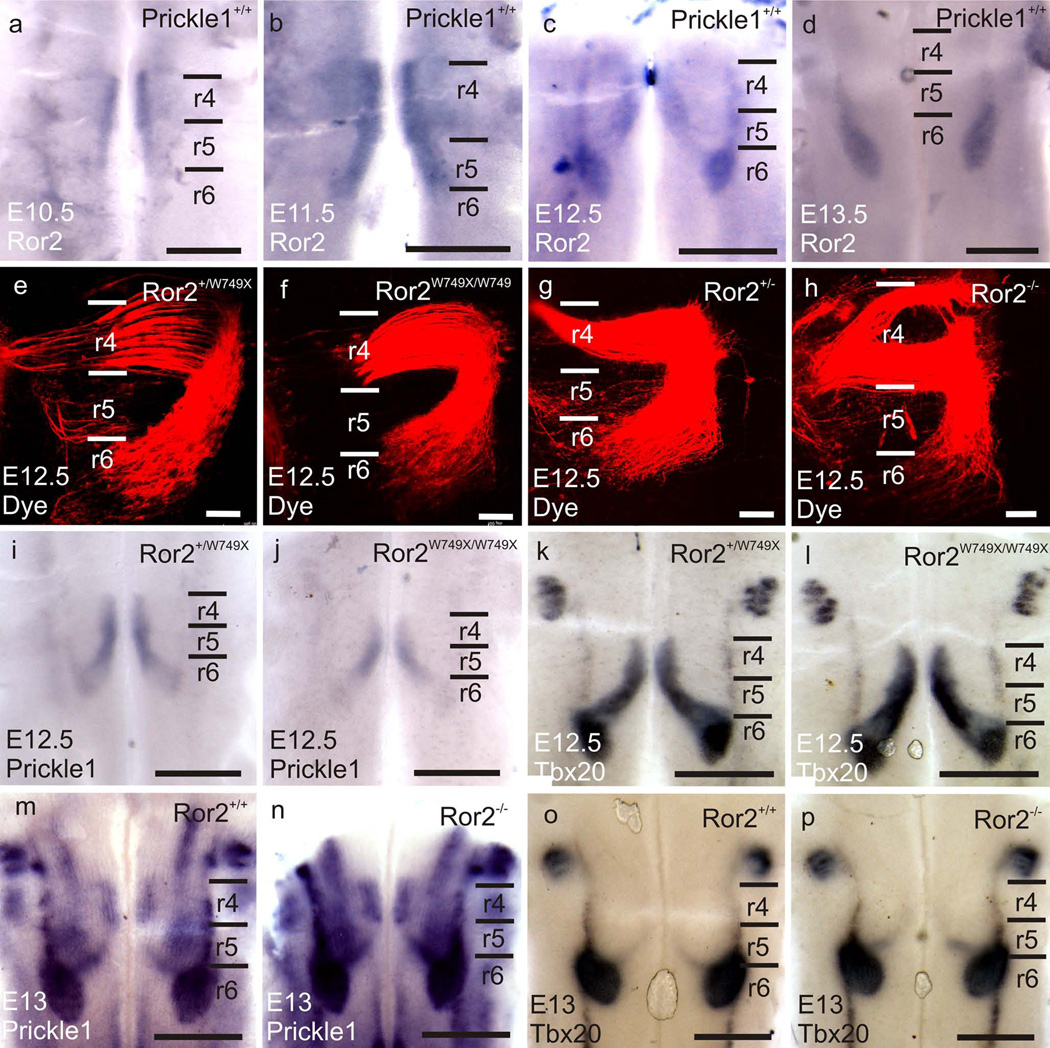
Ror2 is an important receptor of Wnt5a in Wnt5a/PCP signaling cascade, interacting with Wnt5a and Vangl2 (Gao, et al., 2011, He, et al., 2008, Mikels, et al., 2009, Oishi, et al., 2003). Therefore, we hypothesized Ror2 might be essential for FBM caudal migration. Consistent with this hypothesis, our data show that Ror2 was expressed in the hindbrain predominantly by the FBMs from E10.5 to E13.5 (Fig. 6 a–d). We therefore analyzed two Ror2 mutant lines, Ror2W749X/W749X and Ror2−/− (Raz, et al., 2008, Takeuchi, et al., 2000), to see whether FBM migration might be affected.
Fig. 6.
Ror2 mutations do not affect FBM migration. a–d: Ror2 is expressed by the FBMs from E10.5 to E13.5 shown by whole-mount in situ hybridization. e–h: Dye tracing at E12.5 shows the FBMs in Ror2W749/W749X and Ror2−/− migrate normally to r6. Note: the separated FBM in r4 in D is preparation artifacts. i–j: Prickle1 is expressed by the migrating neurons in both wild-type and the Ror2W749X mutant at E12.5. k–l: at E12.5, Tbx20 mRNA expression shows FBMs in both the wild-type and the Ror2W749X mutant migrate caudally to r6. m–n: Prickle1 is expressed by the migrating neurons in both wild-type and the Ror2−/− FBMs at E13. o–p: at E13, Tbx20 mRNA expression shows FBMs in both the wild-type and the Ror2−/− migrate caudally to r6. The scale bar is 500µm in a–d and I-P, and 100µm in e–h
We backfilled the FBMs from the inner ear with different colors of lipophilic dyes from both sides and prepared the hindbrain in open-book as described above. At E12.5, FBMs migrated to r6 normally in both Ror2 mutant lines (Fig. 6 e–h). Since there were no defects in FBM caudal migration, we expected normal Prickle1 expression in the hindbrain. As we predicted, Prickle1 was expressed by the migrating FBMs in Ror2W749X mutants and Ror2 knockouts (Fig. 6 i, j, m and n). In addition, Tbx20 expression was normal during the caudal migration of FBMs in these Ror2 mutants (Fig. 6 k, l, o and p). 3 Ror2W749X/W749X embryos and 3 Ror2−/− embryos analyzed this way did not show any caudal migration defects, and thus we did not pursue further analysis. We conclude that neither the Ror2 point mutation nor Ror2 deletion exerts any noticeable effect on FBM migration, indicating an unexpected flexibility of the use of different components of the PCP pathway in different developing systems.
Discussion
FBM caudal migration in zebrafish and mice is molecularly similar
FBM migration is differentially distributed across vertebrates. Some groups display caudal migration such as sharks, certain bony fish (zebrafish), salamanders and mammals (mouse), whereas others like frogs and birds show no caudal migration (Fritzsch, 1998, Szekely and Matesz, 1993). Since the FBM caudal migration is scattered across vertebrates (Fig. 7 b), either multiple independent events in which the loss of an ancestral migration pattern occurred in lamprey, chicken and frogs; or multiple independent events leading to the gain of novel migration patterns occurred in sharks, zebrafish, salamanders and mammals. If FBM migration in mammals is molecularly comparable to zebrafish, the most parsimonious explanation would be an ancestral evolution of caudal migration in osteognathostomata or even jawed vertebrates including elasmobranchs. Supporting this hypothesis, several genes are used by both zebrafish and mouse for FBM caudal migration, such as Hoxb1, Celsr2 and Vangl2 (Bingham, et al., 2002, Glasco, et al., 2012, Pata, et al., 1999, Qu, et al., 2010, Rohrschneider, et al., 2007, Wada, et al., 2006).
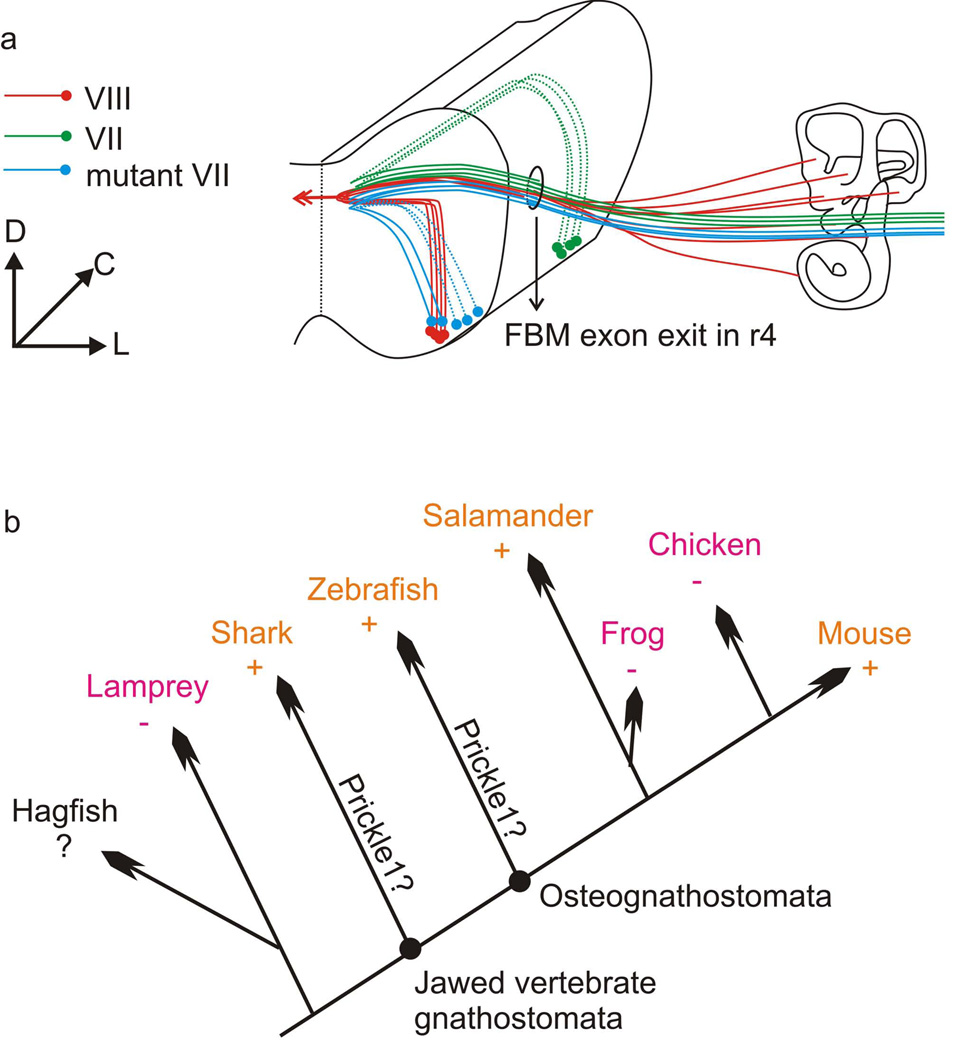
Fig. 7.
a: Diagram showing the migratory route of wild-type and Prickle1C251X/C251X FBMs. Both the olivo-cochlear efferents (VIII, red) and the facial nerve (VII, green) exit at r4. While the wild-type FBMs migrate caudally to r6, the mutant FBMs (blue) stay in r4 and r5 and lie dorsally to the olivo-cochlear efferent nucleus. b: Diagram showing the distribution FBM caudal migration (+) or lack of caudal migration (−) in vertebrates. Orange color highlights the presence of FBM caudal migration in some members, magenta color highlights the absence of FBM caudal migration in all members of a given lineage. Similarities of molecular data on FBM migration in mammals and zebrafish suggest that the common ancestor had such migration. This implies that frogs and birds have independently lost caudal FBM migration. C: caudal; D: dorsal; L: lateral; +: FBM migrates caudally; −: FBM does not migrate caudally; ?: data not available; Prickle1?: further analysis is required to show whether Prickle1 was present in this common ancestor and involved in FBM migration
We show here for the first time that Prickle1 is required in FBM migration in mammals (Fig. 7 a) as in bony fish (Wanner, et al., 2013). Prickle1a and prickle1b are required for FBM caudal migration in zebrafish cell-autonomously and non-cell-autonomously, respectively (Carreira-Barbosa, et al., 2003, Mapp, et al., 2011, Mapp, et al., 2010). However, in mouse, Prickle1 is expressed only in the migrating neurons, not in the surrounding cells, suggesting a cell-autonomous mechanism, similar to prickle1b of zebrafish. We interpret the overall similarity as a plesiomorphic feature of osteognathostomata [sarcopterygian and actinopterygina fish (Fritzsch, et al., 2013)]. Unfortunately, the molecular mechanism of FBM caudal migration in elasmobranchs such as sharks is unknown. If migration in sharks will be shown to also depend on the PCP pathway it would suggest that FBM caudal migration co-evolved with the evolution of jaws (Fig. 7 b). This interpretation suggests that the absence of caudal migration in birds, frogs and several bony fish reflects an independent loss of some molecular features essential for this process. To confirm this independent loss of FBM caudal migration, molecular data from frogs and birds are now needed to show independent loss of molecules crucial for FBM caudal migration. Beyond this ultimate question, identifying what molecule(s) now considered essential for FBM migration are absent in either frog and chicken FBM could provide additional insights into the causality of molecular interactions mediating FBM migration.
Prickle1 affects neuronal migration and possibly neuronal circuit formation
We have previously shown that Prickle1 plays a role in regulating cell survival in the limb (Yang, et al., 2013a). However, in FBMs, while caudal migration is defective, there is no obvious effect on FBM judging by the similar facial nerve territory of control and mutant littermates (Fig. 4). It is possible that misguided neurons in Prickle1 mutants receive proper trophic support for their survival. It is also possible the mis-migrated FBM neurons die after P0. Resolving this issue requires further analysis in conditional knockout mutants which would require targeted deletion of Prickle1 in the FBMs, using the Tbx20-cre or Isl1-cre (Tbx20 and Isl1 are transcription factors required for motor neurons specification), or Hoxb1-cre [specific for r4 (Chen, et al., 2012)] combined with the recently available Prickle1f/f mice (Liu, et al., 2013).
We still do not understand the functional significance of the FBM caudal migration to r6. It is possible that this caudal migration to r6 allows the neurons to receive the uniquely mixed bilateral cortical input from the primary motor cortex (Holstege, et al., 1977) to play a major role in the eye blinking reflex (Holstege, 1991, Nieuwenhuys, et al., 1998). Generating viable r4 specific or FBM specific conditional Prickle1 deletion mutants using different cre lines would allow testing if the proper cortical and subcortical connection to FBMs to mediate eye blinking reflex is forming when FBMs stay within r4–r5 instead of migrating to r6.
In addition to the expression in the FBMs in embryonic brains, Prickle1 is also expressed by postmitotic neurons in the cortical plate of the cortex (Okuda, et al., 2007). This expression suggest that the migration of these neurons in the cortex might be affected, contributing to the neuronal phenotype in flies, zebrafish, mice and men (Mei, et al., 2013, Tao, et al., 2009).
The location of facial accessory nucleus is not affected
In mammals, in addition to the facial nucleus formed at r6 (VII), the FBMs also form an accessory nucleus (VIIa, aka suprafacial nucleus) (Komiyama, et al., 1984, Matsuda, et al., 1979, Nieuwenhuys, et al., 1998, Szekely and Matesz, 1993, Székely and Matesz, 1982). The neurons in the accessory facial nucleus innervate the posterior belly of the digastric muscle while neurons from the posterior trigeminal nucleus innervate the anterior belly of the digastric muscle, which helps to move the hyoid bone. The VIIa is normally located at the rostral r4, immediately caudal to the posterior trigeminal nucleus.
Since the FBMs migrate from r4 to r6 during development, we asked whether the location of the VIIa nucleus is affected. By labeling the FBMs from the posterior belly of digastric, we found that the VIIa remained in r4 in our Prickle1C251X/C251X embryos and their littermate controls (Fig 3).
Different roles of PCP components in FBM migration
Wnt5a, Ror2, Vangl2 and Prickle1 are pivotal players in Wnt/PCP signaling (Gao, et al., 2011, He, et al., 2008, Nomachi, et al., 2008, Oishi, et al., 2003, Wang, et al., 2011, Yang, et al., 2013a), yet they play distinct roles in different developing systems. Wnt5a only has limited function in FBM caudal migration (Vivancos, et al., 2009), and Ror2 is not essential in this process (Fig. 6). It is possible that another Ror family member, such as Ror1, is redundant with Ror2 in this process. There is no Ror1 mRNA expression in FBM at E14.5 (http://eurexpress.org). However, it could be that Ror1 is expressed earlier during FBM caudal migration but down-regulated at E14.5. Further analysis is necessary to examine the role of Ror1 in FBM caudal migration.
The clear involvement of Vangl2 and Prickle1 in FBM caudal migration but not Wnt5a or Ror2 suggests different interaction between these genes in FBM caudal migration and limb development (Gao, et al., 2011, He, et al., 2008, Wang, et al., 2011, Yang, et al., 2013a). Likewise, Vangl2 and Prickle1 seem to use somewhat different mechanisms to affect FBM migration. Compared with Vangl2lp mutants, whose FBMs failed to migrate caudally in both heterozygous and homozygous mutants (Glasco, et al., 2012), Prickle1C251X heterozygotic mutants have a less severe phenotype. More specifically, Prickle1 seems to affect caudal FBM migration in a dose dependent fashion and homozygotic Prickle1C251X mutants have similar phenotypes as heterozygotic Vangl2lp mutants. This suggests that if Prickle1 interacts with Vangl2, it is only partially responsible for mediating Vangl2 signaling in FBM caudal migration. It is possible that other Prickle family members, such as Prickle2, or Testin are also playing a role in FBM caudal migration (Ren, et al., 2013). More importantly, Vangl2 is expressed broadly by the hindbrain in mice, which suggests it might function non-cell autonomously to regulate FBM neuron migration as in zebrafish (Sittaramane, et al., 2013). In contrast, Prickle1 is expressed in the migrating FBM of mice, which supports Prickle1 cell-autonomous function, comparable to zebrafish prickle1b.
Taken together, these results indicate that the interaction between the core proteins in Wnt/PCP pathway is not conserved in different developing systems. Rather, these data suggest that the interactions of PCP proteins can be partially uncoupled and adjusted to the specific requirements of a given system. The nature of these requirements in FBM migration versus limb development requires further analysis. Finally, we suggest that the non-cell autonomous function of prickle1a in zebrafish is a neo-functionalization that evolved after bony fish gene duplication. In contrast, the cell-autonomous Prickle1 function in FBM migration is the ancestral function that is minimally shared among osteognathostomata and possibly among all jawed vertebrates. If demonstrated in elasmobranchs, this would suggest that Prickle1 was recruited only once in the jawed vertebrate ancestor to play a cell-autonomous function in FBM caudal migration that was lost multiple times in different vertebrates (Fig. 7).
Acknowledgement
We would like to express our gratitude to all those who have helped us. We are grateful for Shu Wu who assisted in mouse breeding. We thank Dr. A Chandrasekhar, Dr. M Sander, and Dr. S. Miyata for providing plasmids for in situ probe, and Dr. A. Chandrasekhar for comments on this manuscript. This work was supported by NIH R01 grants (R01 DC005590; P30 DC 010362) to BF, and (NIH 1R01 NS064159-01A1) to AGB.
Footnotes
We have no conflict of interest to declare.
Reference
- Bassuk AG, Wallace RH, Buhr A, Buller AR, Afawi Z, Shimojo M, Miyata S, Chen S, Gonzalez-Alegre P, Griesbach HL, Wu S, Nashelsky M, Vladar EK, Antic D, Ferguson PJ, Cirak S, Voit T, Scott MP, Axelrod JD, Gurnett C, Daoud AS, Kivity S, Neufeld MY, Mazarib A, Straussberg R, Walid S, Korczyn AD, Slusarski DC, Berkovic SF, El-Shanti HI. A Homozygous Mutation in Human PRICKLE1 Causes an Autosomal-Recessive Progressive Myoclonus Epilepsy-Ataxia Syndrome. The American Journal of Human Genetics. 2008;83:572–581. doi: 10.1016/j.ajhg.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bingham S, Higashijima S-i, Okamoto H, Chandrasekhar A. The Zebrafish trilobite Gene Is Essential for Tangential Migration of Branchiomotor Neurons. Developmental Biology. 2002;242:149–160. doi: 10.1006/dbio.2001.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- Chen Y, Takano-Maruyama M, Fritzsch B, Gaufo GO. Hoxb1 controls anteroposterior identity of vestibular projection neurons. PLoS ONE. 2012;7:e34762. doi: 10.1371/journal.pone.0034762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 Guides Axons at the Midline and Is Necessary for Normal Vestibular Function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Duncan J, Kersigo J, Gray B, Fritzsch B. Combining Lipophilic dye, in situ Hybridization, Immunohistochemistry, and Histology. 2011:e2451. doi: 10.3791/2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. Of Mice And Genes : Evolution Of Vertebrate Brain Development. Karger, Basel: SUISSE; 1998. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue™ Maroon, NeuroVue™ Red and NeuroVue™ Green and their use for double and triple labeling of neuronal profile. Brain Research Bulletin. 2005;66:249–258. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Elsevier, Amsterdam PAYS-BAS; 1993. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T. Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evol Dev. 2013;15:63–79. doi: 10.1111/ede.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. International Journal of Developmental Neuroscience. 1997;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Developmental Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasco DM, Sittaramane V, Bryant W, Fritzsch B, Sawant A, Paudyal A, Stewart M, Andre P, Cadete Vilhais-Neto G, Yang Y, Song M-R, Murdoch JN, Chandrasekhar A. The mouse Wnt/PCP protein Vangl2 is necessary for migration of facial branchiomotor neurons, and functions independently of Dishevelled. Developmental Biology. 2012;369:211–222. doi: 10.1016/j.ydbio.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen Y. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G. Descending motor pathways and the spinal motor system: limbic and nonlimbic components. Progress in brain research. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG, Dekker JJ. The organization of the bulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. II. An autoradiographic tracing study in cat. Brain : a journal of neurology. 1977;100:264–286. [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. Journal of Comparative Neurology. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Komiyama M, Shibata H, Suzuki T. Somatotopic representation of facial muscles within the facial nucleus of the mouse. A study using the retrograde horseradish peroxidase and cell degeneration techniques. Brain, behavior and evolution. 1984;24:144–151. doi: 10.1159/000121312. [DOI] [PubMed] [Google Scholar]
- Liu C, Lin C, Whitaker DT, Bakeri H, Bulgakov OV, Liu P, Lei J, Dong L, Li T, Swaroop A. Prickle1 is expressed in distinct cell populations of the central nervous system and contributes to neuronal morphogenesis. Human Molecular Genetics. 2013;22:2234–2246. doi: 10.1093/hmg/ddt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Developmental brain research. 2003;140:223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Mapp OM, Walsh GS, Moens CB, Tada M, Prince VE. Zebrafish Prickle1b mediates facial branchiomotor neuron migration via a farnesylation-dependent nuclear activity. Development. 2011;138:2121–2132. doi: 10.1242/dev.060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp OM, Wanner SJ, Rohrschneider MR, Prince VE. Prickle1b mediates interpretation of migratory cues during zebrafish facial branchiomotor neuron migration. Developmental Dynamics. 2010;239:1596–1608. doi: 10.1002/dvdy.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Uemura M, Takeuchi Y, Kume M, Matsushima R, Mizuno N. Localization of motoneurons innervating the posterior belly of the digastric muscle: a comparative anatomical study by the HRP method. Neuroscience letters. 1979;12:47–52. doi: 10.1016/0304-3940(79)91478-2. [DOI] [PubMed] [Google Scholar]
- Mei X, Wu S, Bassuk AG, Slusarski DC. Mechanisms of prickle1a function in zebrafish epilepsy and retinal neurogenesis. Disease Models & Mechanisms. 2013;6:679–688. doi: 10.1242/dmm.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. Journal of Biological Chemistry. 2009;284:30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Jabs N, Lork DE, Fritzsch B, Sander M. Nkx6. 1 controls migration and axon pathfinding of cranial branchio-motoneurons. Development. 2003;130:5815–5826. doi: 10.1242/dev.00815. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Pasqualetti M, Takio Y, Hirano S, Rijli FM, Kuratani S. Segmental development of reticulospinal and branchiomotor neurons in lamprey: insights into the evolution of the vertebrate hindbrain. Development. 2004;131:983–995. doi: 10.1242/dev.00986. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Human Molecular Genetics. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, Wrana JL. Regulation of Planar Cell Polarity by Smurf Ubiquitin Ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Donkelaar HJ, Nicholson C, Smeets W, Wicht H. The central nervous system of vertebrates. Springer Berlin etc; 1998. [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor Tyrosine Kinase Ror2 Mediates Wnt5a-induced Polarized Cell Migration by Activating c-Jun N-terminal Kinase via Actin-binding Protein Filamin A. Journal of Biological Chemistry. 2008;283:27973–27981. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Okuda H, Miyata S, Mori Y, Tohyama M. Mouse Prickle1 and Prickle2 are expressed in postmitotic neurons and promote neurite outgrowth. FEBS Letters. 2007;581:4754–4760. doi: 10.1016/j.febslet.2007.08.075. [DOI] [PubMed] [Google Scholar]
- Pata I, Studer M, van Doorninck JH, Briscoe J, Kuuse S, Engel JD, Grosveld F, Karis A. The transcription factor GATA3 is a downstream effector of Hoxb1 specification in rhombomere 4. Development. 1999;126:5523–5531. doi: 10.1242/dev.126.23.5523. [DOI] [PubMed] [Google Scholar]
- Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, Damrau C, Murdoch JN, Evans S, Pfaff SL, Formstone C, Goffinet AM, Chandrasekhar A, Tissir F. Atypical Cadherins Celsr1-3 Differentially Regulate Migration of Facial Branchiomotor Neurons in Mice. The Journal of Neuroscience. 2010;30:9392–9401. doi: 10.1523/JNEUROSCI.0124-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Stricker S, Gazzerro E, Clor JL, Witte F, Nistala H, Zabski S, Pereira RC, Stadmeyer L, Wang X, Gowen L, Sleeman MW, Yancopoulos GD, Canalis E, Mundlos S, Valenzuela DM, Economides AN. The mutation ROR2W749X, linked to human BDB, is a recessive mutation in the mouse, causing brachydactyly, mediating patterning of joints and modeling recessive Robinow syndrome. Development. 2008;135:1713–1723. doi: 10.1242/dev.015149. [DOI] [PubMed] [Google Scholar]
- Ren DD, Kelly M, Kim SM, Grimsley-Myers CM, Chi FL, Chen P. Testin interacts with vangl2 genetically to regulate inner ear sensory cell orientation and the normal development of the female reproductive tract in mice. Developmental Dynamics. 2013 doi: 10.1002/dvdy.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider MR, Elsen GE, Prince VE. Zebrafish Hoxb1a regulates multiple downstream genes including prickle1b. Developmental Biology. 2007;309:358–372. doi: 10.1016/j.ydbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Schwabe GC, Trepczik B, Süring K, Brieske N, Tucker AS, Sharpe PT, Minami Y, Mundlos S. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Developmental Dynamics. 2004;229:400–410. doi: 10.1002/dvdy.10466. [DOI] [PubMed] [Google Scholar]
- Simmons D, Duncan J, Crapon de Caprona D, Fritzsch B. Development of the inner ear efferent system. In: Ryugo DK, Fay RR, Popper AN, editors. Auditory and vestibular efferents. New York: Springer Verlag; 2011. pp. 187–216. [Google Scholar]
- Sittaramane V, Pan X, Glasco DM, Huang P, Gurung S, Bock A, Li S, Wang H, Kawakami K, Matise MP, Chandrasekhar A. The PCP protein Vangl2 regulates migration of hindbrain motor neurons by acting in floor plate cells, and independently of cilia function. Developmental Biology. 2013;382:400–412. doi: 10.1016/j.ydbio.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M-R, Shirasaki R, Cai C-L, Ruiz EC, Evans SM, Lee S-K, Pfaff SL. T-Box transcription factor Tbx20 regulates a genetic program for cranial motor neuron cell body migration. Development. 2006;133:4945–4955. doi: 10.1242/dev.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Thomas-MacArthur V, Strutt D. Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Drosophila melanogaster Planar Polarity Specification. PLoS Genet. 2013;9:e1003654. doi: 10.1371/journal.pgen.1003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Szekely G, Matesz C. The efferent system of cranial nerve nuclei: a comparative neuromorphological study. Advances in anatomy, embryology, and cell biology. 1993;128:1–92. doi: 10.1007/978-3-642-77938-1. [DOI] [PubMed] [Google Scholar]
- Székely G, Matesz C. The accessory motor nuclei of the trigeminal, facial, and abducens nerves in the rat. Journal of Comparative Neurology. 1982;210:258–264. doi: 10.1002/cne.902100305. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-Related Gene in Vertebrates Is Essential for Gastrulation Cell Movements. Current Biology. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes to Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Tao H, Manak JR, Sowers L, Mei X, Kiyonari H, Abe T, Dahdaleh NS, Yang T, Wu S, Chen S, Fox MH, Gurnett C, Montine T, Bird T, Shaffer LG, Rosenfeld JA, McConnell J, Madan-Khetarpal S, Berry-Kravis E, Griesbach H, Saneto RP, Scott MP, Antic D, Reed J, Boland R, Ehaideb SN, El-Shanti H, Mahajan VB, Ferguson PJ, Axelrod JD, Lehesjoki A-E, Fritzsch B, Slusarski DC, Wemmie J, Ueno N, Bassuk AG. Mutations in Prickle Orthologs Cause Seizures in Flies, Mice, and Humans. The American Journal of Human Genetics. 2011;88:138–149. doi: 10.1016/j.ajhg.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Suzuki M, Kiyonari H, Abe T, Sasaoka T, Ueno N. Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proceedings of the National Academy of Sciences. 2009;106:14426–14431. doi: 10.1073/pnas.0901332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torban E, Wang H-J, Groulx N, Gros P. Independent Mutations in Mouse Vangl2 That Cause Neural Tube Defects in Looptail Mice Impair Interaction with Members of the Dishevelled Family. Journal of Biological Chemistry. 2004;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Vivancos V, Chen P, Spassky N, Qian D, Dabdoub A, Kelley M, Studer M, Guthrie S. Wnt activity guides facial branchiomotor neuron migration, and involves the PCP pathway and JNK and ROCK kinases. Neural Development. 2009;4:1–16. doi: 10.1186/1749-8104-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Tanaka H, Nakayama S, Iwasaki M, Okamoto H. Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain. Development. 2006;133:4749–4759. doi: 10.1242/dev.02665. [DOI] [PubMed] [Google Scholar]
- Walsh GS, Grant PK, Morgan JA, Moens CB. Planar polarity pathway and Nance-Horan syndrome-like 1b have essential cell-autonomous functions in neuronal migration. Development. 2011;138:3033–3042. doi: 10.1242/dev.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type A. Human Molecular Genetics. 2011;20:271–285. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner SJ, Saeger I, Guthrie S, Prince VE. Facial motor neuron migration advances. Current Opinion in Neurobiology. 2013 doi: 10.1016/j.conb.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte F, Chan D, Economides AN, Mundlos S, Stricker S. Receptor tyrosine kinase-like orphan receptor 2 (ROR2) and Indian hedgehog regulate digit outgrowth mediated by the phalanx-forming region. Proceedings of the National Academy of Sciences. 2010;107:14211–14216. doi: 10.1073/pnas.1009314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Bassuk AG, Fritzsch B. Prickle1 stunts limb growth through alteration of cell polarity and gene expression. Developmental Dynamics. 2013a;242:1293–1306. doi: 10.1002/dvdy.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Jia Z, Bryant-Pike W, Chandrasekhar A, Murray JC, Fritzsch B, Bassuk AG. Analysis of PRICKLE1 in human cleft palate and mouse development demonstrates rare and common variants involved in human malformations. Molecular Genetics & Genomic Medicine. 2013b doi: 10.1002/mgg3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]