Abstract
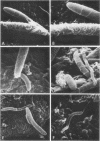
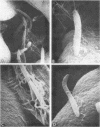
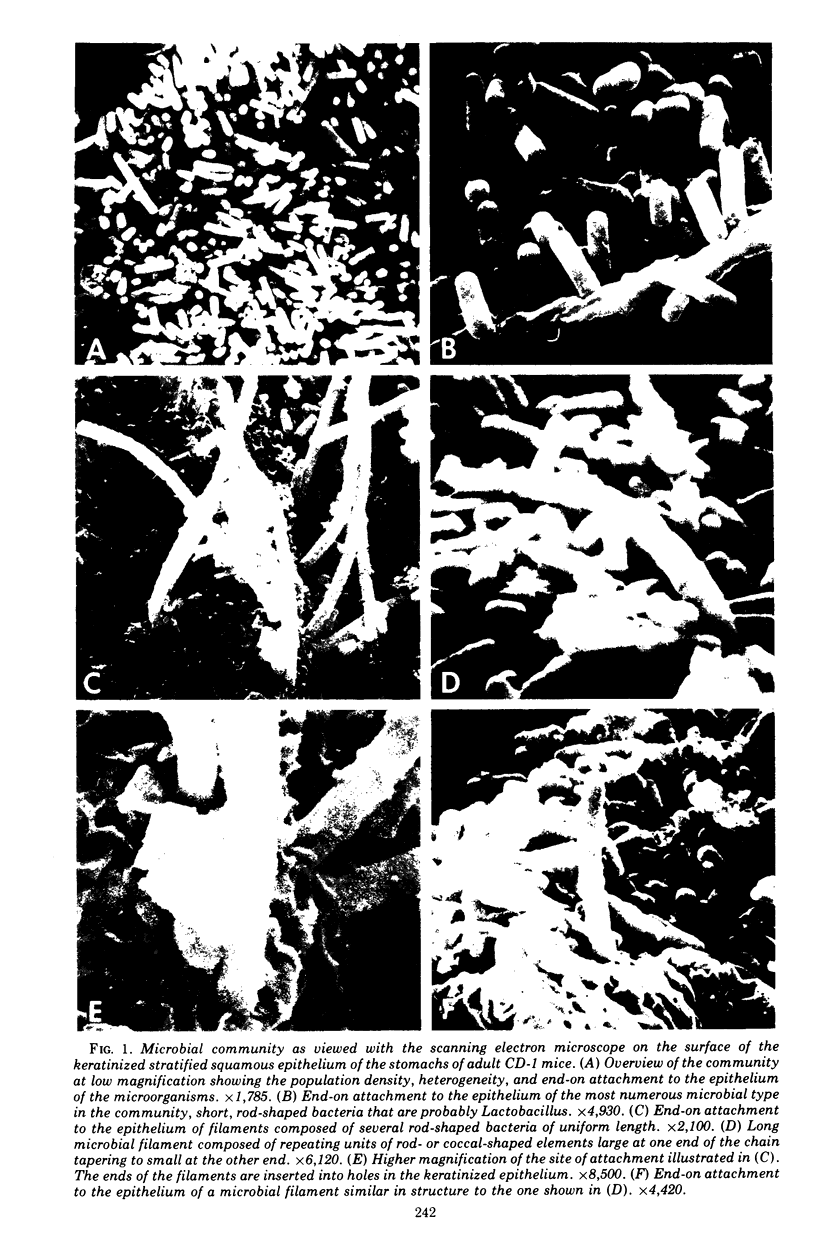
Scanning electron microscopy has been used to visualize the residents of microbial communities populating habitats on epithelial surfaces in the gastrointestinal tracts of mice. In the stomach, bacteria form a dense layer on the stratified squamous epithelium of the nonsecreting area. Microbes of at least three morphological types can be seen in this layer, including short rods with round ends, rods in chains, and tapering filaments composed of repeating units of rod- or coccal-shaped elements varying in size from large at one end of the filament to small at the other end. These three forms all attach by one end to the epithelium. The latter two forms can be found only so attached; in both cases, the end is inserted into a hole or depression in the keratinized epithelium. In the small intestine, a microbe of morphology similar to that of the tapering filaments found in the stomach can be seen attached end-on to the epithelium. Again each filament has one end inserted into a hole in the epithelium. In this case, however, the repeating elements of each filament are all about the same size. In the cecum and colon, predominantly fusiform- and spiral-shaped microbes can be seen mixed together in layers on the epithelium. At least three types of fusiform-shaped microbes can be distinguished on the basis of surface texture, and one type of spiral-shaped microbe can be found. These microorganisms appear to be attached to each other and to the epithelium by weblike filaments. The numerous microbial types present in the various epithelial habitats associate intimately surface-to-surface with each other and with the epithelium. Such surface-surface association may be an important autogenic factor contributing to the stability of the murine gastrointestinal ecosystem.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Mulcahy D., Takeuchi A., Savage D. C. Location and description of spiral-shaped microorganisms in the normal rat cecum. Infect Immun. 1972 Aug;6(2):184–192. doi: 10.1128/iai.6.2.184-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970 Aug 1;132(2):251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C. Bacterial degradation of gastrointestinal mucins. II. Bacterial origin of fecal ABH(O) blood group antigen-destroying enzymes. Gastroenterology. 1968 Feb;54(2):218–224. [PubMed] [Google Scholar]
- Lee A., Gordon J., Dubos R. Enumeration of the oxygen sensitive bacteria usually present in the intestine of healthy mice. Nature. 1968 Dec 14;220(5172):1137–1139. doi: 10.1038/2201137a0. [DOI] [PubMed] [Google Scholar]
- Lee A., Gordon J., Lee C. J., Dubos R. The mouse intestinal microflora with emphasis on the strict anaerobes. J Exp Med. 1971 Feb 1;133(2):339–352. doi: 10.1084/jem.133.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. P., Mata L. J. Bacterial flora associated with the human gastrointestinal mucosa. Gastroenterology. 1970 Jan;58(1):56–61. [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Associations and physiological interactions of indigenous microorganisms and gastrointestinal epithelia. Am J Clin Nutr. 1972 Dec;25(12):1372–1379. doi: 10.1093/ajcn/25.12.1372. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Localization of certain indigenous microorganisms on the ileal villi of rats. J Bacteriol. 1969 Mar;97(3):1505–1506. doi: 10.1128/jb.97.3.1505-1506.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S., Davis C. P. Anaerobic bacteria on the mucosal epithelium of the murine large bowel. Infect Immun. 1971 Oct;4(4):492–502. doi: 10.1128/iai.4.4.492-502.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Zeller J. A. Ultrastructural identification of spirochetes and flagellated microbes at the brush border of the large intestinal epithelium of the rhesus monkey. Infect Immun. 1972 Dec;6(6):1008–1018. doi: 10.1128/iai.6.6.1008-1018.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Smith J. M. The microflora of the pig stomach and its possible relationship to ulceration of the pars oesophagea. J Comp Pathol. 1970 Jul;80(3):359–367. doi: 10.1016/0021-9975(70)90066-6. [DOI] [PubMed] [Google Scholar]
- Yolton D. P., Stanley C., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal alkaline phosphatase activity in mice. Infect Immun. 1971 Jun;3(6):768–773. doi: 10.1128/iai.3.6.768-773.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]