Abstract
Patients with schizophrenia suffer from higher rates of obesity and related morbidity and mortality than the general population. Women with schizophrenia are at particular risk for antipsychotic-induced weight gain, obesity, and related medical disorders such as diabetes and cardiovascular disease. Given preclinical studies revealing the role of the endogenous opioid systems in human appetite and the potential of antipsychotic medications to interfere with this system, we hypothesized that opioid antagonists may be beneficial in arresting antipsychotic-induced weight gain and promoting further weight loss in women with schizophrenia. In the present study, 24 overweight women with a diagnosis of schizophrenia or schizoaffective disorder were randomized to placebo (PLA) or naltrexone (NTX) 25mg/day for 8 weeks. The primary outcome measure was change in body weight from baseline. Patients in the NTX group had significant weight loss (−3.40kg) compared to weight gain (+1.37kg) in the PLA group. Mainly non-diabetic subjects lost weight in the naltrexone arm. These data support the need to further investigate the role of D2 blockade in reducing food reward based overeating. A larger study addressing the weaknesses of this pilot study is currently underway.
Keywords: Naltrexone, Antipsychotics, Obesity, Weight Loss, Schizophrenia
Introduction
Antipsychotic-induced weight gain is an important problem in the clinical care of schizophrenia. Obesity rates are very high in schizophrenia, which in turn contributes to morbidity and early mortality [1-4]. Obesity also contributes to lower quality of life, further stigma, and depression in this population [5] Obesity among schizophrenia populations is in part an iatrogenic problem. Pre-antipsychotic era studies of body weight demonstrate overweight and obesity rates lower than general population [6, 7]. First reports of concern about antipsychotic-induced weight gain began a few years after the introduction of chlorpromazine [8, 9]. For successful holistic treatment of schizophrenia, it is clear that the weight gain problem needs to be resolved. The gold standard of obesity treatment for the general population is behavioral interventions that aim to incorporate healthy nutrition with realistic calorie restriction and physical activity into the daily lives of the participants through behavioral change [10, 11]. Such lifestyle interventions have consistently been shown to halt or reduce antipsychotic-induced weight gain and produce modest weight loss [12, 13]. However, when lifestyle interventions are used in the severe mentally ill population, the magnitude of weight loss achieved is much less than that of non-psychiatric samples. It appears that lifestyle intervention strategies may need to be supplemented by pharmacological strategies that have the potential to counteract the metabolic effects of antipsychotic medications
One of the most likely reasons for antipsychotic-induced weight gain is central histamine 1 (H1) receptor blockade as the antipsychotics which cause the most weight gain are strong blockers of H1 receptors [14, 15]. H1 receptors are involved in appetite regulation in the hypothalamus. Pure H1 blockers increase appetite and are associated with overweight/obesity [16]. Another obvious pharmacological action that might be responsible for weight gain is action on several serotonin (5-HT) receptors, particularly 5HT2c which is blocked by many second generation and several first generation antipsychotics. 5HT2c receptors are involved in appetite regulation [14, 17]. Lorcaserin, a 5HT2c agonist, was recently approved by the FDA as a weight loss agent. Blockade of H1 or 5-HT receptors, however, does not explain why a medication like haloperidol, an almost pure dopamine-2 (D2) blocker, would cause clinically significant weight gain [18, 19]. Weight gain associated with haloperidol is more pronounced for medication naïve first episode samples, where it has been shown to produce on average 7 kg weight gain over the course of a year [19, 20]. Most first episode patients are young, healthy, and have similar cardio-metabolic risk when they enter care to their non-SMI peers in the general population [3]. Their cardio-metabolic risk doubles within the first year of entry into psychiatric care [21].
D2 receptors do not appear to be directly involved in energy metabolism, at least in the appetite regulation component. On the other hand, the dopamine system is an integral part of the human reward system, and D2 receptors are particularly involved in food reward [22]. It can be said that food is a prime substrate for the human reward system. Endorphin release starts at chewing and is particularly pronounced for high sugar, high fat “palatable” foods [23]. These are efficient calories that are easily storable for lean times. Both animals and humans decrease their preference for palatable foods when central opioid receptors are blocked by an opioid receptor blocker [24, 25]. D2 receptors appear to be necessary for endorphin action for food reward [22]. Obese people have been shown to have down-regulated D2 action, and certain D2 receptor alleles, which result in decreased sensitivity of D2 receptors, have been reported to be associated with obesity [26-28]. D2 blockade in animals, when given in doses that simulate human clinical use, increases free food intake, which is reversed by opioid blockers, although opposite animal data exist as well[29]. D2 blockade appears to have an effect similar to reward dilution[30]. Because D2 blockade at various levels is the common property of all antipsychotics in clinical use, one simple explanation is that antipsychotics cause a curbing of the food reward, which in turn might cause patients to eat richer foods more frequently in order to reach similar levels of food reward. Increased circulating endogenous opioids shown to exist in obese females on antipsychotic medications provide partial support to this hypothesis[31]. If this is the case, D2 blockade may be partially responsible for the antipsychotic-induced weight gain. Perhaps, if the opioidergic reward is completely blocked, the compensatory reward eating will stop. Opioid receptor blockers, such as naltrexone, have been studied as weight loss agents and have not been shown to be consistently effective by themselves; however, they may be able to counteract the part of antipsychotic-induced weight gain that might be caused by D2 blockade. A recent study of add-on naltrexone treatment for subjects using olanzapine reported no significant change in weight, however a decrease of fat mass was reported[32].
Based on the above rationale, as a proof of concept, we conducted a double blind, randomized, placebo-controlled trial of the general opioid receptor blocker, naltrexone, as an add-on agent for continuing antipsychotic-induced weight gain among patients with schizophrenia. A low-dose strategy was employed to minimize side effects because higher doses did not produce any additional benefit in other populations[33]. Based on previous studies, we only recruited women to this pilot trial in order to maximize the signal[34]. We hypothesized that naltrexone would be able to stop the antipsychotic-induced weight gain, while subjects who receive placebo would continue the steady increase in their weight or show no effect.
Methods
Twenty-four overweight women between the ages of 18-70 who met DSM-IV criteria for schizophrenia or schizoaffective disorder, based on SCID, were recruited. Subjects were defined as overweight if they had a BMI greater than or equal to 27 kg/m2 and weight gain in the past year above 2% of previous year’s body weight. Subjects were required to be on a stable dose of antipsychotic medication and deemed to be symptomatically stable by the clinical staff in the previous two months. All subjects gave a written informed consent to participate and the Yale HIC approved the protocol.
Subjects were excluded if they 1) Met criteria for current alcohol or other substance dependence, opioid use for any reason, or positive urine drug screen for opiates; 2) Had a history of dementia, mental retardation, or other neurological disorder that may interfere with study ratings; 3) Were not capable of giving informed consent for participation in the this study; 4) Ongoing pregnancy; 5) Known sensitivity to naltrexone; 6) A medical disorder known to cause obesity; 7) Use of sibutramine, topiramate, amphetamines, or over –the-counter weight remedies; or 8) Impaired liver function.
Study design
This was an eight week randomized, double-blind placebo-controlled, parallel, fixed-dose clinical study. Subjects who met inclusion criteria participated in a screening visit where blood and urine were taken and weight and BMI were measured. If laboratory results were within normal limits, the subject continued to a baseline visit where they were randomized to placebo or naltrexone 25 mg/day for eight weeks. Weight was measured with shoes off to the nearest 0.1 kg and height was measured to the nearest 0.1 cm to calculate BMI. At each visit, weight and blood pressure were obtained and subjects were asked about side effects and medication compliance. Questionnaires, interviews, and laboratory testing were performed at baseline, week 4, and week 8. No other behavioral or diet intervention was provided.
Study medicines were prepared by CMHC research pharmacy. Active and placebo capsules were identical. CMHC research pharmacy used a computerized randomization procedure to which investigators and subjects were blind. Compliance is monitored through pill counts.
Blood sampling and Analysis
All blood sampling was done via an indwelling catheter. Plasma glucose concentration was analyzed by the glucose oxidase method (Yellow Springs Instruments, Ohio)[35]. Plasma immunoreactive insulin concentration was determined with a double antibody radioimmunoassay (Diagnostic, Webster, TX)[36]. Serum cholesterol (Total-C, HDL-C) and Triglycerides (TG) were determined by standard enzymatic procedures (Sigma, St. Louis, MO). Assays of HbA1c were performed using an DCA Vantage analyzer (Siemens, Germany)[37].
Interviews and questionnaires
The Positive and Negative Syndrome Scale (PANSS) was utilized to demonstrate that the intervention did not cause any worsening of schizophrenia symptoms [38]. Three Factor Eating Questionnaire (TFEQ)[39], also known as the Eating Inventory is a measure of eating behaviors with three factors: dietary (cognitive) restraint, disinhibition, and hunger. The TFEQ is a frequently used measure in obesity trials and has clinical utility to assess changes during treatment as well as treatment outcomes[40] The Questionnaire on Craving for Sweet or Rich Foods (QCSRF) is a two factor, nine item scale assessing the presence of cravings for rich and sweet foods and has been found to have good psychometric properties [41]. The abbreviated Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q-18) is a brief, self administered questionnaire that was used to monitor quality of life outcomes [42].
Statistical analysis
Analyses were performed with a modified intent-to-treat principle where every randomized participant who received at least one study pill was analyzed in the group to which they were randomized. Descriptive statistics were calculated for all participants at baseline to compare naltrexone and placebo participants using analysis of variance (ANOVA) for normally distributed covariates and chi-square tests for categorical covariates. Clinically relevant group differences were assessed using covariate adjustment in supportive analyses. All analyses were performed using SPSS, version 17.0 for Windows (SPSS, Chicago, IL).
Results
Participant characteristics
Twenty-four women completed the initial screening and were randomized. Three participants (NTX=2, PLA=1) dropped out after randomization due to change of mind, leaving 21 (NTX=10, PLA=11) participants who started the intervention. All 21 subjects completed the study and comprise the evaluable sample. Table 1 presents the baseline characteristics of the sample. Two groups had similar demographics with no statistical difference. However, significantly more patients in the NTX arm had diabetes (6 vs. 1, χ2=5.79, p=.016), thus we performed additional analyses co-varying for diabetes.
Table 1.
Baseline Characteristics of The Sample
| Naltrexone (n=11) | Placebo (n=12) | |
|---|---|---|
| Age (years) | 50.0 ± 9.6 | 41.4 ± 11.6 |
| Weight (Kg) | 116.8 ± 27.8 | 102.0 ± 27.1 |
| Diagnosis | 7- Schizophrenia 4- Schizoaffective |
5- Schizophrenia 7- Schizoaffective |
| Atypical antipsychotic | 7 | 8 |
| PANSS Total Score | 64.7± 17.2 | 56.0± 16.7 |
| Diabetes | 6 | 1 |
| Race | 4- White 7- African American 0- Hispanic |
7- White 4- African American 1- Hispanic |
| Education (years) | 13.4 ± 4.1 | 13.7 ± 3.9 |
Laboratory and anthropometric values
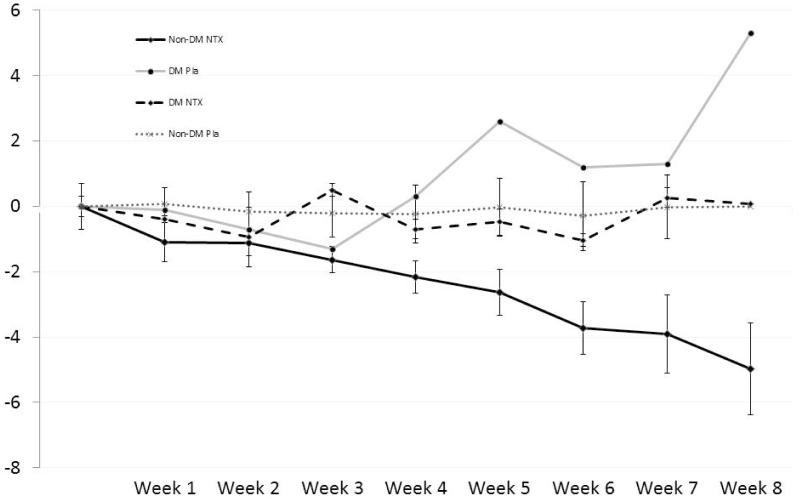
After eight weeks, participants randomly assigned to NTX had significant weight loss (−3.40kg, CI= −5.16,−1.65, F=14.79, p=.001) compared to weight gain in those assigned to PLA (+1.37kg, CI=−.30, 3.03), with diabetic status entered as a covariate. Subjects assigned to NTX had a significant reduction in BMI (−1.37, CI= −2.054,−0.68, F=15.86, p=.001) vs. placebo (+0.57, CI= −0.79,−1.22) after the intervention. On post-hoc analysis weight change remained significantly different between the two groups, even when the analysis was not co-varied by diabetes (−2.45kg CI= −4.86,−0.04 vs. +0.47kg CI= −1.76, +2.70; U=27.00, p=0.049). However, on average, only the non-diabetic subjects assigned to naltrexone lost weight (−4.98kg, CI= −8.74, −1.22), which was significant compared to baseline (Fig 1). Changes in BMI showed a similar pattern. Waist circumference on average was reduced by 3cm in the NTX arm and increased by 1cm in the placebo arm. However, this was not statistically significant between groups.
Figure 1.
Weight Change (Mean kg ± SE) for Diabetic (DM) and Non-Diabetic (Non-DM) subjects on naltrexone (NTX) and Placebo (Pla)
After eight weeks, there were no significant differences in metabolic laboratory parameters from baseline or between groups (Table 2).
Table 2.
Baseline vs. Week 8 Laboratory Values Between Placebo (Pla) and Naltrexone (NTX) groups
| Pla | NTX | |||
|---|---|---|---|---|
| week 0 | week 8 | week 0 | week 8 | |
| Weight | 104.66 (26.8) | 105.14 (28.1)* | 110.51 (19.2) | 108.06 (17.7)* |
| BMI | 40.14 (12.0) | 40.32 (12.5) ** | 41.95 (8.4) | 41.0 (7.7) ** |
| Glucose (mg/dl) | 93.82 (7.9) | 94.11 (7.5) | 113.40 (28.2) | 129.22 (45.2) |
| Insulin (uIU/ml) | 13.84 (10.8) | 12.36 (4.4) | 26.42 (40.0) | 35.51 (42.0) |
| Triglycerides (mg/dl) | 137.80 (48.1) | 121.44 (57.1) | 117.40 (49.4) | 121.22 (49.7) |
| Total Cholesterol (mg/dl) | 193.09 (28.1) | 189.78 (41.2) | 158.0 (32.2) | 175.33 (41.3) |
| HDL Cholesterol (mg/dl) | 48.73 (10.1) | 53.56 (10.1) | 47.5 (5.5) | 49.22 (8.0) |
| LDL Cholesterol (mg/dl) | 123.18 (41.3) | 111.22 (33.5) | 86.80 (26.2) | 104.11 (39.9) |
| Hemoglobin A1c (%) | 5.72 (0.2) | 5.71 (0.2) | 6.41 (0.8) | 6.65 (1.1) |
(p =0.049)
(p = 0.041) All others not significant between groups
QCSRF, TFEQ, PANSS, and Q-LES-Q 18
There were no differences in craving or reinforcement of sweet and rich foods at baseline. Between group analysis of change over 8 weeks did not show any statistical difference. In post-hoc analyses of the QCSRF subscales, NTX but not placebo resulted in a significant reduction in the craving of sweet and rich foods (−3.10±3.92 vs. 0±4.31, z= −2.25, p=.024). Change in reinforcement was not different from baseline for either group after the intervention. There were no significant differences between the groups at any time point in TFEQ cognitive restraint, disinhibition, and susceptibility to hunger subscales. Post-hoc analyses of change were not significant.
There were no differences in psychiatric symptoms or quality of life at baseline or in change over the 8-weeks of treatment.
Discussion
This pilot study demonstrated that naltrexone produced weight loss in a group of female schizophrenia patients who were acutely gaining weight while on antipsychotics. The effect was only seen in patients not currently diagnosed with diabetes mellitus. The medication did not produce any adverse change in psychiatric symptomatology and was well tolerated. All subjects who took at least one dose of study medication completed the study.
While there was significant weight loss in the active naltrexone group, this was not associated with corresponding change in metabolic parameters such as blood glucose and lipids. We might not have the power to observe this change. Also, improvement in metabolic parameters might require longer follow-up.
The mechanism of weight change seemed to be explained by decreased craving for sweet and rich foods. This is compatible with the working hypothesis that has driven this study; individuals on antipsychotic medications experience a reward dilution and eat more food to achieve the same reward. No other part of their eating behavior seemed to be changed. While it is not possible to exclude other mechanisms of weight loss with naltrexone, it is helpful to note that naltrexone alone does not produce appreciable weight loss in obese/overweight individuals who are not on antipsychotic medications[43]. Also it is interesting to note that the partially negative study by Taveira et al recruited only subjects on olanzapine, an antipsychotic with relatively low D2 and very strong H1 blockade[32]. This may indirectly support the working hypothesis of this paper, that naltrexone might counteract the D2 blockade-related portion of antipsychotic induced weight gain. On the other hand, Taviera et al showed a decrease in fat mass, and since both groups lost weight, presumably due to nutritional counseling provided, it might be hard to discern differences in naltrexone and placebo groups[32]. Clearly studies with larger sample sizes are necessary.
At this point it is hard to explain why subjects who have already developed diabetes mellitus did not respond to naltrexone, however it is important to note that there was only one diabetic subject in the placebo arm. To our knowledge neither opioid receptors nor dopaminergic receptors are well studied in diabetic individuals in relation to food intake. This area clearly requires more study.
This study has some shortcomings. Due to the pilot nature of the study, the sample size was small, partly offset by large effect size. Another limitation is that we only recruited women. While women seem to suffer more from antipsychotic-induced weight gain, most people who receive antipsychotics for the treatment of schizophrenia are male. We also did not stratify the study randomization based on diabetes status; thus, we had to control for an uneven distribution statistically. Also, due to the sample size, we were unable to look at the effect of antipsychotic class on outcome in this sample. Finally, the naltrexone dose used blocks about 70% of mu receptors, but may not block all delta and kappa receptors in the brain [44, 45]. It is not clear if a higher dose, such as that used in alcoholism, 50 mg per day, would have provided further benefit. We have recently started a larger NIH funded study to address all the weaknesses of the current study.
There is a need for interventions to address antipsychotic-induced weight gain to prevent morbidity and early mortality in the vulnerable population who have to use these drugs. We have hypothesized that antipsychotic weight gain is partly due to D2 receptor blockade induced dilution of food reward causing compensatory overeating. As a proof the concept we have conducted a randomized, double-blind, placebo-controlled study of naltrexone to block the food reward thus rendering compensatory overeating useless to achieve the same level of food reward. Naltrexone, as predicted, produced weight loss for a group of patients with schizophrenia who were acutely gaining weight, whereas subjects randomized to placebo continued to gain weight. However, the effect was observed only in patients who did not have diabetes. The results provide partial support for our working hypothesis. A larger study which addresses the weaknesses of this pilot study is currently under way [46].
Acknowledgments
Source of Funding
This study was fully funded by a Pilot Project Grant from Women’s Health Research at Yale (WHRY). Dr Tek was also supported by a NARSAD Young Investigator Award. The State of Connecticut, Department of Mental Health and Addiction Services supports all research at the Connecticut Mental Health Center.
Footnotes
Conflicts of Interest
Dr. Tek acknowledges the following sources of support: Grants/research support: NIH/NIDDK, Genentech; and is a member of the Genentech Inc. advisory panel.
Dr. Ratliff is currently employed as an Associate Nutrition Scientist at the Dr Pepper Snapple Group.
Ms. Reutenauer declares no conflicts of interest.
Dr. Ganguli currently has no relevant conflicts of interest, but has in the past received research grant support or honoraria from Jassen pharmaceutical, Lilly, Bristo Myers-Squibb, and Pfizer.
Dr. O’Malley received commercial research grant from NABI Biopharmaceuticals, Eli Lilly, Arkeo, and Pfizer, has ownership interest in Applied Behavioral Research, LLC and an abandoned patent on naltrexone, and is a consultant with Pfizer, Abbott, Alkermes, Ethypharm, GlaxoSmithKline, Lilly, Schering Plough, Lundbeck, Janssen, Gilead, and Hazelden Foundation.
Contributor Information
Cenk Tek, Yale University School of Medicine, Department of Psychiatry
Joseph Ratliff, Yale University School of Medicine, Department of Psychiatry.
Erin Reutenauer, Yale University School of Medicine, Department of Psychiatry.
Rohan Ganguli, University of Toronto, Centre for Addiction and Mental Health
Stephanie S. O’Malley, Yale University School of Medicine, Department of Psychiatry
References
- 1.Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiat. 1999;60:215–220. doi: 10.4088/jcp.v60n0402. [DOI] [PubMed] [Google Scholar]
- 2.Saari KM, Lindeman SM, Viilo KM, et al. A 4-fold risk of metabolic syndrome in patients with schizophrenia: the Northern Finland 1966 Birth Cohort study. The Journal of clinical psychiatry. 2005;66:559–563. doi: 10.4088/jcp.v66n0503. [DOI] [PubMed] [Google Scholar]
- 3.Phutane VH, Tek C, Chwastiak L, et al. Cardiovascular risk in a first-episode psychosis sample: a ‘critical period’ for prevention? Schizophrenia research. 2011;127:257–261. doi: 10.1016/j.schres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA: the journal of the American Medical Association. 2007;298:1794–1796. doi: 10.1001/jama.298.15.1794. [DOI] [PubMed] [Google Scholar]
- 5.Barber JA, Palmese L, Reutenauer EL, et al. Implications of weight-based stigma and self-bias on quality of life among individuals with schizophrenia. J Nerv Ment Dis. 2011;199:431–435. doi: 10.1097/NMD.0b013e318221403d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baganz CN, Norris JM. A study of malnutrition in chronic schizophrenia. The American journal of psychiatry. 1943;97:650–658. [Google Scholar]
- 7.Sharp HC, Baganz CN. A Study of the problem of malnutrition in institutionalized psychotic patients. The American journal of psychiatry. 1940;97:650–658. [Google Scholar]
- 8.Mefferd RB, Jr., Labrosse EH, Gawienowski AM, et al. Influence of chlorpromazine on certain biochemical variables of chronic male schizophrenics. The Journal of nervous and mental disease. 1958;127:167–179. doi: 10.1097/00005053-195808000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Planansky K, Heilizer F. Weight changes in relation to the characteristics of patients on chlorpromazine. J Clin Exp Psychopathol. 1959;20:53–57. [PubMed] [Google Scholar]
- 10.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am. 2005;28:151–70. ix. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Jean-Baptiste M, Tek C, Liskov E, et al. A pilot study of a weight management program with food provision in schizophrenia. Schizophr Res. 2007;96:198–205. doi: 10.1016/j.schres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Daumit GL, Dickerson FB, Wang NY, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013;368:1594–1602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey DE, Zorn SH. The pharmacology of weight gain with antipsychotics. J Clin Psychiatry. 2001;62(Suppl 7):4–10. [PubMed] [Google Scholar]
- 15.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 16.Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring) 2010;18:2398–400. doi: 10.1038/oby.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marston OJ, Heisler LK. Targeting the serotonin 2C receptor for the treatment of obesity and type 2 diabetes. Neuropsychopharmacology. 2009;34:252–253. doi: 10.1038/npp.2008.169. [DOI] [PubMed] [Google Scholar]
- 18.Allison D, Mentore J, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: A randomized controlled trial. J Clin Psychiatry. 2006;67:1253–1260. doi: 10.4088/jcp.v67n0812. [DOI] [PubMed] [Google Scholar]
- 20.Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537–543. doi: 10.1192/bjp.187.6.537. [DOI] [PubMed] [Google Scholar]
- 21.Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophrenia research. 2013;146:64–68. doi: 10.1016/j.schres.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stice E, Yokum S, Zald D, Dagher A. Dopamine-based reward circuitry responsivity, genetics, and overeating. Curr Top Behav Neurosci. 2011;6:81–93. doi: 10.1007/7854_2010_89. [DOI] [PubMed] [Google Scholar]
- 23.Melchior JC, Rigaud D, Chayvialle JA, et al. Palatability of a meal influences release of beta-endorphin, and of potential regulators of food intake in healthy human subjects. Appetite. 1994;22:233–244. doi: 10.1006/appe.1994.1022. [DOI] [PubMed] [Google Scholar]
- 24.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 25.Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 28.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 29.Baptista T, Lacruz A, Acosta A, et al. Naltrexone does not prevent the weight gain and hyperphagia induced by the antipsychotic drug sulpiride in rats. Appetite. 2000;34:77–86. doi: 10.1006/appe.1999.0284. [DOI] [PubMed] [Google Scholar]
- 30.Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Baptista T, Lacruz A, Angeles F, et al. Endocrine and metabolic abnormalities involved in obesity associated with typical antipsychotic drug administration. Pharmacopsychiatry. 2001;34:223–231. doi: 10.1055/s-2001-18034. [DOI] [PubMed] [Google Scholar]
- 32.Taveira TH, Wu WC, Tschibelu E, et al. The effect of naltrexone on body fat mass in olanzapine-treated schizophrenic or schizoaffective patients: A randomized double-blind placebo-controlled pilot study. J Psychopharmacol. 2013;28:395–400. doi: 10.1177/0269881113509904. [DOI] [PubMed] [Google Scholar]
- 33.O’Malley SS, Cooney JL, Krishnan-Sarin S, et al. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- 34.King AC, Cao D, Zhang L, O’Malley SS. Naltrexone reduction of long-term smoking cessation weight gain in women but not men: a randomized controlled trial. Biol Psychiatry. 2013;73:924–930. doi: 10.1016/j.biopsych.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twomey PJ. Plasma glucose measurement with the Yellow Springs Glucose 2300 STAT and the Olympus AU640. Journal of clinical pathology. 2004;57:752–754. doi: 10.1136/jcp.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PKD, Mistry J, Hintz RL. Active IGF-I Assays. Diagnostic Systems Laboratories; Webster: 1996. [Google Scholar]
- 37.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clinical chemistry. 2010;56:44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]
- 38.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 39.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 40.Foster GD, Wadden TA, Swain RM, et al. The Eating Inventory in obese women: clinical correlates and relationship to weight loss. Int J Obes Relat Metab Disord. 1998;22:778–785. doi: 10.1038/sj.ijo.0800659. [DOI] [PubMed] [Google Scholar]
- 41.Toll BA, Katulak NA, Williams-Piehota P, O’Malley S. Validation of a scale for the assessment of food cravings among smokers. Appetite. 2008;50:25–32. doi: 10.1016/j.appet.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritsner M, Kurs R, Gibel A, et al. Validity of an abbreviated quality of life enjoyment and satisfaction questionnaire (Q-LES-Q-18) for schizophrenia, schizoaffective, and mood disorder patients. Qual Life Res. 2005;14:1693–703. doi: 10.1007/s11136-005-2816-9. [DOI] [PubMed] [Google Scholar]
- 43.Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. The Journal of clinical endocrinology and metabolism. 2009;94:4898–906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- 44.Rabiner EA, Beaver J, Makwana A, et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry. 2011;16:826–35. 785. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh SL, Chausmer AE, Strain EC, et al. Evaluation of the mu and kappa opioid actions of butorphanol in humans through differential naltrexone blockade. Psychopharmacology (Berl) 2008;196:143–55. doi: 10.1007/s00213-007-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tek C, Guloksuz S, Srihari VH, et al. Investigating the safety and efficacy of naltrexone for anti-psychotic induced weight gain in severe mental illness: study protocol of a double-blind, randomized, placebo-controlled trial. BMC psychiatry. 2013;13:176. doi: 10.1186/1471-244X-13-176. [DOI] [PMC free article] [PubMed] [Google Scholar]