Abstract
Mitochondrial initiated events protect the neurovascular unit against lethal stresses via a process called preconditioning which independently promotes changes in cerebrovascular tone through shared signaling pathways. Activation of the adenosine triphosphate (ATP)-dependent potassium channels on the inner mitochondrial membrane (mitoKATP channels) is a specific and dependable way to induce protection of neurons, astroglia, and cerebral vascular endothelium. Through the opening of mitoKATP channels, mitochondrial depolarization leads to activation of protein kinases and transient increases in cytosolic calcium (Ca2+) levels that activate terminal mechanisms that protect the neurovascular unit against lethal stress. Release of reactive oxygen species (ROS) from mitochondria have similar protective effects. Signaling elements of the preconditioning pathways also are involved in the regulation of vascular tone. Activation of mitoKATP channels in cerebral arteries causes vasodilation, with cell-specific contributions from endothelium, vascular smooth muscle (VSM), and nerves. Pre-existing chronic conditions, such as insulin resistance (IR) and/or diabetes, prevent preconditioning and impair relaxation to mitochondrial centered responses in cerebral arteries. Surprisingly, mitochondrial activation after anoxic or ischemic stress appears to protect cerebral vascular endothelium and promotes the restoration of blood flow; therefore, mitochondria may represent an important, but underutilized target in attenuating vascular dysfunction and brain injury in stroke patients.
Keywords: cerebral arteries, ischemia, anoxia, ATP-sensitive potassium channels, calcium sparks, endothelium, vascular smooth muscle, neurons, nitric oxide synthase, postconditioning
INTRODUCTION
The neurovascular unit is an integrated system of vascular and parenchymal cells and their environment working together to match blood flow to metabolic demand and to maintain brain homeostasis. Mitochondria are double membrane organelles which generate chemical energy in the form of ATP which is distributed within cells and promotes the varied activities of the diverse cell types comprising the neurovascular unit (endothelium, vascular smooth muscle [VSM], astroglia, perivascular neurons, parenchymal neurons, pericytes, and microglia). Although traditionally understood solely as energy producers and relegated to that role, we now appreciate that mitochondria are involved in diverse adaptive activities such as promotion of basal cellular functions, cellular protection, and regulation of vascular tone. Evidence also shows that mitochondrial morphology and function show plasticity and adaptability and can be affected by many factors [12,37,72,107,137]; on the other hand, mitochondrial-centered mechanisms can promote apoptotic and necrotic cell death [56,89]. The purpose of this review is to critically examine the role of mitochondrial-based mechanisms on the tone of cerebral resistance vessels. Specifically, we will examine how the selective targeting of mitochondria in the several cell types comprising the neurovascular unit leads to changes in cerebral vascular tone. The studies on cerebral vascular control in our laboratory originated from studies of pre- and post-conditioning mechanisms in the neurovascular unit when we realized that identical pathways are involved and effects may be interactive. This discussion is limited to the systemic circulation; mitochondrial mechanisms in the pulmonary circulation are different [35]. We will show that even relatively mild metabolic stress, such as that which occurs with IR, a pre-diabetic condition that impairs preconditioning, can dramatically affect mitochondrial related events in the cerebral vasculature. Ultimately, we will present evidence suggesting that mitochondrial activation may unexpectedly benefit the cerebral vasculature after ischemia/anoxia and thus decrease morbidity and mortality in stroke patients. Several excellent reviews which focus primarily on peripheral endothelium [53,85,138] or VSM [108] have been published recently and emphasize the importance of this emerging research area. However, this is the first review to show the similarities between the preconditioning and vascular control pathways and to explore the integration of mitochondrial based signals ranging from multiple cell types to the final vascular response in normal and disease states.
MITOCHONDRIAL ESSENTIALS
Important structural features of mitochondria are an outer, relatively permeable membrane, a relatively impermeable inner membrane, the intermembrane space, extensions of the inner membrane called cristae, and the intracristae spaces referred to as the matrix [34,68]. Each anatomical component has a specific function which, when integrated, is essential for the optimal production and transfer of energy throughout the mitochondria and into the cytosol. The Krebs cycle is located in the matrix and the complexes associated with the electron transport chain are embedded in the inner mitochondrial membrane. The mitochondrial membrane potential (Δψm) across the inner membrane, which is normally maintained at approximately −180mV, provides the proton difference used to drive the synthesis of ATP by the electron transport chain. Several diagnostic and therapeutic approaches take advantage of this negative membrane potential to target positively charged agents specifically to the mitochondria [26,52,69,114]. Detailed descriptions of ATP production by non-mitochondrial glycolysis as well as the mitochondrial electron transport chain are widely available [36,136].
Individual mitochondria are not static entities, rather they are dynamic and can move from one location to another within cells, form networks with other mitochondria or cellular structures (Figures 1 & 2), and undergo replication and fission/fusion in response to physiological and pathological stimuli [26,34,38,137,145]. Because the number and length of cristae are positively related to ATP generating potential, it appears that changes in the location, number, size, and shape of mitochondria under physiological conditions are determined by the energy needs within a cell [61]. In some cell types, such as VSM, mitochondria do not appear to be mobile in the normal state, but this situation changes during migration following vascular damage [17]. In addition to individual mitochondria forming networks within cells (Figures 1 & 2), the mitochondrial outer membranes are often connected or closely juxtapositioned with the membranes of the endoplasmic/sarcoplasmic reticulum (ER/SR) (Figure 2); cellular structures which are involved in calcium storage and release [145]. The complex interplay among mitochondrial, ER/SR, and cytosolic calcium has been described in several recent reviews [16,23,130]. In VSM, mitochondria and SR often appear to be “bundled” together and this structural feature may account for the lack of significant movement of mitochondria under normal conditions in this vascular cell type (Figure 2) as well as the tight functional interactions between mitochondria and SR. However, we are unaware of any systematic studies comparing the mitochondrial morphology and function in the various cell types within the neurovascular unit.
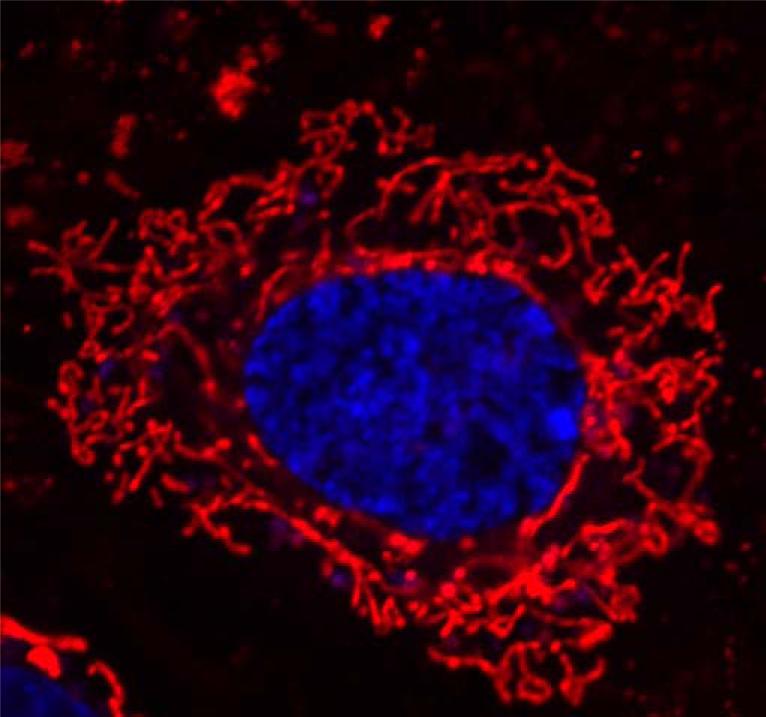
Figure 1.
Mitochondrial morphology within a cerebral vascular endothelial cell. The mitochondria (red) and the nuclei (blue) of live rat brain microvascular endothelial cells were stained using mitochondrial stain (1:10000; lex: 514 nm and lem: 538-681 nm) and Hoechst nuclear stain (1: 1000; lex: 405 nm and lem: 410-585 nm), respectively (ChromeoTM Live Cell Mitochondrial Staining kit, Active Motif, Carlsbad, California). Fluorescence images were obtained using LSM 710 AxioObserver (Carl Zeiss, Hamburg, Germany) with plan-Apochromat 63×/Numerical Aperture 1.4 oil immersion objective.
Figure 2.
Lower and higher magnifications of VSM cells from cerebral arteries from normal rats showing clustering of mitochondria (m) and sarcoplasmic reticulum (SR) in distinct locations. Arteries were fixed in 2.5% glutaraldehyde and post-fixed in 1% osmium tetroxide and embedded in Epon812. Ultrathin sections (80-90 nm) were mounted on formvar-coated nickel grids (200 mesh), air dried, and stained with 4.7% uranyl acetate and lead citrate (at 10 min and 2 min, respectively). The sections were put on grids and viewed using a FEI Tecnai BioTwin 120 keV TEM with a digital imaging setup (Wake Forest University Health Sciences, Winston-Salem, NC).
Mitochondria have their own DNA, distinct from nuclear DNA, and also possess protein synthesizing machinery which produces approximately one-half of their proteins while the other half are encoded by nuclear DNA and imported into mitochondria via several different transport mechanisms. The cytochrome c oxidase complex (Complex IV), which is the last major component of the electron transport chain and is localized to the inner membrane, is a good example of the dual origins of the proteins present in mitochondria since ten subunits are nuclear in origin and three are synthesized within the mitochondria [50,95].
Although it has been suggested that mitochondria contain a variant of nitric oxide synthase (NOS), and thus could produce NO via this enzymatic pathway, there is significant evidence against this view [91,92]. Nonetheless, diffusible NO from non-mitochondrial sources in endothelial cells and neurons [74], or NO arising from mitochondria via non-NOS pathways [94], could affect the functioning of the electron transport chain as well as other mitochondrial functions and could combine with superoxide anion to form peroxynitrite [44,138]. On the other hand, we have shown that NO production from cytosolic NOS occurs due to eNOS phosphorylation and increased cytosolic calcium following activation of mitochondria with diazoxide or BMS-191095 [74].
ROS PRODUCTION AND RELEASE BY MITOCHONDRIA
Mitochondria are constant producers of ROS which have access to locations throughout the cell as well as to adjacent cells. Although generally thought to be an efficient system, a substantial amount of superoxide anion is continuously “lost” or released by the electron transport chain at different sites under normal conditions [36,138]. For example, there is continuous release of superoxide anion in quiescent neurons, astroglia, VSM, and cerebral vascular endothelium [47,74,122]. Mitochondrial ROS can promote preconditioning as well as directly or indirectly affect cerebral vascular tone, as well as influence other cellular functions.
Superoxide anion from mitochondrial and other cellular locations appears to be an important, if not essential, signaling agent involved in the maintenance of basal cell functions such that prolonged suppression of ROS availability is detrimental to cell viability [141]. Superoxide anion acting at sites within the mitochondria or following transport across the inner mitochondrial membrane can influence events throughout the cell. It has been suggested that superoxide anion can leave the mitochondria via voltage dependent anion channels (VDACs) [57,101]. However, VDACs are located in the outer, more porous mitochondrial membrane and it is not clear how superoxide anion would transverse the inner mitochondrial membrane. Alternatively, superoxide anion formed at Complex III may be released into either the matrix or the intermembrane space during certain conditions and then may be transported across the outer mitochondrial membrane by VDACs [60,118]. Superoxide anion also can leave the matrix [66] following enzymatic conversion to hydrogen peroxide through aquaporin-like channels in the inner mitochondrial membrane [8,15,103]. Hydrogen peroxide is considered to be less reactive and has a longer half-life than superoxide anion [120] and can be produced directly by mitochondria under certain conditions [120,143]. A number of factors such as substrate availability, status of the electron transport chain complexes, and the composition of the local environment are able to affect mitochondrial production of ROS [120]. In addition, a physical distortion of the relationship between the two mitochondrial membranes caused by increased shear stress in endothelium leads to enhanced ROS release by mitochondria [143]. It has generally been accepted that mitochondrial depolarization is always accompanied by enhanced ROS release. However, as described in detail below, mitochondrial depolarization and enhanced ROS production are not necessarily linked and can occur separately under a variety of conditions.
The primary sites of superoxide anion production and release are Complex I (NADH-ubiquinone oxidoreductase), Complex II (succinate dehydrogenase, SDH), and Complex III (ubiquinolcyctochrome c oxidoreductase). Complex I and II accept electrons from NADH + H+ and FADH2, respectively, which are transferred to Complex III and finally to Complex IV (cytochrome c oxidase), where the final electron acceptor is oxygen and the final product is water [121,143]. Superoxide anion from these three Complexes is released into the matrix. A number of metabolic poisons are available which inhibit one or more of the respiratory chain complexes: rotenone: Complex I; 3-NPA: Complex II; Antimycin A: Complex III; cyanide: Complex IV; and oligomycin: Complex V. The result of inhibition, especially of Complexes I-III, is enhanced ROS release.
Although “normal” continuous release of ROS from mitochondria appears to play a positive role in the maintenance of basal cellular function, transiently elevated ROS levels can promote selective protein synthesis, preconditioning, and changes in vascular tone. However, chronically but modestly elevated ROS production by mitochondria can lead to cellular dysfunction. For example, we have shown that a mutation of the inner mitochondrial membrane peptidase 2-like (Immp2l) gene leads to chronically enhanced ROS release by mitochondria and subsequently causes reduced vascular dilation to carbachol in mesenteric arteries [100]. Similarly, chronically elevated mitochondrial ROS production due to the metabolic syndrome impairs cerebral vascular function through multiple mechanisms [71]. It also has been reported that a genetic deficiency of MnSOD in mice increases basal superoxide levels in cerebral arteries and aorta [10]. Additionally, Chilian et al. have shown that chronic inhibition of Complex I by rotenone blocks ischemia induced collateral artery growth in the coronary circulation via activation of adenosine monophosphate activated kinase, and the subsequent inhibition of mTOR and p70 ribosomal S6 kinase [119]. Higher levels of cellular ROS, such as occurs during injury, can lead to cell death via both mitochondrial- and non-mitochondrial-pathways, especially in metabolically compromised conditions [57,89].
Production of ROS by mitochondria appears to be an organelle-independent process under most basal conditions, but recent reports indicate that ROS production by mitochondria can either promote ROS production by the extra-mitochondrial NADPH oxidase system, or vice versa, via a positive feedback system [23,29,146]. Thus, the interaction between the mitochondria and cytosolic NADPH oxidase axis leads to cellular damage due to excessive production of ROS by a ROS induced ROS mechanism. The best example of this interaction occurs with the exposure of vascular and non-vascular cells to angiotensin II [29,113]. Deficiency of MnSOD leads to cerebral vascular endothelial dysfunction, especially in aged mice to angiotensin II [21,110].
MITOCHONDRIAL DEPOLARIZATION AND RELATED EVENTS
The most reproducible and robust approach to depolarizing mitochondria experimentally can be achieved by targeting the mitochondrial ATP-sensitive potassium (mitoKATP) channel on the inner mitochondrial membrane with drugs such as BMS-191095 or diazoxide. The physical structure of mitoKATP channels is not yet known with certainty but appears to differ substantially from the previously described plasmalemmal KATP channels [1,43,125,127]. Nonetheless, the pharmacological identification and selectivity to agonists of the mitoKATP channels is convincing to the majority of investigators. Recently, Foster et al. [4] provided evidence that the renal outer medullary potassium channel (ROMK) is a component of the K+ channel of the cardiac mitoKATP. They also showed that overexpression and suppression of ROMK promoted or reduced preconditioning, respectively. Succinate dehydrogenase might also be involved in either the assembly or function of the mitoKATP channel [124,139]. Nonetheless, additional studies are needed to define completely the structure of the mitoKATP channels.
Isolated mitochondria and mitochondria in situ in cultured cells, tissue slices, and in isolated pressurized cerebral arteries depolarize in a dose-dependent manner to selective mitoKATP channel openers such as diazoxide and BMS-191095 [13,47] and mitoKATP channel activity is affected by endogenous factors such as the ADP/ATP ratio [1], peroxynitrite [90,91], superoxide anion [89,91], and cytosolic protein kinase C epsilon (PKCε) [118]. Nonetheless, we expect that other, yet unidentified, physiological and pathological factors will be able to directly or indirectly activate mitochondria including the mitoKATP channel. The classical KATP channel antagonist glibenclamide as well as 5-hydroxydecanoic acid (5-HD), which needs to be metabolized before becoming active [59], block the actions of diazoxide, BMS-191095, and/or PKCε [13,47,118]. Diazoxide, a drug previously used against acute hypertension or hypoglycemia in people, is the most commonly used mitoKATP channel opener [42], but it has the additional effect of inhibiting succinate dehydrogenase (SDH; complex II), especially at high doses [22,82]. Diazoxide also readily crosses the BBB and thus is effective in the brain when given intravenously [96]. Although applications of diazoxide or BMS-191095 depolarize mitochondria, diazoxide, but not BMS-191095, also causes the liberation of ROS [14], which our findings indicate is secondary to SDH inhibition. This view is supported by examination of the effects of the specific inhibitor of SDH, 3-nitropropionic acid (3-NPA), which increases ROS production by mitochondria [13] and also induces preconditioning [63] and changes in vascular tone [71]. Nonetheless, the primary actions of diazoxide on the cells of the neurovascular unit are still specific to mitochondria [14,82], and the associated ROS increase appears to enhance the degree of depolarization [90,91]. In contrast, BMS-191095 is very selective for mitoKATP channels and has no known non-specific effects to complicate the interpretation of the results [14,54,55].
A potential role for mitochondrial calcium activated potassium (mitoKCa) channels in depolarizing mitochondria has been suggested based primarily on the use of the multiple target drug NS1619 [6]. Although NS1619 results in mitochondrial depolarization, it seems likely that, at least in neurons, effects are due to other factors such as inhibition of Complex I and subsequent increased release of ROS [48]. Given the multiple potential sites of action of NS1619 within various cell types, it is also possible that mitochondrial effects to this drug are secondary to non-mitochondrial events. Nonetheless, more research in this area is warranted and the development of more specific agonist would aid these efforts.
MITOCHONDRIAL MEMBRANE POTENTIAL AND ROS PRODUCTION INDEPENDENCE
The use of BMS-191095 has led to findings which challenge accepted views concerning the linkage between mitochondrial depolarization and enhanced mitochondrial ROS release. The selectivity of BMS-191095 for mitoKATP channels and the failure to detect non-specific effects [14,54,55], which complicate the interpretation of results, has shown that it is possible to depolarize mitochondria without eliciting the enhanced release of ROS in a variety of cells including neurons, cerebral vascular endothelium, and cerebral VSM. The previous concept linking mitochondrial membrane potential and ROS release was developed using inhibitors of the electron transport chain which, in addition to increasing ROS release from Complexes I-III, probably depolarized mitochondria via reduced ATP availability necessary for maintaining the negative transmembrane potential. The more recent finding with BMS-191095, which allows dissociation between mitochondrial depolarization and mitochondrial ROS production, is supported by other laboratories [133,137] as well as other experimental approaches in our laboratory [33]. The dissociation between mitochondrial membrane potential and ROS release is not absolute, however, but probably occurs within a limited range of depolarization. The relative independence of mitochondrial membrane potential from ROS production and release, with the subsequent induction of different signaling pathways, underscores the versatility of mitochondria to initiate appropriate and selective cellular responses to varied stimuli.
ROLE OF MITOCHONDRIA IN PRECONDITIONING
Preconditioning represents the condition in which transient exposure of cells to an initiating event leads to protection against subsequent, potentially lethal stimuli (Figure 3) by limiting ROS availability, suppressing large increases in cytosolic levels of free calcium, and/or by other mechanisms which promote cell survivability (Figure 4). The acute signaling mechanisms promoting preconditioning also affect cerebral vascular tone, and the changes in cells following preconditioning resulting from the altered cellular calcium and ROS dynamics probably affect subsequent cerebral artery responses to changing conditions (Figure 5). However, this possibility has not been tested.
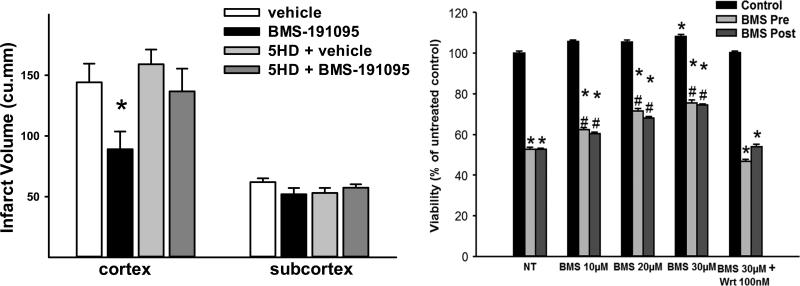
Figure 3.
Examples of preconditioning and postconditioning with mitoKATP channel opener BMS-191095. Left. BMS-191095 protected the rat brain in vivo against 90 min of middle cerebral artery occlusion when given before ischemia [128]. Right. Original data showing that BMS-191095, when given prior to or following OGD, improved survival of cultured rat cerebral endothelial cells. Inhibition of PI3Kinase with wortmannin (Wrt) counteracted beneficial effects of BMS-191095 (unpublished observations). *P<0.05.
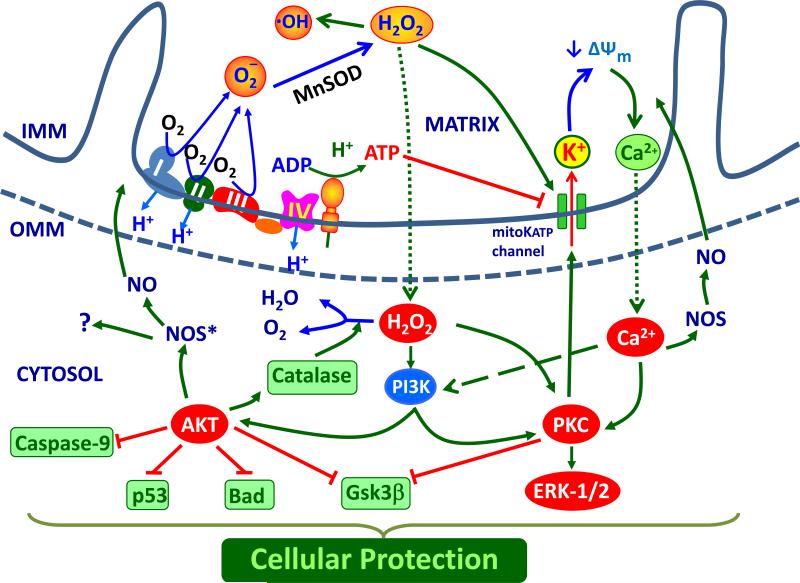
Figure 4.
Schematic illustration showing signaling events (red circles) and ultimate mechanisms (green boxes) resulting in cellular protection following opening of mitoKATP channels or liberation of ROS from the protein complexes which form the electron transport chain. Abbreviations: OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; ΔΨm, mitochondrial membrane potential; O2−, superoxide anion; H2O2, hydrogen peroxide; Ca2+, calcium; ADP, adenosine di-phosphate; ATP, adenosine tri-phosphate; PKC, protein kinase C; Gsk3β, phosphoglycogen synthase kinase 3 beta; PI3K, phosphoinositide 3-kinase; Bad, Bcl-2 associated death promoter, Akt; protein kinase B, eNOS*; phosphorylated endothelial NOS.
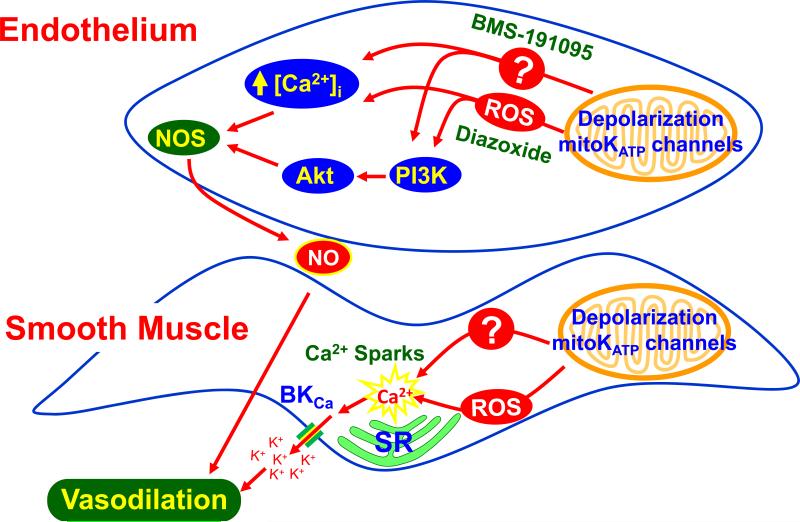
Figure 5.
Schematic illustration showing signaling events (red circles) and ultimate mechanisms (green boxes) resulting in changes in vascular tone following opening of mitoKATP channels or liberation of ROS from the protein complexes which form the electron transport chain. Abbreviations: ΔΨm, mitochondrial membrane potential; O2−, superoxide anion; H2O2, hydrogen peroxide; Ca2+, calcium; ADP, adenosine di-phosphate; ATP, adenosine tri-phosphate; PKC, protein kinase C; Gsk3β, phospho-glycogen synthase kinase 3 beta; PI3K, phosphoinositide 3-kinase; Bad, Bcl-2 associated death promoter, Akt; protein kinase B.
Beginning with our original observation [31], a number of independent studies by other laboratories have established that mitochondrial-centered mechanisms are important initiators of the pre- and post-conditioning response in neurons, astroglia, and cerebral endothelial cells [12,14,30,31,42,46,64,65,96,99,104,109,121,125,128]. Mitochondrial-centered preconditioning also occurs in tissues such as myocardium [70] and skeletal muscle [55] and peripheral endothelium [9,99]. Important mitochondrial specific targets for inducing preconditioning by pharmacological approaches include: (1) potassium channels located on the inner mitochondrial membrane; (2) respiratory chain enzymes; and (3) metabolic substrate restriction.
Although the mitochondrial-initiated mechanisms involved in preconditioning are not fully understood, the activation of protein kinases, transient but modest calcium fluxes, and/or production of ROS appear to be essential signaling events leading to preconditioning (Figure 4). There are two general types of preconditioning: immediate and delayed. Immediate preconditioning occurs within minutes of the initiating stimulus and lasts for several hours before disappearing, whereas delayed preconditioning takes several hours to develop and persists for several days [51,81]. Given these temporal aspects, it seems probable that immediate preconditioning primarily involves changes in the activity or function of enzymes, second messengers, and ion channels already present whereas delayed preconditioning principally is due to de novo protein synthesis such as increased catalase levels [14]. Many subsequent signaling events not only induce preconditioning but also promote changes in vascular tone. Postconditioning, in which the initiating event occurs following the onset of the potentially lethal event [125,144] has also been reported and the mechanisms, especially those involving mitochondria and the PI3-kinase/Akt signaling pathway, appear to be similar to those that promote preconditioning. In cultures of primary rat brain microvascular endothelium, we found that postconditioning was as effective as preconditioning against oxygen-glucose deprivation (OGD) (Figure 3), and that activation of the PI3Kinase/Akt signaling pathway was essential in both types of cellular protection (Figures 3 & 4). The ability of mitochondria from cerebral vascular endothelium to function at the end of OGD, where almost 50% of cells would eventually die without treatment, indicates that mitochondria remain a potentially, useful target in stroke patients.
Although some authors have presented a case for a role of mitochondrial calcium activated potassium (mitoKCa) channels in promoting preconditioning based primarily on the use of the multiple target drug NS1619 [18,27], the experimental evidence is not convincing due to the lack of a specific mitoKCa channel opener and the numerous non-specific effects of NS1619. We were the first to show that NS1619 is both neuroprotective [134] and vasoactive in cerebral [5] arteries, and that preconditioning of neurons by this agent was likely due to inhibition of Complex I or other mechanisms rather than a mitoKCa channel-specific effect [48,49,80,87]. Administration of NS1619 dilates other peripheral arteries [60].
Removal or reductions in key metabolic substrates, such as glucose and oxygen, can induce preconditioning and changes in cerebral vascular tone. For example, transient withdrawal of glucose, the major energy substrate for neurons, as well as removal of amino acids results in mitochondrial depolarization and reduced ATP production and in delayed tolerance against various insults such as OGD, glutamate excitotoxicity, and exogenous hydrogen peroxide toxicity [45]. Adenosine, AMP, ADP, and ATP are vasoactive stimuli in cerebral arteries [116,142].
MITOCHONDRIA AND CEREBRAL VASCULAR TONE
Our studies of preconditioning indicated that many of the signaling events associated with the induction of mitochondrial-targeted cellular protection also affect cerebrovascular tone (Figures 4 & 5). Although it is known that extracellular ATP, from either glycolysis or oxidative phosphorylation, as well as the metabolites adenosine, AMP, and ADP (also preconditioning stimuli) [2,109] can alter cerebrovascular tone via plasmalemmal purinergic receptors [28], only a few studies have examined direct mitochondrial influences on the diameter of cerebral resistance vessels [19,37,71,75,140]. Thus, the study of mitochondrial effects on the cerebral vasculature is a relatively new field. Only a few studies have examined mitochondrial influences on the cerebral vasculature during disease states, but increasing amounts of information are rapidly becoming available in other systemic circulations [54,85,138] and VSM [108]. Although the majority of studies involving mitochondrial influences on the cerebral vasculature have used pharmacological agents, a few studies have used genetically altered mice in which a deficiency of MnSOD has resulted in increased basal levels of superoxide anion and endothelial dysfunction in cerebral arteries especially with exposure to angiotenisn II and aging in male rats [21].
Vascular smooth muscle
Jaggar and colleagues [19,140] were the first to show that activation of mitochondria by diazoxide promoted relaxation of VSM cells in endothelium-denuded cerebral arteries or freshly dissociated VSM via a mechanism primarily involving ROS. Thus, diazoxide application enhanced the generation of ROS from mitochondria, which sequentially caused the activation of ryanodine-sensitive Ca2+ channels on the SR, the generation of Ca2+ transients called “Ca2+ sparks”, and the opening of adjacent large-conductance Ca2+-activated K+ (BKCa) channels on the plasma membrane. The resulting K+ efflux led to VSM hyperpolarization, decreased global intracellular Ca2+, and vasodilation. We have reported similar findings in endothelium denuded arteries with diazoxide [71,74]. The role of mitochondrial ROS resulting from inhibition of SDH in promoting VSM relaxation in cerebral arteries is also shown by application of 3-NPA [71]. However, BMS-191095 has a similar effect in VSM without the involvement of ROS [72,73] (Figure 5). Thus, BMS-191095 does not increase vascular cytosolic or mitochondrial levels of ROS in any of the cell types that we have examined and dilation to BMS-191095 is not affected by ROS scavengers; nonetheless, calcium spark activity increases with BMS-191095 application. The reasons for these differences in findings are unclear but taken together underscore the importance and robustness of mitochondrial mechanisms in promoting relaxation of VSM. We speculate that there is direct electophysiological coupling between mitochondria and SR in VSM and/or that microdomains involving the outer mitochondrial and SR membranes link functional interactions between these two organelles. The close physical association and thus the possibility of direct coupling of mitochondria and SR are clearly seen with electron microscopy in cerebral VSM (Figure 2).
Endothelium
Mitochondrial content in the cerebral vascular endothelium is higher than in endothelium in other peripheral circulations, probably due to the transport requirements of the BBB [107]. We investigated the contribution of mitochondrial factors arising within the endothelium on the integrated response of intact cerebral arteries using several approaches. First, removal of the endothelium altered the vasodilation to diazoxide and BMS-191095, implying that traditional endothelium-derived factors such as NO and prostaglandins contribute to changes in vascular tone [71]. However, the endothelial effects are complicated. In intact blood vessels, inhibition of NOS with L-NAME administration reduced relaxation to diazoxide, indicating a dilator role of NO. In contrast, inhibition of cyclooxygenase (COX) with indomethacin enhanced dilation in diazoxide treated arteries, implying a role for constrictor prostanoids. Indomethacin did not affect vasodilation in endothelium-denuded arteries. Similar results were obtained with BMS-191095 with respect to L-NAME administration [74]. However, unlike diazoxide, indomethacin administration did not enhance dilation to BMS-191095, suggesting that a ROS-mediated mechanism leading to COX activation may account for these differences. Fluorescence and electron spin resonance measurements of NO in intact arteries or cultured cerebral microvascular endothelial cells confirmed the production of NO in response to diazoxide and BMS-191095. In addition, fluorescence measurements showed that a global increase in free cytosolic calcium rather than calcium sparks was temporally associated with increased NO production. We have demonstrated that NO arises from cytosolic rather than mitochondrial sites during normal conditions [92-94]. Thus, BMS-191095 and diazoxide application led to mitochondrial depolarization, activation of eNOS by calcium- and phosphorylation-specific mechanisms, production of NO, and relaxation of VSM (Figure 5). Gutterman and colleagues [143] showed that a physiological stimulus such as increased shear stress is able to directly activate mitochondria in coronary endothelial cells via filamentous connections and dilate coronary arteries via effects of hydrogen peroxide. Whether this endothelium-specific mechanism operates in response to increases in flow or shear stress in cerebral arteries is unclear, since alternative mechanisms promoting either dilation or constriction in cerebral resistance vessels of different sizes and locations have been proposed by Koller and Toth [86]. Mitochondrial depolarization by the protonophore, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP ), in endothelial tubes from mesenteric arteries, has also been shown to activate calcium-activated K+ channels presumably by elevation of endothelial Ca2+ [7]. Even though precise mechanisms may be different in cerebral and coronary arteries, the common feature is that stimulation of mitochondria is able to liberate substances by endothelium which can affect VSM tone.
Mitochondrial numbers and composition in the cerebral vascular endothelium are affected by factors such as ischemic stress (unpublished observations) and hormones such as estrogen [79,123]. Administration of 17beta-estradiol profoundly affects mitochondrial function in cerebral blood vessels via estrogen receptor alpha, thereby enhancing efficiency of energy production and suppressing mitochondrial oxidative stress. These results indicate that ovarian hormones normally act through a distinctive regulatory pathway involving peroxisome proliferator-activated receptor γ coactivator 1 to support cerebral endothelial mitochondrial content and guide mitochondrial function to favor ATP coupling and ROS protection. An interesting observation is that female mice with MnSOD deficiency are largely protected compared with male mice against cerebral vascular dysfunction [21]. The basis for this gender difference is unclear at this time but may reflect yet unknown compensatory mechanisms in the vascular mitochondria of female mice.
Perivascular and parenchymal nerves
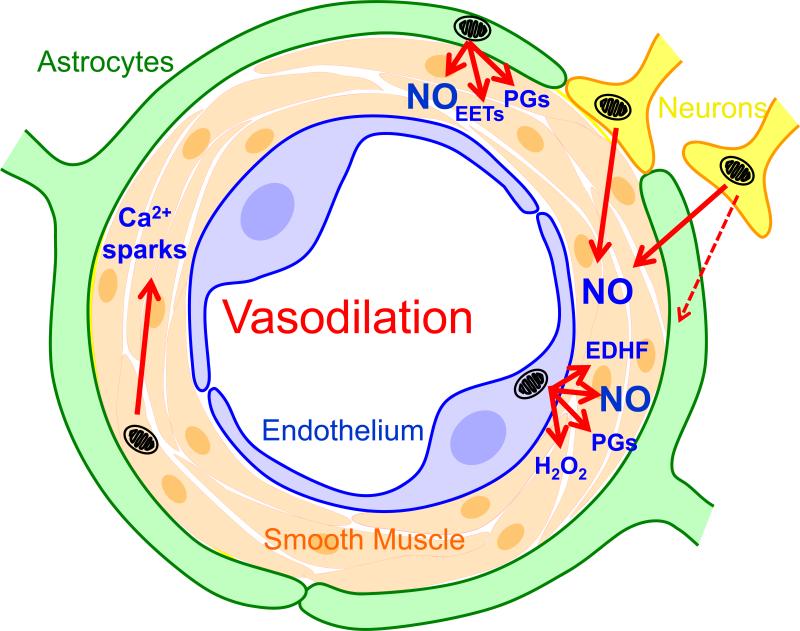
The potential for mitochondrial influences from perivascular nerves or parenchymal neurons on cerebral vascular responses has never been specifically investigated. However, there are a number of factors suggesting an important neuronal contribution from both sources. Mitochondrial density is relatively high in perivascular nerves observed under confocal imaging (unpublished observations) and as seen in our preconditioning studies; mitochondria in neurons are responsive to both diazoxide and BMS-191095 [47]. Cerebral arteries receive innervation from sympathetic, parasympathetic, and nitroxidergic nerves [11,25,131]. These speculations are supported by our preliminary studies. Specific inhibition of neuronal NOS with 7-NI in perivascular nerves, or blockade of perivascular nerves with tetrodotoxin (TTX), inhibits dilation in both intact and denuded cerebral arteries to BMS-191095 [81]. The effects of TTX may be due to interactions between different types of perivascular nerves or between astroglia and perivascular nerves. Electrical activation of perivascular nerves, which might mimic superfusion with diazoxide and BMS-191095, elicits dilation of cerebral arteries which is blocked by TTX [3]. Additionally, BMS-191095 depolarizes mitochondria in cultured cortical neurons and enhances NO production by these cells . Thus, in addition to the endothelium, perivascular nerves, parenchymal neurons, and possibly astroglia as well [67,122], can provide mitochondrially-initiated vasoactive signals to VSM [82-84] for the final determination of integrated changes in cerebral vascular diameter (Figure 6).
Figure 6.
Schematic illustration showing interaction among mitochondrial influences originating from VSM, endothelium, perivascular nerves, and parenchymal astroglia and neurons. Factors produced following activation of mitochondria by physiological, pharmacological, and pathological stimuli in any of these cell types can affect VSM and the interaction of these factors will determine the final, integrated arterial tone. Abbreviations: NO, nitric oxide; PGs, prostaglandins; EDHF, endothelial derived relaxing factor; EETs, epoxyeicosatrienoic acids, K+, potassium ion.
MITOCHONDRIA IN INSULIN RESISTANCE
Insulin resistance often precedes the development of type II diabetes by years or decades, and is considered a relatively silent phase of the metabolic syndrome despite developing vascular dysfunction and moderate arterial hypertension. We and others have characterized many of the general cardiovascular and brain effects of this disease [39-41,60] due to tissue and vascular inflammation and increased basal levels of ROS from both mitochondrial and non-mitochondrial sources [41,42].
We were among the first investigators to demonstrate that several pathological stressors are able to disrupt normal functioning of plasmalemmal KATP channels in cerebral arteries [4,39,40] and therefore it is not surprising that mitoKATP channels would be affected in a similar manner. For example, ischemia/reperfusion as well as nutritional and genetic models of IR reduced function of several types of potassium channels in cerebral VSM, including KATP channels, through mechanisms involving enhanced baseline vascular levels of ROS [39,40]. However, effects of ischemia on KATP channels are transient, so that normal responses return by 4 hours of reperfusion. Thus, ROS scavengers [40], PKC inhibition [39], or anti-inflammation therapies such as statin administration [41] are able to restore normal dilation to potassium channel activators despite continued IR. Even though enhanced ROS from mitochondria is associated with IR, reductions in ROS production from NADPH oxidase results in restored KATP channel function in cerebral arteries. We speculate that ROS induced ROS production, involving the mitochondrial electron transport chain and NADPH oxidase, might be necessary for the full dysfunction of KATP channels in IR. Similarly, a more severe, acute event, namely ischemia/reperfusion is able to reduce KATP channel-dependent dilation in cerebral arteries [4]. Additionally, we have shown that immediate preconditioning which is dependent upon activation of mitoKATP channels is abolished in hearts of IR rats [70]. Thus, ischemic- as well as diazoxide-induced preconditioning fails to protect hearts from ischemia/reperfusion in IR rats whereas both of these approaches limited infarct size in hearts from non-IR rats. In these IR animals, prior to ischemia/reperfusion, a substantial number of the mitochondria in the heart were swollen or showed disruption of the normal pattern for cristae. Moreover, the isolated mitochondria from the hearts of IR animals had reduced responses to diazoxide. An additional finding from this study was that infarct size was enhanced in the hearts from Zucker obese compared with lean rats, which is similar to that which we [106] and others [10] have found in brains of IR, obese mice following MCAO. These results may indicate that normal protective mechanisms initiated at the level of the mitochondria are impaired in many common disease states and thus the brain and other organs are more at risk during ischemic episodes.
Similar to preconditioning, mitochondrial-dependent responses in cerebral arteries are impaired in IR rats [71]. This attenuation of dilation appears to be due to reduced mitochondrial depolarization of VSM as well as reduced ROS generation in response to diazoxide. In addition, decreased NO production/bioavailability, despite increased endothelial NOS expression in IR arteries, appears to contribute to diminished relaxation of VSM. We have found similar impairment of cerebral vascular dilation to BMS-191095 in IR animals [74]. An unexpected result was that, in contrast to mitochondrial activation with diazoxide or BMS-19095, dilator responses to 3-NPA were modestly enhanced in arteries from IR rats [71]. Additionally, co-application of 3-NPA with diazoxide led to more than just an additive dilator effect which was not attenuated in arteries from IR animals. The reason for this effect is unclear, but it seems to indicate that IR interferes with some, but not all vasoactive influences from mitochondria. The potential for the uncoupling of the tight relationship between metabolic need and blood flow in the brain due to cerebral vascular dysfunction associated with IR, as well as the elimination of normal, protective mechanisms involving mitochondria such as preconditioning, may account for the increased risk and severity of neurological diseases and strokes in patients suffering from the metabolic syndrome. Similar to our findings with IR, anti-ROS treatment restores morphological structure in coronary endothelial cells in a mouse model of type 1 diabetes [102]. In addition, Kizhakekuttu et al. [84] provided evidence that mitochondrial derangements lead to reduced endothelium-induced dilation in human arteries. A possible therapeutic approach might involve the early detection and treatment of IR individuals with the aim toward reducing vascular inflammation and mitochondrial dysfunction prior to the further progression of this chronic disease. We have shown that even very short term treatments with a statin, which reduced vascular ROS levels and restored normal KATP channel function without lowering blood cholesterol levels, was effective in IR rats [41].
MITOCHONDRIA AND CEREBRAL ISCHEMIA
Although mitochondrial failure is a major cause of cell death due to ischemia, our recent findings indicate that mitochondria in endothelium may represent a useful target to prevent cell death and restore cerebral blood flow after stroke. Preconditioning before ischemia preserves endothelium-dependent dilation [32] and reduces permeability of the BBB in vivo [96], and post conditioning protects endothelium in vitro against prior OGD (Figure 3). In recent preliminary studies, we examined the effects of experimental stroke on middle cerebral artery responses two days following reperfusion, and found that dilation was greater on the side ipsilateral to the middle cerebral artery occlusion compared with the contralateral, non-stroke side (Figure 7). At this time, dilator and constrictor responses to other, non-mitochondrial stimuli were reduced in middle cerebral arteries on the stroke side. Thus, not only are endothelial cells responsive to mitochondrial-induced postconditioning after anoxic stress, but vascular responsiveness to mitochondrial activators is also enhanced [134]. Therefore, because strokes occur unpredictably, therapeutic targeting of mitochondrial after the onset of ischemic stroke may be a clinically useful approach. Cerebral ischemia, resultant cerebral vascular dysfunction, and parenchymal damage occur unexpectedly following occlusive and hemorrhagic strokes as well as from severe hypotension due to heart attacks and trauma. The only proven therapeutic approach for occlusive strokes is surgical or enzymatic removal of the clot from a cerebral artery, but even after clot removal, patients often suffer from cerebral hypoperfusion, damage to the BBB, and continued parenchymal damage. We believe that activation of mitochondrial mechanisms after the insult may benefit the brain by protecting cerebral vascular cells by preventing further injury to the endothelium and VSM, correcting cerebral hypoperfusion, restoring normal cerebral vascular responsiveness and the BBB, and restricting further neuronal and glia cell death.
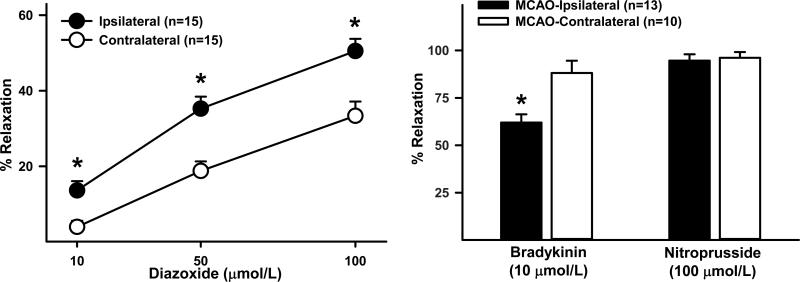
Figure 7.
Original data from Wistar rats showing that dilator responses of isolated, pressurized middle cerebral arteries to diazoxide are substantially enhanced compared to the non-ischemic, contralateral artery two days following 90 minutes of middle cerebral artery occlusion via the filament method. At the same time dilator responses to other endothelial-dependent responses such as bradykinin were reduced. *P<0.05.
CONCLUSIONS, SIGNIFICANCE, AND PERSPECTIVE
We can draw four major conclusions. First, mitochondrial influences are important initiators of preconditioning and postconditioning in the most vulnerable cell types within the neurovascular unit. Thus, targeting mitochondria may be a useful therapeutic approach to protect the cerebral vasculature and brain of people prior to elective surgeries and also following the onset of strokes. Second, individual mitochondrial influences from endothelium, perivascular nerves, and perhaps parenchymal neurons and astroglia are able to contribute to the direct mitochondrial-initiated dilator effects of VSM into a final, integrated change in cerebral vascular tone. Thus, production of vasoactive factors following activation of mitochondria in response to physiological stimuli in one or several of the cells comprising the neurovascular unit may represent the elusive signaling link between metabolic rate and blood flow. Third, important mitochondrial-derived mechanisms such as those that induce preconditioning or cause changes in cerebral vascular tone are disrupted by chronic disease processes such as IR and diabetes, even when relatively mild. Thus, direct or indirect targeting of mitochondria in the early stages of the metabolic syndrome might prove to be an effective treatment to reduce morbidity and mortality in these patients related to the increased risk of cardiovascular disease, strokes, and dementias such as Alzheimer's disease. Fourth, preliminary evidence indicates that mitochondrial mechanisms promoting vasodilation are usually present to some extent following experimental strokes. Thus, targeting mitochondria following a patient's ischemic injury may help correct cerebral hypoperfusion and restore the BBB, and thereby restrict further neuronal and glia cell death.
The study of mitochondrial influences on the vasculature is a rapidly evolving field of investigation; however, there is much research to do in order to understand the intricacies of mitochondrial biology and the mitochondrial mechanisms involved in cerebral vascular control during health and disease. Nonetheless, it seems likely that the manipulation of mitochondrial numbers or function will represent an important therapeutic approach for the future treatment of many chronic and acute disease states in people.
Acknowledgements
Supported by NIH grants HL-077731, HL-030260, HL-065380, and HL-093554. We thank Nancy Busija, M.A., CCC-SLP for editorial assistance. We thank Ken Grant of the Cellular Imaging Shared Resource at Wake Forest University Health Sciences for assistance with electron microscopy.
REFERENCES
- 1.Ardehali H, O'Rourke B. Mitochondrial K(ATP) channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–163. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auchampach JA, Gross GJ. Adenosine A1 receptors, KATP channels, and ischemic preconditioning in dogs. Am J Physiol. 1993;264:H1327–1336. doi: 10.1152/ajpheart.1993.264.5.H1327. [DOI] [PubMed] [Google Scholar]
- 3.Ayajiki K, et al. Evidence for nitroxidergic innervation in monkey ophthalmic arteries in vivo and in vitro. Am J Physiol. 2000;279:H2006–2012. doi: 10.1152/ajpheart.2000.279.4.H2006. [DOI] [PubMed] [Google Scholar]
- 4.Bari F, et al. Global ischemia impairs ATP-sensitive K+ channel function in cerebral arterioles in piglets. Stroke. 1996;27:1874–1881. doi: 10.1161/01.str.27.10.1874. [DOI] [PubMed] [Google Scholar]
- 5.Bari F, Louis TM, Busija DW. Calcium-activated K+ channels in cerebral arterioles in piglets are resistant to ischemia. J Cereb Blood Flow Metab. 1997;17:1152–1156. doi: 10.1097/00004647-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bednarczyk P, et al. Large-conductance Ca2+-activated potassium channel in mitochondria of endothelial EA.hy926 cells. Am J Physiol. 2013;304:H1415–1427. doi: 10.1152/ajpheart.00976.2012. 2013. [DOI] [PubMed] [Google Scholar]
- 7.Behringer EJ, Segal SS. Altered electrical reactivity of endothelial tubes with aging: Role of mitochondria and Ca2+-activated K+ channels. FASEB Journal. 2013;27:679.1. abstract. [Google Scholar]
- 8.Bienert GP, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Biochim Biophys Acta. 2007;1717:1–10. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 9.Broadhead MW, et al. KATP channel activation induces ischemic preconditioning induces ischemic preconditioning of the endothelium in humans in vivo. Circ. 2004;110:2077–2082. doi: 10.1161/01.CIR.0000144304.91010.F0. [DOI] [PubMed] [Google Scholar]
- 10.Brown KA, et al. Effects of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–1946. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- 11.Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161–211. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- 12.Busija DW, et al. Targeting mitochondrial ATP-sensitive potassium channels—A novel approach to neuroprotection. Brain Res Rev. 2004;46:282–294. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Busija DW, et al. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res Bull. 2005;66:85–90. doi: 10.1016/j.brainresbull.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Busija DW, et al. Mitochondrial-mediated suppression of ROS production upon exposure of neurons to lethal stress: Mitochondrial targeted preconditioning. Adv Drug Del Rev. 2008;16:1471–1477. doi: 10.1016/j.addr.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calamita G, et al. The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem. 2005;280:17149–17153. doi: 10.1074/jbc.C400595200. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers S, et al. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium. 2007;42:447–466. doi: 10.1016/j.ceca.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Chalmers S, et al. Mitochondrial motility and vascular smooth muscle proliferation. Arterioscler Thromb Vasc Biol. 2012;32:3000–11. doi: 10.1161/ATVBAHA.112.255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheney JA, et al. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J. Cereb Blood Flow Metab. 2001;21:396–403. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol. 2004;556:755–771. doi: 10.1113/jphysiol.2003.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi DW. Calcium: still center-stage in hypoxic–ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 21.Chrissobolis S, Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension. 2010;55:905–910. doi: 10.1161/HYPERTENSIONAHA.109.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coetzee WA. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther. 2013;140:167–175. doi: 10.1016/j.pharmthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai J, et al. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium. 2005;37:333–340. doi: 10.1016/j.ceca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Dauphin F, MacKenzie ET. Cholinergic and vasoactive intestinal polypeptidergic innervation of the cerebral arteries. Pharmacol Ther. 1995;67:385–417. doi: 10.1016/0163-7258(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 26.Davidson SM. Endothelial mitochondria and heart disease. Cardiovasc Res. 2010;88:58–66. doi: 10.1093/cvr/cvq195. [DOI] [PubMed] [Google Scholar]
- 27.Debska G, et al. A Large-conductance K+ channel openers NS1619 and NS004 as inhibitors of mitochondrial function in glioma cells. Biochem Pharmacol. 2003;65:1827–1834. doi: 10.1016/s0006-2952(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich HH, et al. Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J Vasc Res. 2009;46:253–264. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikalova AE, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domoki F, et al. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke. 1999;30:2713–2718. doi: 10.1161/01.str.30.12.2713. [DOI] [PubMed] [Google Scholar]
- 31.Domoki F, et al. Diazoxide prevents mitochondrial swelling and Ca (2+) accumulation in CAI pyramidal cells after cerebral ischemia in newborn pigs. Brain Res. 2004;1019:97–104. doi: 10.1016/j.brainres.2004.05.088. [DOI] [PubMed] [Google Scholar]
- 32.Domoki F, et al. Diazoxide preserves hypercapnia-induced arteriolar vasodilation after global cerebral ischemia in piglets. Am J Physiol. 2005;289:H368–373. doi: 10.1152/ajpheart.00887.2004. [DOI] [PubMed] [Google Scholar]
- 33.Domoki F, et al. Rosuvastatin induces delayed preconditioning against oxygen-glucose deprivation in cultured cortical neurons. Am J Physiol. 2009;296:C97–105. doi: 10.1152/ajpcell.00366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorn GW, Scorrano L. Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate. Circ Res. 2010;107:689–699. doi: 10.1161/CIRCRESAHA.110.225714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dromparis P, Sutendra G. Michelakis ED: The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl) 2010;88:1003–1010. doi: 10.1007/s00109-010-0670-x. [DOI] [PubMed] [Google Scholar]
- 36.Dröse S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 37.Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 38.Duckles SP, Krause DN. Mechanisms of cerebrovascular protection: oestrogen, inflammation and mitochondria. Acta Physiol. 2011;203:149–154. doi: 10.1111/j.1748-1716.2010.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdős B, et al. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004a;53:1352–1359. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 40.Erdős B, et al. Subtype specific potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 2004b;35:964–969. doi: 10.1161/01.STR.0000119753.05670.F1. [DOI] [PubMed] [Google Scholar]
- 41.Erdős B, et al. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H-oxidase-dependent superoxide anion production. Am J Physiol. 2006;290:H1264–1270. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- 42.Farkas E, et al. Neuroprotection by diazoxide in animal models for cerebrovascular disorders. Vasc Dis Prev. 2006;3:253–263. [Google Scholar]
- 43.Foster DB, et al. Mitochondrial ROMK channel is a molecular component of mitoK(ATP). Circ Res. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 45.Gaspar T, et al. Transient glucose and amino acid deprivation induces delayed preconditioning in cultured rat cortical neurons. J Neurochem. 2006;98:555–565. doi: 10.1111/j.1471-4159.2006.03899.x. [DOI] [PubMed] [Google Scholar]
- 46.Gaspar T, et al. Neuronal preconditioning with the antianginal drug, Bepridil. J Neurochem. 2007;102:595–608. doi: 10.1111/j.1471-4159.2007.04501.x. [DOI] [PubMed] [Google Scholar]
- 47.Gaspar T, et al. ROS-independent preconditioning in neurons via activation of mitoKATP channels by BMS-191095. J Cereb Blood Flow Metab. 2008a;28:1090–1103. doi: 10.1038/sj.jcbfm.9600611. [DOI] [PubMed] [Google Scholar]
- 48.Gaspar T, et al. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J Neurochem. 2008b;105:1115–1128. doi: 10.1111/j.1471-4159.2007.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaspar T, et al. Immediate neuronal preconditioning with NS1619. Brain Res. 2009;1285:196–207. doi: 10.1016/j.brainres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghezzi D, Zeviani M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Adv Exp Med Biol. 2012;748:65–106. doi: 10.1007/978-1-4614-3573-0_4. [DOI] [PubMed] [Google Scholar]
- 51.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 52.Graham D, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 53.Groschner LN, et al. Endothelial mitochondria—less respiration, more integration. Pflugers Arch. 2012;464:63–76. doi: 10.1007/s00424-012-1085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grover GJ, et al. Pharmacologic characterization of BMS-191095, a mitochondrial K(ATP) opener with no peripheral vasodilator or cardiac action potential shortening activity. J Pharmacol Exp Ther. 2001;297:1184–1192. [PubMed] [Google Scholar]
- 55.Grover GJ, et al. Protective effect of mitochondrial KATP activation in an isolated gracilis model of ischemia and reperfusion in dogs. J Cardiovasc Pharmacol. 2003;42:790–792. doi: 10.1097/00005344-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Halestrap AP. The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochem So. Symp. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 57.Han D, et al. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. Arterioscler Thromb Vasc Biol. 2012;32:2531–2539. [Google Scholar]
- 58.Han D, William E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanley PJ, et al. Beta-oxidation of 5-hydroxydecanoate, a putative blocker of mitochondrial ATP-sensitive potassium channels. J Physiol. 2003;547:387–393. doi: 10.1113/jphysiol.2002.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrell JW, Morgan BJ, Schrage WG. Impaired hypoxic cerebral vasodilation in younger adults with metabolic syndrome. Diab Vasc Dis Res. 2013;10:135–142. doi: 10.1177/1479164112448875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill BG, et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem. 2012;93:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holland M, et al. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horiguchi T, et al. Opening of mitochondrial ATP-sensitive potassium channels is a trigger of 3-nitropropionic acid-induced tolerance to transient focal cerebral ischemia in rats. Stroke. 2003;34:1015–1020. doi: 10.1161/01.STR.0000063404.27912.5B. [DOI] [PubMed] [Google Scholar]
- 64.Horiguchi T, et al. The role of nitric oxide in the development of cortical spreading depression-induced tolerance to transient focal cerebral ischemia in rats. Brain Res. 2005a;1039:84–89. doi: 10.1016/j.brainres.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 65.Horiguchi T, et al. Cortical spreading depression-induced tolerance to transient focal cerebral ischemia in halothane anesthetized rats is affected by anesthetic level but not ATP-sensitive potassium channels. Brain Res. 2005b;1062:127–133. doi: 10.1016/j.brainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Jastroch M, et al. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang K, et al. Regulation of gap junctional communication by astrocytic mitochondrial K(ATP) channels following neurotoxin administration in in vitro and in vivo models. Neurosignals. 2011;19:63–74. doi: 10.1159/000323575. [DOI] [PubMed] [Google Scholar]
- 68.Jonckheere AI, Smeitink JA, Rodenburg RJ. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis. 2012;35:211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalyanaraman B. Oxidative chemistry of fluorescent dyes: implications in the detection of reactive oxygen and nitrogen species. Biochem Soc Trans. 2011;39:1221–1225. doi: 10.1042/BST0391221. [DOI] [PubMed] [Google Scholar]
- 70.Katakam PV, et al. Myocardial preconditioning against ischemia-reperfusion is abolished in Zucker obese rats with insulin. Am J Physiol. 2007;292:R920–926. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- 71.Katakam PV, et al. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am J Physiol. 2009;296:R289–298. doi: 10.1152/ajpregu.90656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katakam PVG, Snipes JA, Busija DW. Mitochondrial depolarization enhances Ca2+-sparks generation independent of reactive oxygen species. FASEB J. 2011a;25:819.818. abstract. [Google Scholar]
- 73.Katakam PVG, Snipes JA, Busija DW. Vascular mitochondrial dysfunction associated with insulin resistance impairs activation of Ca2+-sparks and mitochondrial Ca2+ uptake. FASEB J. 2011b;25:1095.1016. abstract. [Google Scholar]
- 74.Katakam PV, et al. Depolarization of Mitochondria in Endothelial Cells Promotes Cerebral Vascular Vasodilation by Activation of Nitric Oxide Synthase. Art Throm Vasc Biol. 2013;33:752–759. doi: 10.1161/ATVBAHA.112.300560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katakam PVG, et al. Mitochondria-Dependent Cerebral Artery Vasodilation is Mediated by the Activation of Neuronal Nitric Oxide Synthase following Mitochondrial Depolarization of Perivascular Nerves. The FASEB Journal. 2012;26:1058.1059. abstract. [Google Scholar]
- 76.Katakam PVG, Busija DW. Mitochondrial Depolarization without Reactive Oxygen Species Production leads to Augmented Cerebral Vascular Relaxation via Diverse Calcium-related events in Smooth Muscle and Endothelium. Stroke. 2012;43:A107. abstract. [Google Scholar]
- 77.Katakam PVG, et al. Mitochondria mediated vasodilation by BMS-191095, a selective mitochondrial KATP channel activator. FASEB J. 2010;24:978.976. abstract. [Google Scholar]
- 78.Katakam PVG, et al. Abnormal diazoxide induced vasodilation in cerebral arteries from Zucker obese rats with insulin resistance. FASEB J. 2007;21:A1262. abstract. [Google Scholar]
- 79.Kemper MF, et al. Endogenous ovarian hormones affect mitochondrial efficiency in cerebral endothelium via distinct regulation of PGC-1 isoforms. J Cereb Blood Flow Metab. 2013;33:122–128. doi: 10.1038/jcbfm.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kicinska A, Szewczyk A. A Large-conductance potassium cation channel opener NS1619 inhibits cardiac mitochondria respiratory chain. Toxicol Mech Methods. 2004;14:59–61. doi: 10.1080/15376520490257482. [DOI] [PubMed] [Google Scholar]
- 81.Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- 82.Kis B, et al. Diazoxide induces delayed preconditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- 83.Kis B, et al. The mitochondrial KATP channel opener BMS191095 induces neuronal preconditioning. Neuroreport. 2004;15:345–349. doi: 10.1097/00001756-200402090-00027. [DOI] [PubMed] [Google Scholar]
- 84.Kizhakekuttu TJ, et al. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Biochem Biophys Res Commun. 2012;422(3):515–521. doi: 10.1161/ATVBAHA.112.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;12:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49:375–389. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korper S, et al. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34+ cell line KG-1a. Exp Hematol. 2003;31:815–823. doi: 10.1016/s0301-472x(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 88.Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29:705–718. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 89.Kubli DA. Gustafsson ÅB: Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:12, 1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lacza Z, et al. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res. 2003a;19:27–36. doi: 10.1016/j.brainres.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 91.Lacza Z, et al. Heart mitochondria contain functional ATP-dependent K+ channels. J Mol Cell Cardiol. 2003b;35:1339–1347. doi: 10.1016/s0022-2828(03)00249-9. [DOI] [PubMed] [Google Scholar]
- 92.Lacza Z, et al. Lack of mitochondrial nitric oxide production in the brain. J Neurochem. 2004;90:942–951. doi: 10.1111/j.1471-4159.2004.02553.x. [DOI] [PubMed] [Google Scholar]
- 93.Lacza Z, et al. Mitochondrial NO and reactive nitrogen species production: Does mtNOS exist? Nitric Oxide. 2006a;14:162–168. doi: 10.1016/j.niox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 94.Lacza Z, et al. Mitochondria produce reactive nitrogen species via an arginine-independent pathway. Free Rad Res. 2006;40:369–378. doi: 10.1080/10715760500539139. [DOI] [PubMed] [Google Scholar]
- 95.Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010 Apr 15;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- 96.Lenzser G, et al. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;105:72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 97.Liu D, et al. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Longhi L, et al. Long-lasting protection in brain trauma by endotoxin preconditioning. J Cereb Blood Flow Metab. 2011;31:1919–1929. doi: 10.1038/jcbfm.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loukogeorgakis SP, et al. Transient limb ischemis induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanisms. Circ. 2007;116:1386–1395. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 100.Lu B, et al. A mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) affects mitochondrial function and impairs fertility in mice. Biol Reprod. 2008;78:601–610. doi: 10.1095/biolreprod.107.065987. [DOI] [PubMed] [Google Scholar]
- 101.Lustgarten MS, et al. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. J Biol Chem. 2003;278:5557–5563. doi: 10.1016/j.bbrc.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53:1783–1794. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marchissio MJ, et al. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol Appl Pharmacol. 2012;64:246–254. doi: 10.1016/j.taap.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Mayanagi K, et al. The mitochondrial K(ATP) channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:348–355. doi: 10.1038/sj.jcbfm.9600345. [DOI] [PubMed] [Google Scholar]
- 105.Mayanagi K, et al. Systemic administration of diazoxide induces delayed preconditioning against transient focal cerebral ischemia in rats. Brain Res. 2007;1168:106–111. doi: 10.1016/j.brainres.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 106.Mayanagi K, et al. Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. J Cerebral Blood Flow Metabol. 2008;28:1927–1935. doi: 10.1038/jcbfm.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McBride HM, Neuspiel M, Wasiak S. Mitochondria: More than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 108.McCarron JG, et al. From Structure to Function: Mitochondrial Morphology, Motion and Shaping in Vascular Smooth Muscle. J Vasc Res. 2013;50(5):357–371. doi: 10.1159/000353883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther. 2012;17:21–33. doi: 10.1177/1074248410396877. [DOI] [PubMed] [Google Scholar]
- 110.Miller JD, et al. MnSOD protects against COX1-mediated endothelial dysfunction in chronic heart failure. Am J Physiol. 2010;298:H1600–H1607. doi: 10.1152/ajpheart.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muller FL, Liu Y, Van Remmem H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 112.Nagy K, et al. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J Neurosci Res. 2004;76:697–704. doi: 10.1002/jnr.20120. [DOI] [PubMed] [Google Scholar]
- 113.Nazarewicz RR, et al. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am J Physiol. 2013;305:H1131–1140. doi: 10.1152/ajpheart.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 115.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capacity of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 116.Pelligrino DA, Vetri F, Xu HL. Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol. 2011;22:229–36. doi: 10.1016/j.semcdb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol. 2007;147:291–299. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perez-Pinzon MA, Dave KR, Raval AP. Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antioxid Redox Signal. 2005;7:1150–1157. doi: 10.1089/ars.2005.7.1150. [DOI] [PubMed] [Google Scholar]
- 119.Pung YF, et al. The Role of Mitochondria Bioenergetics and Reactive Oxygen Species in Coronary Collateral Growth. Am J Physiol. 2013;305:H1275–1280. doi: 10.1152/ajpheart.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Quinlan CL, et al. The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. Methods in Enzymology. 2013;526:189–217. doi: 10.1016/B978-0-12-405883-5.00012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajapakse N, et al. Activation of mitochondrial ATP-sensitive potassium channels prevents neuronal cell death after ischemia in neonatal rats. Neurosci Lett. 2002;327:208–212. doi: 10.1016/s0304-3940(02)00413-5. [DOI] [PubMed] [Google Scholar]
- 122.Rajapakse N, et al. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J Neurosci Res. 2003;73:206–214. doi: 10.1002/jnr.10657. [DOI] [PubMed] [Google Scholar]
- 123.Razmara A, et al. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharmacol Exp Ther. 2008;325:782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rines AK, Bayeva M. Ardehali H: A New pROM King for the MitoKATP Dance ROMK Takes the Lead. Circ Res. 2012;111:392–393. doi: 10.1161/CIRCRESAHA.112.275461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robin E, et al. Postconditioning in focal cerebral ischemia: Role of the mitochondrial ATP-dependent potassium channel. Brain Res. 2011;1375:137–146. doi: 10.1016/j.brainres.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 126.Rutkai, et al. Novel mitochondrial mechanisms mediate enhanced vasodilation of rat middle cerebral arteries to mitochondrial depolarization following ischemia-reperfusion injury. FASEB J. 2013;27:1131.1110. abstract. [Google Scholar]
- 127.Seharaseyon J, et al. Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral Kir gene transfer. J Mol Cell Cardiol. 2000;32:1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- 128.Shimizu K, Lacza Z, Rajapakse N, Horiguchi T, Snipes J, Busija DW. MitoKATP opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am J Physiol. 2002;283:H1005–H1011. doi: 10.1152/ajpheart.00054.2002. [DOI] [PubMed] [Google Scholar]
- 129.Somlyo AV, Somlyo AP. Strontium accumulation by sarcoplasmic reticulum and mitochondria in vascular smooth muscle. Science. 1971;174:955–958. doi: 10.1126/science.174.4012.955. [DOI] [PubMed] [Google Scholar]
- 130.Terao S, et al. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–950. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- 131.Toda N, Okamura T. Nitroxidergic nerve: regulation of vascular tone and blood flow in the brain. J Hypertens. 1996;14:423–34. [PubMed] [Google Scholar]
- 132.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50:307–320. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 133.Tretter L, Adam-Vizi V. Uncoupling without an effect on the production of reactive oxygen species by in situ synaptic mitochondria. J Neurochem. 2007;103:1864–1871. doi: 10.1111/j.1471-4159.2007.04891.x. [DOI] [PubMed] [Google Scholar]
- 134.Veltkamp R, et al. Potassium channel activators protect the NMDA-induced cerebral vascular dilation after combined hypoxia and ischemia in piglets. Stroke. 1998;29:837–843. doi: 10.1161/01.str.29.4.837. [DOI] [PubMed] [Google Scholar]
- 135.Votyakova TV, Reynolds IJ. DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 136.Walker JE. The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans. 2013;41:1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- 137.Wappler EA, et al. Mitochondrial dynamics associated with oxygen-glucose deprivation in rat primary neuronal cultures. PLoS One. 2013;2013;8(5):e63206. doi: 10.1371/journal.pone.0063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive species. Antiox Redox Sig. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta. 2013;1827(5):598–611. doi: 10.1016/j.bbabio.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. Antioxid Redox Signal. 2011;15:1517–30. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.You J, et al. Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol. 1999;277:H893–900. doi: 10.1152/ajpheart.1999.277.3.H893. [DOI] [PubMed] [Google Scholar]
- 143.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 144.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–85. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zick M, Rabl R, Reichert AS. Cristae formation-linking ultrastructure and function of mitochondria. Biochim Biophys Acta. 2008;1793:5–19. doi: 10.1016/j.bbamcr.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 146.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol. 2011;301:H647–53. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]