Abstract
Purpose
Geriatric frailty is a common syndrome of older adults that is characterized by increased vulnerability to adverse health outcomes and influences treatment choice. Pharmacoepidemiologic studies in older adults that rely on administrative claims data are limited by confounding due to unmeasured frailty. A claim-based frailty score may be useful to minimize confounding by frailty in such databases. We provide an overview of definitions and measurement of frailty, evaluated the claim-based models of frailty in literature, and recommend ways to improve frailty adjustment in claims analysis.
Methods
We searched MEDLINE and EMBASE from inception to April 2014, without language restriction, to identify claim-based multivariable models that predicted frailty or its related outcome, disability. We critically appraised their approach, including population, predictor selection, outcome definition, and model performance.
Results
Of 152 reports, three models were identified. One model that predicted poor functional status using health care service claims in a representative sample of community-dwelling and institutionalized older adults showed an excellent discrimination (C statistic, 0.92). The other two models that predicted disability using either diagnosis codes or prescription claims alone in institutionalized or frail adults had limited generalizability and modest model performance. None of the models have been applied to reduce confounding bias in pharmacoepidemiologic studies of drug therapy.
Conclusions
We found little research conducted on development and application of a claim-based frailty index for confounding adjustment in pharmacoepidemiologic studies in older adults. More research is needed to advance this innovative, potentially useful approach by incorporating the expertise from aging research.
INTRODUCTION
Pharmacoepidemiologic studies of mortality in older adults using administrative claims data are mainly criticized for their limited ability to capture important clinical information and prognostic factors that are available to physicians who make treatment decisions.1 Physicians are more likely to withhold treatments to patients who have limited life expectancy or high vulnerability to treatment-related adverse events. As a result, incomplete measurement and adjustment for such patient characteristics can result in confounding bias. For example, vaccinations were less likely to be given to patients who had long-term hospitalizations or skilled nursing stays.2 Users of lipid-lowering agents, non-steroidal anti-inflammatory drugs, and glaucoma drugs were healthier and had lower mortality than non-users among older adults.3–5 The protective association persisted after adjusting for age, sex, and comorbidities.3,5 This suggests that some important prognostic factors were not captured in claims data.
Frailty as an unmeasured confounder
One such prognostic factor that is increasingly recognized by physicians is frailty. A frail older person is typically described as a “slow, weak, and thin” person who appears older than one’s chronologic age. In the geriatrics and gerontology literature, frailty is defined as a state of increased vulnerability and reduced ability to recover homeostasis after a stressful event, thereby leading to adverse outcomes, such as falls, disability, delirium, and mortality.6–11 It typically results from accumulated impairments in multiple physiologic systems.7 Frail patients are at higher risk of treatment-related adverse events due to their reduced physiologic reserve and limited adaptability to a stressful event. As a result, physicians are more likely to use a lower intensity or avoid treatments that may cause serious adverse events and discontinue treatments that may not result in immediate benefits in order to minimize polypharmacy. Several recent practice guidelines even beyond the geriatrics field recommend considering frailty and assessment of risks and benefits in treatment choice in older adults.12–18 A differential use of various medical and surgical interventions by frailty status has been well documented in observational studies that reflect real-world clinical practice (Table 1). How strongly frailty influences treatment choice varies across the types of treatment and associated risks. For instance, the prevalence difference in frailty was larger for high-risk interventions (e.g. chemotherapy and warfarin) than for low-risk interventions (e.g. statin). This difference may be more pronounced in the oldest old population, up to 50% of whom are frail.19 As such, modification of treatment according to frailty status result in confounding bias in pharmacoepidemiologic studies in older adults.
Table 1.
Examples of Differential Use of Medical and Surgical Treatments by Frailty Status
| Treatment or Target Condition | Treatment Options | Frail patients N (%) | Non-frail patients N (%) |
|---|---|---|---|
| Aortic stenosis (Bainey et al.)51 | Total sample (mean age: 82 years) | 68 (100) | 85 (100) |
| Surgical aortic valve replacement | 6 (9) | 27 (32) | |
| Transcatheter aortic valve replacement | 32 (47) | 26 (31) | |
| Medical treatment | 30 (44) | 32 (37) | |
| Bisphosphonate (Sambrook et al.)52 | Total sample (mean age: 83 years) | 326 (100) | 1675 (100) |
| Bisphosphonate | 2 (1) | 76 (5) | |
| No bisphosphonate | 324 (99) | 1599 (95) | |
| Chemotherapy for breast cancer (Aaldriks et al.)53 | Total sample (mean age: 76 years) | 28 (100) | 27 (100) |
| ≥ 4 cycles of chemotherapy | 18 (64) | 21 (78) | |
| < 4 cycles of chemotherapy | 10 (36) | 6 (22) | |
| Coronary artery disease (Purser et al.)54 | Total sample (mean age: 77 years) | 194 (100) | 112 (100) |
| Coronary artery bypass surgery | 23 (13) | 21 (19) | |
| Percutaneous coronary intervention | 70 (38) | 59 (53) | |
| Medical treatment | 91 (49) | 32 (28) | |
| Statin (Gnjidic et al.)55 | Total sample (age: 77 years) | 147 (100) | 1518 (100) |
| Statin | 53 (36) | 659 (43) | |
| No statin | 94 (64) | 859 (57) | |
| Warfarin for atrial fibrillation (Perera et al.)56 | Total sample (mean age: 83 years) | 130 (100) | 77 (100) |
| Warfarin | 30 (23) | 53 (69) | |
| No warfarin | 100 (77) | 24 (31) |
In addition, some emerging evidence suggests that frailty may predict older patients who are more likely to harm from chemotherapy or surgical procedures.20,21 On the other hand, the association between sleep medications and hip fracture among nursing home residents was greater in those with mild frailty (who were ambulatory and had more opportunities to fall) than those with severe frailty (who were almost non-ambulatory).22 This reflects that the safety profile of a treatment may depend on frailty status. Therefore, frailty can be an effect modifier in studies of treatment effectiveness and safety in older adults.
Measurement of frailty
How to operationalize the concept of frailty for research and clinical practice has been a hot topic of geriatrics and gerontology research in the past decade.9 In research, the frailty phenotype7 and the frailty index11 are most widely used among several validated frailty models.23 The frailty phenotype, proposed by Fried et al., is constructed on the biological cycle of frailty that consists of shrinking, weakness, exhaustion, slowness, and low physical activity.7 The frailty index, proposed by Mitnitski et al., counts the number (or proportion) of health deficits from a pre-specified number (usually ≥30) of symptoms, signs, diseases, test abnormalities, and disability in physical, psychological, and social domains.11 The frailty phenotype requires very specific information from self-report and geriatric assessment, whereas the frailty index places few restrictions on the type and number of individual components to define frailty (Table 2). Because of its flexibility in choosing individual items, the frailty index has been successfully applied to various population-based surveys, cohorts, and patient databases that did not contain the same components of health deficits as the original database where it was initially developed.24 This is an important advantage of the frailty index that may be useful in developing a claim-based frailty score. In clinical practice, most physicians recognize frailty based on information from a combination of sources, which include observation of the overall appearance (“eyeball” test), review of comorbidities, medications, and ADL disability, and examination of mobility and muscle strength.7,25
Table 2.
Measurement of Frailty in Geriatrics and Gerontology Research
| Measurement of Frailty Phenotype7 | Measurement of Frailty Index11 | |
|---|---|---|
| Core features of frailty50 |
|
|
| Operationalization | Frailty is quantified as the presence of the following characteristics:
Frailty is defined if 3 of the following items are met; pre-frail if 1 or 2 of the items are met. |
Frailty is quantified as the number (or proportion) of health deficits from a pre-specified number of items (at least 30) of any symptoms, signs, diseases, test abnormalities, and disability that satisfies the following criteria:
Although it is used as a continuous variable, the cutoff of 0.20 or 0.25 has been used. |
| Advantages |
|
|
| Disadvantages |
|
|
In administrative claims data, most of the components that are used to determine frailty are not available or incompletely measured. Thus, two individuals with the same age and seemingly similar comorbidity profile in claims data may have a considerably different level of frailty and mortality risk. Individuals who are near the end of life with advanced frailty may have fewer encounters with health care system, which further limits our ability to capture their health status completely and similarly to those with more encounters. Moreover, there is no specific International Classification of Diseases (ICD) code for frailty. Although physicians commonly consider frailty before recommending treatments, they do not reliably document manifestations of frailty in medical records or submit relevant ICD codes (e.g. abnormality of gait, cachexia, debility, exhaustion, fall, failure to thrive, fatigue, loss of weight, pressure ulcer, senility, or muscle weakness).
The relationship of frailty, disability, and comorbidity and relevance to confounding adjustment
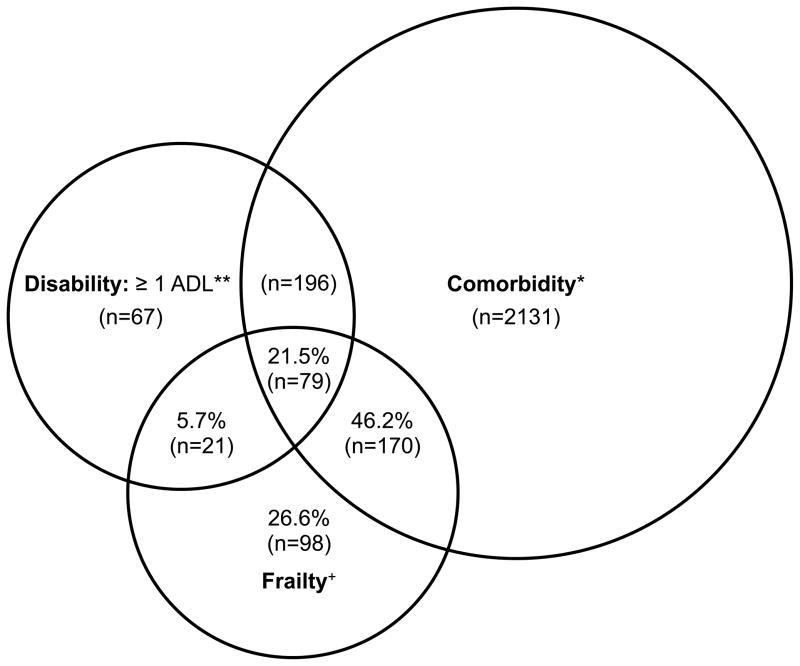
The inter-relationship among frailty, disability, and comorbidity is complex and untangling these overlapping concepts may be difficult.9,26 Disability is defined as difficulty in performing basic or instrumental activities of daily living (ADLs) and viewed as a consequence of frailty and comorbidities.26 In general, individuals with high burden of comorbidities are more likely to become frail and disabled. Nonetheless, it is important to recognize that frailty can identify patients at high risk for poor outcomes who otherwise may not be identified as such based on comorbidity and disability. In the Cardiovascular Health Study, 27% of frail older adults in the community did not have major comorbidities or disability (Figure).7 Frailty was associated with 1.3 to 2.2-fold elevated risk of worsening disability, hospitalization, and mortality, independently of comorbidities and disability.7 This suggests that adjusting for comorbidities and disability may result in residual confounding by frailty. The magnitude of this bias will depend on the prevalence difference in frailty between treated and untreated patients as well as the association between frailty and the outcome of interest that is not captured by comorbidities or disability.
Figure. The Relationship Among Frailty, Comorbidity, and Disability in the Cardiovascular Health Study (Reprinted with permission7).
Percents listed indicate the proportion among those who were frail (n = 368), who had comorbidity and/or disability, or neither. Total represented: 2,762 participants who had comorbidity and/or disability and/or frailty. n of each subgroup indicated in parentheses. +Frail: overall n = 368 frail participants. *Comorbidity: overall n = 2,576 with 2 or more out of the following 9 diseases: myocardial infarction, angina, congestive heart failure, claudication, arthritis, cancer, diabetes, hypertension, chronic obstructive pulmonary disease. Of these, 249 were also frail. **Disabled: overall n = 363 with an ADL disability; of these, 100 were frail.
Existing approaches that may reduce unmeasured confounding by frailty
To date, much progress has been made in risk adjustment methods using summary scores of measured confounding, such as propensity scores or disease risk scores.27–30 The impact of unmeasured confounding by surrogates of frailty (e.g. disability, cognitive and physical function impairment) has been assessed by a sensitivity analysis using an independent dataset and the confounder-outcome associations from the literature.31–34 If a subset of claims data is linked to a validation dataset that contains frailty measures, propensity score calibration or reweighting can be applied to reduce confounding by frailty.35,36 These methods require an external data source with frailty measures. In the absence of such external data, a self-controlled design or an active comparator design can be useful. High-dimensional propensity score analysis37,38 that adjusts for proxies (which may be associated with frailty) and instrumental variable analysis37 may also reduce unmeasured confounding by frailty under some untestable assumptions. In a study of conventional versus atypical antipsychotic use on mortality in nursing home residents, high-dimensional propensity score and instrumental variable analyses resulted in the effect estimates similar to those when additional clinical data, including manifestations of frailty, were adjusted for.37
Claim-based frailty score as an alternative approach
As an alternative approach, a frailty score can be developed and validated from claims data. Although geriatricians and gerontologists have developed several models of frailty for clinical and research use,23,39 little effort has been made to translate these models into pharmacoepidemiologic studies. A successful adaptation of these models of frailty for claims data has a great potential to improve the validity of pharmacoepidemiologic studies in older adults. In the following sections of this review, we critically evaluated the existing claim-based models of frailty in the literature and proposed how to develop and validate a claim-based frailty score for pharmacoepidemiologic studies.
METHODS
We identified claim-based models of frailty in the literature by searching MEDLINE and EMBASE from inception to 4/30/2014, using the following key terms and their variations: frailty, disability, activity of daily living, vulnerability, bias, confounding, case mix, risk adjustment, and administrative claims database. We also searched the reference lists of included papers. Since functional impairment and disability are manifestations of frailty, any papers that reported a multivariable model to predict frailty or disability using claims data were included. In each paper, we examined data source, population characteristics, outcome definition, predictors, and model performance (calibration and discrimination) in the derivation and validation samples.
RESULTS
Of 152 citations identified in literature search, we found three claim-based multivariable models40–43 that predicted frailty or disability in older adults (Table 3). These models varied widely with respect to population source, outcome definition, type of predictors, and model performance. Rosen et al. analyzed the national Veterans Affairs database of long-term care residents to develop a model that used 13 diagnostic categories to predict worsening disability in three basic ADLs over six months.40,41 The model was limited by moderate discrimination (C statistic 0.68–0.70) and uncertain generalizability to females and community-dwelling non-veterans. In a random community-based sample of frail older Canadians identified by a postal screening questionnaire, Dubois et al. developed an index that predicts a summary disability score that consists of basic and instrumental ADLs, mobility, communication, and mental function, based on prescription claims for 11 conditions.42 However, the overall model fit was poor (R2 = 0.12–0.16) and its generalizability to non-frail older adults remains uncertain. The index by Davidoff et al. overcomes most limitations of the earlier indices by using a nationally representative sample of both community-dwelling and institutionalized Medicare beneficiaries.43 Instead of using diagnosis codes or prescription claims, this index used health care service claims (e.g. types of visits, procedures, durable medical equipment) that are both positively and inversely associated with poor functional status. Poor functional status was an approximation of the Eastern Cooperative Oncology Group performance status from basic and instrumental ADLs, strength, stamina, and exercise. This outcome definition seems to overlap with some components of frailty phenotype (weakness, slowness, and low physical activity). Although the discrimination (C statistic 0.92) and calibration of this index were excellent, they might have been overestimated due to the inclusion of individuals who are already institutionalized or on hospice care for terminal illness. None of the three identified models have been applied to reduce confounding bias in pharmacoepidemiologic studies of a drug treatment.
Table 3.
Prediction Models of Frailty and Disability Based on Claims Data
| Model Development | Model Performance | ||||
|---|---|---|---|---|---|
|
| |||||
| Author (Year) | Population | Outcome Definition | Predictors | Derivation Cohort | Validation Cohort |
| \Rosen (2000; 2001)40,41 |
Derivation sample: All long-term care residents in the Veterans Affairs system, US, in 1996 Validation sample: All long-term care residents in the Veterans Affairs system, US, in 1997 |
Worsening disability, defined as ≥ 2 points increase in ADL dependence index (range: 3–15), derived from eating, toileting, and transferring over 6 months |
Diagnosis codes for the following conditions:
|
Characteristics: N=15,639 Mean age: 72 years Male: 96% Outcome: 15% |
Characteristics: N=13,723 Mean age: NR Male: NR Outcome: NR |
|
Discrimination: C-statistic: 0.70 |
Discrimination: C-statistic: 0.68 |
||||
|
Calibration: H-L GOF: P=0.10 Predicted/observed risk Decile 1: 2%/3% Decile 5: 14%/13% Decile 10: 32%/33% |
Calibration: H-L GOF: P=0.02 Predicted/observed risk Decile 1: 2%/3% Decile 5: 14%/14% Decile 10: 29%/29% |
||||
|
| |||||
| Dubois (2010)42 |
Derivation sample: A random sample of frail community-based older adults, Canada Validation sample: Same as derivation sample (a random split of the total population) |
Functional Autonomy Measurement System score (0–87 points), derived from ADL, IADL, mobility, communication, and mental function |
Prescription claims for the following conditions:
|
Characteristics: N=1,000 Mean age: 83 years Male: 38% Outcome: 19 points |
Characteristics: N=402 Mean age: 83 years Male: 38% Outcome: 20 points |
|
Goodness of fit: R2 = 0.16 |
Goodness of fit: R2 = 0.12 |
||||
|
| |||||
| Davidoff (2013)43 |
Derivation sample: A representative sample of community-based and institutionalized Medicare beneficiaries Validation sample: Same as derivation sample (a random split of the total population) |
Functional status (poor versus good), derived from ADL, IADL, strength, stamina, and exercise |
Health care service claims in the following categories:
|
Characteristics: N=7,394 Mean age: 78 years Male: 39% Outcome: 9% |
Characteristics: N=7,394 Mean age: 78 years Male: 38% Outcome: 9% |
|
Discrimination: C-statistic: 0.92 Using the cutoff 0.11, Sensitivity: 0.81 Specificity: 0.92 PPV: 0.50 NPV: 0.98 |
Discrimination: C-statistic: NR Using the cutoff 0.11, Sensitivity: 0.79 Specificity: 0.92 PPV: 0.48 NPV: 0.98 |
||||
|
Calibration: H-L GOF: P=0.32 |
Calibration: H-L GOF: NR |
||||
Abbreviations: ADL, activity of daily living; E&M, evaluation and management; H-L GOF, Hosmer-Lemeshow goodness-of-fit test; IADL, instrumental activity of daily living; NR, not reported.
DISCUSSION
To date, we found very little research conducted on development and application of a claim-based frailty score in pharmacoepidemiologic studies. Two models that were developed from institutionalized or already frail individuals had limited generalizability to the general population of older adults in the community.40–42 Only the model by Davidoff et al. that was developed from a representative population of older adults seemed useful for pharmacoepidemiologic studies.43 Nonetheless, these models focused on predicting disability rather than the accepted definitions of frailty from aging research. Measuring frailty is attractive, because it can identify high-risk older patients even when they are not considered high risk based on comorbidities and disability as well as those who are more likely to benefit (or harm) from a treatment.20,21 In the following paragraphs, we propose two potential approaches to develop a claim-based frailty score that incorporate insights from geriatrics and gerontology research.
Approach 1: Deriving the frailty index directly from claims database
Given the challenges in measuring individual components to define the frailty phenotype from claims data, we believe that creating a frailty index with diagnosis and procedure codes from claims data (as “health deficit”) may be feasible and offer some advantages over the frailty phenotype. The flexibility in choosing individual items for the frailty index can allow selection of pre-specified list of claims in the database at hand, as long as a sufficient number of claims are selected to cover multiple physiologic systems and their prevalence increases gradually with age (without saturating too early). In addition, the health deficits required to construct the frailty index may differ between community-dwelling and institutionalized populations. For instance, the prevalence of hearing loss gradually increases with age in community-dwelling older adults; but the prevalence reaches almost 100% in institutionalized older adults. When counted as a health deficit, hearing loss does not help discriminate the health risk in institutionalized populations. Therefore, it can be included in the frailty index only for community-dwelling population. Another advantage is that the frailty index provides better discrimination in predicting the risk of adverse outcomes than the frailty phenotype.44,45 While the frailty phenotype may be clinically appropriate to identify vulnerable patients for interventions and monitor the change in individual components over time, the frailty index is more suitable for the purpose of confounding adjustment in pharmacoepidemiologic studies of mortality.
Approach 2: Gold-standard-driven development of a claim-based frailty score
As an alternative to deriving the frailty index directly from claims database, we can build a multivariable model that uses claims to predict a “gold standard” frailty (either the frailty phenotype or the frailty index) that is defined from in-person assessment. Below we describe steps to develop and evaluate a claim-based frailty score (Table 4).
Table 4.
Recommendations for Development and Evaluation of a Claim-Based Frailty Score
| Recommendations | |
|---|---|
| Database | Consider population-based epidemiologic studies of aging that have claims and detailed clinical or survey data. Below are some examples:
Consider linking claims data to electronic health records. |
| Population | Develop claim-based frailty scores separately for community-dwelling population and institutionalized population. |
| Definition of “gold standard” frailty | Use the frailty index as a “gold standard” than the frailty phenotype for the purpose of confounding adjustment. Determine a pre-specified list (≥30) of health deficits (symptoms, signs, diseases, test abnormalities, and disability) in multiple physiologic systems available in database. Construct the “gold standard” frailty index as proportion of deficits. |
| Development of claim-based frailty score | Determine a time frame in which potential predictors are measured (e.g. 1 year). Consider the following a priori list of ICD-9-CM diagnosis codes and HCPCS codes that represent clinical manifestations of frail patients.
Consider additional diagnosis codes, prescription claims, and health care service claims to identify predictors that are either positively or negatively associated with the “gold standard” frailty. |
| Evaluation of claim-based frailty score | Validate the performance of a claim-based frailty score against the “gold standard” definition of frailty in an independent hold-out sample, cross-validation, or bootstrap resampling. Test the associations with the following clinical outcomes that are known to be associated with frailty:
Assess the effectiveness of using claim-based frailty score (e.g. restriction, stratification, matching) on bias reduction using a known example of a treatment that has substantial confounding by frailty. |
Abbreviations: HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM, the International Classification of Diseases, Ninth Revision, Clinical Modification
The first step is to find a database that has both claims data and in-person assessment that is needed to define a “gold standard” frailty. A few population-based epidemiologic studies of aging that have been linked to claims data can be valuable data sources (e.g. Adult Changes in Thought Study, Cardiovascular Health Study, Health and Retirement Study, Longitudinal Study of Aging, and National Long-Term Care Survey) (Study main websites were provided in Table 4). Electronic health records (EHR) are a potentially useful source to capture frailty in a real-world population that can be linked to claims. EHR contains clinical data (laboratory, pharmacy, radiology, and physician orders), and narrative clinical notes (medical history, symptoms, and clinical reasoning behind treatment decision making) that are difficult to obtain from claims data. However, there are challenges in measuring a “gold standard” frailty from EHR. As mentioned earlier, most physicians do not use one of the accepted models of frailty but rely on their subjective assessment (“eyeball” test). Although there are a few clinical prediction models based on EHR data,46–48 how to create an EHR-based frailty score remains to be determined. Selective availability of certain clinical data, complex patterns of health care system use and difficulties in mining information from narrative clinical notes pose several technical and analytical challenges, such as missing data problems49 and differential ascertainment or misclassification of confounders. Routine use of clinical templates and advances in natural language processing programs may mitigate these challenges in the future.
The next step is to define a “gold standard” frailty using detailed clinical information in the surveys, epidemiologic cohorts, or EHR. Although both the frailty phenotype and frailty index can be used, we recommend modeling the frailty index that has better discrimination than the frailty phenotype in predicting the outcome risk. Once a “gold standard” frailty is defined, a wide range of claims that are measured within a time frame (e.g. 1 year) – diagnoses, prescriptions, and health care service claims – need to be considered. Based on our understanding about the clinical phenotype or underlying biology of frailty, we should consider certain predictors a priori, such as diagnosis codes, durable medical equipment claims, and nursing or personal care service claims that characterize frail patients (Table 4). In addition to these claims, we need to include claims that are negatively associated with frailty. As shown by Davidoff et al.,43 some health care services (e.g. ambulance, upper gastrointestinal tract endoscopy, and minor skin procedures) are more utilized by frail patients, while preventive services (e.g. screening and vaccination) are less utilized.
The performance (calibration and discrimination) of the derived claim-based frailty score needs to be evaluated in an independent hold-out sample, or using cross-validation or bootstrap resampling to avoid overestimation of model performance. The criteria for a successful frailty measure have been proposed in terms of content validity, construct validity, and criterion validity.50 According to the criteria, a successful frailty measure should be dynamic (changes over time), include multiple determinants, and replicate some of the known relationships with age (more common in advancing age), gender (more common in women), and adverse clinical outcomes (fall, hospitalization, institutionalization, home health service use, and mortality). Finally, a validated claim- based frailty score should be applied to reduce confounding bias in a known example of a treatment that has substantial confounding by frailty.
Areas of uncertainty
First, it may be useful to examine the inherent characteristics of a claim-based frailty score. It is important to study the optimal length of period to capture claims data for development of a claim-based frailty score and the sensitivity of the score to reflect changes in clinical frailty over time. Second, it is unknown which of the two approaches we outlined leads to better confounding adjustment. This can be assessed by comparing C statistics between the derived frailty scores. Third, when the frailty score, comorbidity score, and disability score are created using the same claims database, it is unclear whether the claim-based frailty score can provide additional prognostic information beyond existing confounding summary scores (e.g. comorbidity score, disease risk score, or propensity score). Lastly, the transportability of a frailty score developed from one population to similar populations in different databases that might have different health care utilization and practice patterns should be evaluated. This may be less problematic with our first proposed approach, that is, deriving the frailty index directly from claims data using a pre-selected list of health deficits available in that particular claims database. Our second approach, however, needs validation in independent databases.
CONCLUSIONS
As observational data remain a major source of comparative effectiveness research in older adults, there is a critical need to advance methods to adjust for confounding bias by frailty by incorporating the accumulated expertise from geriatrics and gerontology research. Among different operationalized definitions of frailty used in clinical practice, we believe that the frailty index, the count of health deficits in multiple physiologic systems, is more promising for the purpose of confounding adjustment. A claim-based frailty score should consider including certain a priori list of diagnoses and health care service claims that characterize frail patients, as well as consider additional diagnoses, prescriptions, and health care service claims that are either positively or negatively associated with the frailty. More research is needed to evaluate the performance and transportability of claim-based frailty scores in different databases and additional reduction in confounding bias beyond existing methods.
Acknowledgments
Dr. Kim has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Kim was supported by the Charles A. King Trust Postdoctoral Fellowship award from the Medical Foundation, a division of Health Resources in Action, and KL2 Medical Research Investigator Training award from the Harvard Catalyst, the Harvard Clinical and Translational Science Center, and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (1KL2 TR001100-01). Dr. Schneeweiss is principal investigator of the Harvard-Brigham Drug Safety and Risk Management Research Center funded by the Food and Drug Administration. His work is partially funded by grants and contracts from the Patient Centered Outcomes Research Institute, Food and Drug Administration, and National Heart, Lung, and Blood Institute.
Sponsor’s Role: The sponsor had no role in the design, methods, data collection, analysis and preparation of this paper.
Footnotes
Financial disclosure: Dr. Schneeweiss is a consultant to WHISCON, LLC and to Aetion, Inc., a software manufacturer of which he owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis and Boehringer-Ingelheim unrelated to the topic of this study.
Conflict of Interest: Dr. Kim has no disclosure. Dr. Schneeweiss is a consultant to WHISCON, LLC and to Aetion, Inc., a software manufacturer of which he owns shares. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis and Boehringer-Ingelheim unrelated to the topic of this study.
Author Contributions: Dr. Kim contributed to study concept and design, data acquisition, analysis and interpretation of data, and preparation of manuscript. Dr. Schneeweiss contributed to study concept and design, analysis and interpretation of data, and preparation of manuscript.
References
- 1.Ray WA. Observational studies of drugs and mortality. N Engl J Med. 2005 Dec 1;353(22):2319–2321. doi: 10.1056/NEJMp058267. [DOI] [PubMed] [Google Scholar]
- 2.McGrath LJ, Cole SR, Kshirsagar AV, Weber DJ, Sturmer T, Brookhart MA. Hospitalization and skilled nursing care are predictors of influenza vaccination among patients on hemodialysis: evidence of confounding by frailty. Med Care. 2013 Dec;51(12):1106–1113. doi: 10.1097/MLR.0b013e3182a50297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001 Nov;12(6):682–689. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Sturmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005 May 1;161(9):891–898. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009 Apr 21;119(15):2051–2057. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013 Mar 2;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004 Jun;59(6):M627–632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 9.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacas A, Rockwood K. Frailty in primary care: a review of its conceptualization and implications for practice. BMC medicine. 2012;10:4. doi: 10.1186/1741-7015-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. The Scientific World Journal. 2001 Aug 8;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes M. Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013 Nov;61(11):2020–2026. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014 Feb 5;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 14.European Heart Rhythm A, European Association for Cardio-Thoracic S. Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010 Oct;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 15.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. The lancet oncology. 2007 Dec;8(12):1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 16.Hurria A, Wildes T, Blair SL, et al. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014 Jan;12(1):82–126. doi: 10.6004/jnccn.2014.0009. [DOI] [PubMed] [Google Scholar]
- 17.American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012 Apr;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006 Apr 26;295(16):1935–1940. doi: 10.1001/jama.295.16.1935. [DOI] [PubMed] [Google Scholar]
- 19.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010 Apr;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 20.Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC. Cardiovascular interventions. 2012 May;5(5):489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Puts MT, Santos B, Hardt J, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol. 2014 Feb;25(2):307–315. doi: 10.1093/annonc/mdt386. [DOI] [PubMed] [Google Scholar]
- 22.Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA internal medicine. 2013 May 13;173(9):754–761. doi: 10.1001/jamainternmed.2013.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013 Jun;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005 Dec;53(12):2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 25.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005 Aug 30;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004 Mar;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 27.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005 Jul;14(7):465–476. doi: 10.1002/pds.1062. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011 Mar 15;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 29.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012 Dec;50(12):1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 30.Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res. 2009 Feb;18(1):67–80. doi: 10.1177/0962280208092347. [DOI] [PubMed] [Google Scholar]
- 31.Schneeweiss S, Wang PS. Claims data studies of sedative-hypnotics and hip fractures in older people: exploring residual confounding using survey information. J Am Geriatr Soc. 2005 Jun;53(6):948–954. doi: 10.1111/j.1532-5415.2005.53303.x. [DOI] [PubMed] [Google Scholar]
- 32.Schneeweiss S, Setoguchi S, Brookhart MA, Kaci L, Wang PS. Assessing residual confounding of the association between antipsychotic medications and risk of death using survey data. CNS Drugs. 2009;23(2):171–180. doi: 10.2165/00023210-200923020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Wang PS. Association between SSRI use and hip fractures and the effect of residual confounding bias in claims database studies. J Clin Psychopharmacol. 2004 Dec;24(6):632–638. doi: 10.1097/01.jcp.0000145344.76288.39. [DOI] [PubMed] [Google Scholar]
- 34.Pressley JC, Patrick CH. Frailty bias in comorbidity risk adjustments of community-dwelling elderly populations. J Clin Epidemiol. 1999 Aug;52(8):753–760. doi: 10.1016/s0895-4356(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JC, Marsh T, Lumley T, et al. Validation sampling can reduce bias in health care database studies: an illustration using influenza vaccination effectiveness. J Clin Epidemiol. 2013 Aug;66(8 Suppl):S110–121. doi: 10.1016/j.jclinepi.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturmer T, Schneeweiss S, Avorn J, Glynn RJ. Adjusting effect estimates for unmeasured confounding with validation data using propensity score calibration. Am J Epidemiol. 2005 Aug 1;162(3):279–289. doi: 10.1093/aje/kwi192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huybrechts KF, Brookhart MA, Rothman KJ, et al. Comparison of different approaches to confounding adjustment in a study on the association of antipsychotic medication with mortality in older nursing home patients. Am J Epidemiol. 2011 Nov 1;174(9):1089–1099. doi: 10.1093/aje/kwr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009 Jul;20(4):512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013 Sep;61(9):1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 40.Rosen A, Wu J, Chang BH, Berlowitz D, Ash A, Moskowitz M. Does diagnostic information contribute to predicting functional decline in long-term care? Med Care. 2000 Jun;38(6):647–659. doi: 10.1097/00005650-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Rosen A, Wu J, Chang BH, et al. Risk adjustment for measuring health outcomes: an application in VA long-term care. American journal of medical quality: the official journal of the American College of Medical Quality. 2001 Jul-Aug;16(4):118–127. doi: 10.1177/106286060101600403. [DOI] [PubMed] [Google Scholar]
- 42.Dubois MF, Dubuc N, Kroger E, Girard R, Hebert R. Assessing comorbidity in older adults using prescription claims data. J Pharm Health Serv Res. 2010;1(4):157–165. [Google Scholar]
- 43.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–165. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 45.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008 May;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC. Heart failure. 2013 Jun;1(3):245–251. doi: 10.1016/j.jchf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Rapsomaniki E, Shah A, Perel P, et al. Prognostic models for stable coronary artery disease based on electronic health record cohort of 102 023 patients. Eur Heart J. 2014 Apr;35(13):844–852. doi: 10.1093/eurheartj/eht533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrold J, Byhoff E, Harris P, et al. All hospice patients are not equal: development of a visit-based acuity index. J Palliat Med. 2014 Feb;17(2):135–140. doi: 10.1089/jpm.2013.0109. [DOI] [PubMed] [Google Scholar]
- 49.Schneeweiss S, Rassen JA, Glynn RJ, et al. Supplementing claims data with outpatient laboratory test results to improve confounding adjustment in effectiveness studies of lipid-lowering treatments. BMC Med Res Methodol. 2012;12:180. doi: 10.1186/1471-2288-12-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005 Sep;34(5):432–434. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 51.Bainey KR, Natarajan MK, Mercuri M, et al. Treatment assignment of high-risk symptomatic severe aortic stenosis patients referred for transcatheter AorticValve implantation. Am J Cardiol. 2013 Jul 1;112(1):100–103. doi: 10.1016/j.amjcard.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook PN, Cameron ID, Chen JS, et al. Oral bisphosphonates are associated with reduced mortality in frail older people: a prospective five-year study. Osteoporos Int. 2011 Sep;22(9):2551–2556. doi: 10.1007/s00198-010-1444-6. [DOI] [PubMed] [Google Scholar]
- 53.Aaldriks AA, Giltay EJ, le Cessie S, et al. Prognostic value of geriatric assessment in older patients with advanced breast cancer receiving chemotherapy. Breast. 2013 Oct;22(5):753–760. doi: 10.1016/j.breast.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006 Nov;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 55.Gnjidic D, Le Couteur DG, Blyth FM, et al. Statin use and clinical outcomes in older men: a prospective population-based study. BMJ open. 2013;3(3) doi: 10.1136/bmjopen-2012-002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009 Mar;38(2):156–162. doi: 10.1093/ageing/afn293. [DOI] [PubMed] [Google Scholar]