Abstract
Inducible heat shock proteins (HSP), regulated by heat shock factor-1 (HSF-1), protect against renal cell injury in vitro. To determine whether HSPs ameliorate ischemic renal injury in vivo, HSF-1functional knock-out mice (HSF-KO) were compared with wild-type mice following bilateral ischemic renal injury. Following injury, the kidneys of wild-type mice had the expected induction of HSP70 and HSP25; a response absent in the kidneys of HSF-KO mice. Baseline serum creatinine was equivalent between strains. Serum creatinines at 24 hours reflow in HSF-KO mice were significantly lower than in the wild-type. Histology showed similar tubule injury in both strains after ischemic renal injury but increased medullary vascular congestion in wild-type compared with HSF-KO mice. Flow-cytometry of mononuclear cells isolated from kidneys showed no difference between strains in the number of CD4+ and CD8+ T cells in sham operated animals. At 1 hour of reflow, CD4+ and CD8+ cells were doubled in the kidneys of wild type but not HSF-KO mice. Foxp3+ T regulatory cells were significantly more abundant in the kidneys of sham-operated HSF-KO than wild-type mice. Suppression of CD25+Foxp3+ cells in HSF-KO kidneys with the anti-CD25 antibody PC61 reversed the protection against ischemic renal injury. Thus, HSF-KO mice are protected from ischemic renal injury by a mechanism that depends on an increase in the T regulatory cells in the kidney associated with altered T cell infiltration early in reflow. Hence, stress response activation may contribute to early injury by facilitating T cell infiltration into ischemic kidney.
Introduction
Ischemia induced acute kidney injury (AKI) leads to renal insufficiency and increase in morbidity and mortality in critically ill patients. Ischemic AKI causes morphologic disruption of renal tubule epithelial cells and peritubular alterations including in the microvasculature (1–5). Multiple factors are implicated in modulating ischemia reperfusion injury and recovery of kidneys. These include an inflammatory response and stress or heat shock protein (HSP) responses (6–8).
Several leukocyte subtypes, including neutrophils, macrophages and lymphocytes have been detected in the peritubular vasculature, interstitium and tubules. These leukocyte subpopulations are under investigation to determine their roles in ischemic kidney injury (9–11). There is growing evidence supporting a role for T lymphocytes in ischemic kidney injury. T cell depletion and double knockout of CD4 and CD8 cells are protective in mice subjected to renal ischemia (12). However, combined T and B cell deficiency appears not to be protective in ischemic AKI; isolated B cell deficiency, though, is partially protective (13, 14). This protection is reversed with wild type serum, but not B cells, suggestive of a soluble factor being involved in exerting the B cell effect in AKI (14).
Protection against injury following ischemic preconditioning has been attributed to multiple factors including induction of HSPs (7). Over-expression of HSP70 and 27, likely through their protein chaperone function, protect cultured renal proximal tubule cells against injury from ATP depletion, an established in vitro model of ischemic renal injury (15–17).
The role of HSPs in ischemic renal injury in vivo is still under investigation. Ischemia induces increase in HSPs 70 and 25 in rat kidneys (18–21). Protection against a second insult following a preconditioning injury has been attributed to multiple factors, including induction of HSPs (20, 22–26). The resistance of immature rat renal tubules against anoxic injury is associated with increased heat shock factor -1 (HSF-1) activity and with increased HSP70 expression in immature kidney compared with adult (27). This tolerance to hypoxia was reversed in the presence of heat shock factor (HSF) decoy, which inhibited HSP70 expression.
Binding of activated, trimerized HSF-1 to the upstream heat shock element is fundamental in upregulation of inducible HSPs (28). In models of renal ischemia, HSF-1 is primarily activated by metabolic stresses associated with ATP depletion (18, 19). To understand better the role of HSP induction in in vivo ischemic renal injury, we studied HSF-1 functional knockout mice (HSF-KO). Our hypothesis was that HSP induction by renal ischemia would be inhibited in HSF-KO mice, and that HSF knockout mice would then suffer worse ischemic renal injury.
Results
HSP expression in WT and HSF-KO mice
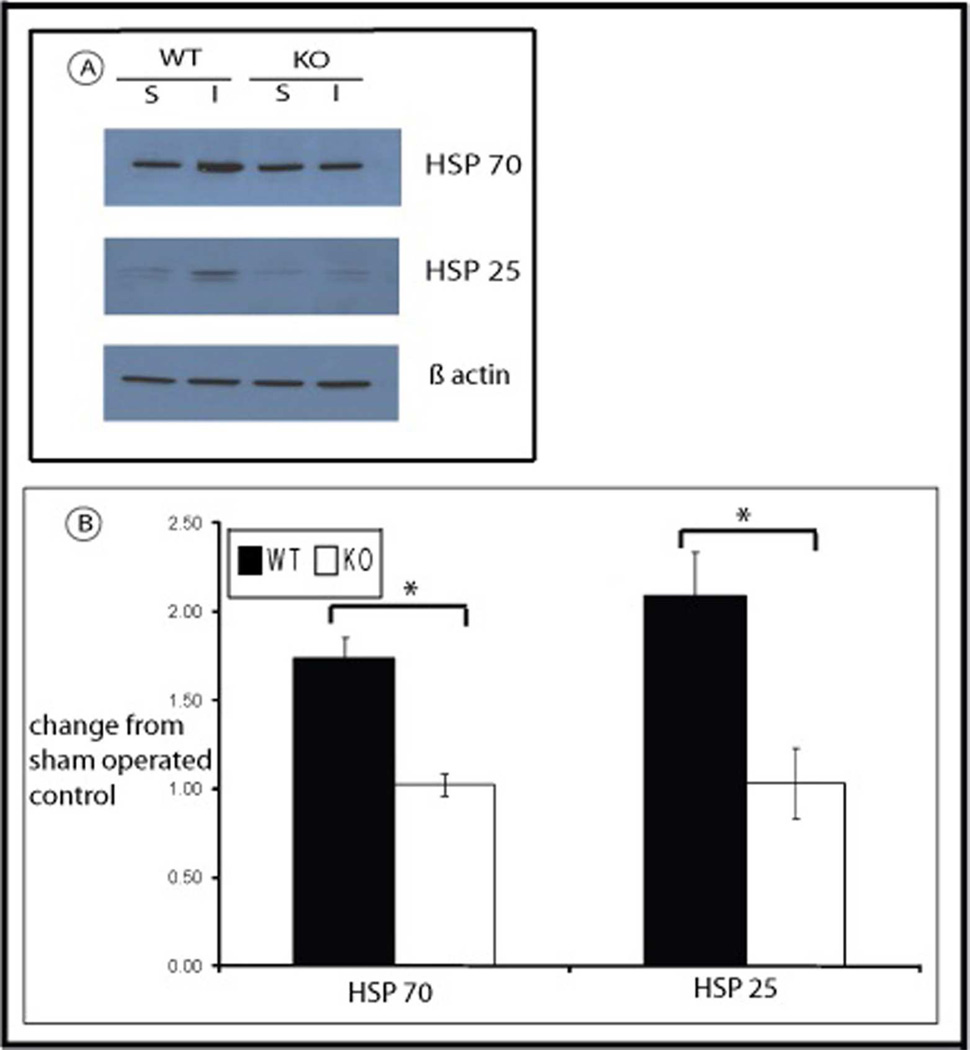
Expression of HSPs 70 and 25 was measured in kidneys from WT and HSF-KO mice following 45 minutes ischemia and recovery for 24 hours and compared with their expression in sham operated control mice. As has been demonstrated previously in rats, mice kidney has a baseline expression of HSP70 and HSP25 (Figure 1; Panel A and B). Following ischemia and reperfusion for 45 minutes and 24 hours respectively, there is significant induction in WT kidneys of both HSPs above baseline levels (77% above baseline sham for HSP70, 94% above sham for HSP25; p=0.01 for both). As is shown in Figure 1, in HSF-KO mice kidneys there also is baseline expression of both HSPs, 70 and 25, equivalent to WT mice kidney. However, unlike the wild type animals, there is no significant induction of these HSPs following ischemia and reperfusion in HSF-KO mice kidney (p=0.9 and 0.7 for HSP70 and HSP25, respectively, compared to non-ischemic sham operated control). This lack of induction of HSPs triggered by ischemia in HSF-KO mice compared with WT mice is significant (p<0.005 for both HSP70 and HSP25 in HSF-KO vs. WT at 24 hrs reflow).
Figure 1.
HSP expression in WT and HSF-KO mice following ischemia reperfusion. Panel A is the representative Western blots of WT and HSF-KO mice kidney tissue stained with antibody against HSP70, HSP25 and actin following sham (S) surgery and ischemia reperfusion injury (I) for 45 minutes and 24 hours respectively. Panel B is the graph of densitometry (mean +/− SEM) of Western blots probed for HSP70 and HSP25, expressed as change from sham conditions, using actin as loading control (n=4 for all conditions). * represents p< 0.05 between groups.
Renal function in WT and HSF-KO mice
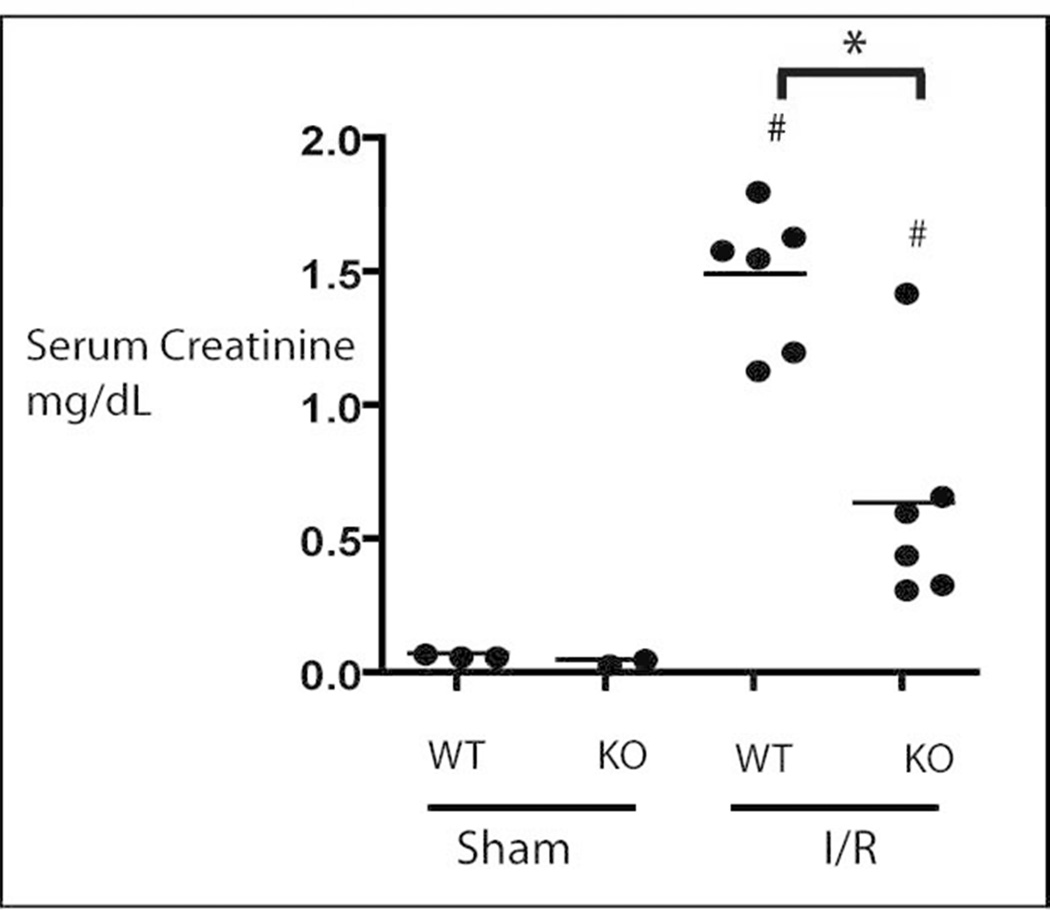
To determine the effect on renal function of ablated induction of HSP 70 and 25 in the HSF-KO mice, serum creatinine was measured in both the HSF-KO and WT animals under each condition (Figure 2). We measured serum creatinine using a Jaffe assay on initial studies. Later studies were done by Jaffe assay and Mass Spectrometry assay to confirm the validity of the Jaffe assay results. While the absolute values of serum Cr differed between the two assays, the pattern and statistically significant difference between experimental groups held true. Serum creatinine of sham WT and HSF-KO mice were comparable (by Jaffe assay 0.22 mg/dL and 0.19 mg/dL, respectively; p=0.19 with n=6 for each, by mass spectrometry 0.07 mg/dL and 0.05 mg/dL, respectively; n= 2–3). Following 45 minutes ischemia and 24 hours recovery, the WT mice manifested renal insufficiency with the expected increase in serum creatinine to 2.1 mg/dL by Jaffe assay and 1.5 mg/dL by mass spectrometry. In HSF-KO mice, subjected to the same duration of ischemia and reperfusion as WT mice, serum creatinine increased only to 0.9 mg/dL by Jaffe assay and 0.6 by mass spectrometry. This difference in serum creatinine following ischemia reperfusion between the WT and HSF-KO mice was statistically significant (p=0.000001 for Jaffe assay and 0.001 for mass spectrometry).
Figure 2.
Serum creatinine in WT and HSF-KO mice. Mice were subjected to sham surgery or renal ischemia injury for 45 minutes and 24 hours reflow (I/R) Shown in figure are mass spectrometry results. N ≥ 6 for all conditions, including sham, by Jaffe assay. * represents p= 0.000001 by Jaffe assay and 0.001 by mass spectrometry between groups. # represents p<0.0004 by Jaffe assay between each sham and injury (I/R) group for both WT and KO animals.
Histology of WT and HSF-KO mouse kidney
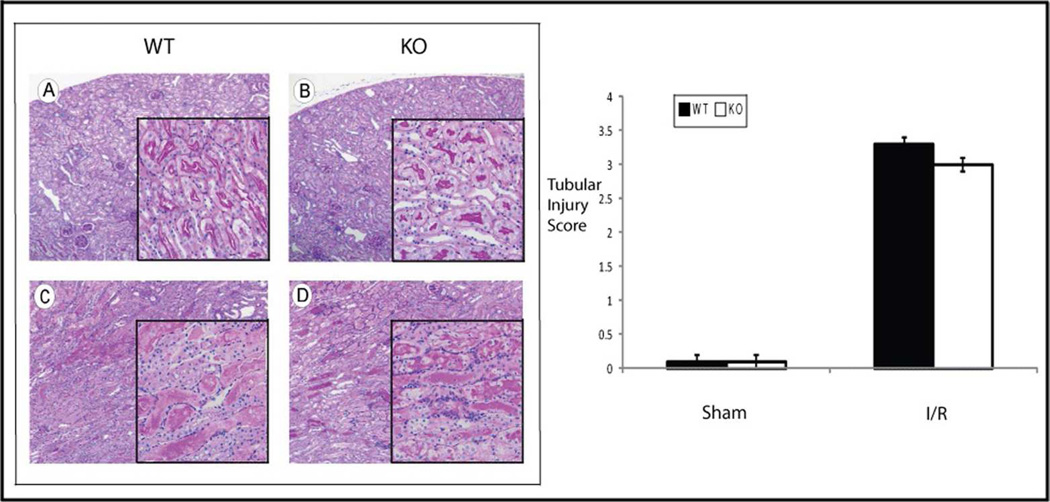
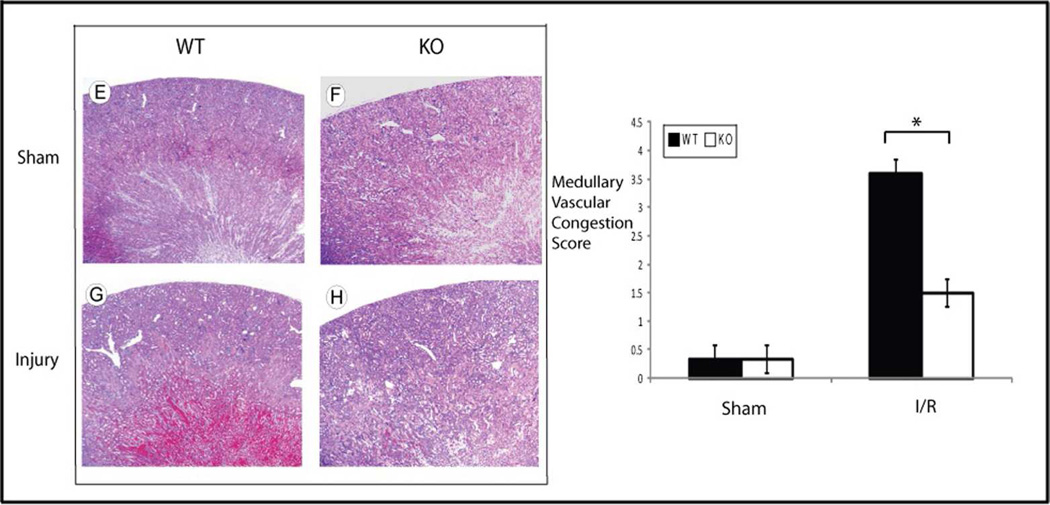
Histology of the WT and HSF-KO kidneys were compared both in the uninjured condition and following ischemic injury. The degree of histological changes was scored by two investigators blinded to the experimental conditions (details in methods), using PAS staining for tubular injury and H&E staining for assessment of medullary vascular congestion. The findings were consistent for an n of 5 in each experimental group. No significant difference was found in the histology score of the WT compared to HSF-KO mice kidney following sham surgery with PAS (WT to HSF-KO p=1.0) or H&E staining (WT to HSF-KO p=1.0) (Figures 3a and 3b; Panels A, B, E and F and graphs). The sham kidneys from both groups displayed only mild fixation artifact in the proximal tubule (in PAS: Figure 3a; Panels A & B and H&E stain: Figure 3b; Panels E & F). Of note, there appeared to be no renal developmental abnormalities or dysplasia in the HSF-KO mice kidneys when compared to the WT kidney. Following ischemia, there appeared to be nearly equivalent proximal tubule injury in both WT and HSF-KO mice kidneys (Figure 3a: Panels C & D). There was no statistical difference in the histology score for proximal tubule injury between the WT and HSF-KO mice kidneys (WT to HSF-KO p=0.3) (Figure 3a graph). However, the typical prominent medullary vascular congestion seen following ischemic renal injury in the WT kidney was essentially absent in the HSF-KO kidneys (Figure 3b: Panels G and H). The histological score for medullary vascular congestion was significantly different between the two strains (WT to HSF-KO p=0.0007) (Figure 3b graph).
Figure 3.
a Histology and tubular injury grading of WT and HSF-KO kidney tissue. Panels A, B, C & D are representative of PAS stained sections of WT and KO mice kidneys subject to sham surgery (Panels A&B) or 45 minutes ischemia and 24 hour reperfusion (Panels C&D). Original images are 10 × and insert images are 40 ×. The graph is the tubular injury score of n=5 for each condition, blinded to the scorers. (No significant difference between WT and HSF-KO after I/R, p=0.3)
b Histology and medullary vascular congestion grading of WT and HSF-KO kidney tissue. Panels E, F, G & H are representative of H&E stained sections of WT and HSF-KO mice kidneys subject to sham surgery (Panels E&F) or 45 minutes ischemia and 24 hour reperfusion (Panels G&H). Images are 4×. The graph is the vascular congestion score of n=5 for each condition, blinded to the scorers. * represents p< 0.0007 between groups.
Mononuclear cells isolated from WT and HSF-KO kidneys
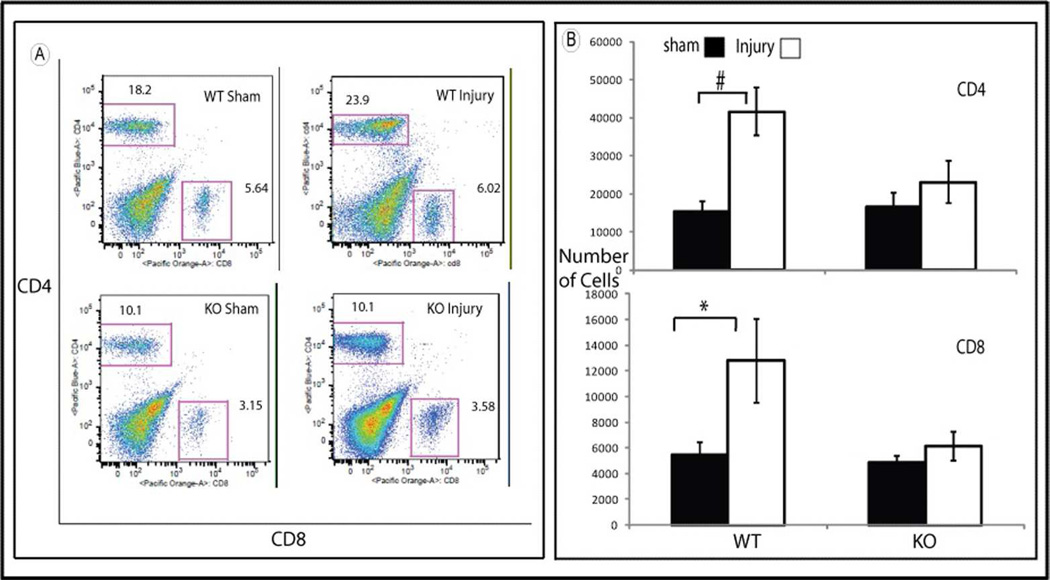
We used flow- cytometry to study the pattern of mononuclear cells isolated from kidneys from both strains of mice. In sham operated control, there was no difference in the number of CD4+ and CD8+ T cells found in WT compared to HSF-KO kidneys (Figure 4, Panels A and B). Following 45 minutes of bilateral ischemia and 1 hour reperfusion, the number of CD4+ and CD8+ cells in WT mice kidneys was more than double the numbers found in uninjured controls (p=0.001 for CD4+, p=0.04 for CD8+). In contrast, there was no significant change in either CD4+ or CD8+ cell numbers (p=0.5 for CD4+ and p=0.6 for CD8+ cells) in the HSF-KO mice kidneys following ischemia compared to uninjured control (Figure 4; Panels A and B).
Figure 4.
Flowcytometry of mononuclear cells isolated from WT and HSF-KO mice kidneys. Panel A is representative flowcytometry of mononuclear cells marked with antibodies against CD4 and CD8 isolated from WT and HSF-KO mice kidney following sham surgery and ischemia followed by 1 hour reperfusion. Panel B is the summation of the flowcytometry data (mean +/− SEM) for CD4 and CD8 positive cells, expressed as number of cells (n= 4 – 8 for all conditions). * represents p<0.05 between groups. # represents p<0.002 between groups.
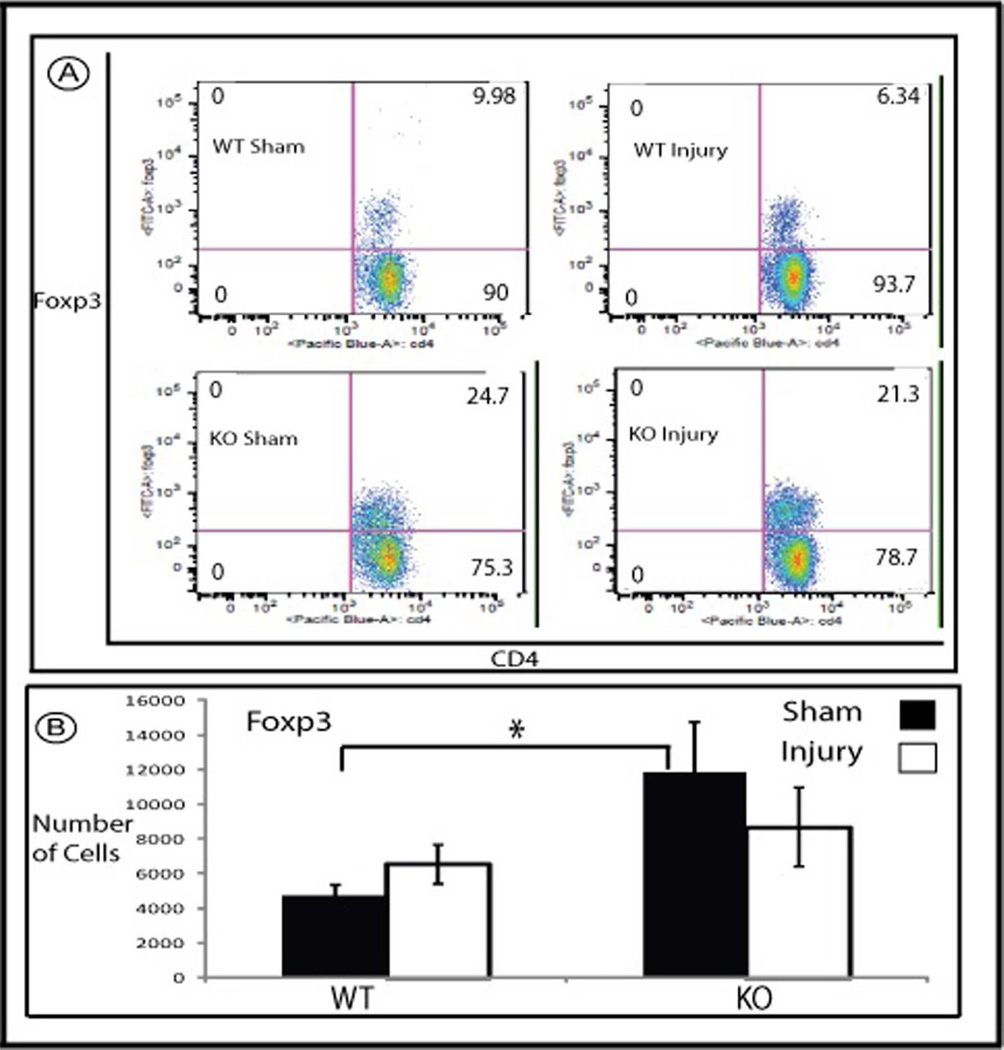
In addition, we found that there is significantly more Foxp3+ T regulatory cells in sham operated mice kidneys of HSF-KO animals compared with WT (Figure 5, p=0.02 WT Sham to HSF-KO Sham). When we examined the intrarenal distribution of FoxP3+ cells using immunohistochemistry, we found no difference in HSF-KO mice kidneys compared to WT. In both, the Foxp3+ cells were found exclusively in the outer medulla in the peritubular interstitium (not shown). There was no significant change in the number of intrarenal Foxp3+ cells early after I/R compared to sham operated kidneys in either strain (Figure 5; Panels A and B).
Figure 5.
Flowcytometry of mononuclear cells isolated from WT and HSF-KO mice. Panel A is representative flowcytometry of mononuclear cells marked with antibodies against Foxp3 and CD4, isolated from WT and HSF-KO mice kidney following sham surgery and ischemia followed by 1 hour reperfusion. Panel B is the summation of the flowcytometry data (mean +/− SEM) for Foxp3 positive cells, expressed as total number of cells (n=6–12 for all conditions). * represents p<0.02 between sham operated WT and HSF-KO.
Treatment with anti-CD25
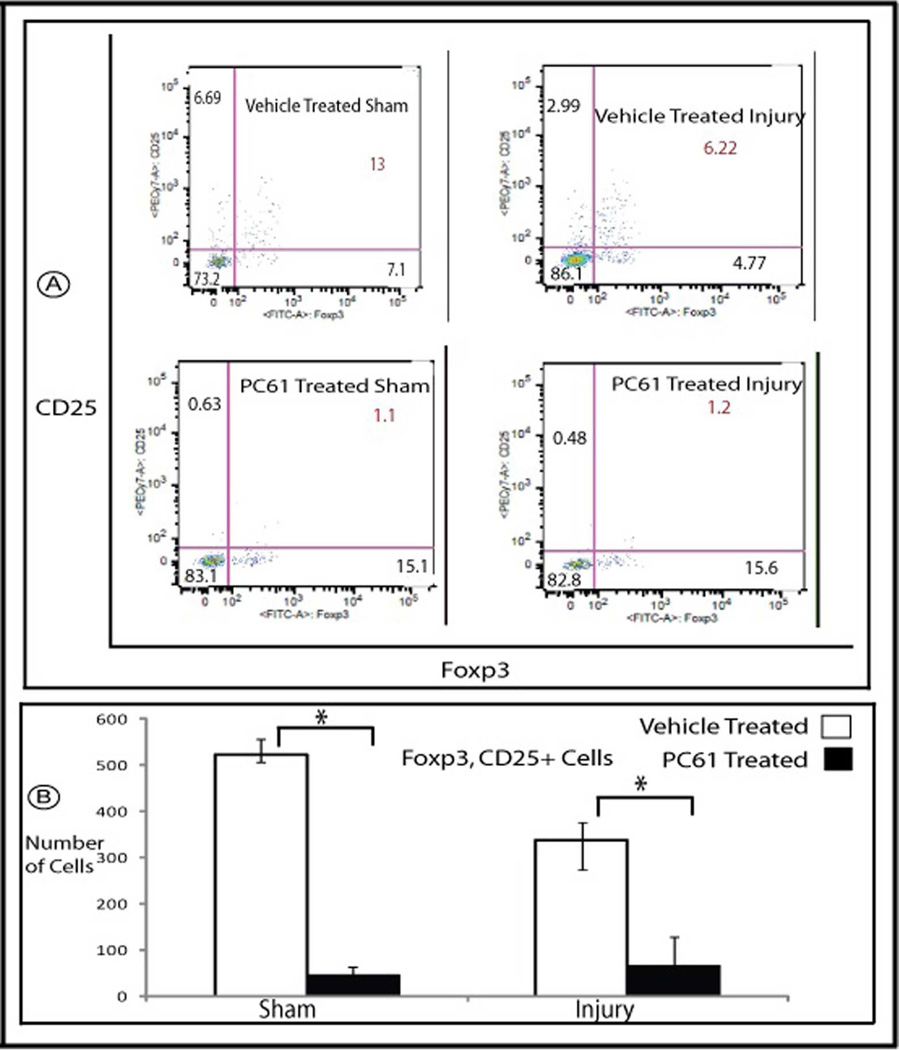
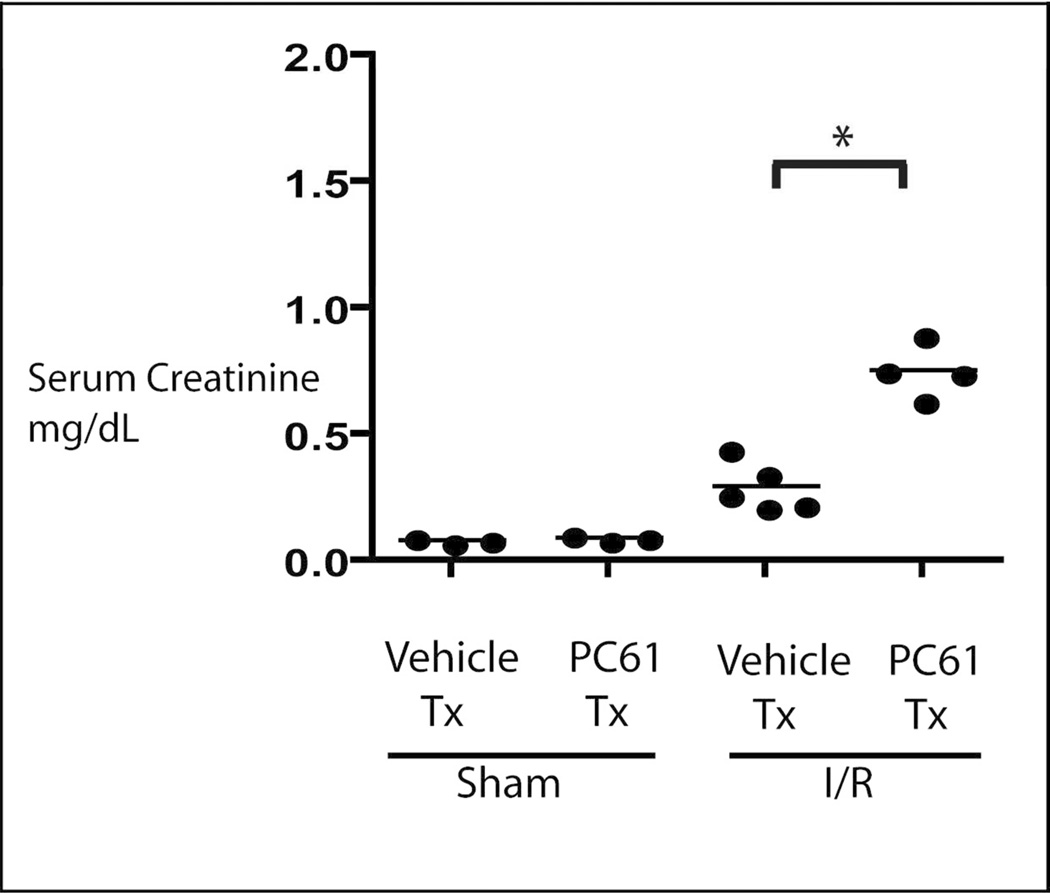
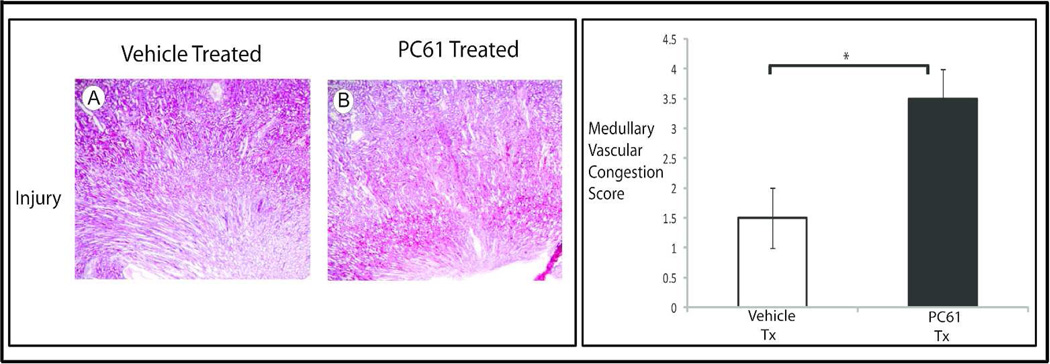
HSF-KO animals were treated with PC61, anti-CD25 antibody, to deplete that subpopulation of T regulatory cells, or vehicle control. Mononuclear cells were isolated from the kidneys following sham surgery, or ischemia for 45 minutes and reperfusion for 24 hours. As shown in figure 6, treatment with PC61 intravenously for 5 days suppressed the renal Foxp3, CD25+ cells significantly in the sham operated condition and also at 24 hours reperfusion following ischemia (PC61 to vehicle control p=0.006). Under sham operated condition, treatment with PC61 did not change the baseline serum creatinine when compared with the vehicle treated animals (PC61 to vehicle treated by Jaffe assay 0.26 and 0.24, respectively; p= 0.2 and by mass spectrometry 0.09 and 0.08, respectively; p= 0.3, Figure 7). CD25+ cell depletion prior to renal ischemia in HSF-KO animals resulted in significantly higher serum creatinine when compared with HSF-KO animals with no CD25+ cell depletion prior to renal ischemia (PC61 and vehicle treated by Jaffe assay were 0.93 and 0.42, respectively; p= 0.02, and by mass spectrometry were 0.75 and 0.29, respectively; p= 0.0003 Figure 7). Renal ischemia following CD25+ cell depletion in the HSF-KO animals also resulted in significantly more medullary vascular congestion, similar to HSF-WT, compared with vehicle treated HSF-KO animals (Figure 8, histology score PC61 to vehicle treated p= 0.009).
Figure 6.
Flowcytometry of mononuclear cells isolated from HSF-KO mice kidney. Panel A is representative flowcytometry of mononuclear cells marked with antibodies against Foxp3 and CD25, isolated from HSF-KO mice kidney following treatment with PC61 or vehicle, and subjected to sham surgery or ischemia followed by 24 hour reperfusion. Panel B is the summation of the flowcytometry data (mean +/− SEM) for Foxp3, CD25 positive cells, expressed as total number of cells (n=5 for all conditions). * represents p<0.006 between PC61 and vehicle treated conditions.
Figure 7.
Serum creatinine in HSF-KO mice with PC61 treatment. HSF-KO mice were treated with PC61 or vehicle, and then subjected to sham surgery or renal ischemia injury for 45 minutes and 24 hours reflow (I/R) Shown in figure are mass spectrometry results, n=5 for all conditions by Jaffe assay as detailed in text. * represents p< 0.0003 for mass spectrometry assay and p< 0.02 for Jaffe assay between groups shown.
Figure 8.
Histology and medullary vascular congestion grading of HSF-KO kidney tissue. HSF-KO mice were treated with PC61 or vehicle and subjected to I/R. Panels A and B are representative of H&E stained sections of HSF-KO mice kidneys treated with vehicle (A) or PC61 (B) and subjected to 45 minutes ischemia and 24 hr reperfusion. Images are 4×. The graph is the vascular congestion score of n=3 for each condition, blinded to the scorers. * represents p< 0.009 between groups.
Discussion
Many in vitro studies from several laboratories provide compelling and consistent evidence that HSPs afford protection to renal epithelia against injury (15, 16, 22–24). Such consistency is not present in results from in vivo models. We believe that this inconsistency arises, in part, because such models have relied principally on preconditioning injury either from heat or ischemia (29–31). In the present study, then, we sought to resolve this issue by taking advantage of the HSF-1 knockout mouse model (32, 33). In this model, HSF-1 is functionally knocked-out such that HSPs are not induced in response to the typical stress of heat, in all tissues examined, including the kidney. Furthermore, in cardiac tissue HSPs are not induced in this model in response to ischemia (34). Our hypothesis, then, was that renal ischemia would not induce the typical HSP elaboration in this knock-out model, and that the functional effect would be worse renal outcome in the HSF-KO mouse.
As we anticipated, neither of the inducible HSPs, HSP70 or 25, extensively studied in the renal ischemia models, were induced by renal ischemia in the HSF-KO mice, unlike the typical induction in the WT mice. At baseline, renal levels of HSP70 and 25 and renal morphology did not differ between the two strains. These results indicate that HSF-1 regulates induction of these HSPs in response to ischemia, but does not regulate constitutive expression of the inducible HSPs. Furthermore, although HSPs have been described to participate in renal development (35, 36), it appears that role is restricted to constitutively expressed HSPs, not stress inducible HSPs, since we found no evident renal developmental abnormalities in the HSF-KO mice by histological examination.
Counter to our hypothesis, we found that the HSF-1 functional KO mice had less renal dysfunction than WT mice subjected to the same duration of I/R injury. This posed a conceptual dilemma, since the results appear to run against nearly all of the in vitro studies, including those from our group studying the effect of blocking HSF function using synthetic HSF decoy (27, 37). So, we then considered potential mechanistic pathways for these apparently discrepant results.
One clue comes from the histological findings. We found no difference between the strains in the typical ischemia induced changes in proximal tubules. However, there is a clear and significant difference between WT and HSF-KO kidneys in microvascular changes following ischemia. Specifically, WT mice manifest the typical dramatic vascular congestion in the renal medulla following ischemia, a finding nearly absent in the HSF-KO mice kidneys. This finding is particularly pertinent, since essentially all studies that have demonstrated protective effects of HSPs in renal ischemia models have focused on effects on renal tubule cell structure, function, or viability (15, 16, 22–24). With the growing knowledge that injury to the microvasculature is an important element in ischemic renal injury (1–5, 38), we considered potential mechanisms where inducible HSPs may play a pathological role in this portion of the kidney following ischemia.
The pathway ripe for exploration involved inflammatory mechanisms of injury, since they may be initiated at the microvasculature level by an ischemic renal insult (3–5). Several groups have established the significant role that immune responses play in acute kidney injury (39–42). In physiological conditions, vascular endothelia serve as a barrier preventing immunological injury to underlying tissue from pro-inflammatory cells and cytokines in the blood (3–5). Microvascular injury, such as occurs with ischemia, leads to vascular endothelial dysfunction and a pro-inflammatory environment (43).
HSPs are known to have a role in immunological mechanisms, including innate immunity. The expression of some toll-like receptors (TLR) in renal epithelia, notably TLR-2 and TLR-4, are increased with AKI (8). Both receptors are activated by HSPs, including HSP 70 (44–46). This interaction shapes the final immune response by mediating the release of cytokines and affecting the cytolytic function of phagocytic cells (46–48).
This background, combined with the unanticipated findings presented here in figures 2 and 3b, led us to examine the pattern of mononuclear cell infiltration into the kidneys of WT and HSF-KO mice. We focused our studies on T cells, since several studies indicate that a T cell response modulates I/R injury in mouse kidney (10–13, 49). The lymphocyte response following renal ischemia has been demonstrated in multiple studies to occur early, and to peak at one hour of reperfusion (9, 50). So we chose to examine the lymphocyte pattern in the kidney at one hour of reflow after ischemia, long before the typical changes in renal histology and function are found after an ischemic insult.
Early after the ischemic insult to WT kidneys, we found increased infiltration of pro-inflammatory mononuclear cells, CD4 positive T helper and CD8 positive T cytotoxic cells, consistent with previous investigations (40–42). No such increase was found in the HSF-KO mice. Of particular interest was the finding that prior to ischemia there are significantly more immunomodulatory Foxp3+ regulatory T cells in the HSF-KO kidneys compared with the WT mice kidneys. This finding appears to be unique to the kidneys in HSF-KO mice, since we did not find significant differences in the number of Foxp3+ cells in blood, spleen or lymph nodes in HSF-KO mice compared to WT (data not shown).
Since regulatory T cells have been shown to contribute to the protective effect seen with ischemic preconditioning in the kidney (10), we sought to determine whether the baseline elevated T regulatory cells that we found in the HSF-KO kidneys play a role in the tolerance to ischemic renal injury seen in these animals. So, we treated HSF-KO animals with PC61, anti-CD25 antibody, to suppress the elevated T regulatory cells in kidneys of these mice, and then examined its effect on renal ischemia reperfusion injury. Suppression of the CD25+ Foxp3+ regulatory T cells in HSF-KO kidneys prior to the renal ischemic insult resulted in significantly worse renal function, partially reversing the protection against ischemia induced renal insufficiency found in this model.
Taken together, these results suggest that the pre-injury milieu of increased Foxp3+ cells is responsible, at least in part, for providing protection to HSF-KO mice kidneys against ischemic injury, and the mechanism may be to blunt infiltration of pro-inflammatory CD4 and CD8 cells. In our studies with PC61 treatment in HSF-KO mice, there was no increase in CD4 and CD8 cells in kidneys (results not shown) at 1 hour of reperfusion following ischemia with suppression of CD25+Foxp3+ regulatory cells. We attributed this to suppression of CD4 and CD8 T cells that are also CD25 positive. Regulatory T cells have also been shown to modulate later infiltration of innate immunity cells, macrophages and neutrophils, into the kidney after ischemia (10, 49). It may be, then, that the higher baseline Foxp3+ cells in HSF-KO kidney also affect this downstream pathway to renal injury, providing additional benefit to kidneys subjected to ischemia. Our study does not address this additional potential mechanism, which could be a fruitful avenue for future exploration of this model.
In sum, then, we found that HSF-KO kidneys at baseline had an increased level of immunomodulatory Foxp3+ regulatory T cells, and that the pro-inflammatory environment that develops in WT kidneys shortly following an ischemic insult does not develop in HSF-KO mice kidneys. In addition, suppression of the regulatory T cells in HSF-KO mice resulted in partial reversal of the inherent protection of this strain against ischemic renal injury.
So taken in the context of our previous in vitro work, although HSPs may provide protection at a cellular level in tubular epithelial cells, the present study demonstrates that the systemic effect of inducible HSPs may not be protective in the setting of an in vivo ischemic insult. How might inducible HSPs regulated by HSF contribute to immune mediated mechanisms of renal injury after ischemia? We suspect HSP expression induced by renal I/R in normal WT mice facilitates antigen presentation, providing increased endogenous targets for the innate immune response.
In addition, a separate HSF1 regulated pathway may contribute to renal injury after ischemia. Studies have demonstrated that several immune responses and inflammation related genes have heat shock response elements, and are directly regulated by HSF-1(51, 52). The increase in constitutive T regulatory cells in the kidneys in HSF-KO mice might, therefore, be a direct result of the non-functional HSF-1 altering the immunological profile in the kidneys of these mice, in a pathway completely separate from HSF-1 regulation of heat shock protein expression. The two separate HSF-1 regulated pathways potentially could then interact to the detriment of kidneys subjected to renal ischemia. In mice with normally functional HSF-1, the combination of increased endogenous targets for innate immune responses (triggered by ischemia and chaperoned by HSPs) along with lower base line T regulatory cells may facilitate early infiltration of pro-inflammatory T cells into the injured kidneys, which extends the damage in the kidney. In contrast, the absence of HSP induction in HSF-KO kidneys might impair antigen presentation and, along with the increased level in baseline regulatory T cells in HSF-KO kidney, result in less pro-inflammatory cell infiltration. Less immune mediated microvascular injury early after ischemia in the HSF-KO mice might then lead to less propagation of renal injury through inflammatory mechanisms. Thus, this altered T cell response in HSF-KO mice kidneys may account for the observed protection against injury in this model.
This mechanism, then, could explain the discrepancy between the well-established in vitro finding of inducible HSPs benefiting both structure and viability of renal tubule cells in ischemia models, and our current findings that ablated HSP induction in vivo results in protection against the full manifestations of renal injury from ischemia. It may be that while inducible HSPs have a distinct beneficial effect localized to the renal tubule cell, the contribution that HSP induction makes to promoting immune mediated pathways to renal injury in vivo is greater. The broader effect of the latter pathway may then supersede the direct beneficial effects of inducible HSPs in preserving renal tubule cell structure, function and viability.
Our study is not the first to report in vivo findings of HSP function in ischemia that apparently conflict with prior in vitro results (53). Supporting prior in vitro studies, proximal tubule cells isolated from transgenic mice that over express human HSP27 were found to be tolerant to H2O2 induced necrosis. However, these same mice manifested worse ischemic renal injury. This worse in vivo renal outcome was attributed to increased pro-inflammatory gene expression, along with increased neutrophil and lymphocyte infiltration into the kidney early (3 hrs) after the ischemic insult. In a follow-up study, selective renal over-expression of HSP27 protected against ischemic renal injury in vivo, in direct contrast to the finding in animals with systemic HSP27 overexpression (54). In the renal specific HSP27 over-expression model, protection against ischemic injury was attributed to decreased neutrophil infiltration of kidneys and reduced pro-inflammatory gene expression.
These two prior reports taken together, then, support our findings and contention that detrimental effects of inducible HSPs, outside of the renal tubule and in the microvasculature, conflict with their beneficial effects within renal tubule cells subjected to an ischemic insult. The inducible HSPs appear to augment an ischemic insult in vivo by facilitating pro-inflammatory mechanisms of renal injury. These studies, then, connect inducible HSPs to pathways of inflammation in renal ischemia/reperfusion injury and recovery. Progressively defining specific beneficial or deleterious effects of individual classes of HSPs, alongside studies of the more generalized stress response regulated by HSF-1, should identify where HSPs can ameliorate injury or promote recovery, as opposed to those pathways through which the stress response might contribute to worse renal outcome.
Materials and Methods
Animal model
Care of the mice before and during the experimental procedures was conducted in accordance with the policies of the Biomedical Resource Center, Medical College of Wisconsin, and the National Institutes of Health guidelines for the care and use of laboratory animals. All protocols had received prior approval by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
HSF-1 functional knock-out (HSF-KO) mice were generated by cross breeding HSF-1 homozygous knock-out male mice with HSF-1 heterozygous female mice, on 129XI/SvJ × BALB/c background, developed and kindly provided to us by Dr. Ivor J. Benjamin, University of Utah School of Medicine Health Sciences (32, 33). HSF-1 wild type mice were generated by cross-breeding HSF-1 heterozygotic male and female mice from the same colony. We performed PCR genotyping using separate primers for HSF-1 WT and functional knock-out DNA sequence to confirm homozygotic HSF-KO and WT status of the animals used in the experiments. Male HSF-1 homozygous knock-out and WT mice were used in all studies.
Ischemia Reperfusion Injury
Surgery was performed at 6 weeks of age. Some animals were given intravenous injection of PC61 (anti-CD25 antibody, clone purchased from the American Tissue Culture Collection, and the antibodies generated and purified in the laboratory) to deplete the CD25+ population of Foxp3 cells, or vehicle, for 5 days as previously described (55). Animals (15–25 g) were anesthetized by intra peritoneal injection of ketamine and medotomidine. Bilateral renal ischemia was induced in mice according to surgical procedures previously described (56). Animals were placed on heated surgical tables and monitored with rectal probes to maintain constant body temperature. Kidneys were exposed with midline incisions and blood supply to the kidneys interrupted with micro-aneurysm clamps for 45 minutes. After the occlusion interval, the clamps were removed, and reflow was verified visually. In sham operations, animals were exposed to the same treatments, but clamps were not applied.
Assessment of renal function and structure
At 24 hours of recovery, animals were anesthetized as described above, and a midline incision was made to expose the kidneys and aorta. Blood samples (minimum 0.5 ml) collected from the aorta into heparinized tubes, were centrifuged for plasma. Plasma creatinine was measured with an enzymatic assay based on the modified Jaffe reaction by an autoanalyzer (ACE, Alfa Wasserman, Fairfield, NJ) to determine the extent of renal injury. Some samples were also assayed by Mass Spectrometry at The University of Alabama at Birmingham, O’Brien Core Facility so as to verify the results obtained from the autoanalyzer using the modified Jaffe reaction. The kidneys were excised promptly and either fixed by immersion in 10% buffered formalin, used for biochemical analysis, or processed for mononuclear cell isolation (see below). The tissues were prepared for routine paraffin embedding and examination by light microscopy using hematoxylin and eosin stains, PAS stain by the histology core at Children’s Research Institute at Medical College of Wisconsin. The degree of histological changes was scored by two investigators blinded to the experimental conditions, using a quantitative scale. Tubular injury was graded using an established scoring scale (57). Briefly, the scoring scales were based on epithelial necrosis and necrotic debris in tubules in outer medulla. Score of 0 represents normal histology, score of 1 represents <10% change, 2 – 10–25% change, 3 – 26–75% change and 4 – >75% change for H&E and PAS staining, similar to that used by other investigators (57). Likewise, we adapted this method to grade the additional manifestation of ischemic injury, medullary vascular congestion; score 0 representing no congestion, 1– <10% congestion, 2 – 10–25% congestion, 3 – 26–75% congestion, 4 – >75% congestion.
Protein expression in kidney tissue
As has been described previously (56), renal tissue taken from HSF-1 function knock-out and WT mice were either snap frozen in liquid nitrogen and stored at −80°C for later processing, or immediately processed as follows: the tissue samples were homogenized using a Potter-Elvehjem homogenizer in 150 mmol/L NaCl, 10 mmol/L Tris (pH 7.5), 1 mmol/L EDTA, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). The samples were centrifuged at 680g for 10 minutes at 4°C and then the homogenates underwent protein determinations as previously described (58). Equal protein aliquots were subjected to Western analysis for HSP25 (antibody SPA 801; Stressgen, Victoria, BC, Canada), HSP72 (antibody SPA 810; Stressgen), and Actin (A5441, Sigma-Aldrich) after electrophoresis on 4–20% precast gradient gels using the Mini-Trans-Blot system (Bio-Rad, Hercules, CA, USA). Immunoreactive protein was detected with enhanced chemiluminescence and quantified by means of densitometry (JImage, NIH) as previously described (15). The linearity of measurements within the experimental range was confirmed by serial dilution and subsequent densitometry.
Mononuclear cell isolation from kidney
Decapsulated kidneys were taken from anesthetized animals following sham surgery, or after 45 minutes renal ischemia and 1 or 24 hours reperfusion (as indicated in results), and incubated in RPMI buffer (11875; Invitrogen) containing 15% FBS and 0.18mg/ml of Collagenase D (11088874103; Roche) at 37°C for 1 hour. Background studies were performed examining the effect of perfusion of the kidneys prior to digestion, compared to no perfusion prior to digestion, on the number of mononuclear cells isolated. No difference was found between the two techniques in the number or pattern of mononuclear cells isolated from the kidney, regardless of whether they were perfused (data not shown). Therefore, further mononuclear cell isolations were performed without prior perfusion of the kidneys. Digested samples were strained with 100 µm filters, washed with DMEM (11995; Invitrogen) and centrifuged twice at 670 g for 5 min. at 4°C. The pellets were resuspended in 44% Percoll (P1644; Sigma) mixture and carefully layered onto 67% Percoll. Following centrifugation at 530 G for 36 min. at 4°C, mononuclear cell layer was collected from the Percoll interface, washed in DMEM and centrifuged twice at 670 G for 7 min. at 4°C. The pellets were resuspended in FACS buffer (PBS with 0.5% FBS), counted on a hemocytometer and prepared for flow cytometry.
Flow cytometry
Isolated mononuclear cells were separated into two groups and incubated with anti-CD16/CD32 Fc receptor blocking antibody for 10 minutes at room temperature prior to incubation with anti-mouse anti- CD4 (MCD0428; Invitrogen), CD8 (MCD0830; Invitrogen) for 30 minutes at 4°C. Samples were then washed with FACS buffer, centrifuged at 670 G at 4°C and then either fixed in 1% paraformaldehyde solution and analyzed immediately or processed for intranuclear staining of Foxp3. The samples for intra nuclear staining were fixed with paraformaldehyde for 30 minutes at 4°C, washed with PBS and centrifuged twice at 670 G for 5 minutes at 4°C and again with 0.1% triton X-100 at 2000 rpm for 5minutes twice at 4°C. The pellets were once again blocked with Fc receptor blocking antibody for 10 minutes at room temperature, incubated with anti-mouse anti-Foxp3(11-5773-82; Ebioscience) for 30 minutes at 4°C. Following fixation with 1% paraformaldehyde once again, samples were analyzed immediately using LSR-II flow cytometer and FACS DIVA software. Each assay included a minimum of 10,000 gated events.
Sample size and statistical evaluation
HSP abundance was measured and compared between HSF-KO and WT mice kidney under each experimental condition (n=4 for each condition). Average values were calculated and expressed in terms of change from the respective level in sham operated control. Serum creatinine was measured and compared between HSF-KO and WT mice (n=6 for each condition), and between HSF-KO mice treated with PC61 or vehicle control (n=5 for each condition) following ischemia for 45 minutes and reperfusion for 24 hours, or sham surgery. Similarly number of total leukocytes that were CD4+, CD8+ and Foxp3+ were measured in the sham and injury condition in HSF-KO and WT mice kidneys (n=6 for each experimental condition). The number of Foxp3+ and CD25+ cells isolated from kidneys of HSF-KO mice treated with PC61 and vehicle control, both after sham operation and following renal ischemia, were measured (n=5 for each condition). Values are expressed as a mean ± standard error of the mean (SEM). Comparison between experimental groups was made using Student’s t-test. Values were considered significantly different if p<0.05.
Acknowledgements
This work was supported by an NIH NIDDK Grant K08-DK075470. Additional funding was provided by a P&F Grant P50 DK079306 and funding from the Children’s Research Institute and the Department Pediatrics, Medical College of Wisconsin, Milwaukee, WI. We thank Dr. Ivor Benjamin for kindly providing the HSF-1 functional knockout mouse model used in this study.
Footnotes
Disclosure: Authors declare no competing interests.
References
- 1.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001 Nov;281(5):F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004 Jan;13(1):1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002 Nov;62(5):1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 4.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003 Aug;285(2):F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 5.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004 Aug;66(2):496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006 Jun;17(6):1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 7.Avner ED, Harmon WE, Niaudet P, Yoshikawa N, editors. Pediatric Nephrology. Springer; 2009. [Google Scholar]
- 8.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009 Jan;130(1):41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007 May 1;178(9):5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 10.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. May;77(9):771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009 Oct;76(7):717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 12.Yokota N, Daniels F, Crosson J, Rabb H. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation. 2002 Sep 27;74(6):759–763. doi: 10.1097/00007890-200209270-00005. [DOI] [PubMed] [Google Scholar]
- 13.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant. 2005 Jun;5(6):1186–1193. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 14.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003 Sep 15;171(6):3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 15.Riordan M, Sreedharan R, Wang S, Thulin G, Mann A, Stankewich M, et al. HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol. 2005 Jun;288(6):F1236–F1242. doi: 10.1152/ajprenal.00438.2004. [DOI] [PubMed] [Google Scholar]
- 16.Van Why SK, Mann AS, Ardito T, Thulin G, Ferris S, Macleod MA, et al. Hsp27 associates with actin and limits injury in energy depleted renal epithelia. J Am Soc Nephrol. 2003 Jan;14(1):98–106. doi: 10.1097/01.asn.0000038687.24289.83. [DOI] [PubMed] [Google Scholar]
- 17.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000 Oct;20(19):7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Why SK, Mann AS, Thulin G, Zhu XH, Kashgarian M, Siegel NJ. Activation of heat-shock transcription factor by graded reductions in renal ATP, in vivo, in the rat. J Clin Invest. 1994 Oct;94(4):1518–1523. doi: 10.1172/JCI117492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eickelberg O, Seebach F, Riordan M, Thulin G, Mann A, Reidy KH, et al. Functional activation of heat shock factor and hypoxia-inducible factor in the kidney. J Am Soc Nephrol. 2002 Aug;13(8):2094–2101. doi: 10.1097/01.asn.0000022008.30175.5b. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KJ, Baird NR, Greene AL. Induction of stress response proteins and experimental renal ischemia/reperfusion. Kidney Int. 2001 May;59(5):1798–1802. doi: 10.1046/j.1523-1755.2001.0590051798.x. [DOI] [PubMed] [Google Scholar]
- 21.Aufricht C, Ardito T, Thulin G, Kashgarian M, Siegel NJ, Van Why SK. Heat-shock protein 25 induction and redistribution during actin reorganization after renal ischemia. Am J Physiol. 1998 Jan;274(1 Pt 2):F215–F222. doi: 10.1152/ajprenal.1998.274.1.F215. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Knowlton AA, Christensen TG, Shih T, Borkan SC. Prior heat stress inhibits apoptosis in adenosine triphosphate-depleted renal tubular cells. Kidney Int. 1999 Jun;55(6):2224–2235. doi: 10.1046/j.1523-1755.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Knowlton AA, Li FH, Borkan SC. Hsp72 expression enhances survival in adenosine triphosphate-depleted renal epithelial cells. Cell Stress Chaperones. 2002 Apr;7(2):137–145. doi: 10.1379/1466-1268(2002)007<0137:heesia>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YH, Borkan SC. Prior heat stress enhances survival of renal epithelial cells after ATP depletion. Am J Physiol. 1996 Jun;270(6 Pt 2):F1057–F1065. doi: 10.1152/ajprenal.1996.270.6.F1057. [DOI] [PubMed] [Google Scholar]
- 25.Emami A, Schwartz JH, Borkan SC. Transient ischemia or heat stress induces a cytoprotectant protein in rat kidney. Am J Physiol. 1991 Apr;260(4 Pt 2):F479–F485. doi: 10.1152/ajprenal.1991.260.4.F479. [DOI] [PubMed] [Google Scholar]
- 26.Schober A, Muller E, Thurau K, Beck FX. The response of heat shock proteins 25 and 72 to ischaemia in different kidney zones. Pflugers Arch. 1997 Jul;434(3):292–299. doi: 10.1007/s004240050399. [DOI] [PubMed] [Google Scholar]
- 27.Sreedharan R, Riordan M, Wang S, Thulin G, Kashgarian M, Siegel NJ. Reduced tolerance of immature renal tubules to anoxia by HSF-1 decoy. Am J Physiol Renal Physiol. 2005 Feb;288(2):F322–F326. doi: 10.1152/ajprenal.00307.2004. [DOI] [PubMed] [Google Scholar]
- 28.Fiorenza MT, Farkas T, Dissing M, Kolding D, Zimarino V. Complex expression of murine heat shock transcription factors. Nucleic Acids Res. 1995 Feb 11;23(3):467–474. doi: 10.1093/nar/23.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel NJ, et al. Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res. 2002 Jun;51(6):722–727. doi: 10.1203/00006450-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Ling H, Edelstein C, Gengaro P, Meng X, Lucia S, Knotek M, et al. Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol. 1999 Sep;277(3 Pt 2):F383–F390. doi: 10.1152/ajprenal.1999.277.3.F383. [DOI] [PubMed] [Google Scholar]
- 31.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002 Jan 18;277(3):2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- 32.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999 Nov 1;18(21):5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998 Mar 27;273(13):7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 34.Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002 Oct 1;21(19):5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupik W, Jasik K, Bembenek J, Widlak W. The expression patterns of heat shock genes and proteins and their role during vertebrate's development. Comp Biochem Physiol A Mol Integr Physiol. Aug;159(4):349–366. doi: 10.1016/j.cbpa.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 37.Riordan M, Garg V, Thulin G, Kashgarian M, Siegel NJ. Differential inhibition of HSP72 and HSP25 produces profound impairment of cellular integrity. J Am Soc Nephrol. 2004 Jun;15(6):1557–1566. doi: 10.1097/01.asn.0000127996.42634.2b. [DOI] [PubMed] [Google Scholar]
- 38.Goligorsky MS. Whispers and shouts in the pathogenesis of acute renal ischaemia. Nephrol Dial Transplant. 2005 Feb;20(2):261–266. doi: 10.1093/ndt/gfh182. [DOI] [PubMed] [Google Scholar]
- 39.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007 Jul;322(1):8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 40.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004 Aug;66(2):486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 41.Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, et al. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004 Aug;66(2):491–496. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]
- 42.Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000 Sep;279(3):F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 43.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 2009 Sep;87(9):859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 44.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, et al. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005 May 27;79(10):1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 45.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002 Feb 1;168(3):1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 46.Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008;(183):111–127. doi: 10.1007/978-3-540-72167-3_6. [DOI] [PubMed] [Google Scholar]
- 47.Osterloh A, Geisinger F, Piedavent M, Fleischer B, Brattig N, Breloer M. Heat shock protein 60 (HSP60) stimulates neutrophil effector functions. J Leukoc Biol. 2009 Aug;86(2):423–434. doi: 10.1189/jlb.0109011. [DOI] [PubMed] [Google Scholar]
- 48.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009 Jun;85(6):905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 49.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009 Aug;20(8):1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007 Jun;71(12):1223–1231. doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 51.Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, et al. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008 Aug;39(2):235–242. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inouye S, Izu H, Takaki E, Suzuki H, Shirai M, Yokota Y, et al. Impaired IgG production in mice deficient for heat shock transcription factor 1. J Biol Chem. 2004 Sep 10;279(37):38701–37709. doi: 10.1074/jbc.M405986200. [DOI] [PubMed] [Google Scholar]
- 53.Chen SW, Kim M, Song JH, Park SW, Wells D, Brown K, et al. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 2009 Mar;75(5):499–510. doi: 10.1038/ki.2008.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim M, Park SW, Chen SW, Gerthoffer WT, D'Agati VD, Lee HT. Selective renal overexpression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. Aug;299(2):F347–F358. doi: 10.1152/ajprenal.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007 Mar 1;178(5):2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 56.Basile DP, Donohoe D, Cao X, Van Why SK. Resistance to ischemic acute renal failure in the Brown Norway rat: a new model to study cytoprotection. Kidney Int. 2004 Jun;65(6):2201–2211. doi: 10.1111/j.1523-1755.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 57.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996 Feb 15;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sreedharan R, Riordan M, Thullin G, Van Why S, Siegel NJ, Kashgarian M. The maximal cytoprotective function of the heat shock protein 27 is dependent on heat shock protein 70. Biochim Biophys Acta. Jan;1813(1):129–135. doi: 10.1016/j.bbamcr.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]