Abstract
Background
Chronic gastrointestinal dysmotility greatly impacts the quality of life. Treatment options are limited and generally symptomatic. Neural autoimmunity is an under-recognized etiology. We evaluated immunotherapy as an aid to diagnosing autoimmune gastrointestinal dysmotility (AGID).
Methods
23 subjects evaluated at the Mayo Clinic for suspected AGID (August 2006-February 2014) fulfilled the following criteria: 1) prominent symptoms of gastrointestinal dysmotility with abnormalities on scintigraphy-manometry; 2) serological evidence or personal/family history of autoimmune disease; 3) treated by immunotherapy on a trial basis, 6-12 weeks (intravenous immune globulin, 16; or methylprednisolone, 5; or both, 2). Response was defined subjectively (symptomatic improvement) and objectively (gastrointestinal scintigraphy/manometry studies).
Key Results
Symptoms at presentation: constipation, 18/23; nausea or vomiting, 18/23; weight loss, 17/23; bloating, 13/23; and early satiety, 4/23. Thirteen patients had personal/family history of autoimmunity. Sixteen had neural autoantibodies and 19 had extra-intestinal autonomic testing abnormalities. Cancer was detected in 3 patients. Pre-immunotherapy scintigraphy revealed slowed transit (19/21 evaluated; gastric, 11; small-bowel, 12; colonic, 11); manometry studies were abnormal in 7/8. Post-immunotherapy, 17 (74%) had improvement (both symptomatic and scintigraphic, 5; symptomatic alone, 8; scintigraphic alone, 4). Nine responders reevaluated had scintigraphic evidence of improvement. The majority of responders who were re-evaluated had improvement in autonomic testing (6 of 7) or manometry (2 of 2).
Conclusions & Inferences
This proof of principle study illustrates the importance of considering an autoimmune basis for idiopathic gastrointestinal dysmotility and supports the utility of a diagnostic trial of immunotherapy.
Keywords: scintigraphy, transit study, autonomic nervous system, autonomic neuropathy, celiac disease, thyroid disease, immunoglobulin
Introduction
Autoimmune gastrointestinal dysmotility (AGID) is a newly recognized clinical entity that is an organ manifestation of autoimmune dysautonomia,(1) and can occur as an idiopathic(2) or paraneoplastic phenomenon.(3) Gastrointestinal hypomotility or hypermotility may present in the setting of generalized dysautonomia,(4) or as a component of a multifocal paraneoplastic autoimmune neurological disorder.(5) The presentation may be gastroparesis, colonic inertia, or intestinal pseudoobstruction (1); rare cases have presented with pyloric obstruction or anal spasm.(5) Symptoms include early satiety, nausea, vomiting, bloating, diarrhea, constipation and involuntary weight loss. The onset may be subacute or insidious, and subtle or overt neurological manifestations may be an accompaniment.
The first IgG biomarker recognized for AGID was the type 1 anti-neuronal nuclear autoantibody (ANNA-1, also called “anti-Hu”).(3) ANNA-1 seropositivity predicts underlying small-cell carcinoma (rarely thymoma) or pediatric neuroblastoma(6); the associated AGID usually responds poorly to therapy.(3) AGID accompanied by autoantibodies targeting ganglionic nicotinic acetylcholine receptors (AChR) containing α3 subunits generally responds favorably to antibody-depleting therapies. Ganglionic AChR antibodies are proven to cause AGID in active and passive immunization animal models.(7-9) Several other neural autoantibody specificities aid the diagnosis of AGID: voltage-gated neuronal potassium channel-complex (VGKC)(1, 10)and calcium-channel antibodies (N-type > P/Q-type), muscle AChR, striational, glutamic acid decarboxylase 65 (GAD65) and peripherin.(1, 11) Gastrointestinal dysmotility is a prominent symptom in 31% of peripherin-IgG seropositive patients.(11)
Symptomatic therapies that are useful in management of AGID include anti-emetics, prokinetic agents (e.g., erythromycin) and cholinesterase inhibitors.(2) Although symptomatic improvement has been recorded following immunotherapy,(1) objective evidence of response has not been documented to date. A trial of immunotherapy has been suggested as a diagnostic tool for patients suspected to have autoimmune neurological disorders,(12) and intravenous methylprednisolone (IVMP) and high dose immune globulin (IVIg) are considered safe and appropriate therapies. However, randomized controlled trials have been conducted only for two immune-mediated neurological diseases (chronic inflammatory demyelinating polyneuropathy and myasthenia gravis).(13, 14) Lacking guidelines, the choice of agent, duration of treatment and indications for changing to a second agent vary with individual practitioners. In this study we evaluated the utility of a diagnostic trial of immunotherapy in patients with suspected AGID. Scintigraphic gastrointestinal transit studies and manometry performed before and after the immunotherapy trial provided objective evidence of efficacy.
Methods
Study Design
The study was a retrospective case series of patients identified through medical chart review.
Study Subjects
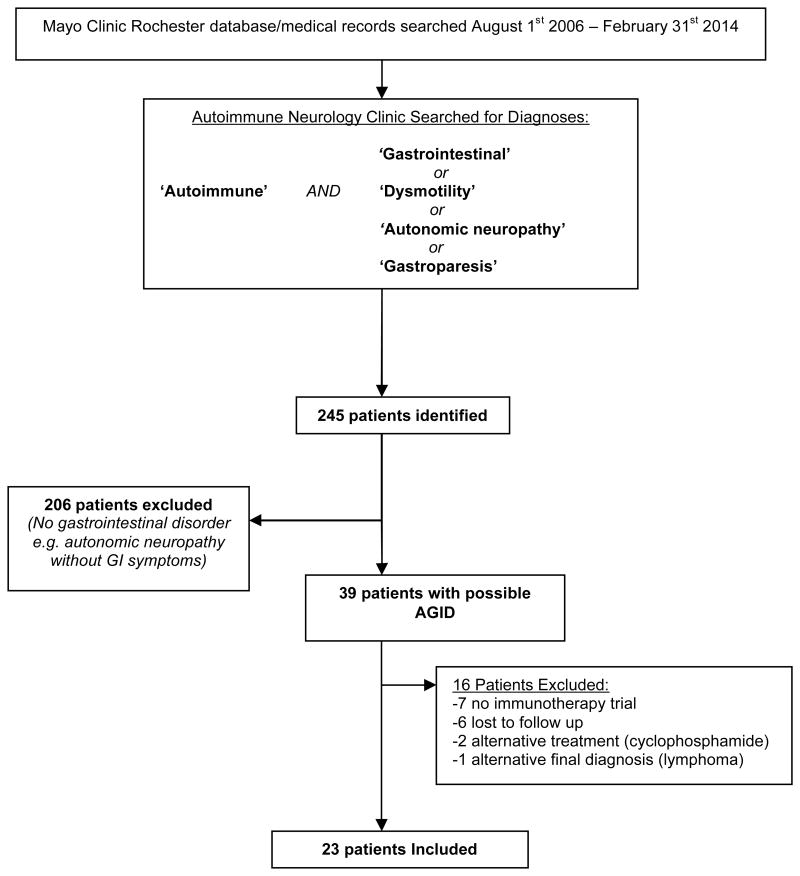
With the goal of better defining the clinical characteristics and response to treatment of AGID patients presenting to Mayo Clinic, Rochester, MN we established a multidisciplinary Autoimmune Gastrointestinal Dysmotility Study Group consisting of physicians from the Department of Neurology (Autoimmune Neurology Section) and the Division of Gastroenterology and Hepatology (Gastrointestinal Motility Section). Twenty three patients of 39 evaluated consecutively (median of 5 patients per year; range, 1-7) for AGID by members of this AGID study group at the Mayo Clinic (August 2006-February 2014) fulfilled inclusion criteria for this study (Figure 1):
Figure 1.
Patient Ascertainment.
Abbreviations: AGID, autoimmune gastrointestinal dysmotility; GI, gastrointestinal.
Prominent symptoms of gastrointestinal dysmotility with confirmatory baseline scintigraphic evidence of gastrointestinal hypomotility, 19 (i.e., slow gastric emptying, delayed transit through small intestine or large intestine), abnormal manometry findings, 3 (gastroduodenal or colonic; consistent with a neuropathy) or symptoms of severe gastrointestinal dysmotility with evidence of pan-dysautonomia (all divisions of the autonomic system affected), 1.
An autoimmune etiology suspected (Table 1) based on clinical suspicion (subacute onset), seropositivity for a neural autoantibody (71%), or personal/family history of autoimmune disease (e.g., thyroiditis, systemic lupus).
Treated by immunotherapy (without changes in other medications) for 4-12 weeks on a trial basis (intravenous high dose immune globulin or methylprednisolone).
Table 1. Clinical features that raise suspicion for AGID.
|
Abbreviations: AGID, autoimmune gastrointestinal dysmotility.
type 1 anti-neuronal nuclear autoantibody (ANNA-1, also called “anti-Hu”)(3), ganglionic nicotinic acetylcholine receptors (AChR) containing α3 subunits(7-9), voltage-gated neuronal potassium channel-complex (VGKC);(1, 10) calcium-channel antibodies (N-type > P/Q-type), muscle AChR, striational, glutamic acid decarboxylase 65 (GAD65) and peripherin.(1, 11)
As immunotherapy is not without risk we generally reserve a trial for patients with severe symptoms refractory to pro-motility agents
Sixteen patients were excluded due to: no immunotherapy trial (mild symptoms or contraindications to treatment), 7; lost to follow up (treatment recommended locally but subsequently did not follow up at our facility), 6; alternative treatment regimen, 2 (both cyclophosphamide) or alternative final diagnosis, 1 (gut lymphoma). Two patients included in this study have been reported previously.(1)
Gastrointestinal Transit Testing
We used a previously described gastrointestinal scintigraphic evaluation.(15) The patient ingests two radioactive tracers: 1) 99mTc mixed in a scrambled egg meal to assess gastric emptying and small bowel transit and 2) a pellet containing 111Indium Cl3 formulated for release in the terminal ileum or proximal ascending colon to measure colonic motility. The progress of both tracers is documented by gamma camera. Gastric emptying rate is calculated as percentage emptied from the stomach at 1, 2 and 4 hours. Small bowel transit is calculated as percentage passed from the small intestine at 6 hours. Colonic motility is assessed at 24 and 48 hours after ingestion, in five regions of interest: 1) ascending colon, 2) transverse colon, 3) descending colon, 4) sigmoid colon/rectum and 5) stool. The estimated geometric center is the average distance traveled by the ingested tracer. Hence, at 24 hours a geometric center of 1 would indicate most of the tracer was in the proximal ascending colon, while a geometric center of 4 would indicate that most had passed to the distal colon. Reference values for each of these indices have been calculated in healthy subjects.(15)
Gastroduodenal manometry study
A guidewire is endoscopically placed beyond the ligament of Treitz, and a manometric catheter is advanced over the guidewire so that pressure sensors are located in the distal antrum, pylorus, and the proximal, mid and distal portions of the duodenum. Recordings are obtained during a 3 hour fasting observation period, followed by a test meal of 500 kcal and 2 hours of postprandial recording. Findings consistent with neuropathy include: antral hypomotility, abnormal frequency or morphology of the phase III activity front, and uncoordinated or irregular bursts of contractility.(16)
Colonic Manometry
The colon is cleansed by ingestion of polyethylene glycol-electrolyte solution after overnight fasting. A manometric-barostat assembly is positioned in the left colon. Compliance and both tonic and phasic contractile responses to a meal and to neostigmine are assessed. Disorganized contractions suggest a neuropathic abnormality.(17)
Autonomic Testing
This involved either an autonomic reflex screen (tests of sudomotor, cardio-vagal and adrenergic functions), a thermoregulatory sweat test or both.(18)
Neural autoantibody testing
All patients underwent a comprehensive neural autoantibody evaluation. This included evaluation for IgG specific for neuronal nuclear antigens (ANNA-1[anti-Hu], 2[anti-Ri] and 3) and neuronal cytoplasmic antigens (amphiphysin-IgG, purkinje cell antibody type 1[anti-Yo], 2 and Tr, collapsin response mediator protein 5 IgG[CRMP-5] and peripherin-IgG).(6, 11, 19, 20) We also assayed antibodies cation channels (neuronal voltage gated calcium channels P/Q and N type, voltage gated potassium channels [α-dendrotoxin-sensitive] and nicotinic acetylcholine receptors extracted from ganglionic neurons and muscle) muscle striational antigens and recombinant glutamic acid decarboxylase antibodies.(6, 21-23) The methods for neural autoantibody testing have been described previously.(6)
Immunotherapies utilized
All 23 study patients completed a 6-12 week therapeutic trial. Fifteen received intravenous immune globulin (0.4 g/Kg), and five received intravenous methylprednisolone (1000 mg daily for 3 days, then once weekly). Two patients received intravenous methylprednisolone for 5 days followed by intravenous immune globulin. One patient developed aseptic meningitis after receiving intravenous immune globulin for three days and subsequently received oral prednisone (60 mg per day) followed by oral mycophenolate for 12 weeks.
Definition of response
Response was defined subjectively by improvement in gastrointestinal symptoms and objectively by improvements in gastrointestinal scintigraphy/manometry studies.
Results
Standard Protocol Approvals, Registrations and Patient Consents
The study was approved by the Mayo Clinic Institutional Review Board (#08-006647). All patients consented to the use of their medical record for research purposes consistent with Minnesota statute 144.335.
Baseline characteristics and demographics
Twenty-one of the 23 patients (91%) were female; 22 patients were Caucasian (96%) and one was Hispanic (4%). The median age at time of treatment was 38 years (range, 16-76). The patients were designated responders (n=17) or non-responders (n=6) based on improvement (subjective or objective) after the trial of immunotherapy. No patients were on maintenance opioid medications at the time of gastrointestinal motility testing and we generally request patients on as needed short acting opioid medications (two patients in this study) to refrain from using them within 48 hours of gastrointestinal motility testing.
Responders (n=17)
Clinical Features
Table 2 summarizes clinical characteristics of the 17 responders. Symptom onset was subacute (<8 weeks) in 9 and insidious (>8 weeks) in 8. The median delay to a diagnostic treatment trial was 19 months (range, 4-123 months). Fifteen of the 17 responders reported constipation. Additionally, 13 nausea/vomiting, 12 had severe weight loss, 9 bloating, 7 abdominal pain, 5 diarrhea, 4 early satiety, and 3 dysphagia. Extra-intestinal autonomic symptoms included: orthostasis, 10 (accompanied by syncope in 5); visual accommodation problems, 4; sensory complaints, 3; dry mouth, 2; sweating problems, 2; and urinary difficulties, 2. Autonomic dysfunction was broadly classified as: pandysautonomia (all divisions of the autonomic nervous system affected), 4; multifocal dysautonomia (multiple divisions of the autonomic system affected), 7; limited dysautonomia (autonomic dysfunction restricted to the gastrointestinal system), 6. Ten patients (59%) had a co-existing autoimmune disorder or family history of autoimmunity in a first degree relative (Table 2).
Table 2a. Clinical Characteristics of 17 patients with immunotherapy-responsive gastrointestinal dysmotility.
| Patient | Age/Sex | Gastrointestinal symptoms | Personal (or family) Hx of autoimmunity |
Feeding Route† |
Dysautonomia type |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI† | Weight loss (Kg) |
Dysphagia | Pain | Early satiety | Nausea/ vomiting |
Bloating | Diarrhea | Constipation | |||||
| 1 | 65/F | 20.1 | ✓ | ✓ | Graves' disease | Oral | Diffuse | ||||||
| 2 | 49/F | 26.12 | ✓ | Celiac disease | Oral | Multifocal | |||||||
| 3 | 57/F | 21.2 | ✓ ( 9.1) | ✓ | ✓ | ✓ | ✓ | ✓ | Hashimoto thyroiditis | Oral | Limited | ||
| 4 | 19/F | 17.4 | ✓ (12.7) | ✓ | ✓ | ✓ | ✓ | ✓ | Celiac disease | Enteral | Multifocal | ||
| 5* | 76/M | 17.7 | ✓ (22.7) | ✓ | ✓ | ✓ | ✓ | - | Oral | Diffuse | |||
| 6 | 71/F | 20.6‡ | ✓ (20.5) | ✓ | ✓ | - | Oral | Diffuse | |||||
| 7 | 36/F | 24.3 | ✓ | ✓ | ✓ | ✓ | ✓ | - | Oral | Limited | |||
| 8 | 26/F | 16.2 | ✓ (13.6) | ✓ | ✓ | ✓ | (Maternal hypothyroidism) | Oral | Limited | ||||
| 9* | 22/F | 18.3 | ✓ (22.7) | ✓ | ✓ | ✓ | ✓ | - | Parenteral | Limited | |||
| 10 | 30/F | 17.4 | ✓ (5) | ✓ | ✓ | ✓ | ✓ | - | Enteral | Multifocal | |||
| 11 | 34/F | 19.9 | ✓ (6) | ✓ | ✓ | ✓ | - | Oral | Multifocal | ||||
| 12 | 44/F | - | ✓ | ✓ | ✓ | (Maternal diabetes) | Oral | Diffuse | |||||
| 13 | 43/F | 15.8 | ✓ (21.4) | ✓ | ✓ | ✓ | ✓ | Lupus | Enteral | Multifocal | |||
| 14 | 34/F | 23.5 | ✓ (6.8) | ✓ | ✓ | Lupus, alopecia areata | Oral | Multifocal | |||||
| 15 | 16/F | 16.7 | ✓ (4.1) | ✓ | - | Enteral | Limited | ||||||
| 16 | 38/F | 18.3 | ✓ (18.2) | ✓ | ✓ | ✓ | ✓ | (crohn's disease, lupus) | Oral | Multifocal | |||
| 17 | 33/F | 33.1 | ✓ | ✓ | ✓ | ✓ | Type 1 diabetes, Hypothyroid | Parenteral | Limited | ||||
Laboratory Investigations
Comprehensive autoimmune serological evaluation revealed a neural-specific autoantibody in 12 of 17 responders (71%). The detected autoantibody specificities included: ganglionic AChR, 6 (median value 0.17 nmol/L, range 0.06 to 5.89 nmol/L [normal range, 0.00 to 0.02 nmol/L]);(4, 7) ANNA-1, 3 (end-point dilutions 1:3840, 1:15360 and 1:61440 [negative is <1:240]);(5) VGKC-complex, 3 (median value 0.15 nmol/L range, 0.10-0.19 nmol/L [negative is 0.00-0.02 nmol/L]);(24) striated muscle, 3 (end-point dilutions, 1:240, 1:240 and 1:960 [negative <1:60]);(25) peripherin, 2 (end-point dilutions, 1:960 and 1:1920 [negative, <1:60]);(11) voltage gated calcium channel N type, 1 (0.07 nmol/L [negative is 0.00-0.02 nmol/L]) and GAD65, 1 (0.17 nmol/L [normal range, 0.00-0.02 nmol/L]).(25) Seven of sixteen patients tested (44%) were seropositive for anti-nuclear antibody (median, 2.4 units; range 1.2-9.1 [normal, <1 unit]). Double stranded DNA antibody was detected in one of the two patients who had systemic lupus erythematosus. Two of 10 patients tested had one or more antibodies to extractable nuclear antigens (SSA, 2; Sm, 1; and ribonucleoprotein, 1). Miscellaneous laboratory testing included elevated sedimentation rate (2 of 14 tested [34 and 50 mm/hour; normal, <29]) and mildly elevated C-reactive protein (1 of 12 tested; value 1.0 mg/dL [normal range, <0.8mg/dL]). Thyroid stimulating hormone (TSH) level was normal in 15 of 15 tested. One patient with co-existing type 1 diabetes had a hemoglobin A1C of 12.7% (normal, <6.5%). Of the remaining patients, fasting glucose was normal in 14 of 15 tested (one patient had a borderline fasting glucose of 126 mg/dL [normal, <126 mg/dL] but a normal hemoglobin A1C of 5.4% [normal range, <6.5%] and was diagnosed with borderline glucose intolerance). No monoclonal immunoglobulin was detected (9 patients tested).
Full Thickness Biopsy Results (n=2)
Small bowel full thickness biopsy in one (patient #17, table 2) in whom no informative neural autoantibodies were found revealed myenteric ganglionitis with CD3+ and CD8+ intraganglional and periganglional lymphocytes. Immunostain for CD117 showed enlarged and hypertrophic interstitial cells of Cajal around the myenteric ganglion. A full thickness biopsy of transverse colon from an ANNA-1 seropositive patient (#9, table 2) who had paraneoplastic AGID revealed segmental ischemic necrosis.
Extra-intestinal Autonomic Testing
Autonomic function testing(18) was abnormal in 14 of 16 (88%) patients who were tested. Thermoregulatory sweat test revealed abnormalities consistent with a small fiber neuropathy in 9 of 12 (75%): reduced sweating in the toes, 4; patchy multifocal reduction in sweating, 3; diffusely reduced sweating, 1; and decreased sweating in both lower extremities, 1. The autonomic reflex screen was abnormal in 12 of 16 patients (75%), with severe abnormalities, 10 (sudomotor, 10; cardiovagal, 9; and adrenergic, 6) and mild abnormalities limited to orthostatic tachycardia, 2.
Cancer Screening and Radiological Findings
Three patients were seropositive for ANNA-1 and had paraneoplastic AGID. An informative neural autoantibody profile led to the search for and finding of cancer in all 3 cases: small-cell lung carcinoma, 1; thymoma, 1; and in situ carcinoma of uterine cervix, 1. Cancer screening, performed in 9 of the remaining 14 patients, was negative (whole body positron emission tomography-computed tomography [PET-CT] or CT). X-rays or computed tomography of the abdomen revealed dilated small bowel loops and/or air fluid levels in 4 of 16 (25%) with radiological reports available.
Scintigraphy and Manometry Studies
Pre-immunotherapy scintigraphy revealed abnormalities in 13 of 15 patients tested (Table 3). Transit was decreased in the following regions: stomach, 7; small bowel, 9 and colon, 8. Gastroduodenal and/or colonic manometry findings were compatible with neuropathy in both of the patients who had normal scintigraphy studies. One of two patients in whom scintigraphy was not performed had evidence of hypomotility by gastroduodenal manometry study; the other had symptoms typical of gastrointestinal hypomotility plus a high serum level of ganglionic AChR antibody (5.36 nmol/L) and evidence of pandysautonomia on extra-intestinal autonomic testing (patient #12, Table 2).
Table 3. Scintigraphy results of 9 patients tested before and after immunotherapy.
| Patient | % emptied from stomach at 4 hrs (NR: 84-98%) | 6 hr small bowel transit (NR: 46-98%) | Geometric center of colonic activity at 24 hrs (NR: 1.6-3.8) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Before | After | Change | Before | After | Change | Before | After | Change | |
|
| |||||||||
| 1* | 77% | 83% | +6% | 90% | 90% | 0% | 0.9 | 2.5 | +1.6 |
| 2* | 94% | 95% | +1% | 1% | 70% | +69% | 1.9 | 1.9 | 0 |
| 3* | 98% | 100% | +2% | 87% | 56% | -31% | 1.5 | 3.2 | +1.7 |
| 4* | 70% | 71% | +1% | 3% | 71% | +68% | 1.1 | 2.1 | +1.0 |
| 6* | 97% | 95% | -2% | 0% | 27% | +27% | 1.1 | 1.6 | +0.5 |
| 7* | 58% | 86% | +28% | 41% | 59% | +18% | 2 | - | - |
| 14* | 28% | 52% | +24% | 40% | - | - | 1.9 | - | - |
| 16* | 53% | 81% | +28% | 31% | 38% | +7% | 2 | - | - |
| 17* | 56% | 61% | +5% | 17% | 7% | -10% | 0.8 | 3.5 | +2.8 |
Abbreviations: NR, normal range.
Patient numbers from Table
Manometry studies revealed abnormalities in at least one region of the gastrointestinal tract in 6 of the 7 responders who were tested. Gastroduodenal manometry revealed abnormalities in 4 of 6. Findings in 3 were compatible with neuropathy: poor conversion to the fed pattern with premature return of the interdigestive migrating motor complex, 1; non-propagated clusters of contractions during fasting and marked antral hypomotility (absence of conversion to the fed pattern), 1; abnormal phase III migrating motor complex-like activity during fasting and failure to convert to the fed pattern following a meal, 1. The fourth patient had common cavity waves suggestive of obstruction. The clinical impression of small bowel obstruction was thought to be secondary to AGID. Colonic manometry, performed in two patients, revealed poor tonic responses to a meal or neostigmine, consistent with colonic motor dysfunction.
Immunotherapy Responses
Following the immunotherapy trial, 13 patients improved symptomatically and four patients had evidence of subclinical improvement (scintigraphy evidence without symptomatic improvement). Examples of symptomatic improvement are outlined in Table 2. Nine of 10 patients with follow-up scintigraphy had evidence of improvement following the immunotherapy trial; three completely normalized (Table 3). Examples of scintigraphic improvements are illustrated in Figure 2. The single patient with no change on their scintigraphy had sufficient clinical improvement to remove their nasogastric tube. Colonic manometry, repeated in one patient, revealed improved motor function. Repeated gastroduodenal manometry in a patient with previously documented neuropathic abnormality, was normal after immunotherapy. Post-immunotherapy autonomic testing demonstrated improvements in 6 of 7 patients who were retested: autonomic reflex screen, 6; thermoregulatory sweat test, 2 (Figure 3). Repeat neural autoantibody titer in the 10 patients retested after immunotherapy was: decreased in 8; and increased in 2 (Table 2).
Figure 2.
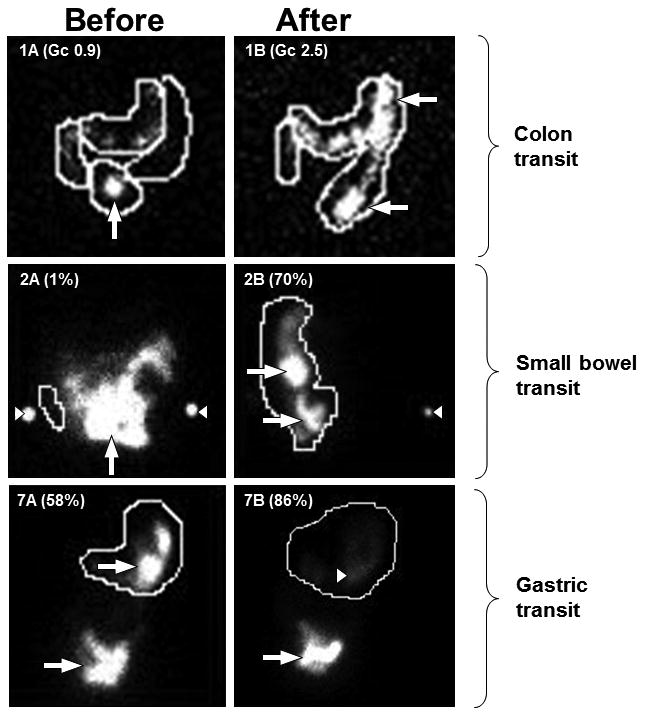
Gastrointestinal scintigraphy studies in three patients before and after a 12-week trial of immunotherapy.
Anterior views of patients #1, #2 and #7 (Table 2 and 3) before (A) and after immunotherapy (B). Patient #1: initial colonic dysmotility demonstrated by retention of radioactivity in the small bowel at 24 hours (1A: white arrow). After high dose intravenous immune globulin therapy, colonic motility was normal; most radioactivity was in the distal colon at 24 hours (1B: white arrows).
Patient #2: radioactivity remaining in the small bowel at 6 hours is consistent with small bowel hypomotility (2A: white standard-arrow). White arrowheads indicate radiolabeled-markers at iliac crests for anatomical localization. After intravenous methylprednisolone therapy, abundant radiolabeled-material in the ascending colon at 6 hours indicates normalization of motility (2B: white standard arrows).
Patient #7: the abundant radioactivity in the stomach at 4 hours (7A: white arrow), indicates gastric hypomotility. After intravenous methylprednisolone therapy, the majority of radioactivity has left the stomach at 4 hours (7B: white standard arrow);the slight blush of radioactive material remaining (white arrowhead) is consistent with normal gastric transit.
Figure 3.
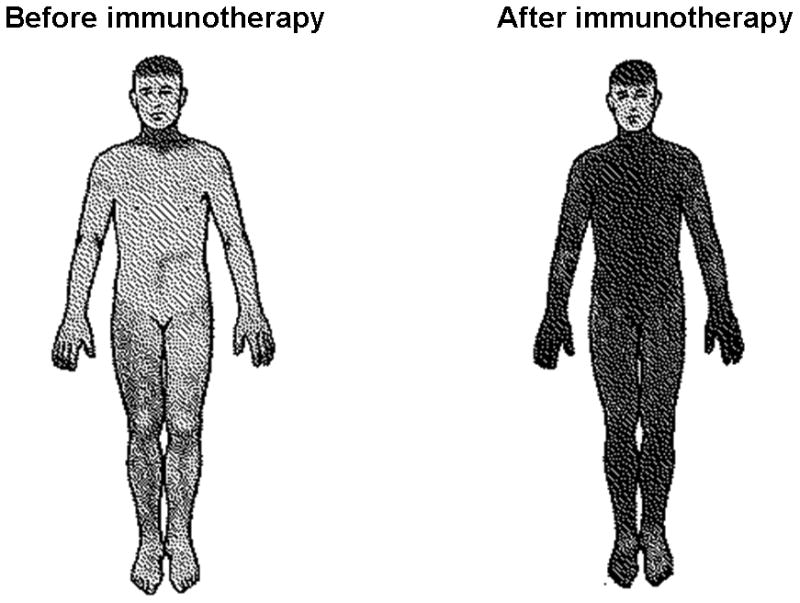
Thermoregulatory sweat-test before and after immunotherapy.
Shaded regions indicate sweating. Heat-induced sweating before immunotherapy is globally reduced, and is normalized after treatment with intravenous immune globulin (patient #1, Table 2 and 3).
Non Responders
Six patients did not improve (subjectively or objectively) after the immunotherapy trial. The responders and non-responders are compared in Table 4. Symptoms were predominantly those of gastroparesis in three, constipation in one and both in two. Neural-specific autoantibodies were detected in five patients (ANNA-1, 1; gAChR, 1; GAD65 antibody, 1; N type calcium channel, 1; striated muscle antibody, 1; and VGKC-complex-IgG, 1). The single patient without neural autoantibodies detected, underwent a trial of immunotherapy for suspected AGID due to co-existing inflammatory arthritis (seronegative but suspected to be autoimmune) in addition to autonomic testing revealing evidence of an extra-intestinal autonomic neuropathy. Body PET did not reveal any cancer in 5 of 5 tested. Scintigraphy revealed abnormal gastric emptying in four, slowed small bowel transit in 3 and slowed colonic transit in 3. Colonic manometry revealed motor dysfunction in the single patient tested. No patients reported symptomatic improvement after immunotherapy (intravenous immune globulin, 5; intravenous methylprednisolone and immune globulin, 1). In the four patients retested by scintigraphy after treatment, no improvement was seen. Neural autoantibodies levels returned to normal after immunotherapy in the patient with ANNA-1, despite the lack of clinical response but were not retested in the other 5 non-responders.
Table 4. Comparison of immunotherapy responders and non-responders.
| Responders (n=17) | Non-responders (n=6) | p value | |
|---|---|---|---|
| Demographics | |||
| Median age (range) at diagnosis | 36 yrs (16-76) | 53.5 yrs (20-72) | 0.22 |
| Clinical Features | |||
| Subacute onset | 9 (53%) | 1 (17%) | 0.18 |
| Personal/family Hx autoimmunity | 10 (59%) | 2 (33%) | 0.34 |
| Paraneoplastic AGID | 3 (18%) | 0 (0%) | 0.54 |
| Laboratory abnormalities | |||
| Antinuclear antibody | 7 (41%) | 3 (50%) | 1.0 |
| Neural specific autoantibody | 12 (71%) | 4 (67%) | 1.0 |
| Extra intestinal autonomic testing | |||
| Abnormalities | 14 of 16 (88%) | 5 of 5 (100%) | 1.0 |
| Post immunotherapy improvement | 6 of 7 (86%) | 1 of 3 (33%) | 0.18 |
| Immunotherapy Treatment | |||
| Median time from onset to immunotherapy | 19 months (4-123) | 71 months (5-201) | 0.13 |
| IVIg (in those with single agent utilized) | 10 of 15 (67%) | 5 of 5 (100%) | 0.27 |
Abbreviations: AGID, autoimmune gastrointestinal dysmotility; Hx, history; IVIg, intravenous immune globulin; yrs, years.
Follow-up of Responders
Immunotherapy was continued beyond the therapeutic trial period in 11 responders: intravenous immune globulin, 7; mycophenolate, 4; oral azathioprine, 4; corticosteroids, 2; and cyclophosphamide, 1. The median follow‐up period after the first visit to our institution was 9 months (range, 1-127).
Discussion
Whilst autoimmunity targeting the autonomic nervous system has been well accepted, particularly as a paraneoplastic phenomenon, documentation of autoimmunity as a basis for GI dysmotility is much more recent. Though this study is limited by its retrospective design, the seventeen responders described in this study suggests that immunotherapy may reverse autoimmune gastrointestinal dysmotility and illustrates the practical importance of considering an autoimmune basis for acquired idiopathic gastrointestinal motility disorders. Symptomatic improvements were generally accompanied by objective evidence of improved gastrointestinal motility and autonomic function on repeated scintigraphic, manometric and autonomic function tests. These supportive tests offer useful surrogate markers of improvement for future randomized controlled clinical trials.
Objective testing with GI transit studies and/or manometry prior to immunotherapy is important to both confirm the presence of dysmotility (avoiding over-treatment of patients without objective abnormalities) and to serve as a measure from which a treatment response can be judged. It is important to discontinue medications that could affect GI transit (e.g. opiates) prior to objective testing. Clues to the diagnosis of AGID that emerged from this study included: detection of one or more neural-specific autoantibodies in 71% of responders; evidence of limited, multifocal or diffuse dysautonomia clinically and on objective autonomic function tests; personal or family history of extra-intestinal autoimmune disorders or risk factors for cancer (past history or family history, or tobacco use). Full thickness biopsies in two immunotherapy responders showed segmental ischemic necrosis in one patient with ANNA-1 associated AGID and lymphocytic myenteric ganglionitis with surrounding hypertrophic interstitial cells of Cajal in a second patient in whom neural autoantibodies were not detected. The inclusion of full thickness gastric or intestinal biopsies has been reported informative for T cell-mediated pathology in ANNA-1 seropositive cases: inflammatory plexitis, neuronal loss(3), and abnormalities of the interstitial cells of Cajal(26). The immunoreactive ganglionic AChR content of the enteric nervous system is greatly reduced in animals with AGID induced experimentally by α3 AChR-IgG, (7, 9, 27) but inflammation has not been seen.
While a thorough evaluation for potential etiologies is important in patients with gastrointestinal dysmotility it should be recognized that autoimmune disorders tend to coexist and thus AGID may exist side by side with other autoimmune disorders which cause dysmotility by alternative mechanisms (e.g. thyroid autoimmunity and diabetes [one patient in our study]). This is illustrated by 9% of patients with the pathogenic and potentially immunotherapy responsive nicotinic ganglionic acetylcholine receptor autoantibody associated autonomic neuropathy having co-existing diabetes.(4) Therefore, in such patients who also have clinical features suggestive of AGID evaluating for neural specific autoantibodies and consideration of a trial of immunotherapy is still appropriate as they may benefit from immunotherapy.
The value of neural-specific IgGs is under-appreciated as an aid to diagnosing autoimmune gastrointestinal dysmotilities, especially in cases of gastroparesis or colonic inertia. However, seronegativity does not exclude the diagnosis of a potentially immunotherapy-responsive gastrointestinal dysmotility, as evidenced by 5 of our 17 responders. Additional autoantibody markers clearly remain to be discovered. A recently reported example is an IgG described in patients with profound diarrhea as prodrome of autoimmune encephalitis. This autoantibody is reactive with the DPPX subunit of the Kv4.2 potassium channel-complex expressed in myenteric plexus).(28) It is conceivable that informative companion autoantibodies may not be detected in cases of AGID mediated by peptide antigen-specific effector T cells (e.g., some paraneoplastic cases). On the other hand, a positive neural-specific autoantibody test may lead to the diagnosis of cancer. The three paraneoplastic cases in our study had an informative autoantibody (ANNA-1), and the predicted small-cell carcinoma was subsequently found in all 3 (bronchial, 2; extrapulmonary [uterine cervix], 1). When suspicion of autoimmunity is high in seronegative cases, the diagnosis of AGID is aided by objective improvement following a trial of immunotherapy (as recommended for patients with suspected autoimmune dementia)(12) in which case consideration of long term maintenance immunotherapy is justified. The small number of patients (particularly in the non-responder group) limited the power to detect any significant difference between the responder and non-responder group. However, there was a trend towards a greater delay from symptom onset to treatment in non-responder patients when compared to responders (71 vs 19 months) and a more insidious (rather than subacute) onset in non-responders which may have contributed to the lack of response. Delay in initiating treatment is associated with lack of immunotherapy response in other autoimmune neurological disorders.(12)
Given the heterogeneous nature of the patients in our study it is essential to exclude other causes prior to considering a trial of immunotherapy. Not all cases of idiopathic gastrointestinal dysmotility should be considered to have AGID, but rather those with at risk (table 1) could be considered for an immunotherapy trial; failure of the trial of immunotherapy is a red flag and we generally do not recommend long-term immunotherapy in these cases. Furthermore, we generally reserve an immunotherapy trial for those who have severe symptoms refractory to symptomatic treatments (7 patients were excluded due to mild symptoms), as immunotherapy is not without risk, particularly in the long-term. As transit study abnormalities and some limited dysautonomia may occur in other diseases (e.g. irritable bowel syndrome) caution is needed when selecting patients for an immunotherapy trial particularly in patients without a neural autoantibody detected (or lacking other features outlined in Table 1). However, the majority of patients in this study had features of small fiber neuropathy on extra-intestinal autonomic testing (e.g. Figure 3) which would not be expected with irritable bowel syndrome or other functional gastrointestinal disorders. Furthermore, these other diseases would not be expected to have clinical or transit study improvements after immunotherapy. Until a randomized controlled immunotherapy trial is done for patients with AGID, evidence of efficacy relies on case reports.(1) Experience from autoimmune paraneoplastic disorders affecting the central and peripheral nervous systems predicts that cancer treatment is of highest importance in managing paraneoplastic AGID.(29) Intravenous immune globulin and glucocorticoids are reported beneficial therapies for AGID, with and without cancer.(1)
The lack of a placebo-treated group and small numbers makes it difficult to determine the natural course of AGID and limited the assessment of statistically significance in this study. Our study was also limited by short follow-up. Our selection of immunotherapy modality was based on patient preference, insurance coverage, neural autoantibody type and the diagnosis of an underlying cancer. Steroid-induced improvement in sense of well-being and placebo-response are potentially confounding factors when symptoms of AGID and dysautonomia are mainly subjective. Although we cannot exclude the possibility that corticosteroids or immune globulin therapy may influence gastrointestinal motility by a mechanism unrelated to autoimmunity, the observations we have documented support the autoimmune hypothesis. There is a need for randomized placebo controlled double-blind trials in AGID.
Table 2b. Clinical Characteristics of 17 patients with immunotherapy-responsive gastrointestinal dysmotility.
| Patient | Manometry abnormalities |
Neural autoantibody detected (titer) |
Cancer detected |
Immunotherapy diagnostic trial |
GI symptom improvement |
Recorded response |
Improved Scintigraphy |
Repeat neural autoantibody after immunotherapy |
Other objective evidence of improvement |
|---|---|---|---|---|---|---|---|---|---|
| 1 | - | gAChR (5.89 nmol/L) | - | IVIg | Yes | Mild | Yes | gAChR (3.69 nmol/L) | Improved autonomic tests |
| 2 | Gastroduodenal: neuropathic | gAChR (0.09 nmol/L) | - | IVMP | No | - | Yes | gAChR (0.19 nmol/L) | - |
| 3 | Colonic: motor dysfunction | Peripherin (1:1920) | - | IVIg | Yes | BM daily compared to once per week | Yes | Peripherin (1:15360) | - |
| 4 | Colonic: motor dysfunction | Peripherin (1:960), striated muscle (1:240) | - | IVIg | Yes | Mild | Yes | - | Improved autonomic tests and manometry study |
| 5* | - | ANNA-1 (1:3840) | Lung, small-cell | IVIg | Yes | Weight gain 55 lb (from 110 to 165 lbs) | - | ANNA-1 (1:240) | - |
| 6 | - | - | - | IVMP | Yes | Normalized | Yes | - | Improved autonomic tests |
| 7 | - | - | - | IVMP | No | - | Yes | - | - |
| 8 | gAChR (0.06 nmol/L) and striated muscle (1:240) | - | IVIg | Yes | Nausea resolved; weight gain 10 lbs | - | gAChR (0.00 nmol/L) and striated muscle (1:60) | Improved autonomic tests | |
| 9* | Gastroduodenal: mechanical dysfunction, attributed to AGID | ANNA-1 (1:15360) VGKC (0.10 nmol/L) | Cervix, small-cell | IVMP followed by IVIg | Yes | Nausea and vomiting resolved | - | ANNA-1 (1:3840) VGKC (0.07 nmol/L) | - |
| 10 | Gastroduodenal: neuropathic | gAChR (0.17 nmol/L) and VGKC (0.15 nmol/L) | - | IVIg followed by prednisone and mycophenolate | Yes | J tube able to be removed | - | gAChR (0.15 nmol/L) | Improved autonomic tests |
| 11 | - | ANNA-1 (1:61440), GAD65 (0.17 nmol/L), VGKC (0.19 nmol/L) | Thymoma | IVMP | Yes | Subjectively reported 80% improvement | - | ANNA-1 (negative) VGKC (0.18 nmol/L) | - |
| 12 | - | gAChR (5.36 nmol/L) | - | IVIg | Yes | Moderate | - | gAChR (2.04 nmol/L) | - |
| 13 | Gastroduodenal: neuropathic | - | - | IVIg | Yes | Weight gain 50lbs; J tube removed | - | - | Improved autonomic tests and manometry study |
| 14 | - | CCN (0.07 nmol/L) | - | IVIg | Yes | Daily vomiting completely resolved. | Yes | - | - |
| 15 | - | gAChR (0.17 nmol/L) and striated muscle (1:960) | - | IVIg | Yes | Nausea less and feeding tube removed | - | gAChR (0.16 nmol/L) | - |
| 16 | - | - | - | IVMP | No | - | Yes | - | - |
| 17 | - | - | - | IVIg | No | - | Yes | - | - |
Abbreviations: AGID, autoimmune gastrointestinal dysmotility; ANNA-1, anti-neuronal nuclear autoantibody type 1 (negative, <1:60) ; BM, bowel motion; BMI, body mass index; CCN, calcium channel N-type (negative, ≤0.02 nmol/L); gAChR, ganglionic acetylcholine receptor (negative, ≤0.02 nmol/L); F, female; GAD65, glutamic acid decarboxylase 65 (negative, ≤0.02 nmol/L; hr, hour; Hx, history; IVIg, intravenous immune globulin; IVMP, intravenous methylprednisolone; Kg, kilogram; M, male; peripherin (negative, <1:60); striated muscle antibodies (negative, <1:60); VGKC, voltage-gated potassium channel-complex antibodies (negative, ≤0.02 nmol/L).
Reported previously(1);
Prior to immunotherapy unless stated;
after immunotherapy (no details available for BMI prior to immunotherapy)
Acknowledgments
Disclosures: Dr Lennon receives royalties for technology relating to aquaporin 4 (AQP4) antibodies for diagnosis of neuromyelitis optica (NMO), is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker, and receives research support from the National Institutes of Health (grant NS065829-01).
Dr. Pittock is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and the National Institutes of Health (NS065829). Dr. Pittock has provided consultation to Alexion Pharmaceuticals, MedImmune LLC, and Chugai Pharma but has received no personal fees or personal compensation for these consulting activities. All compensation for consulting activities is paid directly to Mayo Clinic.
Dr McKeon receives research support from the Guthy-Jackson Charitable Foundation.
Drs. Flanagan, Saito, Murray, Szarka, Foxx-Orenstein, Fox and Fealey have no disclosures.
No author receives royalties from the sale of serological tests by Mayo Medical Laboratories. However, Mayo Collaborative Services, Inc. does receive revenue for conducting these tests.
A non-provisional patent application has been filed by Mayo Clinic for technology relating to “Peripherin-Specific Autoantibodies as a Marker for Neurological and Endocrinological Disease”.
Financial support: This work was supported in part by grant DK71209 from the National Institutes of Health (V.A.L.).
Abbreviations
- AChR
acetylcholine receptor
- AGID
autoimmune gastrointestinal dysmotility
- ANNA-1
anti-neuronal nuclear autoantibody type 1
- GAD65
glutamic acid decarboxylase, 65kD isoform
- IgG
immunoglobulin-G
- IVIg
intravenous immune globulin
- PET-CT
positron emission tomography-computed tomography
Footnotes
Author Involvement: Study concept and design: Dr Flanagan, Pittock and Saito
Acquisition of data: Dr Flanagan, Pittock and Saito
Analysis and interpretation of data: Drs. Flanagan, Saito, Lennon, McKeon, Fealey, Szarka, Murray, Foxx-Orenstein, Fox and Pittock
Drafting of the manuscript: Dr Flanagan and Pittock;
Critical revision of the manuscript for important intellectual content: Drs. Flanagan, Saito, Lennon, McKeon, Fealey, Szarka, Murray, Foxx-Orenstein, Fox and Pittock
Study supervision: Dr Pittock
References
- 1.Dhamija R, Tan KM, Pittock SJ, Foxx-Orenstein A, Benarroch E, Lennon VA. Serologic profiles aiding the diagnosis of autoimmune gastrointestinal dysmotility. Clin Gastroenterol Hepatol. 2008;6:988–992. doi: 10.1016/j.cgh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasha SF, Lunsford TN, Lennon VA. Autoimmune gastrointestinal dysmotility treated successfully with pyridostigmine. Gastroenterology. 2006;131:1592–1596. doi: 10.1053/j.gastro.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology. 1991;100:137–142. doi: 10.1016/0016-5085(91)90593-a. [DOI] [PubMed] [Google Scholar]
- 4.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- 5.Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50:652–657. doi: 10.1212/wnl.50.3.652. [DOI] [PubMed] [Google Scholar]
- 6.Iorio R, Lennon VA. Neural antigen-specific autoimmune disorders. Immunol Rev. 2012;248:104–121. doi: 10.1111/j.1600-065X.2012.01144.x. [DOI] [PubMed] [Google Scholar]
- 7.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111:907–913. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24:7037–7042. doi: 10.1523/JNEUROSCI.1485-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meeusen JW, Haselkorn KE, Fryer JP, et al. Gastrointestinal hypomotility with loss of enteric nicotinic acetylcholine receptors: active immunization model in mice. Neurogastroenterol Motil. 2013;25:84–88. doi: 10.1111/nmo.12030. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles CH, Lang B, Clover L, et al. A role for autoantibodies in some cases of acquired non-paraneoplastic gut dysmotility. Scand J Gastroenterol. 2002;37:166–170. doi: 10.1080/003655202753416821. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain JL, Pittock SJ, Oprescu AM, et al. Peripherin-IgG association with neurologic and endocrine autoimmunity. J Autoimmun. 2010;34:469–477. doi: 10.1016/j.jaut.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010;85:881–897. doi: 10.4065/mcp.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobile-Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet neurology. 2012;11:493–502. doi: 10.1016/S1474-4422(12)70093-5. [DOI] [PubMed] [Google Scholar]
- 14.Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis: a randomized controlled trial. Neurology. 2007;68:837–841. doi: 10.1212/01.wnl.0000256698.69121.45. [DOI] [PubMed] [Google Scholar]
- 15.Charles F, Camilleri M, Phillips SF, Thomforde GM, Forstrom LA. Scintigraphy of the whole gut: clinical evaluation of transit disorders. Mayo Clin Proc. 1995;70:113–118. doi: 10.4065/70.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Szarka LA, Camilleri M. Methods for measurement of gastric motility. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G461–475. doi: 10.1152/ajpgi.90467.2008. [DOI] [PubMed] [Google Scholar]
- 17.Camilleri M, Bharucha AE, di Lorenzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 18.Iodice V, Lipp A, Ahlskog JE, et al. Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry. 2012;83:453–459. doi: 10.1136/jnnp-2011-301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KH, Vernino S, Lennon VA. ANNA-3 anti-neuronal nuclear antibody: marker of lung cancer-related autoimmunity. Ann Neurol. 2001;50:301–311. doi: 10.1002/ana.1127. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49:146–154. [PubMed] [Google Scholar]
- 21.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53:580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 22.Vernino SKT, Lennon VA. Autoimmune autonomic neuropathy and neuromuscular hyperexcitability disorders. In: Rose NR, Folds CDME, et al., editors. Manual of clinical and laboratory immunology. Washington DC: ASM Press; 2002. pp. 1013–1017. [Google Scholar]
- 23.Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73:1161–1166. doi: 10.4065/73.12.1161. [DOI] [PubMed] [Google Scholar]
- 24.Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70:1883–1890. doi: 10.1212/01.wnl.0000312275.04260.a0. [DOI] [PubMed] [Google Scholar]
- 25.McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies: pathology and mechanisms. Acta neuropathologica. 2011;122:381–400. doi: 10.1007/s00401-011-0876-1. [DOI] [PubMed] [Google Scholar]
- 26.Pardi DS, Miller SM, Miller DL, et al. Paraneoplastic dysmotility: loss of interstitial cells of Cajal. Am J Gastroenterol. 2002;97:1828–1833. doi: 10.1111/j.1572-0241.2002.05851.x. [DOI] [PubMed] [Google Scholar]
- 27.Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res. 2004;10:7270–7275. doi: 10.1158/1078-0432.CCR-04-0735. [DOI] [PubMed] [Google Scholar]
- 28.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flanagan EP, McKeon A, Lennon VA, et al. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology. 2011;76:2089–2095. doi: 10.1212/WNL.0b013e31821f468f. [DOI] [PubMed] [Google Scholar]