Abstract
Objective
To combine early, direct assessment of the placenta with indirect markers of placental development to identify pregnancies at greatest risk of delivering small-for-gestational age infants (SGA10).
Methods
We prospectively collected 3D-ultrasound volume sets, uterine artery pulsatility index (UtAPI) and maternal serum of singleton pregnancies at 11–14 weeks. Placental volume (PV), quotient (PQ=PV/gestational age), mean placental and chorionic diameters (MPD and MCD, respectively), and the placental morphology index (PMI=MPD/PQ and adjusts the lateral placental dimensions for quotient) were measured offline. Maternal serum was assayed for placental growth factor (PlGF) and placental protein-13 (PP13). These variables were evaluated as predictors of SGA10.
Results
Of the 578 pregnancies included in the study, 56 (9.7%) delivered SGA10. SGA10 pregnancies had a significantly smaller PV, PQ, MPD and MCD and higher PMI compared to normal pregnancies (P<0.001 for each). Each placental measure remained significantly associated with SGA10 after adjusting for confounders and significantly improved the performance of the model using clinical variables alone (P<0.04 for each) with adjusted AUCs ranging from 0.71 to 0.74. UtAPI did not remain significantly associated with SGA10 after adjusting for confounders (P=0.06). PlGF was significantly lower in SGA10 pregnancies (P=0.02) and remained significant in adjusted models, but failed to significantly improve the predictive performance of the models as measured by AUC (P>0.3). PP13 was not associated with SGA10 (P=0.99).
Conclusions
Direct assessment of placental size and shape with 3-dimensional ultrasound can serve as the foundation upon which to build a multivariable model for the early prediction of SGA.
Keywords: 3D ultrasound, fetal growth restriction, placenta, PlGF, uterine artery Doppler
Introduction
Intrauterine growth restriction (IUGR) is a significant contributor to perinatal morbidity and mortality, including intrauterine fetal demise, newborn encephalopathy, and cerebral palsy1,2 and may have an adverse impact on long-term health outcomes such as cardiovascular disease.3–5 Several studies have indicated, however, that routine prenatal care fails to detect the vast majority of IUGR cases prior to delivery,6,7 preventing clinicians from instituting appropriate fetal surveillance aimed at improving outcomes. In addition, while there are no effective interventions shown to prevent IUGR, any candidate intervention would likely be more effective if implemented earlier in pregnancy to those at greatest risk.
The placenta serves as the key to the transfer of oxygen and nutrition to the fetus. In addition, placental size and shape at delivery are strongly correlated with newborn birth weight.8–11 Nevertheless, there are no standard, validated approaches to evaluating antenatal placental growth during pregnancy as the routine sonographic evaluation of the placenta focuses mainly on its location relative to the internal cervical os.12,13
Advances in three-dimensional (3D) ultrasound technology have allowed for non-invasive measurement the placental volume. In fact, early placental volume has been shown to be significantly associated with IUGR and preeclampsia in several studies.14–19 Moreover, we have previously published pilot data which demonstrated how the relative contributions of both lateral placental growth and placental thickness to the placental volume may provide an enhanced assessment of early placental development and may even improve prediction of adverse pregnancy outcomes such as small for gestational age (SGA).17 Therefore, we set out to further explore the ability of 3D ultrasonographic evaluation of the early placenta to identify pregnancies at greatest risk of IUGR.
In addition, while 3D ultrasound can be used to directly evaluate gross placental size and shape, there are elements of early placental development for which indirect markers may be better suited to evaluate. For example, uterine artery Doppler (UtAD) velocimetry measures the resistance to flow into the uterus, which is significantly impacted by effective trophoblastic invasion and remodeling of the maternal vasculature into a low-resistance system.20 Investigational maternal serum markers may capture other critical components of early placental development such as placental angiogenesis and placental implantation. For example, placental growth factor (PlGF), a member of the vascular endothelial growth factor subfamily, is expressed by trophoblasts and exerts angiogenic effects on the developing placenta and its environment. Placental protein 13, a galectin expressed by the placenta, binds to proteins in the extracellular matrix at the placenta-endometrium interface and assists in placental implantation and maternal artery remodeling. In fact, first trimester serum concentrations of both of these serum markers are significantly decreased in pregnancies destined to develop complications such as preeclampsia.21–27
The objective of this study is to develop a multivariable screening model combining direct and indirect markers of early placental development that can accurately identify pregnancies at increased risk of developing SGA in pregnancy.
Methods
In this prospective cohort study, women carrying singleton pregnancies who presented at 11–14 weeks gestation for nuchal translucency screening at the Hospital of the University of Pennsylvania were recruited and consented during their genetic counseling session according to an IRB-approved protocol (#811129). Singleton gestations with available 3D volume sets, maternal serum, and obstetric outcome data were included in this analysis. Exclusion criteria included multiple gestations, patients presenting after 14 weeks, and patients delivering outside of our institution.
Ultrasound techniques
Enrolled subjects had a 3D volume sweep of the placenta obtained transabdominally (4–8MHz probe, GE Voluson Expert, GE Healthcare, Wisconsin, USA) during their nuchal translucency examination. Sonographers were instructed to maximize their sweep angle and sector width and use the ‘Max’ sweep quality setting (i.e. slower sweep speed) to ensure the sweep included the entire placental mass at high resolution. The volume data set was stored on external hard drives for offline analysis. The fetal CRL was also recorded to confirm the gestational age. Pregnancies without a known last menstrual period (LMP) date or whose LMP was ≥7 days discrepant from the ultrasound dating were re-dated to reflect the CRL. Finally, bilateral uterine artery Doppler velocimetry was performed by identifying the sagittal view of the cervix, gradually moving the transducer laterally to each side, identifying the uterine artery with color Doppler as it crossed the iliac vessels and then interrogating the vessel to obtain the pulsatility index (PI) as a measure of downstream vascular resistance. The mean PI was used for analyses. Each of the sonographers taking part in this study were previously trained and certified in the performance of uterine artery Doppler techniques as part of a prior multi-centered cohort study (Preterm Birth in Nulliparous Women: An Understudied Population at Great Risk-U10, NICHD; ClinicalTrials.gov# NCT01322529).
The stored placenta volume sets were manipulated offline using 4DVIEW (GE, Austria) by a single investigator (NS), who was blinded to pregnancy outcome and using previously described techniques.17 Briefly, placental volume (PV) was measured using Virtual Organ Computer-Aided analysis (VOCAL) to trace the outline of the object of interest in successive planes obtained by rotating the object around the y-axis at 30° rotational intervals. The software then renders the structure and calculates the estimated volume. (Figure 1A) The placental quotient (PQ) was calculated to normalize the PV to gestational age (PQ=PV/days of gestation).
Figure 1. Measurement of the placenta using 3-dimensional ultrasonography.
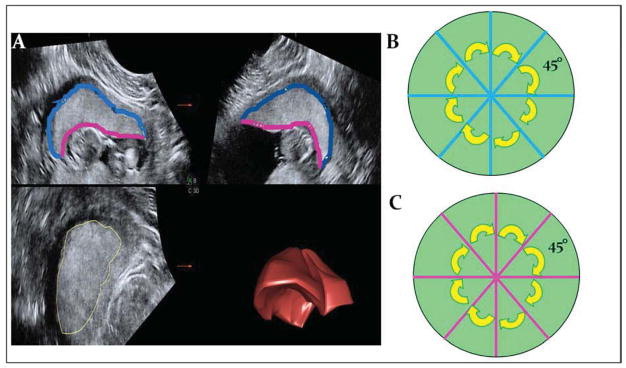
1A shows the sectional plane display of the placental volume set, with quadrants A, B, and C corresponding to the 3 orthogonal planes. Successive tracings in plane A are rendered as a 3-dimensional placental volume in the lower right quadrant (3D). The maternal surface of the placenta is outlines in blue, while the fetal, or chorionic, surface is in pink. When the maternal (blue) and chorionic (pink) surfaces are measured in the A and B planes before and after a 45° rotation around the y-axis, this results in 4 evenly spaced measurements of each surface as depicted in 1B.
Next, to quantify the lateral placental dimensions, we obtained 4 measurements of the maternal placental surface evenly spaced around the circumference by: centering the placenta in all three orthogonal planes, measuring the traced length of the uterine-placental interface in the ‘A’ and ‘B’ planes to obtain yielding two orthogonal placental diameters, rotating the placenta 45° around the y-axis and repeating the two measurements. (Figure 1B) Thus, the mean placental diameter (MPD), the average of these four diameters, represents the lateral placental dimensions and approximates the gross surface area of the myometrial-placental interface.
We then calculated the placental morphology index (PMI=MPD/PQ), which quantifies the contribution of the lateral placental dimensions to the overall placental mass. Thus, the higher the PMI, the greater the relative contribution of the lateral placental dimensions compared to that of the placental thickness. On the other hand, a lower PMI signifies a more significant contribution of placental thickness to the overall placental mass.
Because there are data indicating the importance of the morphology and surface vasculature of the chorionic plate (the fetal surface of the placenta)28,29, we also obtained 4 evenly-spaced measurements of the diameter of the fetal surface of the placenta to obtain a mean chorionic diameter (MCD) using the same rotational approach mentioned above. (Figure 1)
Serum Markers
During the same patient encounter at 11–14 weeks gestation, 5ml of maternal blood was drawn and centrifuged (1200g) at room temperature for 10 minutes. The collected serum was stored at −80°C until analysis. Thawed serum was then assayed for two serum markers involved in early trophoblastic development, PlGF and PP13. Serum concentrations of PlGF and PP13 were measured in duplicate using commercially available ELISA kits (PlGF: R&D Systems, Inc, Minneapolis, MN, USA; PP13: BlueGene Biotech, Shanghai, China) and analyzed as multiple of the median for each gestational age week. Multiples of the median values for each serum biomarker were based on the median serum concentration from the study cohort.
Demographic Variables and Pregnancy Outcomes
Demographic and outcome variables were extracted from the electronic medical record. The variables of interest included: maternal age, ethnicity, pre-pregnancy body-mass index, parity, medical co-morbidities, gestational age at delivery, mode of delivery, birth weight and birth weight percentile30.
Statistical analysis
Placental ultrasound variables and serum markers were analyzed as potential predictive markers of adverse pregnancy outcome. The primary outcome of interest was birthweight ≤10th percentile (SGA10). SGA<5th percentile (SGA5) served as a secondary outcome. Pregnancies were included in the appropriate for gestational age (AGA) group if the birthweight percentile was greater than the SGA outcome being analyzed. The distributions of discrete variables were characterized by proportions and compared by Pearson chi-square or exact methods, as appropriate. The Student’s t test (for normally distributed data) or Mann Whitney U test (for ordinal or non-normally distributed variables) were used to compare continuous variables. Receiver operator characteristic (ROC) curves were used for each significant variable and the area under the curve (AUC) served as a reflection of the overall ability of the variable to discriminate between pregnancies with an adverse outcome and those without.31 The AUC of individual measures as well as combinations of markers were compared using the z-statistic to test the equivalence of two AUCs derived from the same study subjects.32 Finally, bootstrapping techniques with 1000 replications were performed to internally validate the performance of the models by estimating 95% confidence intervals for the AUCs.33 Data analysis was performed using STATA (Version 12, College Station, Tx, USA).
Using previously published data involving our institution,34 we estimated the prevalence of SGA to be 14%. Thus, to be powered to a sensitivity of 70% (+/− 10%) and a type I error of 0.05, we needed to include 577 subjects in the analysis.
Results
Of the 578 pregnancies analyzed, 56 (9.7%) resulted in SGA10 and 28 (4.8%) SGA5. As seen in Table 1, mean maternal age, BMI, and nulliparity were not significantly associated with SGA10, but Black and Asian race and the presence of chronic hypertension were significantly more represented in the SGA10 group compared to AGA pregnancies. In addition, there was a trend towards a higher prevalence of tobacco use among those with SGA10.
Table 1.
Demographic data
| Not SGA10 (N=522) | SGA10 (N=56) | P-value | Not SGA5 (N=550) | SGA5 (N=28) | P-value* | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 30.8 (5.8) | 29.6 (5.8) | 0.12 | 30.8 (5.8) | 29.6 (6.1) | 0.33 |
| Race, N (%) | 0.016 | 0.086 | ||||
| Black | 208 (39.9) | 26 (46.4) | 218 (39.6) | 16 (57.1) | ||
| White/other | 264 (50.6) | 19 (33.9) | 275 (50) | 8 (28.6) | ||
| Asian | 50 (9.6) | 11 (19.6) | 57 (10.4) | 4 (14.3) | ||
| BMI, mean (SD) | 27.1 (6.8) | 26.2 (7.7) | 0.36 | 26.9 (6.8) | 28.5 (9.0) | 0.24 |
| Nulliparity, N (%) | 97 (18.6) | 11 (19.6) | 0.85 | 102 (18.6) | 6 (21.4) | 0.7 |
| CHTN, N (%) | 33 (6.3) | 9 (16.1) | 0.008 | 39 (7.1) | 3 (10.7) | 0.47 |
| Tobacco use, N (%) | 48 (9.2) | 9 (16.7 | 0.1 | 50 (8.1) | 1 (25) | 0.006 |
Placental Measures
Table 2 shows that PV, PQ, MPD, and MCD were all significantly smaller in SGA10 compared to AGA, indicating that a smaller placental mass is a risk factor for SGA. On the other hand, PMI was significantly larger in SGA10 cases, indicating that a relatively wider and flatter placenta was more closely associated with impaired growth compared to a relatively thicker placenta. These associations remained significant after adjusting for confounders. (Table 2)
Table 2.
First trimester ultrasound variables as predictors of SGA10
| Not SGA10 (N=522) | SGA10 (N=56) | P-valuea | Unadjusted AUC (95%CI) | Adjusted AUCb (95%CI) | P-valuec | |
|---|---|---|---|---|---|---|
| PV, cc | 69.8(22) | 55.5(17) | <0.001 | 0.695 (0.625–0.766) | 0.743 (0.678–0.808) | 0.004 |
| PQ | 0.79 (0.2) | 0.63 (0.2) | <0.001 | 0.697 (0.627–0.767) | 0.742 (0.677–0.807) | 0.004 |
| MPD, cm | 11.4 (1.4) | 10.6 (1.6) | <0.001 | 0.632 (0.556–0.707) | 0.705 (0.634–0.777) | 0.04 |
| PMI | 15.2 (3.0) | 17.6 (3.4) | <0.001 | 0.711 (0.644–0.779) | 0.740 (0.673–0.807) | 0.005 |
| MCD, cm | 8.3 (1.0) | 7.6 (0.8) | <0.001 | 0.688 (0.621–0.754) | 0.736 (0.671–0.801) | 0.003 |
| Mean PI | 1.45 (0.5) | 1.64 (0.6) | 0.01 | 0.614 (0.532–0.696) | 0.6883 (0.619–0.758) | 0.064 |
SGA10- birthweight <10th%ile; AUC- area under the curve; PV- placental volume; PQ- placental quotient; MPD- mean placental diameter; PMI- placental morphology index (MPD/PQ); MCD- mean chorionic diameter; PI- pulsatility index (uterine artery Doppler)
T-test;
Adjusted for race, chronic hypertension, and tobacco
P-value comparing the AUC for clinical model (0.652) to the adjusted model using clinical factors plus the ultrasound variable
ROC analysis was used to examine the ability of our models and individual markers to discriminate pregnancies with SGA from pregnancies with appropriately sized infants. A clinical model including the clinical variables alone (i.e. race, chronic hypertension, and tobacco use) yielded an AUC of 0.652 for predicting SGA10. Individual analyses of each placental measure yielded AUCs ranging from 0.63 for MPD to 0.71 for PMI. (Table 2) Importantly, the addition of any placental measure to the background clinical model significantly increased the AUC (P≤0.04 for each placental measure).
In order to compare the test characteristics for each sonographic measure, we identified the cut-off point for each variable that would yield a specificity of ~80% (i.e. false positive rate of ~20%). Table 3 shows the resulting relative risks and test characteristics for each sonographic measure using the chosen cut-off point. Overall, PMI yielded the highest relative risk (3.3; 95%CI: 2.0–5.3) and sensitivity (50.0%, 95%CI: 36.5%–63.5%) for predicting SGA10.
Table 3.
Test characteristics for predicting SGA10
| Cut-off point* | Relative Risk (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | |
|---|---|---|---|---|---|---|
| PV, cc | ≤51.0 | 2.6 (1.6–4.3) | 42.9% (30.0–56.7) | 80.1% (76.3–83.4) | 18.8% (12.6–26.8) | 92.9% (90.0–95.0) |
| PQ | ≤0.59 | 2.6 (1.6–4.2) | 41.1% (28.4–55.0) | 80.8% (77.1–84.1) | 18.7% (12.5–26.9) | 92.7% (89.9–94.9) |
| MPD, cm | ≤10.0 | 1.8 (1.0–3.0) | 30.4% (19.2–44.3) | 81.2% (77.6–84.4) | 14.8% (9.1–22.9) | 91.6% (88.6–93.9) |
| PMI | ≥17.4 | 3.3 (2.0–5.3) | 50.0% (36.5–63.5) | 79.5% (75.7–82.8) | 20.7% (14.4–28.7) | 93.7% (90.9–95.7) |
| MCD, cm | ≤7.5 | 2.5 (1.6–4.2) | 42.9% (30.0–56.7) | 79.3% (75.5–82.7) | 18.2% (12.2–26.0) | 92.8% (89.9–95.0) |
| Mean PI | ≥1.88 | 1.9 (1.2–3.2) | 35.7% (23.7–49.7) | 80.0% (76.3–83.3) | 16.3% (10.4–24.2) | 92.0% (89.0–94.2) |
SGA10- birthweight <10th%ile; PPV- positive predictive value, NPV-negative predictive value; PV- placental volume; PQ- placental quotient; MPD- mean placental diameter; PMI- placental morphology index (MPD/PQ); MCD- mean chorionic diameter; PI- pulsatility index (uterine artery Doppler)
Cut-off point refers to the value of the ultrasound parameter below or above which the screening test was deemed positive.
When examining SGA5, the only significant clinical variables were race and tobacco use. (Table 1) A logistic model with these two clinical factors yielded an AUC of 0.686. Once again, each of the placental measures was significantly associated with SGA5 even after adjusting for these confounders. Also, the addition of each placental measure to the clinical model yielded significantly higher AUCs compared to the clinical model alone (P≤0.04) with the highest adjusted AUC being for MCD (0.804). (Table 4) Once again, PMI yielded the highest relative risk (3.7; 95%CI: 1.8–7.6) and sensitivity (50%; 95% CI: 31.1–68.9) at ~80% specificity, although MCD and PQ performed similarly. (Table 5)
Table 4.
First trimester ultrasound variables as predictors of SGA5
| Not SGA (N=550) | SGA (N=28) | P-valuea | Unadjusted AUC (95%CI) | Adjusted AUCb (95%CI) | P-valuec | |
|---|---|---|---|---|---|---|
| PV, cc | 69.3 (22) | 52.6 (16) | <0.001 | 0.725 (0.636–0.814) | 0.793 (0.723–0.862) | 0.02 |
| PQ | 0.78 (0.2) | 0.6 (0.2) | <0.001 | 0.733 (0.646–0.820) | 0.797 (0.728–0.866) | 0.02 |
| MPD, cm | 11.4 (1.6) | 10.2 (1.7) | 0.001 | 0.688 (0.589–0.784) | 0.784 (0.716–0.852) | 0.04 |
| PMI | 15.3 (3.0) | 17.9 (3.6) | <0.001 | 0.724 (0.635–0.814) | 0.776 (0.698–0.853) | 0.03 |
| MCD, cm | 8.3 (1.0) | 7.4 (0.8) | <0.001 | 0.734 (0.652–0.816) | 0.804 (0.734–0.874) | 0.003 |
| Mean PI | 1.46 (0.5) | 1.62 (0.6) | 0.14 | 0.598 (0.487–0.708) | 0.717 (0.628–0.807) | 0.28 |
SGA5- birthweight <5th%ile; AUC- area under the curve; PV- placental volume; PQ- placental quotient; MPD- mean placental diameter; PMI- placental morphology index (MPD/PQ); MCD- mean chorionic diameter; PI- pulsatility index (uterine artery Doppler)
T-test;
Adjusted for race and tobacco;
P-value comparing the AUC for clinical model (0.686) to the adjusted model using clinical factors plus the ultrasound variable
Table 5.
Test characteristics for predicting SGA5
| Cut-off point* | Relative Risk (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | |
|---|---|---|---|---|---|---|
| PV, cc | ≤51.0 | 3.0 (1.5–6.2) | 46.4% (28.0–65.8) | 79.1% (75.4–82.4) | 10.1% (5.7–17.1) | 96.7% (94.4–98.1) |
| PQ | ≤0.59 | 3.2 (1.6–6.6) | 46.4% (28.0–65.8) | 80.0% (76.4–83.2) | 10.6% (6.0–17.7) | 96.7% (94.5–98.1) |
| MPD, cm | ≤10.0 | 2.6 (1.3–5.4) | 39.3% (22.1–59.3) | 81.1% (77.5–84.2) | 9.6% (5.1–16.8) | 96.3% (94.1–97.8) |
| PMI | ≥17.7 | 3.7 (1.8–7.6) | 50.0% (31.1–68.9) | 80.2% (76.6–83.4) | 11.4% (6.6–18.7) | 96.9% (94.8–98.2) |
| MCD, cm | ≤7.40 | 3.3 (1.6–6.8) | 46.4% (28.0–65.8) | 80.5% (76.5–83.7) | 10.8% (6.1–18.1) | 96.7% (94.5–98.0) |
| Mean PI | ≥1.9 | 1.6 (0.7–3.5) | 28.6% (14.0–48.9) | 80.0% (76.4–83.2) | 6.8% (3.2–13.3) | 95.7% (93.3–97.3) |
SGA5- birthweight <5th%ile; PPV- positive predictive value, NPV-negative predictive value; PV- placental volume; PQ- placental quotient; MPD- mean placental diameter; PMI- placental morphology index (MPD/PQ); MCD- mean chorionic diameter; PI- pulsatility index (uterine artery Doppler)
Cut-off point refers to the value of the for the ultrasound parameter below or above which the screening test deemed positive or negative.
Uterine artery Doppler
Uterine artery mean PI was significantly higher in SGA10 compared to AGA pregnancies. (Table 2) The AUC for uterine artery mean PI alone was 0.614 and showed a trend towards a significant improvement when added to the clinical model (p=0.06). Uterine artery Doppler mean PI was not significantly associated with SGA5 (P=0.14). (Table 4)
Maternal Serum Markers
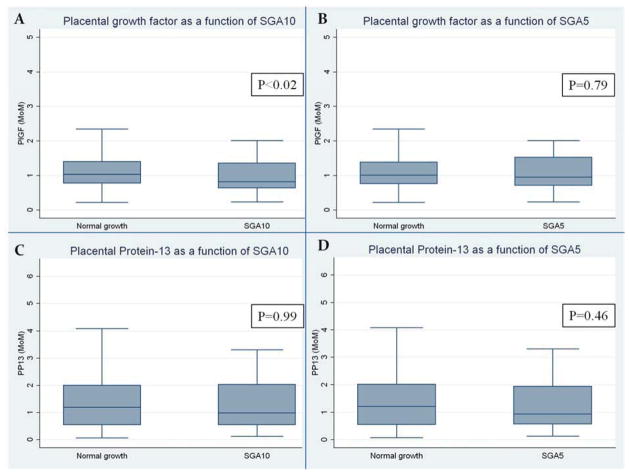
The median maternal serum PlGF (MoM) was significantly lower in SGA10 (0.82 MoM, IQR:0.63–1.37) compared to AGA pregnancies (1.03 MoM, IQR:0.78–1.41; p<0.02). (Figure 2) The incidence of SGA10 was 13.3% (37/278) among those with a PlGF ≤1 MoM compared with 6.3% (19/300) among those with a PlGF>1 MoM (relative risk: 2.1; 95%CI: 1.2–3.6). This association between PlGF and SGA10 remained significant after adjusting for confounders (P=0.005), with an adjusted AUC of 0.683 for the model. However, the addition of PlGF to the clinical model did not significantly improve the overall prediction of SGA10 (P=0.3). In addition, there was no association between PlGF and SGA5 (P=0.79). There was no association between median PP13 (MoM) and SGA10 (P=0.99) or SGA5 (P=0.46). (Figure 2)
Figure 2. Maternal serum markers and SGA.
Box plots display how median PlGF values, as measured in multiples of the median (MoM), were significantly lower in SGA10 pregnancies compared to pregnancies with normal fetal growth (A) but were not different when analyzing GA5 pregnancies (B). PP13 multiple of the median values were no different in SGA10 pregnancies (C) or SGA5 pregnancies (D).
Combined Models
Mean PI did not retain its significant association with SGA10 when included in models including any of the placental measurements (P≥0.3 for each placental measure). PlGF remained significantly associated with SGA10 when added to models containing a placental measure; however, there was no statistically significant improvement in the AUC in any of the models (P>0.3 for each model). (Table 6) As noted above, PlGF was not significantly associated with SGA5 in our cohort and was, therefore, not included in the final combined model.
Table 6.
Combined prediction models for predicting SGA10
| Adjusted AUC with PlGF | P-valuea | Adjusted AUC without PlGF | P-valueb | |
|---|---|---|---|---|
| PV | 0.753 | 0.06 | 0.743 | 0.4 |
| PQ | 0.753 | 0.06 | 0.742 | 0.3 |
| MPD | 0.723 | 0.003 | 0.705 | 0.4 |
| PMI | 0.75 | 0.065 | 0.74 | 0.4 |
| MCD | 0.745 | 0.03 | 0.736 | 0.6 |
SGA10- birthweight <10th%ile; AUC- area under the curve; PlGF- placental growth factor; PV- placental volume; PQ- placental quotient; MPD- mean placental diameter; PMI- placental morphology index (MPD/PQ); MCD- mean chorionic diameter
P-value for PlGF in the model adjusted for race, chronic hypertension, tobacco use and the placental ultrasound measurement.
P-value comparing the AUCs for the adjusted models with and without including PlGF
Thus, while the most parsimonious prediction models for SGA10 in our cohort included race, chronic hypertension, tobacco use, one of the sonographic placental measures, and PlGF as a dichotomous variable, the contribution of PlGF was likely limited as it did not lead to a significant increase in the overall AUC. (Table 6) To predict SGA5, the model with the best performance included race, tobacco use, and one of the placental measures to achieve AUC values of 0.78–0.80. (Table 4) Bootstrap techniques confirmed the precision of the AUCs of the multivariable models by confirming the 95% confidence intervals, this supporting the internal validity of these models for predicting SGA in this cohort.
Discussion
Our results demonstrate that direct placental evaluation using 3-dimensional ultrasonographic placental measurements can significantly improve the early prediction of SGA. Furthermore, these direct placental measurements perform better than indirect placental markers such as uterine artery Doppler and maternal serum PlGF and PP13.
Several investigators have demonstrated that early placental volume correlates with pathophysiologic surrogates of placental function such as biochemical analytes and uterine blood flow and is significantly associated with pregnancy outcomes.19,35–39 However, while our study corroborates the association between small placental volume and SGA, we also set out to explore the potential for other gross features of the early placental mass to serve as relevant indicators of its early development. Interestingly, our results indicate that obtaining first trimester placental diameters may have a similar predictive value as the volumetric measurements. In fact, the mean chorionic diameter, or MCD, taken along the fetal surface of the chorionic plate, achieved the same adjusted AUCs as the placental volume measurement. While we used our rotational 3D approach to ensure that the 4 diameters of the fetal surface were taken through the center of the placenta and at 45° intervals, it may be that a standardized and validated 2-dimensional (2D) approach to measuring these diameters would be simpler and more feasible to perform and still yield a similar predictive value to a 3D approach.
Two-dimensional placental measures have been proposed as potentially useful predictors of adverse outcomes. In fact, several investigators have generated a ‘placental profile’ that combines 2D measures of placental diameter and thickness and serum markers and achieved excellent positive predictive values.40–44 However, these studies have primarily focused on extremely high risk pregnancies with a very high prevalence of adverse outcome. While this may still be clinically relevant information, this cannot be used to support 2D placental measures as a clinically useful screening tool in a lower risk or unselected population. In fact, McGinty et al.45 applied a similar placental profile to a low risk population with a prevalence of SGA of 6%. They found that sonographic appearance of the placental morphology and measures of placental thickness were not useful. However, they did find that a small placental length, taken straight through the placental thickness, was significantly associated with SGA with an odds ratio: 2.8 (95%CI: 1.1–6.9), although adjustment for demographic variables was not reported.
Interestingly, our previous work has also shown that 2D placental diameter measurements in the second trimester were statistically associated with SGA.46 Moreover, those data showed that taking the mean of two, orthogonal 2D placental diameter measurements yielded a statistically significant improvement in the prediction of SGA compared to a single measurement. Nevertheless, the overall prediction, even in combination with fetal biometric parameters, was still suboptimal for clinical use. Further work is warranted to determine if the placental diameter techniques used in the current study can be further improved and adapted to 2D scanning to allow for a standardized and clinically useful tool that can be a point-of-care test for early identification of at-risk pregnancies.
In our cohort, placental measures were better predictors of SGA than indirect markers of placental development such as mean uterine artery Doppler pulsatility index. While mean PI was significantly associated with SGA10, the significance of this association did not persist in the adjusted models. Furthermore, mean PI was not significantly associated with SGA5, although this may have been partially due to the smaller number of SGA5 cases. Thus, while uterine artery Doppler velocimetry has been the focus of numerous studies investigating early prediction of adverse outcomes, our results would indicate that its role in the early prediction of SGA is limited.
To supplement the direct sonographic assessment of gross placental development, we investigated two maternal serum markers of placental angiogenesis (PlGF) and implantation (PP13). Similar to other investigations, our study demonstrated that low PlGF was indeed significantly associated with SGA10, even after adjusting for the relevant clinical variables. However, in our cohort, PlGF levels did not significantly improve the performance of the multivariable model as measured by the adjusted AUCs. One possible explanation for the poor performance of PlGF may be related to the early gestational age of our serum collection. Some of the more significant associations found between PlGF and adverse pregnancy outcomes have measured PlGF in mid-gestation rather than at 11–14 weeks.24,25,47,48 Although one longitudinal study22 demonstrated a significant association between PlGF and SGA as early as the first trimester, a second could not detect such differences until the second trimester.47 In addition, because our observed prevalence of SGA10 (9.6%) was less than the expected 14%, we were underpowered to detect the 70% sensitivity for predicting SGA10. However, post-hoc power analysis showed that our sample size would still detect a sensitivity of 82.5%.
PP13, the other serum marker we investigated, showed no significant associations with SGA in our cohort. This is in line with the conclusions of a recent systematic review, which found significant variability in the performance of this analyte and concluded that, despite early promising data, it does not appear that PP13 is of clinical utility.49
Our results demonstrate that direct placental evaluation using ultrasonographic measurements can shed light on early placental development, help identify patients at increased risk for developing SGA, and could serve as the basis for multivariable prediction models. Further research should focus on identifying additional biomarkers that can supplement placental measurements to further improve the prediction model.
Acknowledgments
Research funding for this study was provided from:
1R03HD069742-01A1 (NS)
The Penn Presbyterian George L. and Emily McMichael Harrison Fund for Research in Obstetrics and Gynecology (NS)
Footnotes
The authors report no conflict of interest.
Reprints will not be available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ACOG Practice Bulletin: Intrauterine growth restriction. Washington, DC: 2000. Reaffirmed 2009. [Google Scholar]
- 2.American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy and Cerebral Palsy, American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Neonatal encephalopathy and cerebral palsy: Defining the pathogenesis and pathophysiology. Washington: American College of Obstetricians and Gynecologists; 2003. [Google Scholar]
- 3.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–62. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–9. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Bais JM, Eskes M, Pel M, Bonsel GJ, Bleker OP. Effectiveness of detection of intrauterine growth retardation by abdominal palpation as screening test in a low risk population: an observational study. Eur J Obstet Gynecol Reprod Biol. 2004;116:164–9. doi: 10.1016/j.ejogrb.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Jahn A, Razum O, Berle P. Routine screening for intrauterine growth retardation in Germany: low sensitivity and questionable benefit for diagnosed cases. Acta Obstet Gynecol Scand. 1998;77:643–8. doi: 10.1034/j.1600-0412.1998.770611.x. [DOI] [PubMed] [Google Scholar]
- 8.Salafia CM, Charles AK, Maas EM. Placenta and fetal growth restriction. Clin Obstet Gynecol. 2006;49:236–56. doi: 10.1097/00003081-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Salafia CM, Maas E, Thorp JM, Eucker B, Pezzullo JC, Savitz DA. Measures of placental growth in relation to birth weight and gestational age. Am J Epidemiol. 2005;162:991–8. doi: 10.1093/aje/kwi305. [DOI] [PubMed] [Google Scholar]
- 10.Salafia CM, Zhang J, Charles AK, et al. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008;22:229–39. doi: 10.1111/j.1365-3016.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams LA, Evans SF, Newnham JP. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ. 1997;314:1864–8. doi: 10.1136/bmj.314.7098.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACOG Practice Bulletin No. 101: Ultrasonography in pregnancy. Obstet Gynecol. 2009;113:451–61. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- 13.AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2010;29:157–66. doi: 10.7863/jum.2010.29.1.157. [DOI] [PubMed] [Google Scholar]
- 14.Hafner E, Philipp T, Schuchter K, Dillinger-Paller B, Philipp K, Bauer P. Second-trimester measurements of placental volume by three-dimensional ultrasound to predict small-for-gestational-age infants. Ultrasound Obstet Gynecol. 1998;12:97–102. doi: 10.1046/j.1469-0705.1998.12020097.x. [DOI] [PubMed] [Google Scholar]
- 15.Hafner E, Schuchter K, van Leeuwen M, Metzenbauer M, Dillinger-Paller B, Philipp K. Three-dimensional sonographic volumetry of the placenta and the fetus between weeks 15 and 17 of gestation. Ultrasound Obstet Gynecol. 2001;18:116–20. doi: 10.1046/j.1469-0705.2001.00489.x. [DOI] [PubMed] [Google Scholar]
- 16.Odibo AO, Goetzinger KR, Huster KM, Christiansen JK, Odibo L, Tuuli MG. Placental volume and vascular flow assessed by 3D power Doppler and adverse pregnancy outcomes. Placenta. 2011;32:230–4. doi: 10.1016/j.placenta.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz N, Coletta J, Pessel C, et al. Novel 3-dimensional placental measurements in early pregnancy as predictors of adverse pregnancy outcomes. J Ultrasound Med. 2010;29:1203–12. doi: 10.7863/jum.2010.29.8.1203. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo G, Capponi A, Pietrolucci ME, Capece A, Arduini D. First-trimester placental volume and vascularization measured by 3-dimensional power Doppler sonography in pregnancies with low serum pregnancy-associated plasma protein a levels. J Ultrasound Med. 2009;28:1615–22. doi: 10.7863/jum.2009.28.12.1615. [DOI] [PubMed] [Google Scholar]
- 19.Schuchter K, Metzenbauer M, Hafner E, Philipp K. Uterine artery Doppler and placental volume in the first trimester in the prediction of pregnancy complications. Ultrasound Obstet Gynecol. 2001;18:590–2. doi: 10.1046/j.0960-7692.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- 20.Jauniaux E, Jurkovic D, Campbell S, Hustin J. Doppler ultrasonographic features of the developing placental circulation: Correlation with anatomic findings. Am J Obstet Gynecol. 1992;166:585–7. doi: 10.1016/0002-9378(92)91678-4. [DOI] [PubMed] [Google Scholar]
- 21.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–34. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Kusanovic JP, Than NG, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199:122, e1–e11. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonen R, Shahar R, Grimpel YI, et al. Placental protein 13 as an early marker for pre-eclampsia: a prospective longitudinal study. BJOG. 2008;115:1465–72. doi: 10.1111/j.1471-0528.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 24.Espinoza J, Romero R, Nien JK, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326, e1–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–9. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 26.Cowans NJ, Spencer K, Meiri H. First-trimester maternal placental protein 13 levels in pregnancies resulting in adverse outcomes. Prenat Diagn. 2008;28:121–5. doi: 10.1002/pd.1921. [DOI] [PubMed] [Google Scholar]
- 27.Chafetz I, Kuhnreich I, Sammar M, et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35, e1–7. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Salafia CM, Yampolsky M, Misra DP, et al. Placental surface shape, function, and effects of maternal and fetal vascular pathology. Placenta. 2010;31:958–62. doi: 10.1016/j.placenta.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yampolsky M, Salafia CM, Shlakhter O, Haas D, Eucker B, Thorp J. Centrality of the umbilical cord insertion in a human placenta influences the placental efficiency. Placenta. 2009;30:1058–64. doi: 10.1016/j.placenta.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 31.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 32.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 33.Efron B. Better Bootstrap Confidence Intervals. Journal of the American Statistical Association. 1987;82:171–85. [Google Scholar]
- 34.Srinivas SK, Sammel MD, Stamilio DM, et al. Periodontal disease and adverse pregnancy outcomes: is there an association? Am J Obstet Gynecol. 2009;200:497, e1–8. doi: 10.1016/j.ajog.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Hafner E, Metzenbauer M, Dillinger-Paller B, et al. Correlation of first trimester placental volume and second trimester uterine artery Doppler flow. Placenta. 2001;22:729–34. doi: 10.1053/plac.2001.0721. [DOI] [PubMed] [Google Scholar]
- 36.Hafner E, Metzenbauer M, Hofinger D, et al. Comparison between three-dimensional placental volume at 12 weeks and uterine artery impedance/notching at 22 weeks in screening for pregnancy-induced hypertension, pre-eclampsia and fetal growth restriction in a low-risk population. Ultrasound Obstet Gynecol. 2006;27:652–7. doi: 10.1002/uog.2641. [DOI] [PubMed] [Google Scholar]
- 37.Metzenbauer M, Hafner E, Hoefinger D, et al. Three-dimensional ultrasound measurement of the placental volume in early pregnancy: method and correlation with biochemical placenta parameters. Placenta. 2001;22:602–5. doi: 10.1053/plac.2001.0684. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo G, Capponi A, Cavicchioni O, Vendola M, Arduini D. First trimester uterine Doppler and three-dimensional ultrasound placental volume calculation in predicting pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2008;138:147–51. doi: 10.1016/j.ejogrb.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler T, Evans PW, Anthony FW, Godfrey KM, Howe DT, Osmond C. Relationship between maternal serum vascular endothelial growth factor concentration in early pregnancy and fetal and placental growth. Hum Reprod. 1999;14:1619–23. doi: 10.1093/humrep/14.6.1619. [DOI] [PubMed] [Google Scholar]
- 40.Costa SL, Proctor L, Dodd JM, et al. Screening for placental insufficiency in high-risk pregnancies: is earlier better? Placenta. 2008;29:1034–40. doi: 10.1016/j.placenta.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Proctor LK, Toal M, Keating S, et al. Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2009;34:274–82. doi: 10.1002/uog.7308. [DOI] [PubMed] [Google Scholar]
- 42.Toal M, Chaddha V, Windrim R, Kingdom J. Ultrasound detection of placental insufficiency in women with elevated second trimester serum alpha-fetoprotein or human chorionic gonadotropin. J Obstet Gynaecol Can. 2008;30:198–206. doi: 10.1016/S1701-2163(16)32756-6. [DOI] [PubMed] [Google Scholar]
- 43.Toal M, Chan C, Fallah S, et al. Usefulness of a placental profile in high-risk pregnancies. Am J Obstet Gynecol. 2007;196:363, e1–7. doi: 10.1016/j.ajog.2006.10.897. [DOI] [PubMed] [Google Scholar]
- 44.Viero S, Chaddha V, Alkazaleh F, et al. Prognostic value of placental ultrasound in pregnancies complicated by absent end-diastolic flow velocity in the umbilical arteries. Placenta. 2004;25:735–41. doi: 10.1016/j.placenta.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45.McGinty P, Farah N, Dwyer VO, et al. Ultrasound assessment of placental function: the effectiveness of placental biometry in a low-risk population as a predictor of a small for gestational age neonate. Prenat Diagn. 2012;32:620–6. doi: 10.1002/pd.3870. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz N, Wang E, Parry S. Two-dimensional sonographic placental measurements in the prediction of small-for-gestational-age infants. Ultrasound Obstet Gynecol. 2012;40:674–9. doi: 10.1002/uog.11136. [DOI] [PubMed] [Google Scholar]
- 47.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–82. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 48.Tjoa ML, van Vugt JM, Mulders MA, Schutgens RB, Oudejans CB, van Wijk IJ. Plasma placenta growth factor levels in midtrimester pregnancies. Obstet Gynecol. 2001;98:600–7. doi: 10.1016/s0029-7844(01)01497-1. [DOI] [PubMed] [Google Scholar]
- 49.Schneuer FJ, Nassar N, Khambalia AZ, et al. First trimester screening of maternal placental protein 13 for predicting preeclampsia and small for gestational age: in-house study and systematic review. Placenta. 2012;33:735–40. doi: 10.1016/j.placenta.2012.05.012. [DOI] [PubMed] [Google Scholar]