Abstract
Objective
To assess if maternal factors associated with fetal lean and fat mass differ between sexes.
Study Design
Secondary analysis of a prospective cohort delivering via scheduled Cesarean from 2004–2013. Maternal blood was collected prior to surgery for metabolic parameters. Placental weight and neonatal anthropometrics were measured within 48 hrs. Anthropometric differences between sexes were assessed with Student’s t-test. Multiple stepwise regression analysis assessed the relationship between independent maternal variables and neonatal lean body mass (LBM), fat mass (FM) or percent (%) fat as dependent variables in males and females combined and separately.
Results
We analyzed 360 women with normal glucose tolerance and wide range of pregravid body mass index (BMI, 16–64 kg/m2) and their offspring (N=194 males and 166 females). Males had more FM (mean difference 40 ± 18 g, P=0.03) and LBM (mean difference 158 ± 34 g, P<0.0001) than females. Percent body fat and measured maternal variables did not differ between sexes. In both sexes, placental weight had the strongest correlation with both neonatal LBM and FM, accounting for 20–39% of the variance. In males, maternal height, BMI and weight gain were significant predictors of both lean and fat mass. In females, plasma interleukin-6 and C-reactive protein were respectively independently associated with percent body fat and lean body mass.
Conclusion
Our findings suggest that the body composition and inflammatory environment of the mother modulate the metabolic fitness of neonates, as predicted by fat and lean mass, in a sex-specific manner.
Keywords: inflammation, maternal BMI, neonatal anthropometry, placenta
Introduction
It is well-established that babies born small- or large-for-gestational age are at a higher risk of developing cardiovascular disease, obesity and metabolic deficiencies in later life1;2. Understanding factors that influence fetal growth in utero are, thus, of clinical interest in determining a child’s long-term health. Maternal nutrition (i.e. diet and body composition) and placental transport capability are key influences on fetal growth and are strongly associated with birth weight3–5. However, it is increasingly understood that birth weight is not the only marker of perturbations in fetal growth. It was previously reported that offspring of obese mothers have increased fat mass, but not lean mass6 in addition to increased insulin resistance in offspring7, suggesting that fetal adiposity is sensitive to maternal nutrition and potentially underlies long-term metabolic fitness.
There is mounting evidence that the fetus responds to the maternal environment in a sex-specific manner. Males are born heavier and longer to well-nourished mothers8. This suggests that male growth may be more sensitive to nutrient supply during pregnancy. Indeed, when mothers are poorly nourished, males tend to be more affected than females, showing greater degrees of growth restriction (or fat deposition)9–11 and increases in cardiovascular disease risk in later life12; this sensitivity may be due to a mis-match in the supply and demand of nutrients. These findings suggest that growth of male fetuses is more sensitive to maternal nutrition throughout pregnancy and female fetuses may be more able to adapt to minor nutritional differences.
Maternal pre-pregnancy and early pregnancy body composition (fat and fat free mass or clinically BMI) indicate long-term maternal nutrition and are thought to be better predictors of fetal outcome than weight gain, a marker of nutrition during pregnancy13–15. Lampl et al. showed that birth weights of male offspring were more highly correlated with maternal weight and height than female birth weights8. While Lampl used birth weight as the primary outcome, the sex-specific effects of maternal anthropometrics on the growth of fetal fat and lean mass are unknown. We hypothesize that maternal anthropometric variables are correlated with neonatal body composition in a sexdependent manner. To test this hypothesis, we analyzed a cohort of over 300 healthy women with normal glucose tolerance in pregnancy undergoing scheduled caesarean delivery. Neonatal body composition was calculated from skinfold measurements collected within 48 hrs. of birth.
Materials and Methods
Data Collection
This is a secondary analysis of a prospective cohort delivering via scheduled Cesarean from 2004–2013. The indications for the vast majority of these were elective repeat Cesareans or breech presentation. Our exclusion criteria included pregnancies complicated by gestational diabetes, pre-eclampsia, chronic hypertension and illegal drug use, multifetal gestations and infants with anomalies. Information on maternal demographics (via maternal report), height and weight (via clinical records) was obtained following written informed consent as approved by the Institutional Review Board at MetroHealth Medical Center. Maternal blood (following overnight fast) was collected prior to surgery for metabolic parameters. Following delivery, the placenta was weighed. Within 48 hrs of birth, neonatal anthropometrics were measured and recorded by a trained research nurse. Birth weight was measured on a calibrated scale and a measuring board was used for length measurements. The flank skinfold was assessed in the mid-axillary line directly above the iliac crest. Neonatal body composition estimates were made using the following validated equation17: fat mass = 0.39055 (birth weight, kg) + 0.0453 (flank skinfold, mm) − 0.03237 (length, cm) + 0.54657. Lean body mass was calculated as birth weight minus fat mass. Percent body fat was calculated as fat mass/birth weight × 100.
Metabolic Measurements
Maternal fasting glucose was measured on the YSI Glucose Analyzer (YSI Life Sciences, Yellow Springs, OH). Plasma insulin (Millipore, Billerica, MA), C-reactive protein (Alpha Diagnostic, San Antonio, TX) and interleukin-6 (R&D Systems, Minn, MN) levels were measured by immunoassay following manufacturers’ directions.
Analysis
Total pregnancy weight gain was calculated using weight at first antenatal visit (if prior to 12 weeks) and last recorded pregnancy weight (GA >35 weeks). Net maternal gestational weight gain was calculated as total pregnancy weight gain minus neonatal birth weight and placental weight. Statistical modeling was used to determine maternal anthropometrics and metabolic parameters that predict neonatal growth in males and females. Spearman correlation analysis was used to assess the association between neonatal anthropometrics and maternal and placental variables in males and females separately. Variables found to be correlated with neonatal anthropometrics were included in the stepwise regression model. Forward stepwise regression analysis assessed the relationship between independent maternal variables and neonatal lean body mass (LBM), fat mass (FM) or percent (%) fat as dependent variables in males and females separately. Semipartial correlation coefficients were calculated for each dependent variable in the resulting models. All statistical analyses were run using STATA 10.1 (StataCorp LP, College Station, TX).
Results
We analyzed 360 women with normal glucose tolerance (based on a 1hour 50g glucose challenge test result <135 mg/dl or, if positive, a 100g oral glucose challenge according to Carpenter and Coustan16) and wide range of pregravid BMI (16–64 kg/m2) and their offspring (N=194 males and 166 females). As shown in Table 1, weight, length and placental weight were higher in males than females at birth. Males also had more FM (mean difference 40 ± 18 g, P=0.03) and LBM (mean difference 158 ± 34 g, P<0.0001) than females as measured by skinfolds. Adiposity (% body fat) was similar between the sexes. No differences in maternal characteristics were detectable between males and females.
Table 1.
Maternal and neonatal characteristics
| Males | Females | |||||
|---|---|---|---|---|---|---|
| N | Mean ± SD | Min – Max | N | Mean ± SD | Min – Max | |
| Maternal | ||||||
| Gestational Age (weeks) | 191 | 38.8 ± 0.7 | 36.0 – 41.0 | 164 | 38.8 ± 0.6 | 36.0 – 40.0 |
| Parity | 194 | 1.7 ± 1.0 | 0.0 – 6.0 | 166 | 1.7 ± 1.1 | 0.0 – 6.0 |
| Age (y) | 194 | 28.1 ± 5.6 | 18.0 – 46.0 | 166 | 27.3 ± 5.9 | 18.0 – 42.0 |
| Race (%AA/HISP/CAUC) | 193 | 38/12/49 | 164 | 37/10/53 | ||
| Pre-pregnancy Weight (kg) | 194 | 81.2 ± 23.3 | 42.3 – 156.8 | 165 | 82.6 ± 24.6 | 43.1 – 164.5 |
| Height (m) | 194 | 1.6 ± 0.1 | 1.5 – 1.8 | 166 | 1.6 ± 0.1 | 1.4 – 1.9 |
| Pre-pregnancy BMI (kg/m2) | 194 | 30.8 ± 8.4 | 16.0 – 55.2 | 165 | 31.3 ± 8.9 | 16.9 – 64.3 |
| Late Pregnancy BMI (kg/m2) | 194 | 36.8 ± 7.9 | 20.8 – 57.5 | 166 | 36.6 ± 8.4 | 21.2 – 69.2 |
| Net Weight Gain (kg) | 188 | 11.7 ± 8.3 | −5.3 – 42.9 | 153 | 10.5 ± 8.5 | −14.9 – 37.1 |
| Insulin (µU/ml) | 190 | 18.3 ± 8.9 | 4.1 – 63.3 | 157 | 17.7 ± 9.8 | 3.0 – 73.4 |
| Glucose (mg/dL) | 189 | 77.9 ± 9.0 | 54.0 – 118.0 | 156 | 77.0 ± 9.5 | 54.0 – 107.0 |
| HOMA-IR | 189 | 3.6 ± 2.1 | 0.7 – 16.0 | 156 | 3.5 ± 2.3 | 0.5 – 19.4 |
| CRP (ng/ml) | 124 | 9345 ± 7221 | 435 – 28482 | 86 | 9334 ± 6891 | 795 – 26721 |
| IL6 (pg/ml) | 111 | 3.8 ± 3.0 | 0.7 – 18.8 | 92 | 3.3 ± 1.7 | 0.9 – 8.5 |
| Triglycerides (mg/dL) | 85 | 184 ± 78 | 56 – 541 | 67 | 195 ± 75 | 72 – 425 |
| Neonatal | ||||||
| Birth weight (kg) | 194 | 3.4 ± 0.5 | 1.9 – 5.0 | 165 | 3.2 ± 0.4* | 2.1 – 4.8 |
| Length (cm) | 192 | 49.3 ± 2.1 | 39.8 – 54.4 | 164 | 48.5 ± 2.0* | 42.4 – 57.1 |
| Fat Mass (kg) | 184 | 0.44 ± 0.19 | 0.02 – 1.12 | 156 | 0.40 ± 0.15* | 0.07 – 0.87 |
| Lean Mass (kg) | 184 | 2.9 ± 0.3 | 1.9 – 3.9 | 156 | 2.8 ± 0.3* | 2.1 – 3.9 |
| % Body Fat | 184 | 12.5 ± 3.7 | 1.1 – 23.8 | 156 | 12.2 ± 3.3 | 2.9 – 21.0 |
| Placental Weight (g) | 188 | 685.8 ± 172.7 | 294.3 – 1316.5 | 155 | 648.5 ± 148.9* | 298.0 – 1189.5 |
| Fetal:Plwt | 188 | 5.1 ± 0.9 | 2.9 – 8.9 | 154 | 5.1 ± 1.0 | 3.1 – 11.4 |
| Insulin (µU/ml) | 183 | 7.5 ± 4.4 | 1.9 – 30.2 | 155 | 7.8 ± 4.5 | 0.8 – 26.3 |
| Glucose (mg/dL) | 184 | 66.6 ± 12.6 | 27.0 – 122.0 | 157 | 66.5 ± 11.5 | 33.0 – 111.0 |
| HOMA-IR | 182 | 1.2 ± 0.8 | 0.3 – 6.6 | 154 | 1.3 ± 0.8 | 0.1 – 5.3 |
SD: standard deviation of the mean; AA: African American; Hisp: Hispanic; Cauc: Caucasian; HOMA-IR: homeostasis model assessment-estimated insulin resistance; CRP: C-reactive protein; IL6: interleukin-6.
P<0.05 vs male by Student’s t-test
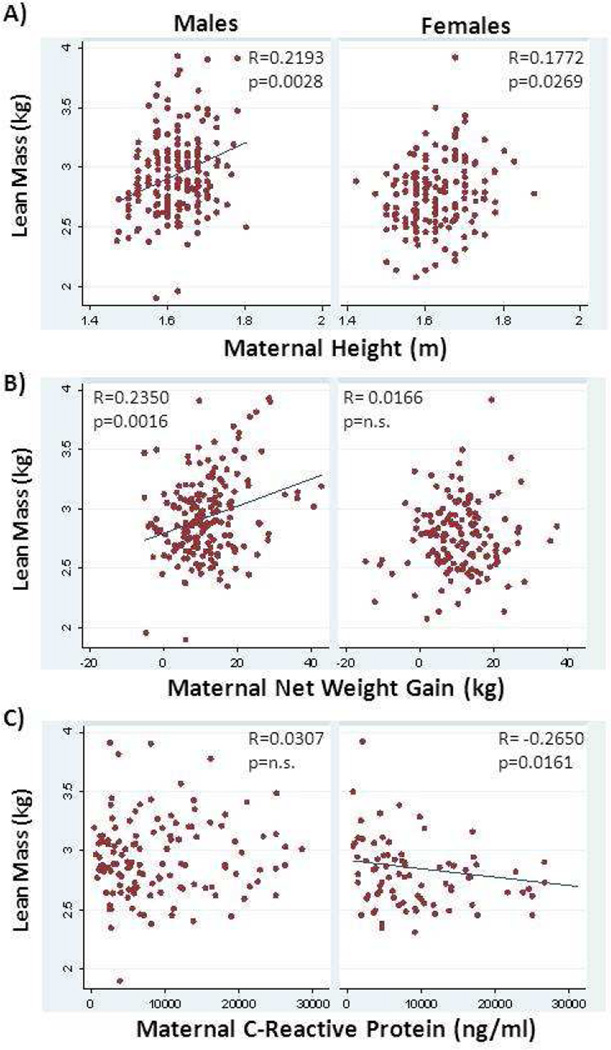
Univariate analysis using Spearman rank correlation assessed the associations between body composition (FM, LBM, adiposity) at birth and maternal and placental variables, in all infants and in males and females separately (see Supplementary Table 1 for correlation coefficients). In both males and females, placental weight was significantly associated with fat mass, lean mass and % body fat. Maternal height (Figure 2A) and gestational age were associated with lean body mass in both males and females, but all other maternal variables displayed sex-specific associations with neonatal body composition.
Figure 2.
Association between neonatal lean body mass as calculated from skin fold thickness and maternal factors. Maternal height was significantly associated with lean body mass in both male and female neonates before adjustment for covariates (A). Net weight gain was positively correlated with lean body mass in male, but not female neonates (B). Maternal plasma C-reactive protein levels were negatively related to lean body mass in female, but not male, neonates (C). Spearman’s correlation coefficient and unadjusted p value are shown.
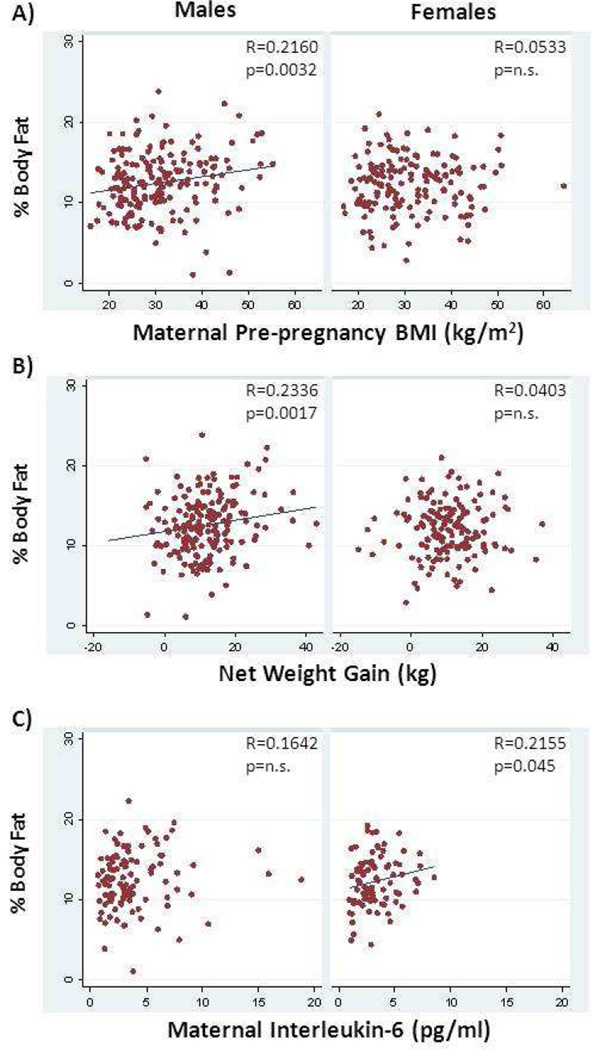
In males only, fat mass and adiposity were correlated with gestational age, pre-pregnancy weight, pre-pregnancy BMI (Figure 1A) and net weight gain (Figure 1B). Fat mass in males was also correlated with maternal height while % body fat was associated with maternal triglycerides. Lean mass in males was associated with maternal age, pre-pregnancy weight and net weight gain (Figure 2B). Adiposity (% body fat) in females was correlated with maternal insulin, HOMA-IR and interleukin-6 levels (Figure 1C). Female lean body mass was negatively associated with maternal C-reactive protein levels (Figure 2C).
Figure 1.
Association between neonatal percent body fat as calculated from skin fold thickness and maternal factors. Maternal pre-pregnancy body mass index (BMI) (A) and net weight gain (B) were associated with body fat in male, but not female neonates. Maternal plasma interleukin-6 levels were significantly associated with neonatal percent body fat in females only (C).. Spearman’s correlation coefficient and unadjusted p value are shown.
We did not find any significant correlations between paternal BMI and neonatal body composition in combined or separate analyses of males and females (data not shown). Furthermore, neonatal body composition did not differ by maternal race/ethnicity within or between sexes (data not shown).
Based upon univariate analysis, variables found to be significantly correlated with neonatal anthropometrics in males and females combined or analyzed separately were included in forward stepwise regression analysis for LBM, FM and % body fat as dependent variables in their respective groups: males and females combined (Suppl. Table 2) and separate (Table 2). In the combined analysis (males and females), placental weight was responsible for 30–37% of the variance in neonatal lean mass, fat mass or % body fat. Maternal pre-pregnancy BMI accounted for only 1% of variance in fat mass and % body fat. Gestational age and maternal net weight gain were minor, but consistent predictors for all body composition measures. Maternal height and age were also minor predictors of lean body mass variance when males and females were combined. When males and females were modeled separately (Table 2), again placental weight had the strongest correlation with neonatal LBM, FM and adiposity accounting for 20–39% of the variance in both sexes. Maternal variables altogether accounted for less than 10% of the variance in neonatal body composition. Similar to the combined regression analysis (Suppl Table 2), in males, maternal anthropometrics were significant predictors of both lean and fat mass. Conversely, in females, only maternal indicators of systemic inflammation were predictive of lean mass (CRP) or adiposity (IL6). Gestational age was a minor (accounting for 2–4% of variance), but consistently significant predictor of fat and lean mass in males more so than females.
Table 2.
Semipartial correlation coefficients for maternal factors that affected body composition in male and female infants at birth based on stepwise regression modeling
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Factor | sr2 | P value | Factor | sr2 | P value | ||
| LBM | LBM | ||||||
| n=175 | Placental Weight | 0.39 | <0.0001 | n=81 | Placental Weight | 0.26 | <0.0001 |
| Gestational Age | 0.04 | <0.0001 | Maternal CRP | 0.05 | 0.016 | ||
| Net weight gain | 0.03 | 0.001 | Gestational Age | 0.04 | 0.037 | ||
| Maternal Height | 0.02 | 0.003 | |||||
| FM | FM | ||||||
| n=175 | Placental Weight | 0.35 | <0.0001 | n=145 | Placental Weight | 0.34 | <0.0001 |
| Net weight gain | 0.04 | <0.0001 | |||||
| Pre-pregnancy BMI | 0.02 | 0.009 | |||||
| Gestational Age | 0.02 | 0.020 | |||||
| % Fat | % Fat | ||||||
| n=175 | Placental Weight | 0.31 | <0.0001 | n=85 | Placental Weight | 0.20 | <0.0001 |
| Net weight gain | 0.03 | 0.004 | Maternal IL6 | 0.03 | 0.098 | ||
| Gestational Age | 0.02 | 0.013 | |||||
| Pre-pregnancy BMI | 0.02 | 0.013 | |||||
Sr2: Semipartial correlation coefficients estimate the degree of variance explained by each factor
Supplementary Table 2 also displays results of the stepwise regression modeling in all infants (males and females combined) without maternal plasma metabolites. We were not able to collect maternal plasma on all subjects and this analysis was performed to assess the effect of reduction in subject number on the final models. The missing data did not significantly affect the analysis when males and females were combined (Suppl Table 2), nor did it affect the analysis of males alone (Suppl. Table 3). When female infants were modeled without the plasma metabolite variables (Suppl. Table 3), only placental weight predicted fat mass or % body fat; lean body mass in females was additionally predicted by maternal height (3% of variance).
Comment
The key finding of this study was that the growth and adiposity of male and female fetuses are uniquely sensitive to maternal nutritional and inflammatory signals. We confirmed our hypothesis that male offspring displayed greater sensitivity to maternal anthropometrics i.e. maternal pre-pregnancy BMI and weight gain. Unexpectedly, the only maternal factors measured predicting female body composition were markers of inflammation (plasma CRP and IL-6). These results have expanded the current knowledge of sex-differences in fetal growth to better understand the maternal influences on fetal body composition.
We found that the growth of male fetuses, but not females, was dependent on maternal pre-pregnancy BMI and height together with net weight gain during pregnancy. Maternal weight gain and pre-pregnancy anthropometrics were equally predictive of male fetal growth when placental weight and gestational age are accounted for. This appears to contradict the idea that pre-pregnancy nutrition is of greater importance than weight gain during pregnancy14;15; rather, we find that both long-term and short-term nutritional indicators are key influences on fetal fat, lean mass growth and adiposity (% body fat) in males. These relationships may differ, however, when studying complicated pregnancies (i.e. women with gestational diabetes) or when women are grouped by body habitus. Maternal height is thought to be a marker of early life nutrition – the result of both genetic potential and nutritional supply in utero and adolescence8;17;18. Neonatal lean mass is associated with maternal height19. Offspring of shorter and heavier women have a higher birth weight and fat mass6;19–22. Consistent with these studies, lean body mass of male offspring was positively related to maternal height in our cohort, while fat mass and adiposity was dependent on maternal BMI. We speculate that lean tissue growth depends upon genetic potential and long-term maternal nutrition (reflected in maternal height) and is fueled by additional nutrients in utero (weight gain). Fat mass development and adiposity may be more dependent on short-term maternal nutrition before and during pregnancy, chiefly factors affecting placental gene expression and nutrient transport. Males may be particularly sensitive to maternal nutrition due to their faster growth in utero and their higher requirement for dietary lipids during development9;23 as compared to females; however, this begs the question: what maternal factors predict female growth?
The only maternal variables predicting female growth in this cohort were markers of maternal inflammation. Maternal plasma C-reactive protein (CRP) levels at the time of delivery were negatively related to female, but not male, lean body mass at birth. CRP is an acute phase protein that is upregulated in response to release of inflammatory cytokines (i.e. IL-6)24. Additionally, we found that maternal plasma interleukin (IL)-6 levels were positively related to female adiposity; this particular relationship became less significant in the multiple regression model (P=0.098) when combined with placental weight. In general, we find that high levels of maternal inflammation are associated with less lean mass and higher adiposity in female offspring which may impede their metabolic fitness later in life. Lean mass (particularly muscle) is critical for insulin sensitivity and energy expenditure25, and the amount of muscle you are born with is highly correlated with your adult lean mass26. Interestingly, we have previously found that reduced maternal muscle mass is associated with placental inflammation27, suggesting a vicious cycle whereby an inflammatory environment in utero leads to reduced lean mass and increased adiposity in female offspring, decreased muscularity as adults and greater risk of inflammation in their own pregnancies. It is not clear why only female fetuses were sensitive to maternal inflammatory markers. Torche et al. have found that in women who experienced traumatic events during pregnancy (i.e. natural disaster)28, female offspring were more likely to be affected than males, and the sex ratio was skewed to favor female offspring28. This is consistent with studies showing that severe nutritional deprivation alters the sex ratio – fewer males are born under these extreme circumstances9;29. Our cohort was composed of healthy, uneventful pregnancies of women with varying nutritional histories, but within normal physiological boundaries, thus our findings represent the early signs of fetal sensitivity to the maternal environment, rather than severe deficiencies leading to alterations in sex ratios.
Placental weight was the primary factor that explained the greatest variance in fetal growth in both sexes (from 20–40%). The strong association between placental weight and birth weight has previously been shown30, but we observed that placental weight is a key predictor of both lean, fat mass and % body fat. Placental weight is associated with maternal obesity31, but in our study, maternal BMI and placental weight were independently associated with fetal growth, suggesting that big placentas do not explain all of the growth effects of maternal obesity.
Our study was a retrospective analysis of prospectively collected data, and was of a cross-sectional nature. We captured data at the end of pregnancy and so cannot comment on the role of maternal inflammation in early pregnancy, or the growth trajectories of the fetuses. Though our analyses were based upon detailed measurements of neonatal anthropometrics, we had limited numbers of mother-child pairs in our study (~350). A weakness of this study is that the anthropometric measures were not based on total body electrical conductivity (TOBEC) or air displacement plethysmography (PeaPod). Some data was missing on the mothers due to inability to obtain plasma samples on all women. However, our supplemental analyses performed without these data supported our conclusions that male and female growth was differentially sensitive to maternal variables. Our cohort was ethnically diverse, however we did not measure socioeconomic status and therefore cannot comment on the role of SES in these outcomes. This study is the first to examine sex differences in neonatal body composition relative to maternal variables. Though we did not focus on paternal variables in this study, we found that paternal BMI had no association with neonatal body composition.
We conclude that lean and fat mass growth in utero is sensitive to both short- and long-term maternal nutritional markers and to inflammatory indicators in a sex-specific manner. These findings support the notion that the future health and metabolic fitness of the offspring from healthy, uncomplicated pregnancies of a diverse population may be established by the body composition and inflammatory environment of the mother.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the National Institute of Child Health & Development (R00HD062841 to PFOG and R01HD22965-19 to PMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
The information in this manuscript was presented at the 34th Annual Scientific Meeting of the Society for Maternal Fetal-Medicine, New Orleans, LA February 5–8, 2014
Reference List
- 1.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 2.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ. 1996;312(7028):410–414. doi: 10.1136/bmj.312.7028.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576(Pt 3):935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23(1):271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195(4):1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22(4):431–443. doi: 10.1002/ajhb.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivers JP, Crawford MA. Maternal nutrition and the sex ratio at birth. Nature. 1974;252(5481):297–298. doi: 10.1038/252297a0. [DOI] [PubMed] [Google Scholar]
- 10.Alwasel SH, Abotalib Z, Aljarallah JS, Osmond C, Alkharaz SM, Alhazza IM, et al. Sex differences in birth size and intergenerational effects of intrauterine exposure to Ramadan in Saudi Arabia. Am J Hum Biol. 2011;23(5):651–654. doi: 10.1002/ajhb.21193. [DOI] [PubMed] [Google Scholar]
- 11.Sojo L, Garcia-Patterson A, Maria MA, Martin E, Ubeda J, Adelantado JM, et al. Are birth weight predictors in diabetic pregnancy the same in boys and girls? Eur J Obstet Gynecol Reprod Biol. 2010;153(1):32–37. doi: 10.1016/j.ejogrb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. Am J Clin Nutr. 2003;77(4):960–966. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- 13.Stuebe AM, Forman MR, Michels KB. Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes (Lond) 2009;33(7):743–752. doi: 10.1038/ijo.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catalano PM. Obesity and pregnancy--the propagation of a viscous cycle? J Clin Endocrinol Metab. 2003;88(8):3505–3506. doi: 10.1210/jc.2003-031046. [DOI] [PubMed] [Google Scholar]
- 15.Sanin Aguirre LH, Reza-Lopez S, Levario-Carrillo M. Relation between maternal body composition and birth weight. Biol Neonate. 2004;86(1):55–62. doi: 10.1159/000077586. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 17.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339(8788):283–287. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruel MT, Rivera J, Habicht JP, Martorell R. Differential response to early nutrition supplementation: long-term effects on height at adolescence. Int J Epidemiol. 1995;24(2):404–412. doi: 10.1093/ije/24.2.404. [DOI] [PubMed] [Google Scholar]
- 19.Elshibly EM, Schmalisch G. Relationship between maternal and newborn anthropometric measurements in Sudan. Pediatr Int. 2009;51(3):326–331. doi: 10.1111/j.1442-200X.2008.02756.x. [DOI] [PubMed] [Google Scholar]
- 20.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2009 doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, Inskip HM, et al. Parental determinants of neonatal body composition. J Clin Endocrinol Metab. 2007;92(2):523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C. Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab. 2007;92(10):3904–3911. doi: 10.1210/jc.2007-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326(7401):1245–1246. doi: 10.1136/bmj.326.7401.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li SP, Liu TY, Goldman ND. cis-acting elements responsible for interleukin-6 inducible C-reactive protein gene expression. J Biol Chem. 1990;265(7):4136–4142. [PubMed] [Google Scholar]
- 25.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 26.Baker J, Workman M, Bedrick E, Frey MA, Hurtado M, Pearson O. Brains versus brawn: an empirical test of Barker's brain sparing model. Am J Hum Biol. 2010;22(2):206–215. doi: 10.1002/ajhb.20979. [DOI] [PubMed] [Google Scholar]
- 27.O'Tierney PF, Lewis RM, McWeeney SK, Hanson MA, Inskip HM, Morgan TK, et al. Immune response gene profiles in the term placenta depend upon maternal muscle mass. Reprod Sci. 2012;19(10):1041–1056. doi: 10.1177/1933719112440051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torche F, Kleinhaus K. Prenatal stress, gestational age and secondary sex ratio: the sex-specific effects of exposure to a natural disaster in early pregnancy. Hum Reprod. 2012;27(2):558–567. doi: 10.1093/humrep/der390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford MA, Doyle W, Meadows N. Gender differences at birth and differences in fetal growth. Hum Reprod. 1987;2(6):517–520. doi: 10.1093/oxfordjournals.humrep.a136581. [DOI] [PubMed] [Google Scholar]
- 30.Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, Sun W, et al. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008;22(3):229–239. doi: 10.1111/j.1365-3016.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 31.Swanson LD, Bewtra C. Increase in normal placental weights related to increase in maternal body mass index. J Matern Fetal Neonatal Med. 2008;21(2):111–113. doi: 10.1080/14767050701866963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.