To the Editor,
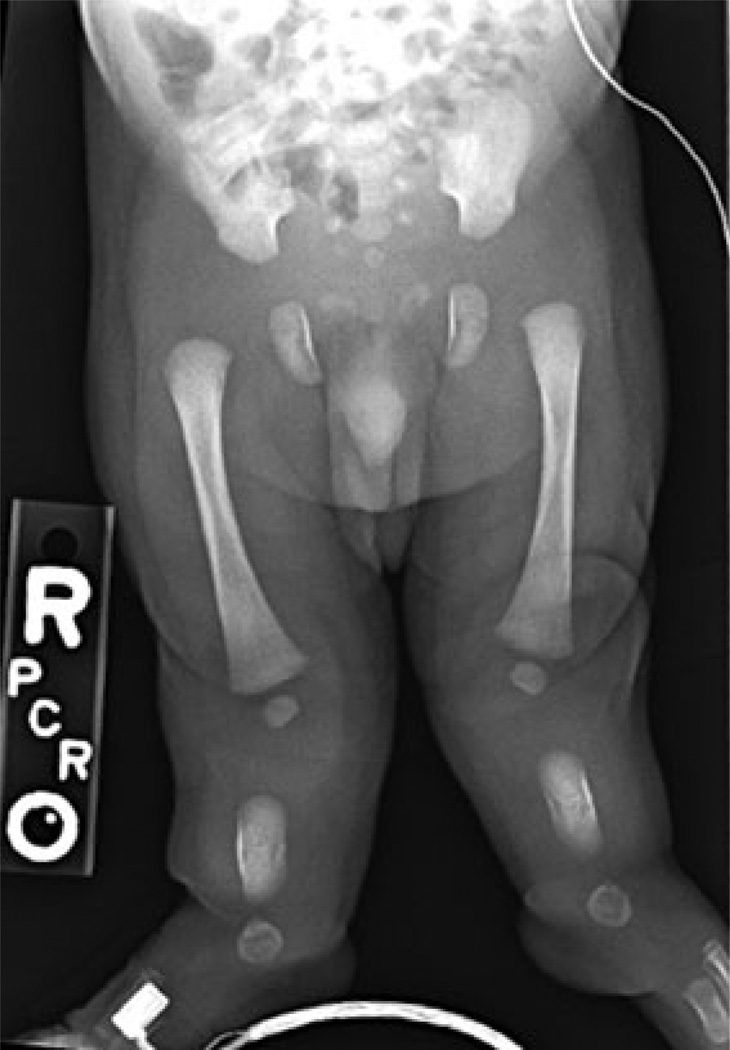
Only 18 patients with fluorescence in-situ hybridization (FISH) confirmed interstitial 6p deletions have been reported and defined based on chromosomal location. Proximal, interstitial deletions (6p22–6p24) are associated with heart, kidney, eye and brain defects, craniofacial anomalies, and psychomotor and developmental delay (1). We have identified a young infant with a 6p22–6p24 deletion with hypereosinophilic syndrome (HES), lower extremity hemimelia with mesomelic shortening, and sensorineural hearing loss. The male child was the term product of an uncomplicated pregnancy delivered by cesarean section due to skeletal abnormalities identified on prenatal ultrasound. His parents are healthy, non-consanguineous, with no family history of genetic disorders. The child’s intrauterine growth was appropriate for gestational age. At birth mild facial anomalies were noted including plagiocephaly, up slanting palpebral fissures, depressed nasal bridge, long philtrum, high anteriorly arched palate, and posteriorly rotated ears with over folded helices. He also had left hip dysplasia and left undescended testicle. Echocardiogram showed a patent foramen ovale vs. small secundum atrial septal defect, which closed spontaneously. Renal ultrasound revealed mild bilateral nonprogressive pelviectasis. Computed tomography demonstrated malformed bilateral semicircular canals resulting in sensorineural hearing loss. He had bilateral tibial and fibular hemimelia and significant lower extremity mesomelia (Fig. 1).
Figure 1.
Lower extremity radiograph taken at birth demonstrating bilateral tibial and fibular hemimelia and lower extremity mesomelia.
Chromosome 6p deletion was delineated by array comparative genomic hybridization from whole blood showing an interstitial loss in copy number within chromosome 6p22.3 detected with 64 clones from position 20, 019, 758-21, 784, 966 encompassing at least 1.76 Mb. The region of loss was independently verified by molecular cytogenetic FISH using the RP11-86017 probe (BlueGnome, Cambridge, UK) specifically for 6p22.3 (ARUP Laboratories, Salt Lake City, UT, USA). Further genetic evaluation of the cause of the patient’s deafness showed no apparent mutations in the GJB2 (connexin 26) gene associated with non-syndromic familial deafness (DNFA3), indicating these genes were not likely the cause of his hearing loss. Parental genetic testing showed no evidence of microdeletions or copy number variant on chromosome 6p22.3 by FISH analysis.
At 3 months of age, the child developed eczematous patches on his cheeks, scalp, and upper extremities. By 10 months of age, the rash spread over both lower extremities. Initial white blood count showed a leukocytosis of 20,000 cells/µl, with 7200 cells/µl eosinophils. As shown in Table 1, repeat studies at 11 months of age showed a total white cell count of 59,920 cells/µl, with 5393 cells/µl neutrophils, 5992 cells/µl lymphocytes, and 47,936 cells/µl eosinophils. Hemoglobin, hematocrit, and platelet counts were normal (Table 1). The eosinophilia not did appear to be the result of parasitic infection as stool evaluation for ova and parasites as well as IgG titers for strongoloides and toxicara was negative. Additional laboratory evaluation revealed an ANA less than 1:40, IgE 20 IU/ml, tryptase level 3.4 µg/l, and immunoglobulin profiles for IgG, IgA, and IgM that were appropriated for age (Table 1). Lymphocyte subset enumeration was normal with 2581 cells/µl total CD3 T cells, CD4/CD8 T-cell ratio of 3.3, 1122 cells/µl total NK cells, and 1909 cells/µl total CD19 B cells. Expression of CD11a, CD11b, CD11c, and CD18 was normal as determined using flow cytometry analysis of peripheral blood monocytes and neutrophils (National Jewish Health Center, Denver, CO, USA). IL-5 level was elevated at 8.3 pg/ml (normal <4.5 pg/ml) (Viracor-IBT Laboratories, Lee’s Summit, MO, USA). Skin biopsy of the rash showed superficial dermatitis with perivascular infiltration of both lymphocytes and eosinophils. Echocardiogram and abdominal ultrasound were performed to evaluate for infiltrative processes in the liver, spleen, and heart, and the results of these studies were normal.
Table 1.
Laboratory results obtained during initial evaluation
| Test (normal values for age) | Patient’s results (age 11 months) |
|---|---|
| White blood cell count (5980–14,990 cells/µl) | 59,920 cells/µl |
| Hemaglobin (8.9–12.7 g/dl) | 11.8 g/dl |
| Platelet count (206,000–597,000 cells/µl) | 419,000 cells/µl |
| Absolute neutrophil count (1200–9000 cells/µl) | 5393 cells/µl |
| Absolute lymphocyte count (2600–10,400 cells/µl) | 5992 cells/µl |
| Absolute eosinophil count (0–500 cells/µl) | 47,936 cells/µl |
| IgG (294–1069 mg/dl) | 247 mg/dl |
| IgA (16–84 mg/dl) | 21 mg/dl |
| IgM (41–149 mg/dl) | 61 mg/dl |
| IgE (<10 IU/ml) | 20 IU/ml |
| Absolute CD3 (1600–6700 cells/µl) | 2581 cells/µl |
| CD4:CD8 ratio (1.38–4) | 3.3 |
| Absolute CD4 (1000–46,000 cells/µl) | 1909 cells/µl |
| Absolute CD8 (400–2100 cells/µl) | 600 cells/µl |
| Absolute CD56 (200–1200 cells/µl) | 1122 cells/µl |
| Absolute CD19 (600–2700 cells/µl) | 1719 cells/µl |
| Stool ova and parasite | Negative × 3 |
| Toxocara antibody | <1.00 |
| Strongyloides antibody | <1.00 |
| B12 Level (<5 yr not established; for 5–9 yr olds 250–1205 pg/ml) | 1271 pg/ml |
| Tryptase (1.9–13.5 µg/l) | 3.4 µg/l |
| IL-5 (<4.5 pg/ml) | 8.2 pg/ml |
| ANA (<1:40) | <1:40 |
Bone marrow examination performed at 11 months of age was hypercellular with active and progressive trilineage maturation and marked eosinophilia. There was no evidence of increased numbers of mast cells, granulomas, metastatic tumor, or increased numbers of blasts cells. All stages of eosinophils were represented ranging from eosinophilic myelocytes to bi-lobed and segmented eosinophils. Obvious dysplastic changes were not seen. Megakaryocyte representation was normal. There was no marrow fibrosis as evaluated by reticulin staining. Immunoperoxidase studies with CD117 showed only sparsely scattered basophils. Flow cytometry analysis of leukocytes in the bone marrow showed no abnormalities in expression of CD3, CD5, CD7, CD4, CD8, CD56, CD19, CD20, CD22, CD10, HLA-DR, CD11b,CD11c, TdT, CD13, CD14, CD15, CD117, CD33, CD64, CD45, CD34, CD38, CD41a, or CD235a. Lymphocytes comprised 7.8% of all nucleated cells, granulocytes 77.3%, monocytes 1.4%, nucleated RBC 12.8%, and myeloblasts 0.7%. There was a distinct predominance of eosinophilic myeloid precursor (64.3%). To evaluate for lymphocytic variant HES, an assessment of T-cell clonality within the bone marrow was performed using spectratyping. Polymerase chain reaction using forward and reverse primers for T-cell receptor gamma and beta showed no evidence of oligoclonality (Quest Diagnostics, San Juan Capistrano, CA, USA). FISH analysis of the bone marrow specimen showed no evidence of myeloproliferative HES based on lack of fusion of the PDGFRA and FLP1LI loci at 4q12, rearrangement of the PDGFRB locus at 5q32, or rearrangement of the FGFR1 locus at 8p12 (2). Vitamin B12 level, a marker of myeloid eosinophilia, was mildly elevated for children over age 5 yr at 1271 pg/ml (Quest Diagnostics).
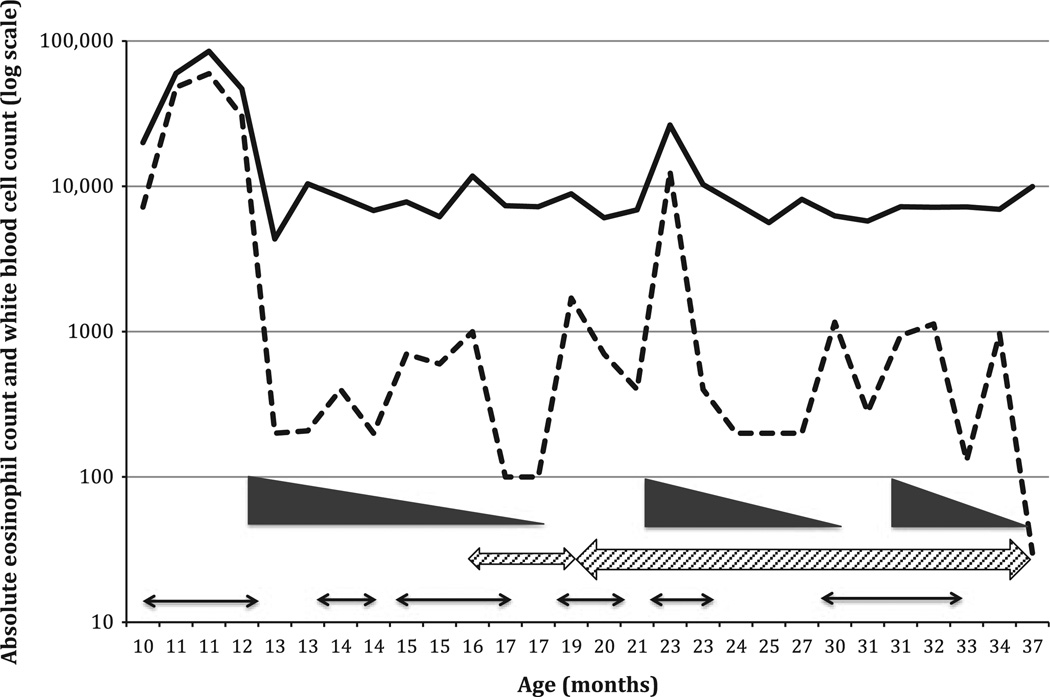
Treatment of HES was initiated at 12 months of age, and the response to treatment is shown in Fig. 2. Initially prednisone was started at 1 mg/kg daily and eosinophilia declined to 200 cells/µl after 3 wk but rebounded when steroids were weaned to 0.35 mg/kg daily. With rebound in eosinophilia, there was exacerbation of the eczematous rash and recurrence of fever. Due to recurrence of symptoms when the prednisone dose was weaned below 0.2 mg/kg every other day, a daily dose of hydroxyurea was added at 12 mg/kg/dose. As pediatric dosing for hydroxyurea for treatment of HES has not been established, dosing was based upon levels used in the treatment of infants and children with sickle cell anemia (3). Toxicity monitoring included complete blood count, comprehensive metabolic panel, and uric acid levels every 4 wk, and clinical exams were performed every 2 months. None of the adverse events previously associated with hydroxyurea, such as neutropenia, thrombocytopenia, anemia, nail changes, or headache, were observed (3). He has not required prednisone since 34 months of age, and hydroxyurea is continued at an increased dose of 16 mg/kg daily. This treatment regimen resulted in resolution of the eosinophilia and rash.
Figure 2.
Therapy induced changes in total white cell and eosinophil counts. Total cell counts shown on the y-axis as log10 scale of cells/µl and the x-axis shows patient’s age in months. Total white cell count is shown as the solid line and total eosinophil count as the dotted line. Timing of prednisone therapy (1 mg/kg and weaned) is shown as the solid wedges; rash is shown as the thin arrows, and hydroxyurea as the hashed arrows. At 19 months of age, hydroxyurea was increased from 12 mg/kg/day to 16 mg/kg/day (wider hashed arrow).
Our patient represents a novel clinical phenotype of chromosome 6p22.3 deletion which includes HES, lower extremity hemimelia, and sensorineural hearing loss. The known phenotypes of 6p deletion syndrome have ranged from autism spectrum disorder, facial abnormalities, cardiac defects, and hearing loss, but this child is the first reported case with hemimelia and HES (1). No other causes of eosinophilia could be identified such as parasitic infection, leukemia, or autoimmunity. Based on current classification schemas for HES, this patient best fits an idiopathic, undefined variant where there are no signs of organ infiltration, no evidence of myeloproliferation, or T-cell activation or oligoclonal expansions (2, 4). The only finding consistent with the myeloproliferative form of HES was a mildly elevated vitamin B12 level, as there was no evidence of anemia, thrombocytopenia, elevation in tryptase, or hepatosplenomegaly (4, 5). Assessment for PDGFRA and FLP1LI fusion within the bone marrow specimen was also negative (6). His clinical and laboratory findings were also inconsistent with lymphocytic variant HES. Analysis of T-cell receptor gene recombination using PCR-based spectratyping is the most sensitive way to assess oligoclonal T-cell expansions in blood or bone marrow (7). However, analysis for oligoclonality within both the gamma/delta and alpha/beta T-cell populations failed to reveal any dominant T-cell expansions. T-cell subset analysis showed no evidence of activation in the blood. Abnormal T-cell CD4/CD8 ratios are commonly seen in lymphocytic variant HES, and there was no evidence of abnormal expression of CD38, HLA-DR, or CD22 on T cells within the bone marrow (4). IL-5 level, while elevated, was not in the range expected to be seen in myeloproliferative or lymphocytic variant HES (8). In spite of these findings, there were gaps in the evaluation of HES in our patient as the lymphocyte infiltration within the skin biopsy was not evaluated for T-cell clonality and levels of thymus, and activation-regulated chemokine (CCL17), a marker for lymphocytic variant HES, was not measured (9, 10). However, CCL17 levels in young infants are higher than in adults and older children, and elevated levels in infants with HES have not been established. Therefore, the results of this assay may not provide additional predictive value as a biomarker of HES.
The clinical manifestations of HES in our patient was benign. Apart from fever and rash, there was no other clinical findings associated with the eosinpophilia and, after 3 yr, no evidence of organ infiltration. The patient responded well to conservative therapy with corticosteroids and hydroxyurea (2, 6). As evident in Fig. 2, rebound in the rash and eosinophil count occurred when prednisone was weaned necessitating the use of hydroxyurea as a steroid sparing agent. There are almost no reports of the use of hydroxyurea in very young infants with HES. However, using a dosing regimen commonly used for infants with sickle cell disease, our patient tolerated this medication well showing no evidence of hydoxyurea toxicity at the 16/mg/kg/day dose. He continues to show an excellent therapeutic response as measured by resolution of the rash and normalization of his eosinophil count. He was successfully weaned off prednisone and has been maintained on hydroxyurea monotherapy. It is possible there are genes within 6p that play a critical role in activation and proliferation of myeloid elements within the bone marrow. Currently, the precise roles these genes play in the mechanisms of 6p mitigated eosinophil proliferation are unknown. Similarly, transcriptional elements that map to 6p that regulate growth factors for eosinophil development are unknown. The expanding use of array technologies may provide additional insight into the role 6p genes play in myeloid development. As more children with HES and 6p deletions are identified, new insight into how this region of 6p delineates genotype to phenotype relationship in the 6p22–6p24 syndrome will be discovered. Such studies are needed in order to determine the long term implications of these deletions in affected children.
Acknowledgments
This study was supported 1R01 AI 100147-01, by the Robert A. Good Endowment and a grant from the Jeffrey Model Foundation.
J.W. Sleasman received research support from the National Institutes of Health and CSL Behring.
Footnotes
Conflict of interest
No competing financial interests exist for the other authors.
References
- 1.Mirza G, Williams RR, Mohammed S, et al. Refined genotype-phenotype correlations in cases of chromosomal 6p deletion syndromes. Eur J Hum Genet. 2004;12:718–728. doi: 10.1038/sj.ejhg.5201194. [DOI] [PubMed] [Google Scholar]
- 2.Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117:1292–1302. doi: 10.1016/j.jaci.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Strouse J, Lanzkron S, Beach M, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122:1332–1342. doi: 10.1542/peds.2008-0441. [DOI] [PubMed] [Google Scholar]
- 4.Simon HU, Rothenberg ME, Bochner BS, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126:45–49. doi: 10.1016/j.jaci.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zittoun J, Farcet JP, Marquet J, Sultan C, Zittoun R. Cobalamin (vitamin B12) and B12 binding proteins in hypereosinophilic syndromes and secondary eosinophilia. Blood. 1984;63:779–783. [PubMed] [Google Scholar]
- 6.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319.e3–1325.e3. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kou ZC, Puhr JS, Wu SS, et al. Combination antiretroviral therapy results in a rapid increase in T cell receptor variable region beta repertoire diversity within CD45RA CD8 T cells in human immunodeficiency virus-infected children. J Infect Dis. 2003;187:385–397. doi: 10.1086/367674. [DOI] [PubMed] [Google Scholar]
- 8.Ravoet M, Sibille C, Gu C, et al. Molecular profiling of CD3(−)CD4(+) T-cells from patients with the lymphocytic variant of hypereosinophillic syndrome reveals targeting of growth control pathways. Blood. 2009;114:2969–2983. doi: 10.1182/blood-2008-08-175091. [DOI] [PubMed] [Google Scholar]
- 9.de Lavareille A, Roufosse F, Schmid-Grendelmeier P, et al. High serum thymus and activation-regulated chemokine levels in the lymphocytic variant of the hypereosinophillic syndrome. J Allergy Clin Immunol. 2002;110:476–479. doi: 10.1067/mai.2002.127003. [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa T, Nagao M, Hiraguchi Y, et al. Measurement of thymus and activation-regulated chemokine/CCL17 in children with atopic dermatitis: elevated normal levels in infancy and age-specific analysis in atopic dermatitis. Pediatr Allergy Immunol. 2009;20:633–641. doi: 10.1111/j.1399-3038.2009.00851.x. [DOI] [PubMed] [Google Scholar]