Abstract
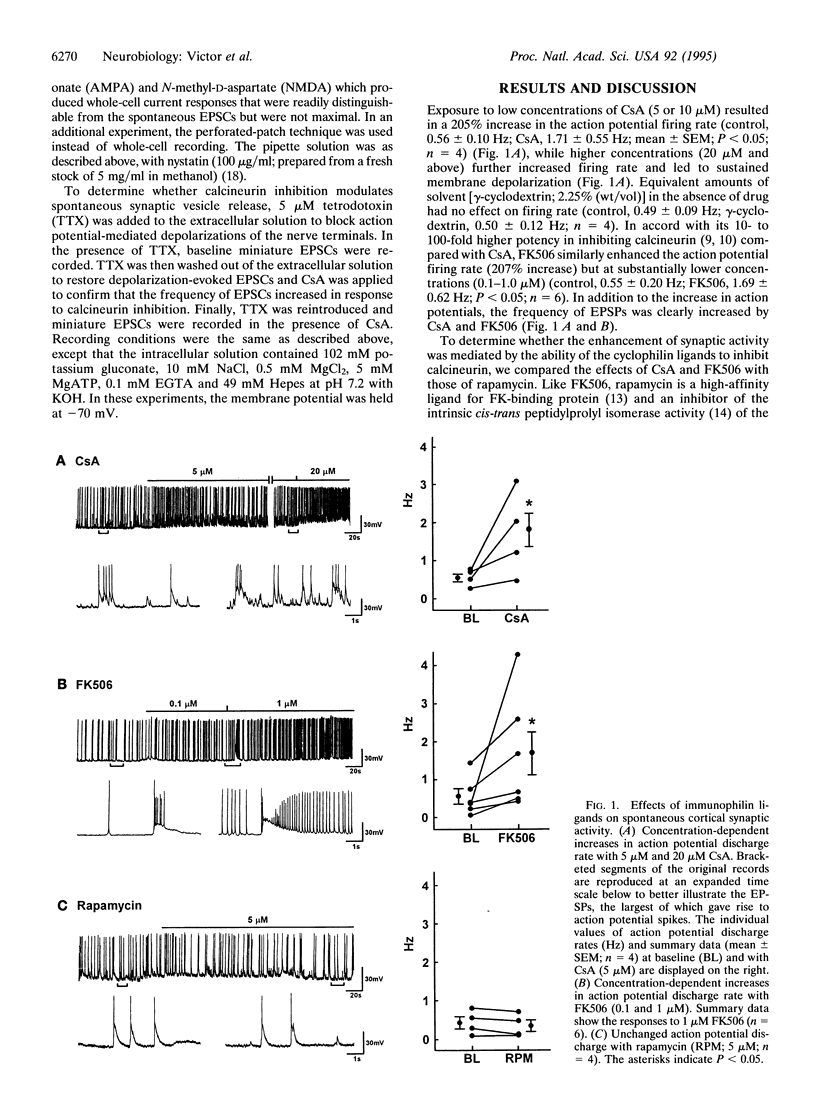
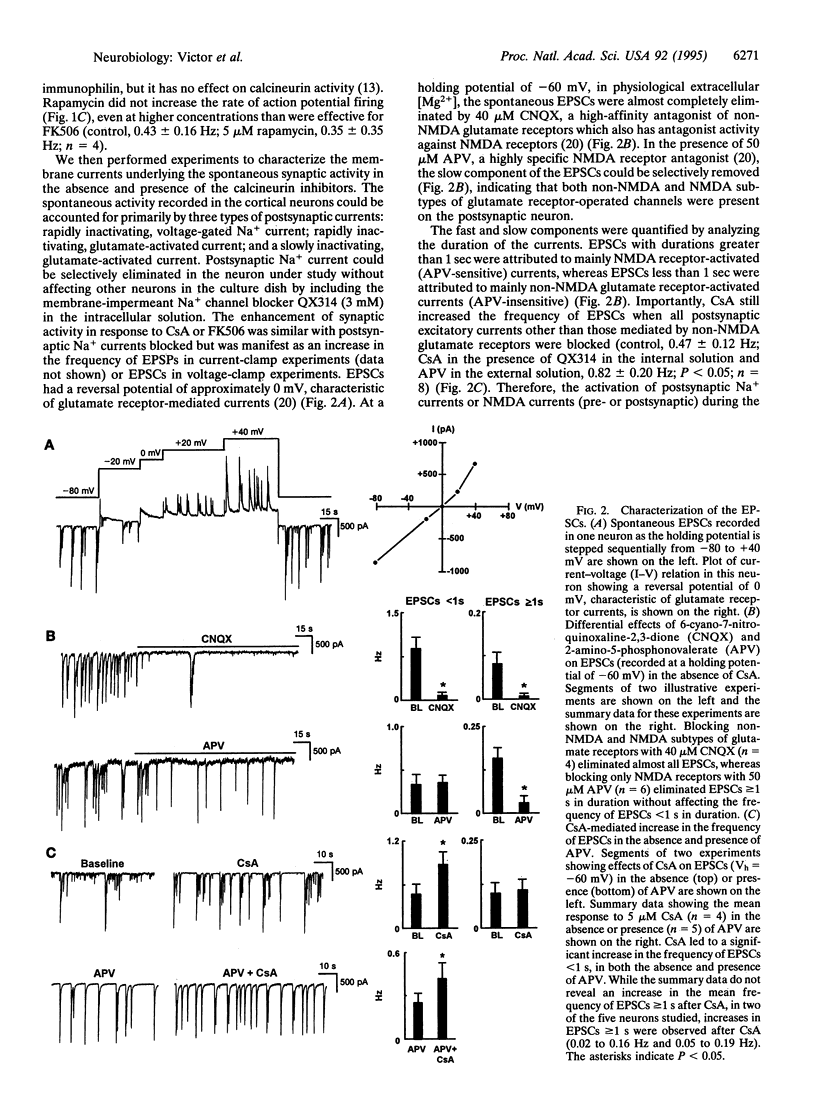
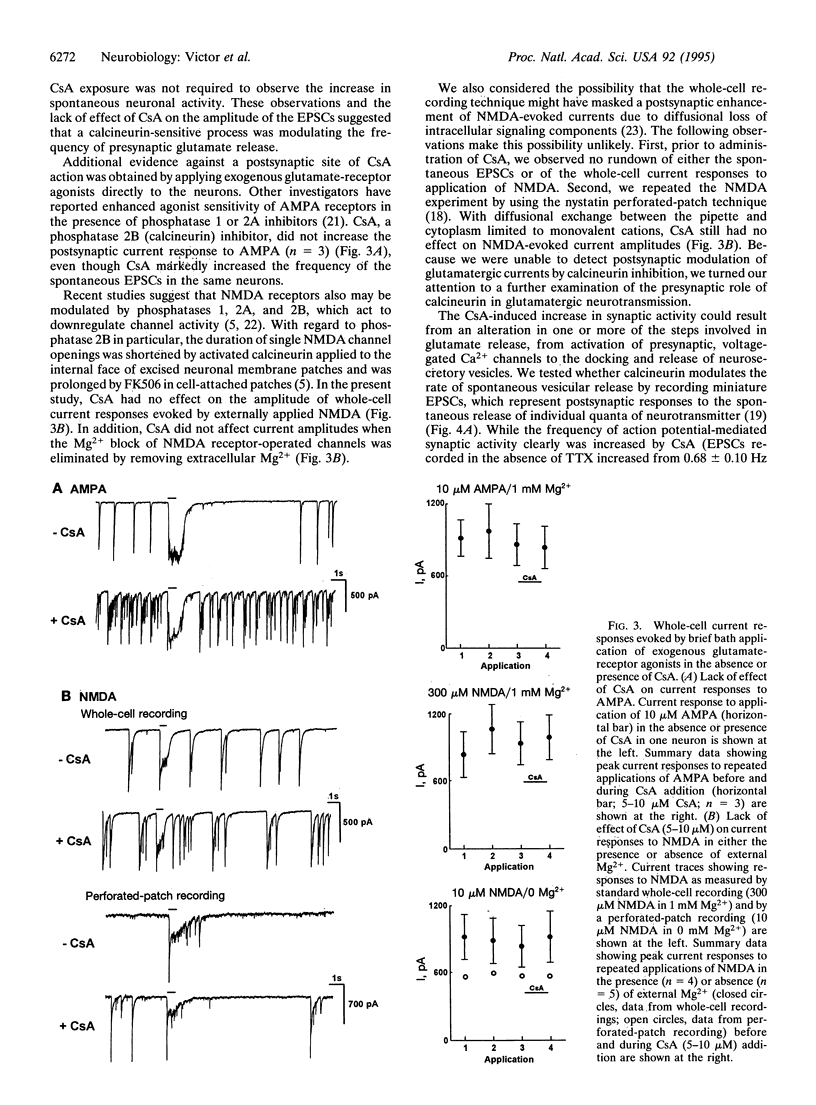
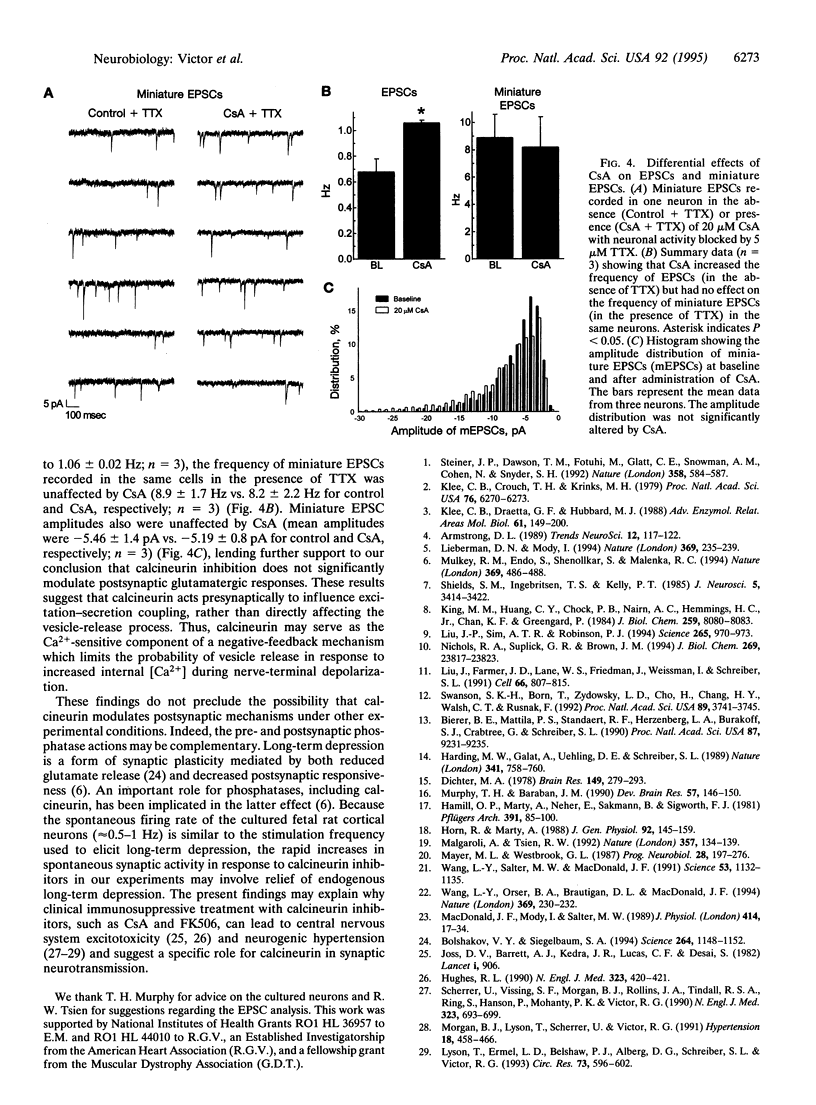
Synaptic plasticity is modulated by Ca(2+)-induced alterations in the balance between phosphorylation and dephosphorylation. Recent evidence suggests that calcineurin, the Ca(2+)-calmodulin-dependent phosphatase (2B), modulates the activity of postsynaptic glutamate receptors. However, in rat cortex, calcineurin is enriched mainly in presynaptic, not postsynaptic, fractions. To determine if calcineurin modulates glutamatergic neurotransmission through a presynaptic mechanism, we used whole-cell patch clamp experiments to test effects of two specific calcineurin inhibitors, cyclosporin A (CsA) and FK506, on synaptic activity in fetal rat cortical neurons. The rate of spontaneous action-potential firing was markedly increased by either CsA or FK506 but was unaffected by rapamycin, a structural analog of FK506 which has no effect on calcineurin. In voltage-clamp experiments, CsA increased the rate but not the amplitude of glutamate receptor-mediated, excitatory postsynaptic currents, suggesting an increased rate of glutamate release. CsA had no effect on the amplitude of currents evoked by brief bath application of selective glutamate receptor agonists, providing further evidence for a pre- rather than postsynaptic site of action. In conclusion, these data indicate that calcineurin modulates glutamatergic neurotransmission in rat cortical neurons through a presynaptic mechanism.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. L. Calcium channel regulation by calcineurin, a Ca2+-activated phosphatase in mammalian brain. Trends Neurosci. 1989 Mar;12(3):117–122. doi: 10.1016/0166-2236(89)90168-9. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov V. Y., Siegelbaum S. A. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994 May 20;264(5162):1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- Dichter M. A. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978 Jun 30;149(2):279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. L. Cyclosporine-related central nervous system toxicity in cardiac transplantation. N Engl J Med. 1990 Aug 9;323(6):420–421. doi: 10.1056/NEJM199008093230616. [DOI] [PubMed] [Google Scholar]
- Joss D. V., Barrett A. J., Kendra J. R., Lucas C. F., Desai S. Hypertension and convulsions in children receiving cyclosporin A. Lancet. 1982 Apr 17;1(8277):906–906. doi: 10.1016/s0140-6736(82)92171-7. [DOI] [PubMed] [Google Scholar]
- King M. M., Huang C. Y., Chock P. B., Nairn A. C., Hemmings H. C., Jr, Chan K. F., Greengard P. Mammalian brain phosphoproteins as substrates for calcineurin. J Biol Chem. 1984 Jul 10;259(13):8080–8083. [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Lieberman D. N., Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994 May 19;369(6477):235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Liu J. P., Sim A. T., Robinson P. J. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994 Aug 12;265(5174):970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lyson T., Ermel L. D., Belshaw P. J., Alberg D. G., Schreiber S. L., Victor R. G. Cyclosporine- and FK506-induced sympathetic activation correlates with calcineurin-mediated inhibition of T-cell signaling. Circ Res. 1993 Sep;73(3):596–602. doi: 10.1161/01.res.73.3.596. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Tsien R. W. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature. 1992 May 14;357(6374):134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Morgan B. J., Lyson T., Scherrer U., Victor R. G. Cyclosporine causes sympathetically mediated elevations in arterial pressure in rats. Hypertension. 1991 Oct;18(4):458–466. doi: 10.1161/01.hyp.18.4.458. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Endo S., Shenolikar S., Malenka R. C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994 Jun 9;369(6480):486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Murphy T. H., Baraban J. M. Glutamate toxicity in immature cortical neurons precedes development of glutamate receptor currents. Brain Res Dev Brain Res. 1990 Dec 1;57(1):146–150. doi: 10.1016/0165-3806(90)90195-5. [DOI] [PubMed] [Google Scholar]
- Nichols R. A., Suplick G. R., Brown J. M. Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate. J Biol Chem. 1994 Sep 23;269(38):23817–23823. [PubMed] [Google Scholar]
- Scherrer U., Vissing S. F., Morgan B. J., Rollins J. A., Tindall R. S., Ring S., Hanson P., Mohanty P. K., Victor R. G. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990 Sep 13;323(11):693–699. doi: 10.1056/NEJM199009133231101. [DOI] [PubMed] [Google Scholar]
- Shields S. M., Ingebritsen T. S., Kelly P. T. Identification of protein phosphatase 1 in synaptic junctions: dephosphorylation of endogenous calmodulin-dependent kinase II and synapse-enriched phosphoproteins. J Neurosci. 1985 Dec;5(12):3414–3422. doi: 10.1523/JNEUROSCI.05-12-03414.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J. P., Dawson T. M., Fotuhi M., Glatt C. E., Snowman A. M., Cohen N., Snyder S. H. High brain densities of the immunophilin FKBP colocalized with calcineurin. Nature. 1992 Aug 13;358(6387):584–587. doi: 10.1038/358584a0. [DOI] [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Orser B. A., Brautigan D. L., MacDonald J. F. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994 May 19;369(6477):230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Salter M. W., MacDonald J. F. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991 Sep 6;253(5024):1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]



