Abstract
Cycloaddition reactions involving tetrazine have proven to be powerful bioorthogonal tools for various applications. Conceivably, sequential and selective labeling using tetrazine-based reactions can be achieved by tuning the reaction rate. By varying the substituents on tetrazine, cycloaddition rate variations of over 200 fold have been achieved with the same dienophile. Coupled with the availability of different dienophiles, such as norbornene, the reaction rate difference can be over 14,000 folds. These substituted tetrazines can be very useful for selective labeling under different conditions.
Introduction
In the bioorthogonal labeling field, selective labeling via copper-free click chemistry1–5 is finding increasing applications. There are many pairs of excellent click reagents used in this area including strain-promoted azide-alkyne cycloadditions (SPAAC)6–10 and inverse-electron demand Diels–Alder reactions (IEDDAR) involving trans-cyclooctene(TCO)11–13 and tetrazine. The selective labeling of two (or more) positions in a single biomolecule is more challenging than coupling only one reagent. In order to achieve multiplexing, one approach could bethrough the use of reagents with distinct reaction rates. Cycloaddition reactions involving strain-promoted azide and alkyne typically have second order rate constants of no more than 1.9 M−1s−1.14 The IEDDA involving 1,2,4,5-tetrazines with TCO, alkyne, or norbornene, which have been used for DNA modifications and protein labeling, were developed by the Fox group and the Weissleder group and have very fast reaction rates (second order rates of up to 22000 M−1s−1).11–13 With the extremely fast reaction rates involving tetrazine, it gives the chance to tune the reaction rate to allow for staged labeling and yet still have a fairly fast reaction rate for each reaction to finish within a reasonable period of time. Thus we are interested in looking for ways to fine tune the tetrazine-based reaction rates for staged labeling and multiplexing applications.
In our previous work, we studied the reaction between 3,6-substituented-1,2,4,5-tetrazines (1) and bicycle[6.1.0]nonyne (BCN, 2) and found this reaction rate to be tunable (Figure 1).15Thus we used the BCN-tetrazine pair as our model reaction set. Again, it should be noted that tetrazine can react very quickly with various alkenes and alkynes and thus a “tool set” for fast labeling can be developed by using the tetrazine cycloaddition chemistry.16 Herein, we present a comprehensive follow up study, in which we optimized the procedure for tetrazine synthesis and investigated the reactivity between substituted 1,2,4,5-tetrazines (1) and BCN (2) (Fig. 1).
Figure 1.
1,2,4,5-Tetrazine (1) as electron-poor diene (tetrazine, 1) and bicycle[6.1.0]nonyne (BCN, 2) as a dienophile
Results and Discussion
Synthesis of asymmetric 3,6-disubstitued-1,2,4,5-tetrazine
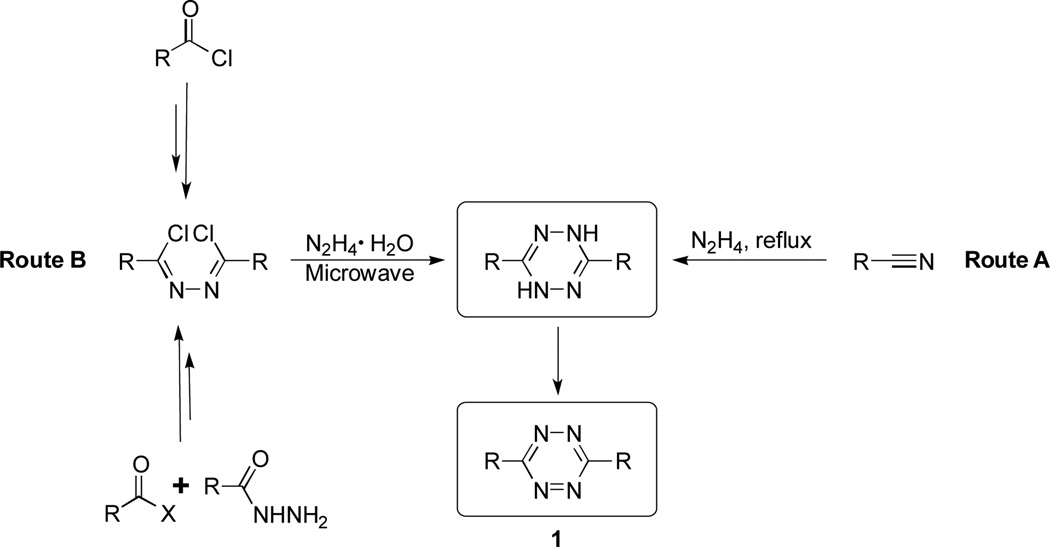
The study requires the preparation of tetrazines with various substitutions. Especially important are the asymmetrically substituted tetrazines, which afford a greater degree of tunability as compared to only using symmetric tetrazines. Generally, 3,6-disubstitued-1,2,4,5-tetrazines can be synthesized from commercially available nitriles and hydrazine hydrate (Scheme 1, Route A) in a one-pot procedure.17–21 Unfortunately, this method is substituent dependent and only suitable for some symmetric examples. Asymmetric tetrazines with strong electron withdrawing groups either cannot be made by this method or can only be made with extremely low yields and reproducibility.22–25
Scheme 1.
Synthetic routes of 3,6-substitued-1,2,4,5-tetrazines.
For the synthesis of asymmetric tetrazines, we optimized the conditions and improved the yield upon our previous reported synthetic method (Scheme 1, Route B).26, 27 The formation of the 1,2,4,5-tetrazine-ring system involves condensation of 1,2-dichloromethylene hydrazines with hydrazine monohydrate under microwave conditions. The dihydrotetrazine, which is dissolved in acetic acid, can be oxidized by sodium nitrite at 0 °C. This optimized procedure allows the preparation of a variety of tetrazines (1, Table 1) with moderate yields (up to 50 %), short reaction time, and easy purifications.
Table 1.
3,6-Substituted-1,2,4,5-tetrazines (1) prepared*
 |
We examined whether microwave could facilitate the conversion step of 1,2-dichloromethylene hydrazines (3) to 3,6-substituted-1,2,4,5-tetrazines (1). As shown in Table 2, the reaction time were shortened from up to 24 hr to 30 min with an improvement of reaction yield in the range of about 20 % when microwave was used.
Table 2.
Reaction yield and time differences between traditional conditions and microwave conditions for conversion of 1,2-dichloromethylene hydrazines to 3,6-substituted-1,2,4,5-tetrazines.
| Product | Yield[*] (reaction time) |
Yield[**] (reaction time) |
|---|---|---|
 |
61% (8 h) | 81% (30 min) |
 |
58% (8 h) | 73% (30 min) |
 |
66% (16 h) | 80% (30 min) |
 |
52% (5 h) | 65% (30 min) |
Yields under traditional reflux conditions.
Yields under microwave conditions.
All yields were Isolated yields after column chromatography.
Kinetic Studies
[4+2] Cycloaddtion with electron-poor tetrazines led to the expected clean pyridazines products. The critical determining factor of reaction rate is the LUMOdiene–HOMOphil gap.28–30 Our previous work15 analyzed the effect of electron-withdrawing substituents on decreasing the LUMO energy of the diene, leading to a decrease in the LUMOdiene – HOMOphil gap and consequently an increase in the reaction rate. Again the highly strained alkyne (BCN 2) was used as a model dienophile. All reactions proceeded cleanly with N2 as byproduct. Side reactions were not observed and yields were generally high (Table 3). The reaction between a substituted tetrazine (1) and BCN (2) leads to significant changes in the UV-vis spectrum of tetrazine due to its conversion to a pyridazine product. Hence, the reaction rate constants can be determined by monitoring the decrease of the UV intensity at 290~300 nm of the reaction mixture (Table 3).
Table 3.
Reactions of 3,6-substituented-1,2,4,5-tetrazines (1) with BCN (2); rate constants at ambient temperature in MeOH.
 | |||
|---|---|---|---|
| Tetrazine (R1 / R2) | Product Number |
Yield (%)* |
Second order rate constant (M−1s−1) |
 |
5 | 97 | 125 |
| 6 | 98 | 118 | |
| 7 | 96 | 23 | |
| 8 | 95 | 10 | |
 |
9 | 97 | 3.6 |
| 10 | 94 | 2.7 | |
| 11 | 92 | 0.58 | |
| 12 | 94 | 1.4 | |
 |
13 | 92 | 2.3 |
Isolated yields after column chromatography.
We first examined the reaction between BCN and 3,6-diphenyl-1,2,4,5-tetrazine (1b) in methanol (HPLC-grade) as a reference. The rate constant was found to be 3.6 M−1s−1 (Table 3). Introduction of pyridin-2-yl to the tetrazine structure increased the reaction rate because of strong electron withdrawing property of the pyridyl group. For example, the second order rate constant of 3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine (1a) was 118 M−1s−1, which represents a 37-fold improvement in reaction rate compared to the reaction of diphenyltetrazine (1b). In the case of an asymmetric tetrazine, the reaction of 3-(4-fluorophenyl)-6-(pyridin-2-yl)-1,2,4,5-tetrazine (1n) with BCN showed a rate constant of 23 M−1s−1, which is lower than that of 1a, presumably because the 4-fluorophenyl group has less electron withdrawing ability than pyridine. However, it is still 7-fold faster than the reference reaction with 1b. To further tune the reaction rate, 4-(trifluoromethyl)phenyl groups and pyrimidine ring were attached to the tetrazine ring to give 3,6-bis(4-(trifluoromethyl)phenyl)-1,2,4,5-tetrazine (1k) and 3-(pyrimidin-2-yl)-6-(4-(trifluoromethyl)phenyl)-1,2,4,5-tetrazine (1p), respectively. The second order rate constants of these two tetrazines with BCN in MeOH at ambient temperature were found to be 10 and 125 M−1s−1, respectively. The rate for 3-(pyrimidin-2-yl)-6-(4-(trifluoromethyl)-phenyl)-1,2,4,5-tetrazine (1p) is the fastest so far, which represents a 40-fold improvement over that of the reaction involving diphenyltetrazine.
Since increasing the electron density on the tetrazine is expected to increase the LUMOdiene–HOMOphil gap by increasing the diene LUMO energy, the reaction rate should be slower than reference reaction when the tetrazine has an electron-donating group. Indeed, we test three tetrazines with electron-donating substituents (1c, 1d, 1e), and found the reaction rate constants to be 2.3 M−1s−1, 0.58M−1s−1, and 1.4 M−1s−1, respectively in MeOH.
We also found the reaction rate between 1,2,4,5-tetrazine and BCN being moderately sensitive to solvent property (Table 4). For example, the reaction of 3,6-bis(4-fluorophenyl)-1,2,4,5-tetrazine (1i) has a slightly higher reaction rate (2.7 M−1s−1) when performed in MeOH than in acetonitrile (1.4 M−1s−1). Another interesting finding is the reaction rate of 1i being slightly lower than that of 1b (3.6 M−1s−1) in MeOH. Though we expected the reaction of 1i be faster than 1b since fluorine is an electron-withdrawing group (σp = 0.06). The answer could be in the balance between resonance effect and inductive effect. The same solvent-sensitivity is found in the reaction of 3,6-bis(4-hydroxylphenyl)-1,2,4,5-tetrazine (1d) and 3,6-bis(4-methoxyphenyl)-1,2,4,5-tetrazine (1e). The reaction rate for 1d and 1e in MeOH is 0.58 M−1s−1 and 1.4 M−1s−1, respectively. However, in acetonitrile, the rates are 0.36 M−1s−1 and 0.16M−1s−1. Water could be included as a significant part of the solvent with some attenuation of reaction rates.
Table 4.
Solvent effect on rate constants between 3,6-substituented-1,2,4,5-tetrazines (1) with BCN (2) at ambient temperature.
| MeOH Second order rate constant (M−1s−1) |
CH3CN Second order rate constant (M−1s−1) |
|
|---|---|---|
 |
3.6 | 1.2 |
| 2.7 | 1.4 | |
| 0.58 | 0.36 | |
| 1.4 | 0.16 |
Norbornene as a dienophile
Norbornene is another click partner for electron-poor tetrazines,31–35 and has been used in DNA modification and protein labeling. The reaction with norborene is much slower than with TCO or strained alkyne BCN. The Carell group performed a systematic kinetic study of the reactivity of norbornene derivatives and demonstrated that norbornenes with an non-electron-withdrawing substituent in the exo position are favored in the reaction.36 Tetrazines with electron-withdrawing substituents were chosen for our kinetic studies. All reactions were performed in MeOH at 1:10 ratio (tetrazine: 1 mM; norborene: 10 mM). From the kinetic data (Table 5), we can see the trend of reactivity being the same as for strained alkyne BCN. However, the reaction rate constants are far smaller than that of the reaction between substituted tetrazines and BCN. For instance, the reaction rate constant of the reference (1b) is 8.5 × 10−3 M−1s−1, which means this reaction could be completed in 2 days at1 mM tetrazine concentration. The fastest reaction rate observed is that of the asymmetric tetrazine (1p), which has a pyrimidin-2-yl group and a potential “handle” for further tethering. The reaction was finished in 2 hour (1 mM tetrazine concentration). This is also the reason for asymmetric pyridimdin-2-yl tetrazine being the most popular tetrazine used in the norbornene system based on literature reports.34, 37
Table 5.
Reactions of 3,6-substituented-1,2,4,5-tetrazines (1) with norbornene-2-carboxaldehyde (4); rate constants at ambient temperature in MeOH.
 | |
|---|---|
| Tetrazine (R1 / R2) | Second order rate constant (M−1s−1) |
 |
8.5× 10−3 |
| 0.039 | |
| 0.056 | |
| 0.65 | |
 |
0.61 |
From Table 5, we can see that the norborene system has relatively slow reaction rates. All rate constants are at least 200 fold slower than the BCN system. However, compared to Staudinger ligation (2.4 × 10−3 M−1s−1) and SPAAC (in the range of 2 ~ 70 × 10−3M−1s−1),14 the reaction rates for the tetrazine-norborenen system are still considered fast and can still be used for click labeling applications. Thus, among all the reactions examined in this, there is an over 14,000-fold separation in reactions rates (Entry 1 in Table 3 and Entry 1 in Table 5). Such a large separation in reaction rates should allow for the selection of reaction pairs suitable for staged labeling of more than one site in the same biomacromolecule. If the trans-alkenes are brought into the picture, there is an even greater reaction rate separation, which should provide a great deal of flexibility in selecting reaction pairs for labeling studies.
Conclusion
Tetrazine cycloaddition as a powerful bioorthogonal tool can be used for selective labeling studies by using reaction pairs with large separations in reaction rates. By tuning the substituents of the tetrazine moiety, a large separation of reaction rates can be achieved. We believe that the availability of reactions pairs with tunable reaction rates will be very useful in click-labeling applications.
Experimental
Materials and Methods
All reagents and solvents were of reagent grade or were purified by standard methods before use. Column chromatography was carried out on flash silica gel (Sorbent 230–400 mesh). TLC analysis was conducted on silica gel plates (Sorbent Silica G UV254). NMR spectra were recorded at 400 MHz for 1H on a Bruker instrument. Chemical shifts (δ values) and coupling constants (J values) are given in ppm and hertz, respectively, using solvents (1H NMR) as the internal standard. Symmetric tetrazines1a–1e, and BCN (2) were synthesized according to literature procedures. General synthesis procedure and characterization data of 3,6-substituted-1,2,4,5-tetrazines 1f–1p, compound 6, and compound 9 were already reported by our group.15, 26
Kinetics measurements of the reaction of 3,6-substituted-1,2,4,5-tetrazines 1 with BCN 2 or norbornene-2-carboxaldehyde 4
UV/Vis kinetic measurements: Separate solutions of pure tetrazines (1) and pure BCN (2, >95–98 % by 1H-NMR) or norbornene-2-carboxaldehyde (4, 95%, Sigma-Aldrich), were prepared in HPLC-grade MeOH at room temperature. The stability of tetrazines (1) in MeOH was examined by monitoring their absorption at 295–330 nm. Solutions containing 1 (25 µM) and 10–18 fold excess of BCN (2) or norbornene-2-carboxaldehyde (4) were pipetted into quartz cuvettes and thoroughly mixed. All kinetic runs were the results of triplicates. Curve fitting was conducted in Prism5 software.
General procedure for strain-promoted inverse electron demand Diels-Alder reactions with 1,2,4,5-tetrazine
To a solution of tetrazine (1) in MeOH (1 mL, HPLC-grade), BCN (2) or norbornene-2-carboxaldehyde (4) in MeOH (1 mL, HPLC-grade) was added. Reactions were stirred at room temperature for 5 to 30 min. The progress of the reaction was monitored by TLC. Upon completion, the reaction mixture was directly loaded on the flash column chromatography for purification.
((6aS,7S,7aR)-1-(Pyrimidin-2-yl)-4-(4-(trifluoromethyl)phenyl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2-d]pyridazin-7-yl)methanol (5)
Compound 5 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 20 : 1) to give a yellow solid (Yield, 97 %). 1H NMR (MeOD): 0.95–1.10 (m, 3H), 1.65–1.75 (m, 2H), 2.10–2.32 (m, 2H), 2.80–2.90 (m, 2H), 2.93–3.10 (m, 2H), 3.68 (d, 2H, J = 6.8 Hz), 7.67 (t, 1H, J = 5.0Hz), 7.78 (d, 2H, J= 8.0 Hz), 7.90 (d, 2H, J= 8.0 Hz), 9.04 (d, 2H, J = 5.0 Hz). HRMS (ESI): m/z calcd for C23H21F3N4O [M+H]+ 427.1746, found 427.1744.
((6aR,7S,7aS)-1-(4-Fluorophenyl)-4-(pyridin-2-yl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2-d]pyridazin-7-yl)methanol (7)
Compound 7 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 20 : 1) to give a yellow solid (Yield, 96 %). 1H NMR (MeOD): 1.00–1.13 (m, 3H), 1.60–1.70 (m, 2H), 2.10–2.20 (m, 2H), 2.80–2.90 (m, 2H), 2.93–3.10 (m, 2H), 3.67 (d, 2H, J = 7.2 Hz), 7.32 (dd, 2H, J1 = 8.8 Hz; J2 = 8.8 Hz), 7.50–7.60 (m, 3H), 7.81 (d, 1H, J = 7.6 Hz), 8.06 (dd, 1H, J1 = 1.6 Hz, J2 = 8.0 Hz), 8.74 (d, 1H, J = 1.6 Hz). HRMS (ESI): m/z calcd for C23H22FN3O [M+H]+ 376.1825, found 376.1818.
((6aR,7s,7aS)-1,4-Bis(4-(trifluoromethyl)phenyl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2-d]pyridazin-7-yl)methanol (8)
Compound 8 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 50 : 1) to give a yellow solid (Yield, 95 %). 1H NMR (MeOD): 1.09–1.20 (m, 3H), 1.60–1.70 (m, 2H), 2.10–2.20 (m, 2H), 2.80–2.90 (m, 2H), 2.93–3.05 (m, 2H), 3.68 (d, 2H, J = 7.2 Hz), 7.76 (d, 4H, J = 8.0 Hz), 7.90 (d, 4H, J = 8.0 Hz). HRMS (ESI): m/z calcd for C26H22F6N2O [M+H]+ 493.1715, found 493.1721.
((6aR,7s,7aS)-1,4-Bis(4-fluorophenyl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2–d]pyridazin-7-yl)methanol (10)
Compound 10 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 50 : 1) to give a yellow solid (Yield, 94 %). 1H NMR (CDCl3): 0.80–0.90 (m, 3H), 1.30–1.40 (m, 2H), 1.40–1.70 (m, 2H) 2.40–2.50 (m, 2H), 2.70–2.80 (m, 2H), 2.88–2.95(m, 2H), 3.44 (d, 2H, J = 6.8 Hz), 7.17–7.22 (m, 4H) 7.49–7.53 (m, 4H). HRMS (ESI): m/z calcd for C24H22F2N2O [M+H]+ 393.1778, found 393.1762.
4,4'-((6aR,7s,7aS)-7-(Hydroxymethyl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2-d]pyridazine-1,4-diyl)diphenol (11)
Compound 11 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 50 : 1) to give a yellow solid (Yield, 92 %). 1H NMR (DMSO-D6): 0.65–0.76 (m, 3H), 1.35–1.50 (m, 2H), 2.25–2.32 (m, 2H), 2.63–2.70 (m, 2H), 2.80–3.86(m, 2H), 3.20 (dd, 2H, J1 = 5.6 Hz, J2 = 5.6 Hz), 4.40(t, 1H, J = 5.6 Hz) 6.91 (d, 2H, J = 8.4 Hz), 7.33 (d, 2H, J = 8.4 Hz), 9.77 (s, 1H). HRMS (ESI): m/z calcd for C24H24N2O3 [M+H]+ 389.1865, found 389.1862.
((6aR,7s,7aS)-1,4-Bis(4-methoxyphenyl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2-d]pyridazin-7-yl)methano(12)
Compound 12 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 50 : 1) to give a yellow solid (Yield, 94 %). 1H NMR (CDCl3): 0.80–0.90 (m, 3H), 1.30–1.40 (m, 2H), 1.55–1.85 (m, 2H) 2.40–2.50 (m, 2H), 2.70–2.80 (m, 2H), 2.88–2.95 (m, 2H), 3.42 (d, 2H, J = 6.8 Hz), 7.02 (d, 4H, J = 8.8 Hz) 7.48 (d, 4H, J = 8.8 Hz). HRMS (ESI): m/z calcd for C26H28N2O3 [M+H]+ 417.2178, found 417.2180.
4,4'-((6aR,7s,7aS)-7-(Hydroxymethyl)-6,6a,7,7a,8,9-hexahydro-5H-cyclopropa[5,6]cycloocta[1,2-d]pyridazine-1,4-diyl)bis(2-nitrophenol) (13)
Compound 13 was prepared according to the general procedure and purified by column chromatography (DCM:MeOH, 50 : 1) to give a yellow solid (Yield, 92 %). 1H NMR (DMSO): 0.70–0.86 (m, 3H), 1.35–1.50 (m, 2H), 2.30–2.40 (m, 2H), 2.65–2.70 (m, 2H), 2.80–3.90 (m, 2H), 3.23 (d, 2H, J = 6.0 Hz), 7.31 (d, 2H, J = 8.4 Hz), 7.73 (d, 2H, J = 8.4 Hz), 8.02 (s, 2H). HRMS (ESI): m/z calcd for C24H22N4O7 [M+H]+ 479.1567, found 479.1570.
Supplementary Material
Acknowledgements
Financial support from the NIH (GM086925, GM084933, and CA159567) and the Georgia State University Molecular Basis of Disease Program (MBD) through a fellowship to DZW is gratefully acknowledged.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sletten EM, Bertozzi CR. Angew. Chem., Int. Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best MD. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 4.Lim RK, Lin Q. Chem. Commun. 2010;46:1589–1600. doi: 10.1039/b925931g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stöckmann H, Neves AA, Day HA, Stairs S, Brindle KM, Leeper FJ. Chem. Commun. 2011;47:7203–7205. doi: 10.1039/c1cc12161h. [DOI] [PubMed] [Google Scholar]
- 6.Ning X, Guo J, Wolfert MA, Boons GJ. Angew. Chem., Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J. Am. Chem. Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poploukhtine AA, Mbua NE, Wolfert MA, Boons GJ, Popik VV. J. Am. Chem. Soc. 2009;131:15769–15776. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debets MF, van Berkel SS, Schoffelen S, Rutjes FP, van Hest JC, van Delft FL. Chem. Commun. 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 10.Jewett JC, Sletten EM, Bertozzi CR. J. Am. Chem. Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor MT, Blackman ML, Dmitrenko O, Fox JM. J. Am. Chem. Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew. Chem., Int. Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debets MF, van Hest JC, Rutjes FP. Org. Biomol. Chem. 2013;11:6439–6455. doi: 10.1039/c3ob41329b. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Wang D, Dai C, Hamelberg D, Wang B. Chem. Commun. 2012;48:1736–1738. doi: 10.1039/c2cc16716f. [DOI] [PubMed] [Google Scholar]
- 16.Sauer J, Heldmann DK, Hetzenegger J, Krauthan J, Sichert H, Schuster J. Eur. J. Org. Chem. 1998;1998:2885–2896. [Google Scholar]
- 17.Karver MR, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2011;22:2263–2270. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauer J, Heldmann DK. Tetrahedron Lett. 1998;39:2549–2552. [Google Scholar]
- 19.Neunhoeffer H, Wiley PF. The Chemistry of Heterocyclic Compounds, Chemistry of 1 2 3-Triazines and 1 2 4-Triazines, Tetrazines, and Pentazin. Wiley-Interscience; 2009. [Google Scholar]
- 20.Yang J, Karver MR, Li W, Sahu S, Devaraj NK. Angew. Chem., Int. Ed. 2012;124:5312–5315. [Google Scholar]
- 21.Robins LI, Carpenter RD, Fettinger JC, Haddadin MJ, Tinti DS, Kurth MJ. J. Org. Chem. 2006;71:2480–2485. doi: 10.1021/jo052577a. [DOI] [PubMed] [Google Scholar]
- 22.Pinner A. Ber. Dtsch. Chem. Ges. 1893;26:2126. [Google Scholar]
- 23.Lang SA, Johnson BD, Cohen E. J. Heterocycl. Chem. 1975;12:1143–1153. [Google Scholar]
- 24.Skorianetz W, Kovats ES. Helv. Chim. Acta. 1971;54:1922–1939. [Google Scholar]
- 25.Larsen C, Binderup E, Moller J. Acta Chem. Scand. 1967;21:2855–2858. [Google Scholar]
- 26.Wang D, Chen W, Zheng Y, Dai C, Wang L, Wang B. Heterocycl. Commun. 2013;19:171–177. [Google Scholar]
- 27.Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. J. Am. Chem. Soc. 2012;134:792–795. doi: 10.1021/ja209325n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balcar J, Chrisam G, Huber FX, Sauer J. Tetrahedron Lett. 1983;24:1481–1484. [Google Scholar]
- 29.Molz T, König P, Goes R, Gauglitz G, Meier H. Chem. Ber. 1984;117:833–839. [Google Scholar]
- 30.Sauer J. In: Compr. Heterocycl. Chem. II. Alan RK, Charles WR, Eric FVS, editors. Oxford: Pergamon; 1996. pp. 901–955. [Google Scholar]
- 31.Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. J. Am. Chem. Soc. 2010;132:7838–7839. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devaraj NK, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansell CF, Espeel P, Stamenović MM, Barker IA, Dove AP, Du Prez FE, O’Reilly RK. J. Am. Chem. Soc. 2011;133:13828–13831. doi: 10.1021/ja203957h. [DOI] [PubMed] [Google Scholar]
- 34.Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Nat. Chem. 2012;4:298–304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker IA, Hall DJ, Hansell CF, Du Prez FE, O'Reilly RK, Dove AP. acromol. Rapid Commun. 2011;32:1362–1366. doi: 10.1002/marc.201100324. [DOI] [PubMed] [Google Scholar]
- 36.Vrabel M, Kölle P, Brunner KM, Gattner MJ, López-Carrillo V, de Vivie-Riedle R, Carell T. Chem. Eur. J. 2013;19:13309–13312. doi: 10.1002/chem.201301838. [DOI] [PubMed] [Google Scholar]
- 37.Hansell CF, Espeel P, Stamenovic MM, Barker IA, Dove AP, Du Prez FE, O’Reilly RK. J. Am. Chem. Soc. 2011;133:13828–13831. doi: 10.1021/ja203957h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.