Abstract
Diet has profound effects on animal longevity and manipulation of nutrient sensing pathways is one of the primary interventions capable of lifespan extension. This often is done through caloric restriction (CR) and a variety of CR mimics have been identified that produce life extending effects without adhering to the rigorous CR dietary regimen. Glycerol is a dietary supplement capable mimicking CR by shifting metabolism away from glycolysis and towards oxidative phosphorylation. Glycerol supplementation has a number of beneficial effects, including lifespan extension, improved stress resistance, and enhanced locomotory and mitochondria activity in older age classes. Using rotifers as a model, we show that supplements of 150–300 mM glycerol produced 40–50% extension of mean lifespan. This effect was produced by raising glycerol concentration only three times higher than its baseline concentration in rotifer tissues. Glycerol supplementation decreased rotifer reliance on glycolysis and reduced the pro-aging effects of glucose. Glycerol also acted as a chemical chaperone, mitigating damage by protein aggregation. Glycerol treatment improved rotifer swimming performance in older age classes and maintained more mitochondrial activity. Glycerol treatment provided increased resistance to starvation, heat, oxidation, and osmotic stress, but not UV stress. When glycerol was co-administered with the hexokinase inhibitor 2-deoxyglucose, the lifespan extending effect of glycerol was enhanced. Co-administration of glycerol with inhibitors like 2- deoxyglucose can lower their efficacious doses, thereby reducing their toxic side effects.
Keywords: lifespan, glycerol, longevity, 2-deoxyglucose, swimming
Introduction
Aging is an evolutionarily conserved, regulated process that is controlled by a complex signaling network (Gems & Partridge 2013). This network consists of multiple parallel pathways, so that interactions are likely (Amrit and May 2010). A key discovery from work on laboratory model organisms has been that lifespans can be extended and health and function improved, by simple interventions like diet and drugs that influence metabolism. Nutrient sensing is one of the key systems that organisms use to match their metabolism to environmental conditions (Libert and Pletcher 2007). Slowing metabolism when food is scarce and accelerating it when food is abundant is a common adaptation shared by many animals (de Magalhaes et al. 2012).
Diet has profound effects on longevity, and caloric restriction (CR) without malnutrition extends lifespan in most animals thus far tested (Testa et al. 2014). CR of 20–40% extends lifespan in many organisms (Sinclair 2005, Bordone and Guarente 2005, Mair and Dillin 2008, Fontana et al. 2010), including rotifers (Gribble and Mark Welch 2013, Snell 2014). The comparative approach using a variety of model organisms has been productive in advancing understanding of the mechanisms of aging (Austad 2009, Deweerdt 2012). Besides restricting caloric intake, a less studied approach to extending lifespan is shifting metabolism from greater reliance on glycolysis towards more reliance on oxidative phosphorylation (Wei et al. 2009, Johnson et al. 2013). Metabolic shifts probably mimic CR by modulating the same nutrient sensing pathways that produce CR lifespan extension, but the mechanisms are not understood (Wei et al. 2009). Campisi (2013) has argued that metabolic shifts may link processes like aging and cancer, and a well known element of age-related tumor progression is the metabolic shift to glycolysis (Warburg effect).
There are a variety of approaches to reducing utilization of pro-aging carbon sources like glucose. One is by inhibiting glycolysis, and some inhibitors, like the hexokinase inhibitor 2-deoxyglucose, can have significant lifespan extending affects (Ingram and Roth 2011). An alternative approach is to provide alternate substrates that suppress glycolysis and favor oxidative phosphorylation. One such substrate is glycerol. Glycerol supplementation has a number of beneficial effects, including lifespan extension, improved stress resistance, and enhanced mitochondria activity (Wei et al. 2009).
In this work, we explore the benefits of glycerol supplementation on aging using rotifers as a model animal. Rotifers have long been employed in aging studies (Snell 2014), and they are particularly well suited for studies of dietary supplementation because of their short lifespan and ease of culture. We demonstrate that modest supplements of 150–300 mM glycerol can produce 40–50% extension of mean lifespan. This occurs because glycerol shifts rotifer metabolism away from glycolysis and towards greater reliance on oxidative phosphorylation. Glycerol also acts as a chemical chaperone, mitigating damage by protein aggregation. Besides extending lifespan, glycerol treatment improves swimming performance in older age classes and maintains more mitochondrial activity. Glycerol treatment provides increased protection from a variety of stressors. The inhibitor 2-DG enhances the glycerol effect, suggesting that it could be co-administered to obtain greater lifespan extension with fewer toxic side effects.
Methods
Rotifer cultures
All experiments were conducted using the rotifer species Brachionus manjavacas originally collected from the Azov Sea region of Russia. This strain has been cultured in the laboratory since 1983 with periodic resting egg production, collection, and storage. The B. plicatilis originated from Spain (Smith and Snell 2013) and B. calyciflorus from Florida (Snell et al. 1991). For each experiment, resting eggs were hatched in 25 mL of 15 ppt ASW (artificial sea water, Instant Ocean) under constant fluorescent illumination (2000 lux) at 25°C. The resting eggs hatched after 18 to 20 hours into a physiologically uniform cohort of neonates. All animals were fed Tetraselmis suecica cultured in F medium (Guillard 1983). Algae were grown in a 560 mL chemostat with ¼ daily replacement under constant illumination (2000 lux) at 25°C. To simplify life table experiments, rotifers were also treated with 20 μM 5-fluoro-2-deoxyuridine (FDU) to prevent the hatching of amictic eggs (Snell et al. 2012).
Experimental Design and Treatments
All chemical treatments were first tested in a 3-day reproductive range-finding test (Snell et al. 2012) to determine the highest dose that does not decrease reproduction. Based on these tests, the following concentrations were used in the life table experiments: 20 μM 2-deoxyglucose (2-DG), 20 μM bromopyruvate, 222 mM glyceraldehyde, 10 μM lonidamine, and 10 μM metformin. Glycerol exposures ranged from 13.7 mM (0.1%) to 274 mM (2%).
Full cohort life tables were performed with 120 female rotifers per treatment. Animals were kept in 24-well plates with 5 females per well in 1 mL medium. Medium contained 6×105 cells/mL T. suecica diluted in 15 ppt ASW, 20 μM FDU, and appropriate test compound. Plates were monitored daily for mortality until all animals were dead, and all animals were transferred to fresh medium on day 8. Plates were maintained at 22°C in low light for the duration of the experiment. Data is reported as mean, median and maximum lifespan (age of 95% mortality).
A few life table experiments with reproduction also were conducted without FDU using 24 female rotifers per treatment, each isolated singly in wells of a 24-well plate. Each well had 1 mL of medium containing 2×105 cells/mL T. suecica and the appropriate test compound. Offspring were counted and removed daily, and the original parthenogenetic mothers were transferred to fresh medium on day 6. These plates were also maintained at 22°C in low light.
Quantification of Glycerol in Tissue
The concentration of glycerol present in rotifer tissue was quantified using a glycerol colorimetric assay (Caymen Chemical, #10010755). Animals were first cultured for three days in the glycerol treatment, 6×105 cells/mL T. suecica, and 20 μM FDU. Treatments were 0%, 13.7, 34, 67, 103, 137, 171, 206, 240, and 274 mM glycerol. After three days, the animals were washed to remove algae, and 100 animals were collected in a 0.6 mL tube, with 3 replicates per treatment. Tubes were placed on ice, causing animals to stop swimming, fall to the bottom, and the liquid was pipetted off the top. Animals were re-suspended in 20 μL DI H2O and manually homogenized using a plastic pestle for about a minute. 10 μL of the crude rotifer homogenate was added to wells of a 96 well plate, and the colorimetric assay was then performed following kit protocol. Absorbance was measured at 542 nm, and values were compared to a standard curve to calculate the glycerol concentration (mg/L) in the tissue samples.
Rotenone Dose-Response Experiment
We tested the effects of glycerol on sensitivity to rotenone, an oxidative phosphorylation inhibitor. Female rotifers were cultured for four days in 6×105 cells/mL T. suecica, 20 μM FDU, and 274 mM glycerol. Control animals were not exposed to glycerol. Animals were then transferred to 24-well plates at 25°C with 6×105 cells/mL T. suecica, 20 μM FDU, and rotenone. Each well contained 1mL medium and 10 rotifers, with 40 animals per treatment. Rotenone treatments ranged from 0 to 0.5 μM in 0.1 μM increments. Survival was recorded 72 hours after rotenone exposure, and these data were used to construct a dose-response curve. Rotenone dose-response curves were compared between control animals and rotifers treated with 274 mM glycerol to determine whether glycerol caused a shift in rotenone sensitivity.
Estimation of Rotifer Swimming Speed
To quantify swimming speed, approximately 40 female rotifers were cultured for 10 days in 24-well plates with 2 to 4 animals per well in 6×105 cells/mL T. suecica, 20 μM FDU, and the appropriate chemical treatment. Plates were maintained at 22°C in low light. Treatments were 100 mM glycerol, 200 mM glycerol, 100 mM glycerol + 20 μM 2-deoxyglucose, and an untreated control. After 10 days, the swimming speed of 15 randomly chosen females from each treatment was recorded at 15X magnification using a PixeLink camera attached to a stereomicroscope. For each video, one animal was placed on a slide in 15 uL of 15 ppt ASW and swimming recorded for 30 seconds. Videos were converted to image sequences using VirtualDub (www.virtualdub.org), and ImageJ was used to remove background noise from the sequences. The ImageJ plugin MTrack2 (http://valelab.ucsf.edu/~nico/IJplugins/MTrack2.html) was used to track animal movement and calculate speed in pixels per frame. This measurement was converted to millimeters per second using the video capture speed of the camera (50 frames per second) and the conversion 102.3 pixels per mm.
Estimation of Mitochondrial Activity
MitoTracker® Red (Invitrogen) was used to estimate mitochondrial activity of rotifers. Female rotifers were cultured in 24-well plates in 6×105 cells/mL T. suecica, 20 μM FDU, and the appropriate chemical treatment for four days. Plates were maintained at 22°C in low light, and treatments were 274 mM glycerol, and an untreated control. Before staining, animals were rinsed in 15 ppt ASW for 1 hour to remove algae from their stomachs and reduce auto-fluorescence. Animals were then transferred to 0.5 mL fresh 15 ppt ASW and 5 μM MitoTracker and incubated at 22°C in the dark for 30 minutes. After staining, the animals were rinsed with 15 ppt ASW, anesthetized with 1mL club soda, fixed with 20 μL of 20% formalin, and rinsed again. To ensure that there was no non-specific binding of MitoTracker, 15 animals from each treatment were anesthetized, fixed with formalin, and incubated at room temperature for four hours before staining and imaging. This allowed us to compare mitochondrial staining in live and dead rotifers. Images were taken at 200X magnification and 5 ms exposure with an Alexa 486 nm filter using a Zeiss Imager.Z1 microscope. ImageJ was used to measure the average pixel intensity of each rotifer and subtract the background. For each treatment, 15 animals were imaged and the mean pixel intensity was calculated.
Stressor Challenge Experiments
To test the effects of glycerol and glycolysis inhibitors on different types of stress resistance, five different stressor challenge experiments were performed using starvation, osmotic shock, heat shock, UV exposure, and oxidative stress. First, female rotifers were cultured for four days in 24-well plates with 1 mL medium and 5 animals per well. Medium consisted of 6×105 T. suecica, 20 μM FDU, and the appropriate chemical treatment. Plates were maintained at 22°C in low light. Treatments were 100 mM glycerol, 20 μM 2-deoxyglucose, 20 μM bromopyruvate, 100 mM glycerol + 20 μM 2-deoxyglucose, and an untreated control. After four days, the animals were removed from the algae containing medium, and challenged with one of the five stressors.
The osmotic shocked animals were transferred from 15 to 60 ppt ASW for one hour. Heat shocked animals were exposed to 40°C for one hour. UV stressed animals were transferred to a petri dish with 5 mL and exposed to 20 min of UV-B radiation, 25 cm from an 8 watt source (UVP, model UVM-28 EI) with an intensity peak at 302 nm. After the stressors were applied, these animals were transferred to 24-well plates at 25°C with 1 mL 15 ppt ASW, 20 μM FDU, and no algae, to follow post-stress survival for 72 h. In the starvation treatment, animals were deprived of all food for 72 h. In the oxidative stress treatment, rotifers were exposed to 0.1 μM juglone for 72 h. These stressor treatments were all chosen to produce considerable mortality in the control after 72 hours, but not 100% mortality. All plates contained 10 female rotifers per well with a total of 120 animals per treatment. After 72 hours, the plates were scored for survival.
Statistics
Test concentrations in the reproductive range finding tests were compared by ANOVA with Dunnett’s test to compare mean fecundity in treatments to control using the statistical package JMP 8 (SAS Institute). Survival curves were compared using the JMP 8 reliability and survival analysis, with a Wilcoxson’s test calculated to compare survival curves of control and treatments. Rotenone LC50s were calculated from linear regressions of dose-response curves estimated with JMP 8. Swimming speed and MitoTracker staining intensities were compared by ANOVA with Dunnett’s test to compare treatments to control. Survival in the stressor challenge treatments was compared to control using Fisher’s Exact Test with Bonferroni correction for multiple comparisons.
Results
Glycerol concentrations of 685 mM or higher had a negative effect on rotifer reproduction (Figure 1). After 48 h exposure, significantly lower reproduction was observed when there was 685 mM glycerol (5%) in the medium, but not 343 mM. At 1028 mM glycerol, no reproduction was observed after 48 or 72 h. This dose-response relationship set the upper boundaries for exploring the positive effects of glycerol supplementation on rotifer lifespan while avoiding toxic effects.
Figure 1.
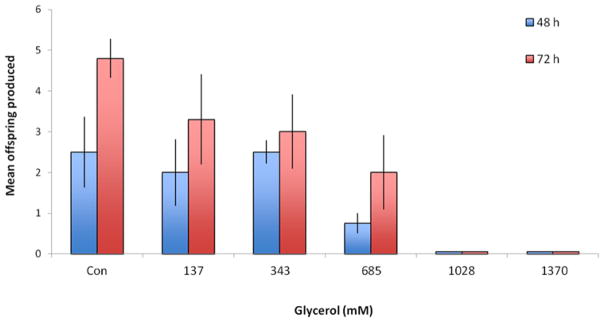
Reproductive ranging finding test for glycerol toxicity to B. manjavacas. Mean offspring produced are the number of females produced by a single parthenogenetic mother in 48 or 72 h at 25°C.
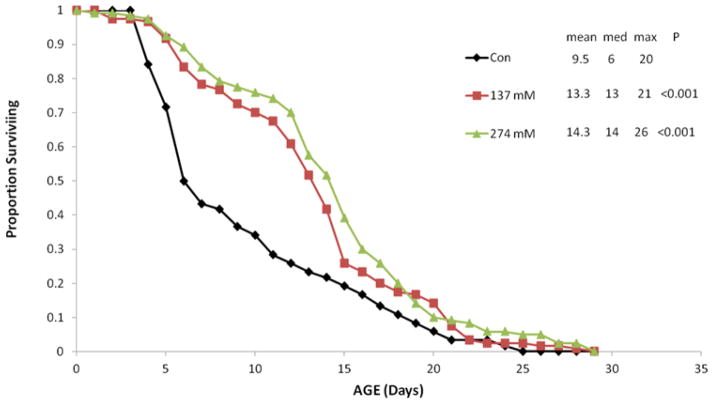
Glycerol supplementation of 137 or 274 mM in the medium significantly extended rotifer lifespan (Figure 2). Mean lifespan at 137 mM glycerol was 40% longer than the control and 51% longer in the 274 mM treatment. Median lifespan (50% mortality) was 117% and 133% longer in 137 and 274 mM glycerol, respectively. In contrast, the age when only 5% of the cohort remained alive (0.05lx), was only 5% longer in the 137 mM glycerol treatment and 30% at 274 mM glycerol. Comparison of the pattern of age-specific mortality in controls versus 137 mM glycerol illustrates that glycerol treatment shifts peak mortality from day 7 in the control to day 15 (Figure 3). This shifts the peak of age-specific mortality from the reproductive phase of the life cycle in the control to the post-reproductive phase in the glycerol treatment.
Figure 2.
Survival curves for B. manjavacas continuously exposed to 0, 137 or 274 mM glycerol. Proportion surviving represents the fraction of an initial cohort of 120 rotifers surviving to the indicated age. Mean, median and maximum lifespan (5% surviving) are reported in days. P refers to the Wilcoxson test probability that the treatment survival curves are similar to control.
Figure 3.
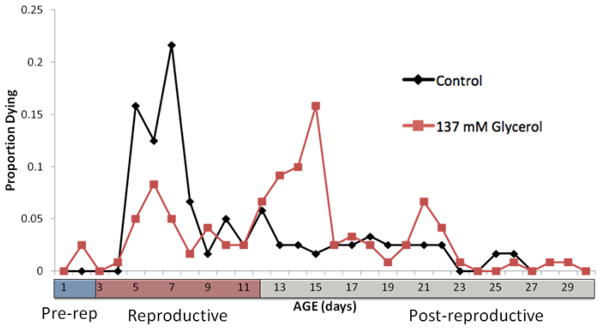
Age-specific mortality of B. manjavacas continuously exposed to 0 or 137 mM glycerol. Proportion dying represents the fraction of an initial cohort of 120 rotifers dying in the indicated age interval. Pre-rep is the number of days in the mean lifespan spent in the pre-reproductive stage, repro is the reproductive stage, and post-rep is number of days spent post- reproductive.
Since rotifers are dosed by adding glycerol to their medium, it is important to know how much glycerol actually is absorbed into rotifer tissues. The relationship between glycerol exposure and concentration in rotifer tissues is linear from 0 to 274 mM glycerol supplementation (Figure 4). In the absence of glycerol supplementation, rotifers have about 45 mg/L glycerol in their tissues from normal metabolic activity. Significant rotifer life extension was observed at 137 mM glycerol supplementation (Figure 1), which produces a 3.3-fold increase in glycerol concentration in rotifer tissues over baseline (Figure 4). This demonstrates that the efficacy dose for life extension (150 mM) is only about 20% of the reproductive toxicity dose (715 mM). Moreover, these observations provide guidelines for investigating the life extension effects of glycerol in other animal species. Our expectation is that life extension effects may be observed when glycerol is provided as a dietary supplement so that baseline tissue concentration is raised about 3-fold.
Figure 4.
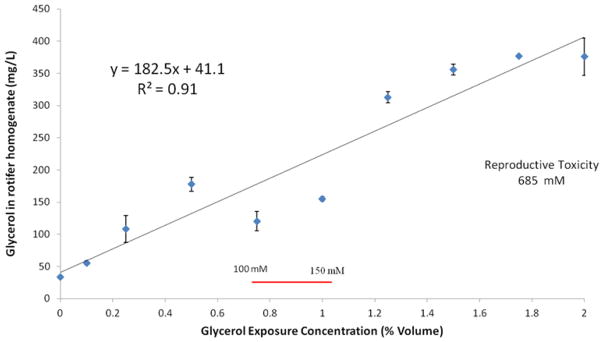
Dose-response curve for glycerol exposure and glycerol concentration in rotifer tissue. Exposure concentrations are reported as percent volume glycerol in the medium. Glycerol in rotifer tissue homogenate is in mg/L. The 100–150 mM line indicates the range of glycerol concentrations where rotifer lifespan extension is observed.
Glycerol supplementation up to 274 mM in the medium had no negative effect on rotifer reproduction (Figure 5). Female lifetime fecundity (Ro) ranged from 13.2 offspring in controls to 18.8 in the 274 mM glycerol treatment. Fecundity in the 274 mM treatment was actually 42% greater than control, which is significant by t-test (df = 46, P = 0.02).
Figure 5.
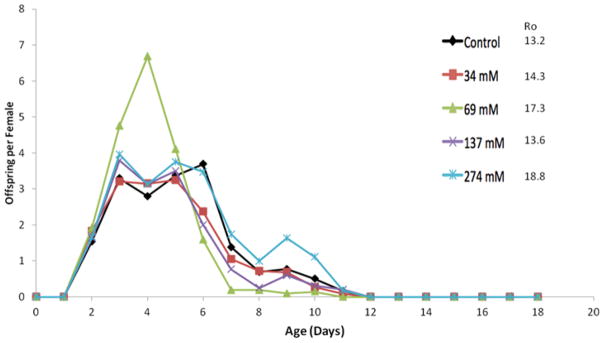
Age-specific reproduction with glycerol treatment. Offspring per female refers to the average number of offspring produced daily by a cohort of 48 females. Ro is the mean total offspring produced by a female over her lifetime.
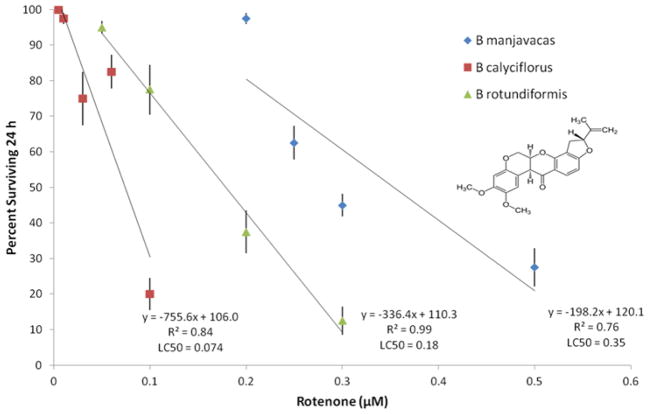
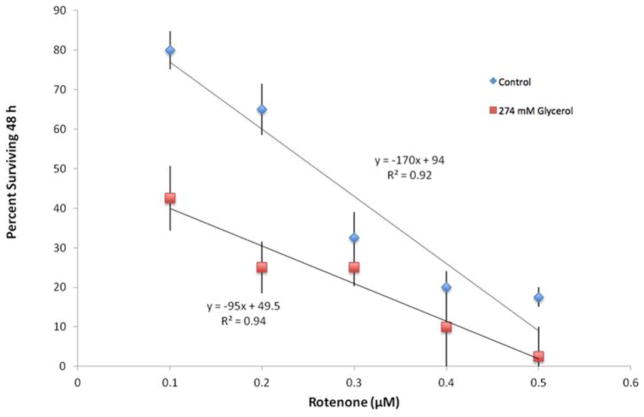
Sensitivity to rotenone varies among three brachionid species, with B. calyciflorus exhibiting the greatest sensitivity (LC50 = 0.074 μM) and B. manjavacas the most resistant (LC50 = 0.35 μM, Figure 6). When the dose-response curves of control and 274 mM glycerol supplemented rotifer cultures are compared in B. manjavacas, the glycerol treatment is more sensitive to rotenone, especially at lower concentrations (Figure 7). This pattern of inhibition demonstrates that glycerol supplemented rotifer cultures are less dependent on glycolysis and more dependent on aerobic respiration. In other words, glycerol supplementation shifts rotifer metabolism away from glycolysis and toward oxidative phosphorylation. Direct inhibition of glycolysis with 20 μM of the hexokinase inhibitor 2-deoxyglucose does not change with glycerol supplementation (data not shown). This suggests that glycerol supplementation does not directly affect glycolysis as much as aerobic respiration.
Figure 6.
Dose-response curves to rotenone of B. manjavacas, B. rotundiformis, and B. calyciflorus. Each data point represents the mean percent survival of 4 replicate populations of 10 rotifers after 24 h exposure to rotenone. Vertical lines represent standard errors for each rotenone concentration. Trend lines are least-squares fit linear regressions. The concentrations where there is 50% mortality (LC50) are calculated from the respective regression equations.
Figure 7.
Rotenone dose-response curves of B. manjavacas in the absence (control) and presence of 274 mM glycerol. Each data point represents the mean percent survival of 4 replicate populations of 10 rotifers after 48 h exposure to rotenone. Vertical lines represent standard errors for each rotenone concentration. Trend lines are least-squares fit linear regressions.
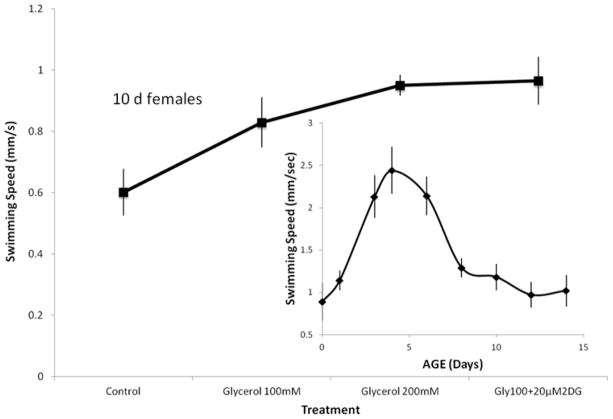
Brachionid rotifers swim continuously throughout their lives, so rotifer swimming speed is a measure of their vitality. Newborn B. manjavacas females swim at about 0.8 mm/s (Figure 8). Swimming speed increases with maturity, peaking at 2.5 mm/s at age 4 days, which corresponds to the age of maximum reproduction. As females age, swimming speed declines, so that by age 12 days it falls to about 1 mm/s. As senescence proceeds, swimming slows further so that rotifers eventually cannot maintain themselves in the water column, they sink to the bottom and die. When rotifers are supplemented with 100 mM glycerol, their swimming speed at age 10 days is 37% greater than controls (P = 0.02, Figure 8). Supplementation with 200 mM glycerol preserved swimming vitality by 60% greater than controls (P<0.01). The slightly greater preservation of swimming speed in older age classes was achieved with a treatment that combined 100 mM glycerol with 20 μM 2-deoxyglucose, which yielded 61% faster swimming at age 10 days (P < 0.01).
Figure 8.
Estimating differences in swimming speed of 10 d old rotifers exposed to various glycerol treatments. Each data point is the average swimming speed (mm/s) of 15 rotifers in each treatment. Vertical lines indicate standard error.
The activity of rotifer mitochondria was quantified using MitoTracker (data not shown). Females were treated for two days, exposed to MitoTracker, and then imaged at 200X magnification for epifluorescence. Those treated with 274 mM glycerol had 77% higher MitoTracker binding than controls (P = 0.02). The positive effect of glycerol vanished if rotifers were dead for four hours prior to incubating in MitoTracker.
Supplementation of the culture medium with 20 μM 2-deoxyglucose or bromopyruvate significantly extended mean rotifer lifespan by 22% (Figure 9), with no effect on reproduction (data not shown). However, supplements of 10 μM lonidamine or metformin had no significant effect on lifespan. Similarly, supplementing the medium with 150 μM D-glucose, L-glucose, or pyruvate had no significant effect on rotifer lifespan, nor did 222 mM glyceraldehyde (data not shown).
Figure 9.
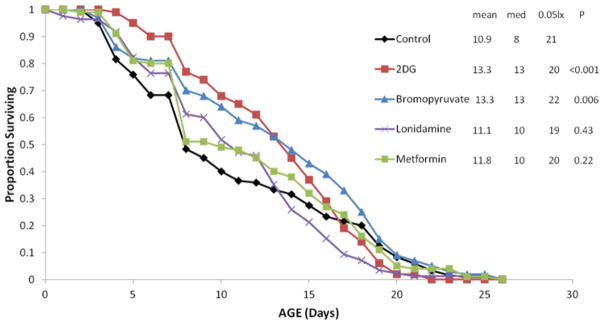
Survival curves for B. manjavacas exposed to hexokinase inhibitors (2-DG, bromopyruvate, or lonidamine) or the drug metformin. Proportion surviving represents the fraction of an initial cohort of 120 rotifers surviving to the indicated age. Mean, median and maximum lifespans (5% surviving) are reported in days. P refers to the Wilcoxson test probability that the treatment survival curves are similar to control.
Glycerol supplementation of the culture medium was protective for a variety of stressors. Rotifers were challenged with one of five stressors, starvation, heat, oxidation, UV, or osmotic stress, and their survival followed for 72 h (Figure 10). Supplementation of rotifer medium with 2% glycerol enhanced resistance to starvation so that 57.5% of animals survived after 72 h, in comparison to 15.8% in controls. If rotifers were exposed to 42°C for 1 h, survival after 72 h in the glycerol treatment was 42.5% compared to 3.3% in controls. Continuous exposure to 0.1 μM juglone caused oxidative stress, leading to 56.7% survival in the glycerol treatment versus 43.3% survival in controls. Glycerol exposure did not confer significant resistance to 20 min UV exposure, producing 15% survival versus 11.4% in the control. Glycerol exposure provided significant protection from osmotic stress (60 ppt for 1 h), with 25% survival in the glycerol treatment compared to 1.5% in controls. Treatment of rotifers with 20 μM 2-deoxyglucose or bromopyruvate did not confer significant resistance to any of the five stressors. Likewise, adding 2-deoxyglucose to glycerol supplementation did not significantly enhance protection from the stressors.
Figure 10.
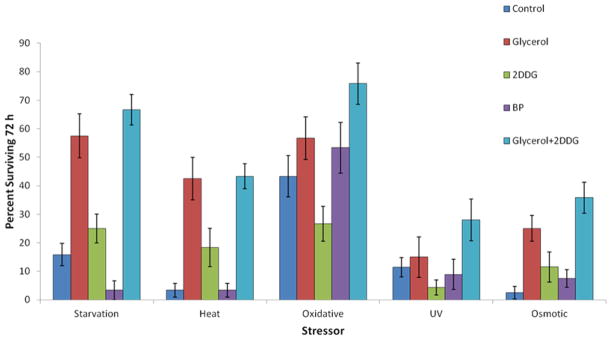
Effect of stressor challenges on B. manjavacas survival. Test rotifers were exposed to 100 mM glycerol, 20 μM 2-DG, 20 μM bromopyruvate, or 100 mM glycerol+20 μM 2-DG for four days before the application of each stressor. The starvation test denied rotifers food for 72 h. The stress tests applied stressors for 20 min (UV), or 1 hour (osmotic and heat), and the oxidative stress test exposed rotifers to 0.1 μM juglone continuously for 72 h. Proportion surviving is the fraction of a cohort of 120 rotifers surviving 72 h after stressor exposure. Variance in each treatment is indicated by the standard error bars on top of each column.
A life table experiment was conducted to investigate interactions between glycerol supplementation and exposure to 2-deoxyglucose. Exposure to 100 mM glycerol did not significantly extend mean rotifer lifespan (P = 0.20), but exposure to 20 μM 2-deoxyglucose caused significant life extension (9%, P = 0.04) (Figure 11). The combined treatment of 100 mM glycerol and 20 μM 2-deoxyglucose significantly extended rotifer lifespan compared to the control (12%, p <0.001) and compared to glycerol treatment alone (7%, P = 0.05)..
Figure 11.
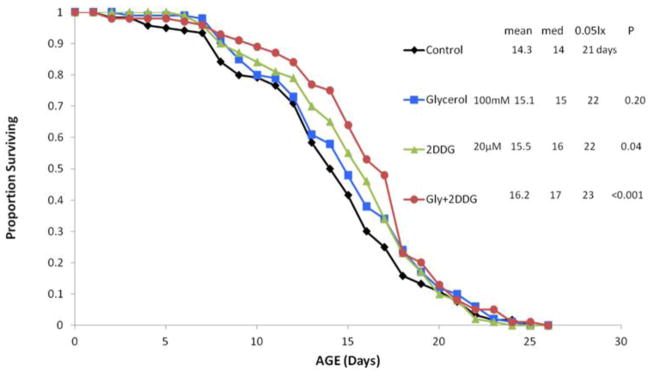
Interaction between glycerol and 2-DG. Rotifers were exposed to 100 mM glycerol, 20 μM 2-DG, or 100 mM glycerol+20 μM 2-DG. Proportion surviving represents the fraction of an initial cohort of 120 rotifers surviving to the indicated age. Mean, median and maximum lifespans (5% surviving) are reported in days. P refers to the Wilcoxson test probability that the treatment survival curves are similar to control.
Discussion
One of our principal findings is that treatment of rotifers with 150–300 mM glycerol extends lifespan 40–50%. This dose is only about three times the baseline tissue concentration of glycerol and produces a positive effect at about 20% of that causing reproductive toxicity. Insight into the mechanism by which glycerol treatment extends lifespan is provided by the experiment with rotenone inhibition. Rotenone is a broad spectrum insecticide and piscicide that selectively inhibits electron transfer from electron transfer chain complex I to ubiquinone. It is useful for probing cellular bioenergetic pathways to estimate the relative contributions of glycolysis and aerobic respiration (Wu et al. 2007). Rotenone selectively inhibits oxidative phosphorylation and 2-DG inhibits glycolysis. Comparing dose-response curves for rotenone in the presence and absence of glycerol provides evidence that glycerol makes rotifers more sensitive to rotenone inhibition because they are more heavily utilizing aerobic respiration. In contrast, the lack of modification of the 2-DG dose-response curve by glycerol suggests that it has little effect on glycolysis.
In addition to shifting metabolism away from glycolysis, glycerol acts as a chemical chaperone, protecting cells from the accumulation of protein aggregates (Deocaris et al. 2008). The mechanism of glycerol action is not completely understood, but it is thought that glycerol stabilizes native proteins by altering the solvent properties of water, protein polarity and diffusion, and reducing side chain reactions that lead to misfolding and aggregation (Diamant et al. 2001). Treatment of human cells with 200–400 mM glycerol induced re-folding of heat-denatured luciferase and helped maintain cell proliferation in the presence of oxidative stress (Deocaris et al. 2008). Glycerol treatment also induced expression of mtHSP70, which accounted for some of its beneficial effect. Deocaris et al. also tested the effect of glycerol on C. elegans and found that it reduced the accumulation of age-related lipofuscins and improved thermo-tolerance, but it failed to extend lifespan. The doses of glycerol in the Deocaris et al. study (200–400 mM) are similar to those we found efficacious for extending rotifer lifespan (100–300 mM glycerol). There are some indications that it may be difficult to achieve these tissue concentrations in mice and humans by oral dosing because of the rapid metabolism of glycerol (Bai et al. 1998). Perhaps achieving such high plasma concentrations is not necessary for human lifespan extension. As we argue below, maybe the lifespan extending effects of glycerol can be achieved by simply maintaining plasma glycerol concentration about three times above baseline values. According to Nelson et al. (2011), this is only about 327 μM in humans and is quite feasible with oral dosing. Clearly there are species differences in glycerol uptake and metabolism, so more work needs to be done before optimal glycerol dosing is determined.
Some rotifer species are capable of shifting metabolism from aerobic to anaerobic pathways because they are adapted to live in low oxygen environments. For example, various rotifer species have been found in low oxygen environments in meromictic lakes (Miracle and Vicente 1983). Moreover, B. plicatilis can maintain high population densities even when oxygen is <1 mg/l (Esparcia et al. 1989). This tolerance of hypoxia is likely due to the ability of this rotifer to shift to anaerobic metabolism by activating lactate and glucose-succinate pathways in hypoxic conditions (Esparcia et al. 1992). Calorically restricted B. plicatilis were significantly more tolerant of extreme hypoxia (7.5–11 h at <0.1% O2) than ad libitum fed rotifers (Ozaki et al. 2010). The CR rotifers up-regulated the glycolytic enzymes glyceraldehyde-3 phosphate dehydrogenase and enolase which enhanced their capacity for anaerobic metabolism and contributed to their greater tolerance of hypoxia. Oxidative stress was induced in Brachionus sp. by exposure to 0.1 mM H2O2 for 24 h. This treatment caused more than a 3-fold up-regulation of hsp-20 mRNA production, as well as significantly increased glutathione peroxidase, reductase, and transferase enzyme activity (Rhee et al. 2011). We presented data in this paper suggesting that rotifer lifespan is extended by reducing glycolysis and enhancing aerobic respiration. We accomplished this by supplementation with glycerol, which may have increased the availability of pyruvate for direct incorporation into the oxidative phosphorylation pathway. Glycolysis was further reduced when rotifers were exposed to 2-DG to inhibit hexokinase activity. Because of their complimentary modes of action, co-administration of both glycerol and 2-DG produced greater life extension than either alone.
The effect on lifespan of shifting of metabolism from anaerobic to aerobic pathways has been explored in other model organisms. Exposure of C. elegans to 2% (112 mM) D-glucose in the agar medium shortened lifespan by about 20% by inhibiting activity of the DAF-16/FOXO and HSF-1 transcription factors (Lee et al. 2009). The life-shortening effect of glucose is likely mediated through changes in glycerol metabolism through the aquaporin gene aqp-1, which encodes a glycerol channel. Nematodes exposed to 1% glycerol in medium, decreased lifespan 23–36%, similar to the glucose effect. Glycerol feeding suppressed aqp-1 expression, which is a regulator of insulin/IGF-1 signaling. Yeast responded oppositely to nematodes when given glucose or glycerol as a sole carbon source (Brisson et al., 2001). Yeast grown on glucose triggered catabolite repression, stimulated glycolysis, and inhibited respiration. In contrast, growth on glycerol relieved catabolite repression and stimulated respiration by conversion of glycerol to pyruvate. If nematodes had responded similarly to glycerol as yeast, Lee et al. (2009) thought that glycerol should lengthen, not shorten, C. elegans lifespan. These authors asserted that glucose and glycerol are metabolically interchangeable in C. elegans, which is clearly not true for yeast. Indeed, we observed life extension in rotifers by glycerol dietary supplementation, which is precisely the response that was observed yeast. Pathways of carbohydrate metabolism clearly differ between nematodes and rotifers, and provides a cautionary tale about assuming that findings in C. elegans are representative of all animals.
Glycerol accumulation is associated with extended lifespan in yeast as evidenced by sch9 mutants accumulating glycerol (Wei et al. 2008). In yeast, Tor1 is a key regulator of a nutrient sensing pathway that switches from reliance primarily on aerobic respiration to glycolysis and glycerol biosynthesis. Increased glycerol production is a key metabolic change that produces lifespan extension in yeast. Down-regulation of the Tor1 pathway mimics caloric restriction by lowering the glucose content of the medium. Effects of glycerol were investigated by examining mutants that accumulated glycerol rather than ethanol. In these experiments, glycerol was produced endogenously rather than supplied externally as a supplement in the medium. Long-lived mutants had higher expression of several genes involved with glycerol biosynthesis. Deletion of glycerol synthesis genes reversed life extension and resistance to heat, osmotic and oxidative stress.
However, supplementation of the growth medium with 0.1 or 1% glycerol did not result in life extension for wild type yeast. Glycerol, unlike glucose or ethanol, did not adversely affect the life extension effect induced by caloric restriction. This suggests that carbon source substitution may be an alternative to caloric restriction as a strategy for delaying aging. Glycerol seems to slow aging in rotifers as it does in yeast, and may have similar effects in other animals.
Brachionid rotifers are planktonic herbivores that swim continuously throughout their lifespan. Swimming speed is age-dependent and is therefore a general measure of animal vitality (Snell et al. 2012). Besides extending lifespan, glycerol treatment also preserved rotifer vitality in older age-classes, maintaining swimming speed 60% higher than in controls. The mechanism by which glycerol extends vitality and promotes healthy aging is not known and is likely to be a profitable area of research.
2-deoxyglucose is a glucose analog that is a promising candidate as a CR mimic (Lane et al. 1998). 2-DG is easily taken up by cells and competitively inhibits glucose utilization because it blocks glycolysis at its first step, inhibiting hexokinase activity. This results in decreased energy production, mimicking the effect of reduced caloric intake. It also shifts energy production towards heavier reliance on aerobic respiration. Our data showed that exposure of rotifers to 20 μM 2-DG consistently extended lifespan by about 20%. When C. elegans was treated with 5 mM 2-DG in agar medium, 17% mean lifespan extension was observed compared to control (Schultz et al. 2007). Impairing glycolysis with 2-DG increases ROS formation 3-fold and produces mitohormesis (Ristow and Zarse 2010). This promoted lifespan extension and increased resistance to oxidative stress induced by exposure to 50 mM paraquat. In contrast, supplementation with 5 mM glucose decreased nematode mean lifespan about 6%.
2-deoxyglucose restricts glycolysis in a wide variety of animals, including mammals (Ingram and Roth 2011). Evidence of its efficacy is provided by rats, which experienced significant life extension when fed a diet supplemented 0.6% 2-DG by weight every other week (Ingram and Roth 2011). Despite these early encouraging results, short-term toxicity studies and a long-term survival study found that doses of 0.2–0.4% 2-DG produced cardiotoxicity in both Fischer-344 and Brown–Norway rats (Minor et al., 2010). These results motivated our search for compounds that can be co-administered with 2-DG, lowering the dose required to achieve efficacy. Our data suggest that co-administering 140 mM glycerol and 20 μM 2-DG produces greater lifespan extension in rotifers than either compound alone, without any toxic effects.
Caloric restriction remains one of the most effective interventions for lifespan extension (Fontana et al. 2010, Testa et al. 2014). One hypothesis about how caloric restriction works is that it suppresses glycolysis and enhances aerobic metabolism, a metabolic state that typically reduces proteotoxicity (Hipkiss 2011a). Excessive glycolysis has been implicated as a major cause of proteotoxic stress through the generation of glycating metabolites like methylglyoxal and other low molecular weight carbonyl compounds (Hipkiss 2011b). Others also have advocated the extension of longevity by enhancing cytoprotective functions like detoxification, innate immunity, oxidative stress response, and proteostasis (Shore and Ruvkun 2013).
Rapamycin is a macrolide drug that specifically inhibits the activity of TOR kinase. It has been approved for human use as an immunosuppressant, cancer chemotherapy, and to prevent restenosis of arteries after cardiac angioplasty. Rapamycin also is broadly effective against aging and aging-related diseases (Menzies and Rubinsztein 2010, Johnson et al. 2013a), and can extend lifespan in yeast, nematodes, Drosophila and mice (Harrison et al. 2009, Blagosklonny 2010, Biedov et al. 2010, Sharp 2011, Miller et al. 2011, Wilkinson et al. 2011). TOR senses cellular nutrient and energy levels and regulates cell survival, growth, proliferation, motility, protein synthesis and transcription (Hay and Sonneberg 2004, Stanfel et al. 2009). It is promising as a CR mimic, targeting downstream sites in nutrient regulation pathways (Ingram and Roth 2011, Testa et al. 2014). Rapamycin exposure caused a metabolic shift from glucose utilization to fatty acid oxidation in L6 rat muscle cells without caloric restriction (Sipula et al. 2006). Skeletal muscle cells exposed to 15 nmol/l rapamycin for 24 h increased fatty acid oxidation by 60% by increasing activities of carnitine palmitoyltransferases I and II, the primary intracellular regulatory enzymes of the fatty acid oxidation pathway, while glycolysis was concomitantly reduced 40%. This kind of metabolic switching between fatty acid and glucose metabolism for energy production in muscle is a normal physiological response to accommodate short-term changes glucose and fatty acid availability (Sipula et al. 2006). Increased fatty acid oxidation at the expense of glucose utilization also is characteristic of a fasting metabolic state. Intraperitoneal injections of 8 mg rapamycin/kg every other day beginning at weaning extended female and male mice lifespans 38 and 25%, respectively (Johnson et al. 2013b). Rapamycin inhibition of mTOR partly alleviated a mitochondrial disease in this mouse model, which was partially explained by rapamycin inducing a metabolic shift toward amino acid catabolism and away from glycolysis, reducing the buildup of glycolytic intermediates. Unfortunately, rapamycin has a number of adverse side effects (Johnson et al. 2013a, Testa et al. 2014), but some of these may be mitigated by the co-administration of other compounds.
Biological signaling is intrinsically complex, typically involving nonlinear pathways, feedback loops, and compensatory mechanisms (de Magalhaes et al. 2012). Consequently, employing combinations of compounds to target multiple pathways is likely to be a more productive approach to improve efficacy and minimize toxicity (Hopkins 2008). We have screened a variety of small molecule inhibitors for life extending ability in rotifers (Snell et al. 2014), many of these can be considered CR mimics since they inhibit similar pathways as CR (Testa et al. 2014). Inhibition of AKT, IGF-1R, AMPK, PI3K signaling had no effect on rotifer lifespan, but inhibition of TOR and JNK gene expression both yielded significant life extension of 20–40%. In contrast, several experiments exposing rotifers to 20 μM resveratrol failed to produce any significant life extension. Exposure to combinations of a few dietary antioxidants also produced modest rotifer life extension of 10–20% (Snell et al. 2012). In this paper, we report that treating rotifers with 10 μM of the diabetes drug metformin had no lifespan extending effect.
The relationship between stress resistance and longevity has been well documented in yeast (Longo and Fabrizio 2002), invertebrates (Johnson et al. 1996), birds (Harper et al. 2011) and mammals (Harper et al. 2007). What is less clear is why activation of these stress resistance pathways often has life extending effects. It has been suggested that the linkage between stress resistance and longevity indicates that accumulation of molecular damage may play a key role in aging (Longo and Fabrizio 2002). Moreover, aging may be slowed by enhancing systems that repair or resist damage, like the xenobiotic metabolizing enzyme glutathione-S-transferase (Ayyadevara et al. 2005). However, recent work has suggested that accumulation of molecular damage may not be the whole story (Gems and Partridge 2013). These authors believe that rather than being caused by the accumulation of damage, aging may result from over-activity of growth and reproduction processes (hyperfunction) that were beneficial earlier in life. Exposure of rotifers to glycerol significantly extended lifespan and provided some protection from starvation, high temperature, oxidative and osmotic stress, but not UV damage. Our data suggest that glycerol supplementation shifts metabolism away from glycolysis and towards oxidative phosphorylation. Independently suppressing glycolysis with the hexokinase inhibitor 2-DG did not provide protection from any of the five stressors. The mechanism of glycerol action in life extension and enhanced stress resistance is poorly understood. Glycerol exposure may reduce accumulation of molecular damage or suppress some as yet undetermined hyperfunctionality, or both.
Whether a compound has therapeutic value or not is largely determined by its dose. Much is known about how various doses of glycerol impact humans because of its use in sports drinks to maintain hydration (Frank et al. 1981, Nelson et al. 2011). Every compound has a characteristic dose-response curve for efficacy and adverse effects (see Figure 12). The therapeutic dose is that narrow window of concentration that maximizes efficacy while minimizing adverse effects. Based on our observations in rotifers and human dose-response studies, we can predict a therapeutic dose of glycerol for testing its lifespan extension potential in humans. For example, normal serum concentrations of glycerol in adult humans are 0.05–0.1 mM/L (Lin 1977). Based upon our findings in rotifers, we could assume that a dose with potential human lifespan extending effects would be approximately three times base line plasma glycerol concentration. Nelson et al. (2011) found that a dose of 0.032 g glycerol/kg lean body mass in human subjects produced a plasma concentration of 0.327 mM/L, which is 3.3 times baseline glycerol concentration. Higher doses resulted in plasma saturation and glycerol appearing in the urine. Glycerol has a density of 1.261 g/cm3, so the dose to raise the plasma glycerol of a 70 kg person to 3.3 times baseline is 1.78 ml glycerol. Since glycerol is cleared from plasma in about two hours, this dose would have to be repeated three times daily to maintain a 3-fold elevated plasma glycerol concentration (Koenigsberg et al. 1995). This dose is only 8% of that recommended by van Rosendale et al. (2010) for trained athletes to achieve hyperhydration. These calculations illustrate that achieving a glycerol dose for testing the possibly of lifespan extending effects in humans may be feasible.
Figure 12.
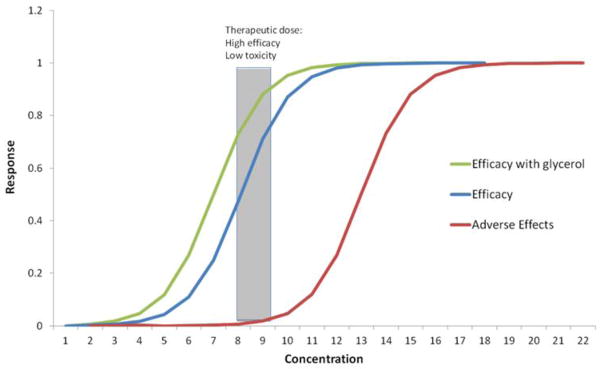
Illustration of theoretical response curves for adverse effects (toxicity) and efficacy. X-axis is the concentration of a test compound and Y-axis is the response level. Low concentrations elicit low responses (toxicity or efficacy) and high concentrations elicit greater responses until saturation. Optimal therapeutic dose is the concentration range where there is high efficacy and low toxicity.
Highlights.
Glycerol mimics CR by shifting metabolism away from glycolysis and towards oxidative phosphorylation.
Glycerol extended lifespan, improved stress resistance, and enhanced locomotory and mitochondria activity.
Using rotifers, we showed that supplements of 150–300 mM glycerol produced 40–50% lifespan extension.
Glycerol treatment provided increased resistance to starvation, heat, oxidation, and osmotic stress, but not UV stress.
Glycerol co-administered with 2-deoxyglucose enhanced the lifespan extending effect of glycerol.
Acknowledgments
We are grateful for the support of the National Institute of Aging, grant R01 AG037960-02 for this work. Kristen Gribble and David Mark Welch made important comments that improved the paper. We also express our appreciation for the expert technical assistance provided by Leilani Barry, Shelby Rittweger, Brett Rabeneck, and Corey Rennolds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Amrit FRG, May RC. Younger for Longer: Insulin signalling, immunity and ageing. Current Aging Science, 2010. 2010;3:166–176. doi: 10.2174/1874609811003030166. [DOI] [PubMed] [Google Scholar]
- Austad SN. Is there a role for new invertebrate models for aging research? J Gerontol A Biol Sci Med Sci. 2009;64A:192–194. doi: 10.1093/gerona/gln059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, et al. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4 hydroxynonenal. Aging Cell. 2005;4:257–71. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Bai C, Biwersi J, Verkman AS, Matthay MA. A mouse model to test the in vivo efficacy of chemical chaperones. J Pharmacol Toxicol Methods. 1998;40:39–45. doi: 10.1016/s1056-8719(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, et al. Mechanisms of lifespan-extension by rapamycin in Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;15:683–688. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- Brisson D, Vohl MC, St-Pierre J, Hudson TJ, Gaudet D. Glycerol: a neglected variable in metabolic processes? Bioessays. 2001;23:534–542. doi: 10.1002/bies.1073. [DOI] [PubMed] [Google Scholar]
- Deocaris CC, Takano S, Priyandoko D, Kaul Z, Yaguchi T, Kraft DC, Yamasaki K, Kaul SC, Wadhwa R. Glycerol stimulates innate chaperoning, proteasomal and stress-resistance functions: implications for geronto-manipulation. Biogerontology. 2008;9:269–282. doi: 10.1007/s10522-008-9136-8. [DOI] [PubMed] [Google Scholar]
- Deweerdt S. Looking for a master switch. Nature. 2012;492:S10–S11. doi: 10.1038/492S10a. [DOI] [PubMed] [Google Scholar]
- Diamant S, Eliahu N, Rosenthal D, Goloubinoff P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem. 2001;276:39586–39591. doi: 10.1074/jbc.M103081200. [DOI] [PubMed] [Google Scholar]
- Esparcia A, Miracle MR, Serra M. Brachionus plicatilis tolerance to low oxygen concentrations. Hydrobiologia. 1989;186:331–337. [Google Scholar]
- Esparcia A, Serra M, Miracle MR. Relationships between oxygen concentration and patterns of energy metabolism in the rotifer Brachionus plicatilis. Comparative Biochemistry and Physiology B – Biochemistry and Molecular Biology. 1992;103:357–362. [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MSB, Nahata MC, Hilty MD. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy. 1981;1:147–160. doi: 10.1002/j.1875-9114.1981.tb03562.x. [DOI] [PubMed] [Google Scholar]
- Gems D, Partridge L. Genetics of longevity in model organisms: Debates and paradigm shifts. Annu Rev Physiol. 2013;75:621–44. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- Gribble KE, Mark Welch DB. Life-span extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera) J Gerontol A: Biol Sci. 2013;68:349–358. doi: 10.1093/gerona/gls170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Berg CJ Jr, editor. Culture of Marine Invertebrates. Hutchinson Ross; Stroudsburg, PA: 1983. [Google Scholar]
- Harper JM, Slamon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J Exp Biol. 2011;214:1902–1910. doi: 10.1242/jeb.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hipkiss AR. Energy metabolism and ageing regulation: Metabolically driven deamidation of triosephosphate isomerase may contribute to proteostatic dysfunction. Ageing Research Reviews. 2011a;10:498–502. doi: 10.1016/j.arr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Hipkiss AR. Energy metabolism, proteotoxic stress and age-related dysfunction – Protection by carnosine. Molecular Aspects of Medicine. 2011b;32:267–278. doi: 10.1016/j.mam.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Roth GS. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Experimental Gerontology. 2011;46:148–154. doi: 10.1016/j.exger.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Lithgow GJ, Murakami S. Hypothesis: interventions that increase the response to stress offer the potential for effective life prolongation and increased health. J Gerontol A Biol Sci Med Sci. 1996;51:B392–B395. doi: 10.1093/gerona/51a.6.b392. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013a;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hul J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, morgan PG, Sedensky MM, Kaeberlein M. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh Syndrome. Science. 2013b;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg PS, Martin KK, Hlava HR, Riedesel ML. Sustained hyperhydration with glycerol ingestion. Life Sci. 1995;57:646–653. doi: 10.1016/0024-3205(95)00316-x. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of calorie restriction. J Anti-Aging Med. 1998;1:327–37. [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metabolism. 2009;10:379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Pletcher SD. Modulation of longevity by environmental sensing. Cell. 2007;131:1231–1234. doi: 10.1016/j.cell.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Lin ECC. Glycerol utilization and its regulation in mammals. Ann Rev Biochem. 1977;46:765–795. doi: 10.1146/annurev.bi.46.070177.004001. [DOI] [PubMed] [Google Scholar]
- Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes JP, Wuttke D, Wood SH, Plank M, Vora C. Genome-environment interactions that modulate aging: Powerful targets for drug discovery. Pharmacol Rev. 2012;64:88–101. doi: 10.1124/pr.110.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F, Rubinsztein D. Broadening the therapeutic scope for rapamycin treatment. Autophagy. 2010;6:286–87. doi: 10.4161/auto.6.2.11078. [DOI] [PubMed] [Google Scholar]
- Miller R, Harrison D, Astle C, Baur J, Boyd A, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Smith DL, Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK, Mattison JA. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243:332–339. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle MR, Vicente E. Vertical distribution and rotifer concentrations in the chemocline of meromictic lakes. Hydrobiologia. 1983;104:259–267. [Google Scholar]
- Nelson JL, Harmon ME, Robergs RA. Identifying plasma glycerol concentration associated with urinary glycerol excretion in trained humans. Journal of Analytical Toxicology. 2011;35:617–673. doi: 10.1093/anatox/35.9.617. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Kaneko G, Yanagawa Y, Watabe S. Calorie restriction in the rotifer Brachionus plicatilis enhances hypoxia tolerance in association with the increased mRNA levels of glycolytic enzymes. Hydrobiologia. 2010;649:267–277. [Google Scholar]
- Rhee JS, Kim RO, Choi HG, Lee J, Lee YM, JS, Lee Molecular and biochemical modulation of heat shock protein 20 (Hsp20) gene by temperature stress and hydrogen peroxide (H2O2) in the monogonont rotifer, Brachionus sp. Comparative Biochemistry and Physiology, Part C. 2011;154:19–27. doi: 10.1016/j.cbpc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Experimental Gerontology. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Sharp ZD. Aging and TOR: interwoven in the fabric of life. Cell Mol Life Sci. 2011;68:587–97. doi: 10.1007/s00018-010-0542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore DE, Ruvkun G. A cytoprotective perspective on longevity regulation. Trends in Cell Biology. 2013;23:409–420. doi: 10.1016/j.tcb.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipula IJ, Brown NF, Perdomo G. Rapamycin-mediated inhibition of mammalian target of rapamycin in skeletal muscle cells reduces glucose utilization and increases fatty acid oxidation. Metabolism Clinical and Experimental. 2006;55:1637–1644. doi: 10.1016/j.metabol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Smith HA, Snell TW. Rapid evolution of sex frequency and dormancy as hydroperiod adaptations. J Evol Biol. 2012;25:2501–2510. doi: 10.1111/j.1420-9101.2012.02614.x. [DOI] [PubMed] [Google Scholar]
- Snell TW, Moffat BD, Janssen C, Persoone G. Acute toxicity tests using rotifers 4. effects of cyst age, temperature, and salinity on the sensitivity of Brachionus calyciflorus. Ecotoxicology and Environmental Safety. 1991;21:308–317. doi: 10.1016/0147-6513(91)90070-6. [DOI] [PubMed] [Google Scholar]
- Snell TW, Johnston RK, Fields AM. Antioxidants can extend lifespan of Brachionus manjavacas (Rotifera), but only in a few combinations. Biogerontology. 2012 doi: 10.1007/s10522-012-9371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Johnston RK, Rabeneck B, Zipperer C, Teat S. Joint inhibition of TOR and JNK pathways interacts to extend the lifespan of Brachionus manjavacas (Rotifera) Exp Gerontology. 2014 doi: 10.1016/j.exger.2014.01.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW. Rotifers as models for the biology of aging. International Review of Hydrobiology. 2014 doi: 10.1002/iroh.201301707. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303:E488–E495. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa G, Biasi F, Poli G, Chiarpotto E. Calorie restriction and dietary restriction mimetics: A strategy for improving healthy aging and longevity. Current Pharmaceutical Design. 2014;20:1–28. doi: 10.2174/13816128113196660699. [DOI] [PubMed] [Google Scholar]
- van Rosendal SP, Osborne MA, Fassett RG, Coombes JS. Guidelines for glycerol use in hyperhydration and rehydration associated with exercise. Sports Med. 2010;40:113–129. doi: 10.2165/11530760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Madia F, Hu I, Ge H, Li LM, Longo VD. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5(5):e1000467. doi: 10.1371/journal.pgen.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–82. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]