Abstract
A large body of evidence using experimental animal models shows that the nicotinic cholinergic system is involved in the control of movement under physiological conditions. This work raised the question whether dysregulation of this system may contribute to motor dysfunction and whether drugs targeting nicotinic acetylcholine receptors (nAChRs) may be of therapeutic benefit in movement disorders. Accumulating preclinical studies now show that drugs acting at nAChRs improve drug-induced dyskinesias. The general nAChR agonist nicotine, as well as several nAChR agonists (varenicline, ABT-089 and ABT-894) reduce L-dopa-induced abnormal involuntary movements or dyskinesias up to 60% in parkinsonian nonhuman primates and rodents. These dyskinesias are potentially debilitating abnormal involuntary movements that arise as a complication of L-dopa therapy for Parkinson’s disease. In addition, nicotine and varenicline decrease antipsychotic-induced abnormal involuntary movements in rodent models of tardive dyskinesia. Antipsychotic-induced dyskinesias frequently arise as a side effect of chronic drug treatment for schizophrenia, psychosis and other psychiatric disorders. Preclinical and clinical studies also show that the nAChR agonist varenicline improves balance and coordination in various ataxias. Lastly, nicotine has been reported to attenuate the dyskinetic symptoms of Tourette’s disorder. Several nAChR subtypes appear to be involved in these beneficial effects of nicotine and nAChR drugs including α4β2*, α6β2* and α7 nAChRs (the asterisk indicates the possible presence of other subunits in the receptor). Overall, the above findings, coupled with nicotine’s neuroprotective effects, suggest that nAChR drugs have potential for future drug development for movement disorders.
Keywords: Ataxia, L-Dopa-induced dyskinesias, Nicotine, Nicotinic acetylcholine receptors, Tardive dyskinesia, Tourette’s syndrome
1. Introduction
The control of movement under physiological conditions is extremely complex and involves the integrated input from numerous central and peripheral nervous system circuits. Multiple neurotransmitter systems have been implicated in locomotion including the glutamatergic, dopaminergic, serotonergic, GABAergic, histaminergic, adrenergic, noradrenergic and peptidergic systems. Extensive preclinical studies also demonstrate a prominent role for the nicotinic cholinergic system in the regulation of motor activity. Systemic administration of nicotine to rodents induces complex changes in locomotor activity, with an acute transient depression followed by a persistent stimulation (Balfour, Benwell, Birrell, Kelly, & Al-Aloul, 1998; Benwell & Balfour, 1992; Clarke, 1990; Stolerman, 1990). These findings raised the idea that aberrant nicotinic cholinergic function may contribute to movement disorders and that treatment with drugs targeting nAChRs may be beneficial.
nAChRs are ligand-gated ion channels composed of five membrane-spanning subunits of which there are multiple subtypes in both the peripheral and central mammalian nervous system (Albuquerque, Pereira, Alkondon, & Rogers, 2009; Millar & Gotti, 2009). These channels are composed of combinations of α (α1–α7), β (β1–β4) and other subunits (δ, γ), but may also consist of only α subunits that express the acetylcholine or agonist binding site (Albuquerque, et al., 2009; Millar & Gotti, 2009). The nAChRs in the CNS vary somewhat from those in the rest of the body, with the receptors in the periphery primarily composed of α1β1γδ, α3β4* and α7 subunits while the principle ones in the brain are the α4β2*, α6β2* and α7 receptors (the asterisk indicates the presence of other possible subunits in the receptor complex) (Quik & Wonnacott, 2011). Although α7 receptors are present both in the central and peripheral nervous system, drugs targeting these receptors do not appear to have appreciable effects in the periphery. CNS nAChRs may also contain the α2, α3, and/or α5 subunits; however, these are present to a much lesser degree (Quik & Wonnacott, 2011).
Here, we review some of the more recent developments supporting a role for the nicotinic cholinergic system and the use of α4β2*, α6β2* and α7 nAChR drugs in several movement disorders including drug-induced dyskinesias, ataxia and Tourette’s syndrome.
2. Role for nicotine and nAChR agonists in reducing L-dopa-induced dyskinesias
An accumulating literature indicates that nicotine and nAChR drugs may be beneficial in reducing the abnormal involuntary movements or dyskinesias that arise with L-dopa treatment for Parkinson’s disease. Although L-dopa is the gold standard treatment for Parkinson’s disease motor symptoms, its use leads to the development of side effects, including dyskinesias (Huot, Johnston, Koprich, Fox, & Brotchie, 2013; Iravani, McCreary, & Jenner, 2012). L-dopa-induced dyskinesias (LIDs) may occur after only a few months of therapy and arise in the majority of patients with extended treatment (Ahlskog & Muenter, 2001). They can be quite incapacitating and are a major drawback in Parkinson’s disease management. Currently there are only limited therapeutic options for LIDs (Meissner, et al., 2011; Obeso, et al., 2010; Schapira, 2009; Schapira & Jenner, 2011; Wichmann, DeLong, Guridi, & Obeso, 2011). New treatment strategies to reduce LIDs are therefore a key research focus.
Drugs targeting numerous neurotransmitter systems have been shown to attenuate LIDs in animal models of Parkinson’s disease including those interacting at the dopaminergic, serotonergic, glutamatergic, opioid, GABAergic and other systems (Blandini & Armentero, 2012; Brotchie & Jenner, 2011; Cenci, 2007; Duty, 2012; Fox, Chuang, & Brotchie, 2009; Gasparini, Di Paolo, & Gomez-Mancilla, 2013; Huot, et al., 2013; Iravani, et al., 2012; Quik, Mallela, Ly, & Zhang, 2013; Rylander, 2012; Sgambato-Faure & Cenci, 2012). In addition, more recent studies indicate that alterations in nicotinic cholinergic mechanisms modulate LIDs.
Initial work investigating a role for the nicotinic cholinergic system on LIDs was done in MPTP-lesioned nonhuman primates. Lesioned monkeys exhibit parkinsonian motor symptoms very similar to those in Parkinson’s disease and develop abnormal involuntary movements with L-dopa treatment analogous to those in L-dopa-treated Parkinson’s disease patients (Duty & Jenner, 2011; Iderberg, Francardo, & Pioli, 2012; Jenner, 2009). Nicotine administration to L-dopa-treated monkeys led to ~60% decrease in LIDs (Fig. 1). This nicotine-mediated attenuation in LIDs endures across time (months), suggesting that nicotine would effectively reduce LIDs in patients with prolonged treatment (Quik, et al., 2007; Quik, Mallela, Chin, et al., 2013; Quik, Mallela, Ly, et al., 2013). The antidyskinetic effect of nicotine required several weeks to develop but also remained for weeks after its removal (Fig. 1) (Quik, et al., 2007; Quik, Mallela, Ly, et al., 2013), indicating that long term molecular and cellular changes underlie the nicotine-mediated decline in LIDs. Nicotine administration reduced LIDs equally well in L-dopa-naïve or L-dopa-primed monkeys, that is, animals with established LIDs (Quik, Mallela, Ly, et al., 2013). This is an important observation for Parkinson’s disease therapy, as it suggests that nicotine treatment may be initiated in patients after LIDs develop with no loss of efficacy.
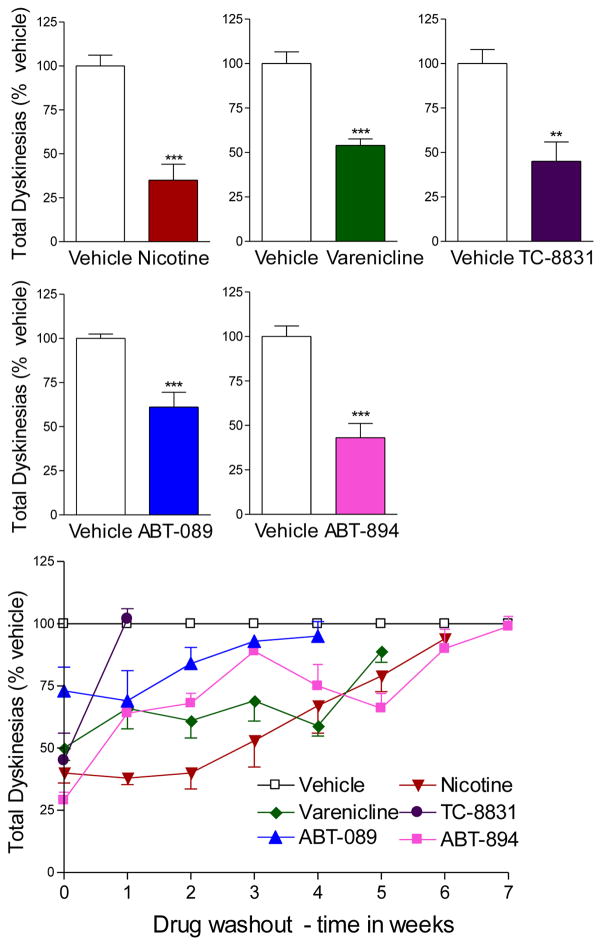
Fig. 1.
Nicotine and nAChR drugs reduce L-dopa-induced dyskinesias in parkinsonian monkeys. MPTP-lesioned monkeys were treated with L-dopa (10 mg/kg) and carbidopa (2.5 mg/kg) twice daily until stably dyskinetic. They were then given the indicated drugs immediately prior to L-dopa gavage. The bars are representative of the maximal decline in LIDs with the optimal drug dose as follows: nicotine, 300 μg/ml in the drinking water; varenicline, 0.03 mg/kg orally; TC-8831, 0.05 mg/kg orally; ABT-089, 0.1 mg/kg orally; and ABT-894, 0.01 mg/kg orally. The drug doses of nicotine, varenicline, ABT-089 and ABT-894 were within the range of those used in clinical trials. Several weeks of treatment were required for a maximal antidyskinetic effect. Tolerance did not develop to any of the nAChR drugs with months of treatment. Drug washout (lower panel) led to a return of LIDs to values similar to those in vehicle-treated monkeys. Values are the mean ± SEM of 5–12 animals per group. Significance of difference from vehicle-treated, **p < 0.01, ***p < 0.001 using Student’s t-test. Data taken in modified form from (D. Zhang, et al., 2014; D. Zhang, et al., 2013).
The antidyskinetic effect of nicotine was also investigated in rodent models because their use offers the advantage that they allow for studies to investigate varying treatment paradigms and molecular mechanisms. Nicotine reduced LIDs when administered by any one of several methods, including oral application, injection and slow-release minipump, with no decline in its effectiveness with time. Moreover, nicotine treatment attenuated LIDs to a similar extent in L-dopa-treated parkinsonian rats and mice, indicating the effect occurred across species (Bordia, Campos, McIntosh, & Quik, 2010; L. Huang, Grady, & Quik, 2011; M. Quik, C. Campos, & S. R. Grady, 2013b; Quik, et al., 2007; Quik, Mallela, Ly, et al., 2013).
In addition to these preclinical studies, a small clinical trial has been conducted to evaluate the antidyskinetic potential of nicotine in Parkinson’s disease patients. Oral nicotine (designated NP002) administration to 50 patients for several months significantly reduced a variety of outcome measures related to LIDs (http://www.neuraltus.com/pages/news.html).
2.1. Nicotine exerts its anti-dyskinetic effect by acting at nAChRs
Nicotine’s mechanism of action to reduce LIDs is an important question because such knowledge may yield more selective therapies. Two approaches have been used to evaluate the nAChRs through which nicotine exerts its effects. One of these involves the use of nAChR null mutant mice. Since β2 containing nAChRs (α4β2* and α6β2* subtypes) are widespread throughout the brain and highly expressed in the nigrostriatal pathway, initial studies were done with mice lacking the β2 subunit. Baseline LID levels were reduced in such mice while nicotine did not decrease LIDs, indicating that β2* nAChRs are necessary for both the occurrence of LIDs and the antidyskinetic effect of nicotine (L. Huang, et al., 2011). Follow up studies with mice lacking the α6 nAChR subunit yielded very similar results suggesting a key involvement of α6β2* nAChRs (Quik, Park, et al., 2012). Nicotine had no antidyskinetic effect in mice lacking α4β2* nAChRs, although there was no change in baseline LIDs in such null mutant mice. These combined data suggest that both α6β2* and α4β2* nAChRs populations play a major role in LIDs but in somewhat distinct manners. Similar studies showed that baseline LIDs were actually increased in α7 nAChR null mutant mice, while nicotine treatment still reduced LIDs (M. Quik, C. Campos, & S. Grady, 2013a). Thus, in contrast to the β2* nAChRs, α7 nAChRs appear to exert an inhibitory control on the occurrence of LIDs. The distinct regulation of LIDs by β2* as compared to α7 nAChRs may not be that unexpected as α7 and β2* nAChRs exhibit very different pharmacological and functional characteristics (Changeux, 2010; Giniatullin, Nistri, & Yakel, 2005; Picciotto, Addy, Mineur, & Brunzell, 2008; Quik, Perez, & Bordia, 2012; Wonnacott, Sidhpura, & Balfour, 2005). Since multiple nAChR subtypes differentially regulate LIDs, diverse nAChR subtype drugs may be effective in reducing LIDs. This idea is further suggested from the results of studies which show that the status of nAChR receptors depends on the extent of nigrostriatal damage and/or L-dopa treatment regimen (Quik, Polonskaya, Kulak, & McIntosh, 2001; Quik, Sum, et al., 2003)(Quik, Bordia, et al., 2003).
A second approach to discern the nAChRs implicated in nicotine’s therapeutic effect involves pharmacological studies in parkinsonian animal models. These data support the results with null mutant mice and show that compounds acting at α4β2*/α6β2* nAChRs reduce LIDs. Varenicline, A-85380, sazetidine, TC-2696, TI-10165, TC-8831 and TC-10600 all attenuated LIDs to varying extents in parkinsonian rats (Fig. 1) (L. Z. Huang, Campos, Ly, Carroll, & Quik, 2011; Quik, Campos, Bordia, et al., 2013). Additionally, varenicline and TC-8831 decreased LIDs ~50% in nonhuman primates, although a limitation with these drugs was the development of emesis (Johnston, et al., 2013; D. Zhang, et al., 2013). ABT-089, a partial α4β2*/α6β2* nAChR agonist, and ABT-894, a full α4β2* and α6β2* agonist, two drugs that have both been approved for use in clinical trials and show little emesis, have also been tested for their ability to reduce LIDs in parkinsonian monkeys (Anderson, et al., 2009; Apostol, et al., 2012; Bain, et al., 2013; Decker, et al., 1997; Ji, et al., 2007; Marks, Wageman, Grady, Gopalakrishnan, & Briggs, 2009; Rowbotham, et al., 2012; Sullivan, et al., 1997). ABT-089 and ABT-894, decreased LIDs 40% and 60%, respectively, without worsening parkinsonism, causing emesis or inducing other side effects (D. Zhang, et al., 2014). Currently available nAChR drugs all act at both α4β2* and α6β2* receptors, and thus it has not been possible to evaluate a role for the individual subtypes in LIDs using a pharmacological approach. α7 nAChR drugs remain to be tested.
In summary, drugs targeting α4β2*/α6β2* nAChRs reduced LIDs up to 60% in parkinsonian monkeys for extended periods of time. Since ABT-089 and ABT-894 have previously been evaluated in phase 2 clinical trials for other indications, these drugs could readily be transitioned to the clinic to evaluate their ability to reduce LIDs.
2.2. Mechanism of action of nicotine to decrease LIDs
A mechanism whereby agonists that act at β2* nAChRs improve L-dopa-induced AIMs may involve nAChR desensitization. It is well known that acetylcholine and nAChR agonists lead to an initial receptor activation that is rapidly followed by molecular alterations that result in channel closing and an effective block of receptor function or desensitization (Buccafusco, Beach, & Terry, 2009; McCarthy, Zhang, Neff, & Hadjiconstantinou, 2011; Picciotto, et al., 2008). The idea that such a molecular mechanism may underlie LIDs is supported by several findings. First, the nAChR antagonist mecamylamine reduces LIDs in parkinsonian rats to a similar extent as the desensitizing agonist nicotine (Bordia, et al., 2010). Second, treatment of nicotine via injection, which involves transient pulsatile administration, or via minipump that involves chronic slow nicotine exposure and desensitization, both result in similar declines in LIDs (Bordia, et al., 2010). Lastly, sazetidine and varenicline, two agonists that reduce LIDs in rats (L. Z. Huang, et al., 2011), are postulated to exert their effects via nAChR desensitization or inactivation (Hussmann, et al., 2012; Rezvani, et al., 2012; Rollema, et al., 2010; Zwart, et al., 2008).
2.3. Localization of CNS nAChRs that mediate antidyskinetic effects of nAChR drugs
The cellular localization and brain regions expressing the nAChRs involved in the nicotine-mediated antidyskinetic effect is an important question. Lesion studies indicate that the striatum makes a major contribution to the nAChR-mediated decline in LIDs. Both nicotine and nAChR drugs most effectively reduce LIDs in mice, rats or monkeys with moderate nigrostriatal damage, that is, when the dopaminergic system is still partially intact (L. Huang, et al., 2011; L. Z. Huang, et al., 2011; Quik, Mallela, Chin, et al., 2013). These results suggest that α6β2* and/or α4β2* nAChRs on nigrostriatal dopamine terminals play an important role in the nAChR-mediated decline in LIDs (Fig 2). However, since nicotine does reduce LIDs to some extent even with severe nigrostriatal damage, nAChRs on non-dopaminergic neurons may also contribute. Alternatively, α4β2* nAChRs in the thalamus, cortex and cerebellum, regions linked to motor control and coordination, may be important. Additionally, α7 nAChRs on cortical afferents to the striatum and/or α7 receptors in other CNS regions may contribute to the antidyskinetic effect of nicotine (Fig. 2). The observation that nicotine most effectively reduces LIDs with moderate nigrostriatal damage, suggests that nAChR drugs may be most effective in reducing LIDs in early stage Parkinson’s disease. However, since multiple compensatory mechanisms may arise with slow progressive nigrostriatal damage and long term L-dopa treatment in Parkinson’s disease, it may be difficult to predict efficacy. This is especially true since the association between the severity of dyskinesia, extent of nigrostriatal damage and length of L-dopa treatment is variable in animal models. For instance, LIDs do not necessarily develop in L-dopa-treated lesioned animals and the level of LIDs is not always dependent on the extent of nigrostriatal damage (Cenci, Lee, & Bjorklund, 1998; Guigoni, et al., 2005; Pearce, Heikkila, Linden, & Jenner, 2001; Togasaki, et al., 2001; Y. Zhang, et al., 2013).
Fig. 2.
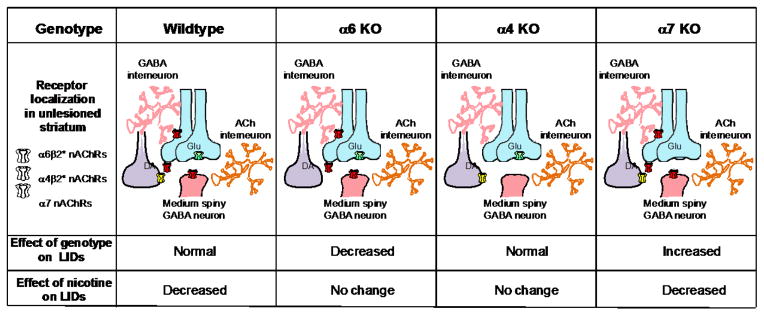
Studies with nAChR null mutant mice indicate that receptors containing α4, α6 or α7 nAChR subunits all play a role in the occurrence of LIDs. Studies with genetically modified mice, nAChR subtype-specific antibodies and drugs, as well as nAChR subunit chimeras and concatamers show that the principle receptors in striatum are the α6β2*, α4β2* and α7 subtypes (Millar & Gotti, 2009; Quik & Wonnacott, 2011). The α6β2* nAChRs are present primarily on dopaminergic terminals originating in the substantia nigra. The α4β2* nAChRs are expressed on dopaminergic terminals and also on GABA interneurons and medium spiny neurons in striatum. By contrast, striatal α7 nAChRs are primarily localized to glutamatergic afferents from the cortex. Experiments with α4, α6 and α7 nAChR subunit knockout mice show that α6β2* and α7 nAChRs are involved in the development of LIDs, as the absence of these receptors decreases and increases baseline LID expression, respectively. In addition, the α6β2* and α4β2* nAChR subtypes are involved in the antidyskinetic effect of nicotine since deletion of the α6 and α4 nAChR subunit prevents nicotine from decreasing LIDs. Data taken in modified form from (L. Huang, et al., 2011; Quik, Campos, & Grady, 2013b; Quik, Park, et al., 2012).
In summary, α4β2* and α6β2* nAChRs on dopamine terminals in the nigrostriatal pathway contribute to the expression of LIDs. Other striatal α4β2* and α7 nAChRs may also be involved, as well as nAChRs in other brain regions. The idea that multiple nAChRs in various brain regions are important is consistent with studies showing that the striatal dopaminergic system is tightly interconnected with numerous neurotransmitter systems involved in movement.
2.4. Anti-dyskinetic effect of non-nAChR drugs
As mentioned earlier, changes in numerous neurotransmitter systems contribute to the etiology of LIDs. These most likely modulate LIDs via multiple molecular mechanisms including alterations in presynaptic dopaminergic function, enhanced activation of the direct dopaminergic pathway, aberrant release of L-dopa via serotonergic neurons, excessive glutamatergic signaling, modulation of opioid activity and others (Blandini & Armentero, 2012; Cenci, 2007; Gasparini, et al., 2013; Huot, et al., 2013; Iravani, et al., 2012; Rylander, 2012). Consistent with this multifactorial origin of LIDs, drugs targeting the glutamatergic, serotonergic, opioid, adenosine and other systems have all been reported to attenuate LIDs to varying extents in parkinsonian animal models (Blandini & Armentero, 2012; Brotchie & Jenner, 2011; Duty, 2012; Fox, 2013; Fox, et al., 2009; Huot, et al., 2013; Samadi, Bedard, & Rouillard, 2006; Sgambato-Faure & Cenci, 2012). Since multiple mechanisms underlie LIDs, it is not unexpected that the reduction in LIDs produced by a drug targeting any one neurotransmitter system is generally not complete (Huot, et al., 2013). Interestingly, recent studies in parkinsonian animal models show that combined treatment with drugs known to partially reduce LIDs yields additive or synergistic declines (Bezard, et al., 2013; Dupre, et al., 2008; Hill, et al., 2004; Iderberg, Rylander, Bimpisidis, & Cenci, 2013; Kobylecki, Hill, Crossman, & Ravenscroft, 2011). These observations imply that the optimal therapy to alleviate LIDs may involve drug combinations or the use of drugs that target several neurotransmitter systems.
3. Nicotine and the nAChR agonist varenicline decrease antipsychotic-induced tardive dyskinesias in animal models
Recent preclinical studies also suggest that nicotine and nAChR agonists improve another class of drug-induced abnormal involuntary movements, the tardive or late dyskinesias that arise with chronic antipsychotic use. Antipsychotics are a very important class of drugs approved for the management of schizophrenia and bipolar disorder, and are also used off-label for depression, autism, attention deficit hyperactivity disorder, obsessive compulsive disorder and post-traumatic stress disorder (Gershanik & Gomez-Arevalo, 2011; Maher & Theodore, 2012; Tarsy, Lungu, & Baldessarini, 2011; Zupancic, 2011). These drugs are dopamine receptor antagonists, and are thought to induce their therapeutic effect by dampening excess dopaminergic activity (Seeman, 2010; Tarsy, et al., 2011; Turrone, Remington, Kapur, & Nobrega, 2003). However, antipsychotics not only modulate aberrant dopaminergic activity linked to psychiatric disorders, but also affect motor function. Thus, although antipsychotics are very valuable clinically, their continued use results in side effects including tardive dyskinesia, which afflicts ~25% of treated patients (Saha, Chant, Welham, & McGrath, 2005). These dyskinesias consist of repetitive abnormal involuntary movements primarily of the face and limbs that may become severe and eventually debilitating (Correll, Leucht, & Kane, 2004; Tarsy, et al., 2011). There is currently little treatment other than antipsychotic dose modification. The use of second-generation antipsychotics has been reported to cause less tardive dyskinesia (Peluso et al., 2012). However, randomized clinical trials show that the decrease is less than anticipated (Correll & Schenk, 2008; Peluso, Lewis, Barnes, & Jones, 2012; Tarsy, et al., 2011; Woods, et al., 2010).
Because of the success of nicotine and nAChR drugs in reducing LIDs, studies were conducted to investigate their potential to reduce tardive dyskinesia in rodents. Nicotine treatment attenuated haloperidol-induced abnormal movements or vacuous chewing movements (VCMs) in both a rat and mouse model of tardive dyskinesia, with a maximal reduction in VCMs of 50% (Fig. 3) (Bordia, Carroll, & Quik, 2013; Bordia, McIntosh, & Quik, 2012). The nicotine-mediated decline in haloperidol-induced VCMs was independent of the mode of administration of haloperidol, that is, injection or a subdermal-slow release pellet. It was also independent of the nicotine treatment regimen with similar declines in VCMs whether nicotine was given orally or via subcutaneous minipump (Fig. 3A, B, C). These data suggest that either oral nicotine administration or the nicotine patch may be useful clinically. Nicotine reduced haloperidol-induced VCMs when given before VCMs had developed and was also effective against established VCMs, with no tolerance following continued use. The general nicotinic agonist varenicline, which interacts with high affinity at α4β2* and α6β2* nAChRs, as well as α3β4* and α7 nAChRs (Bordia, Hrachova, Chin, McIntosh, & Quik, 2012; Coe, et al., 2005; Mihalak, Carroll, & Luetje, 2006; Rollema, et al., 2007), also reduced haloperidol-induced VCMs (Fig. 3D) (Bordia, et al., 2013; Bordia, McIntosh, et al., 2012). These data provide proof-of-principle that nicotine exerts its effect via an interaction at nAChRs. Varenicline reduced VCMs to a greater extent than nicotine, a finding that may relate to varenicline’s ability to interact with 5-HT receptors (Creed-Carson, Oraha, & Nobrega, 2011; Creed, Hamani, Bridgman, Fletcher, & Nobrega, 2012; Lummis, Thompson, Bencherif, & Lester, 2011; Naidu & Kulkarni, 2001).
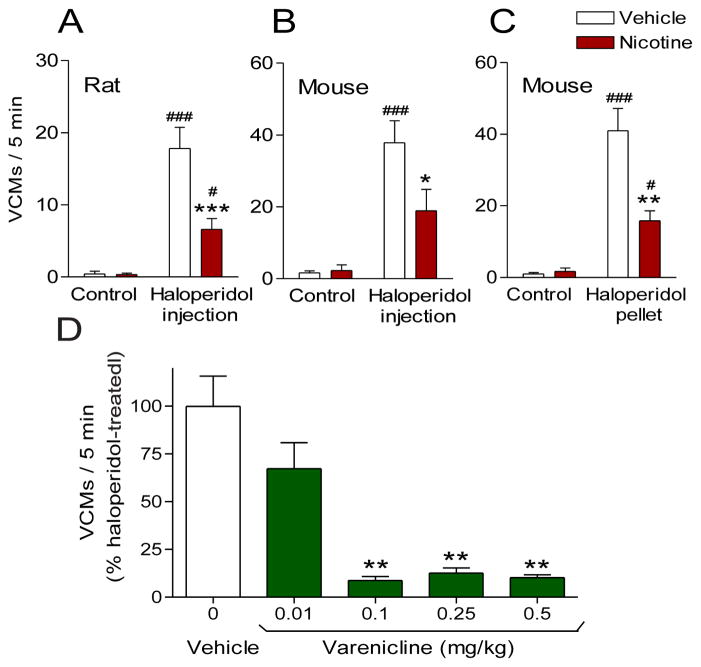
Fig. 3.
Nicotine treatment reduces vacuous chewing movements (VCMs) in a well-established rodent model of tardive dyskinesia. Rats or mice were pretreated with vehicle or nicotine given via minipump (A) or the drinking water (B and C). Two weeks later, they were treated with haloperidol via injection or surgically implanted with sub-dermal pellets that may mimic depot delivery of haloperidol in humans. Both forms of haloperidol treatment led to the development of VCMs, a motor side effect of antipsychotic therapy. (A–C) Chronic nicotine treatment reduced VCMs compared to vehicle-treated animals in rats and mice with either mode of haloperidol administration (injection or pellet), demonstrating the robustness of this effect. (B) The general nAChR agonist varenicline also reduced haloperidol-induced VCMs, indicating the reduction in VCMs is nAChR-mediated. Values are the mean ± SEM of 6–12 animals per group. Significance of difference from the vehicle-treated control, #p < 0.05, ###p < 0.001: from the vehicle-haloperidol-treated group, *p < 0.05, **p < 0.01, ***p < 0.001 using two-way ANOVA followed by a Bonferroni post hoc test or using one-way ANOVA followed by a Dunnett’s post hoc test. Data taken in modified form from (Bordia, et al., 2013; Bordia, McIntosh, et al., 2012).
As mentioned above, antipsychotics are one of the principle therapies for the treatment of schizophrenia. It is well documented that schizophrenic patients are very heavy smokers and may consume several packs of cigarettes per day, that is, be exposed to relatively large quantities of nicotine. This raises the question whether schizophrenics who are heavy smokers exhibit less tardive dyskinesia since animal studies show that nicotine reduces dyskinesias. However, a search of the literature yielded conflicting results on the link between smoking and tardive dyskinesia in humans. An early study indicated that the prevalence of tardive dyskinesia in chronic psychiatric outpatients was significantly higher in smokers than in nonsmokers (Yassa, Lal, Korpassy, & Ally, 1987). In another study, patients who smoked received significantly higher doses of neuroleptics but did not have more frequent or more severe tardive dyskinesia, suggesting that smoking may suppress the occurrence of neuroleptic-induced tardive dyskinesia (Menza, Grossman, Van Horn, Cody, & Forman, 1991). By contrast, Nilsson and coworkers found that dyskinetic men had higher daily cigarette consumption than men without dyskinesia, but the former group also had greater exposure to neuroleptics, higher frequencies of psychiatric morbidity and alcohol dependence, thus confounding results (Nilsson, Waller, Rosengren, Adlerberth, & Wilhelmsen, 1997). A more recent report showed that the prevalence of tardive dyskinesia did not significantly differ between smokers and non-smokers (X. Y. Zhang, et al., 2011). A carefully controlled, double-blind clinical trial appears necessary to understand the relationship between smoking (nicotine intake) and tardive dyskinesia in the clinic.
In summary, studies using rodent models of tardive dyskinesia clearly demonstrate a decline in antipsychotic-induced abnormal movements with nicotine use. Well-controlled clinical trials are the next step to determine the effectiveness of nicotine and/or nAChR drugs to ameliorate anti-psychotic-induced tardive dyskinesia in patients.
4. Nicotine and varenicline treatment attenuate ataxia
The idea that enhanced nicotinic cholinergic stimulation may reduce ataxia stems from both preclinical and clinical studies. Ataxia is characterized by a lack of coordination of voluntary muscle movements involving the cerebellum as well as other CNS regions (van de Warrenburg, et al., 2014). Multiple types of ataxia have been identified including spinocerebellar ataxia, Friedreich’s ataxia and Fragile X associated ataxia. A variety of genetic factors, including missense, inframe deletions, and frameshift insertions/deletions or trinucleotide repeat expansions underlie their pathophysiology, which may involve mitochondrial and other cellular deficits (Gonzalez-Cabo & Palau, 2013; Koeppen & Mazurkiewicz, 2013; Rub, et al., 2013). Treatment has proved challenging and is generally symptomatic only.
Preclinical studies investigating the effect of nicotine and nAChR drugs in animal models of ataxia show that acute intracerebellar nicotine or the α4β2* nAChR agonist RJR-2403 dose dependently attenuated ethanol-induced ataxia (Al-Rejaie & Dar, 2006). Intracerebellar administration of the α4β2* nAChR antagonist dihydro-β-erythroidine attenuated this effect, indicating that cerebellar α4β2* nAChRs play a role (Taslim, Al-Rejaie, & Saeed Dar, 2008). In addition, nicotine and the nAChR agonist varenicline reduced ataxia in rats with a lesion of the olivocerebellar pathway via a nAChR-mediated mechanism (Wecker, et al., 2013). α7 nAChR drugs also decreased the occurrence of ethanol-induced ataxia, indicating that multiple nAChR populations modulate ataxia (Taslim & Saeed Dar, 2011).
The suggestion that nAChR drugs may be useful for the treatment of ataxia in patients initially stemmed from studies using drugs that enhance CNS cholinergic activity. This includes work with physostigmine, a centrally acting acetylcholinesterase inhibitor, which is well known to increase brain acetylcholine levels. Physostigmine treatment improved the symptoms of spinocerebellar degenerations and various inherited ataxias in open label and double-blind randomized trials, possibly via an interaction with the nicotinic cholinergic system (Kark, Blass, & Spence, 1977; Kark, Budelli, & Wachsner, 1981; Rodriguez-Budelli, Kark, Blass, & Spence, 1978). This effect appears to be selective for specific ataxias as physostigmine did not improve symptoms in patients with autosomal dominant cerebellar ataxia and idiopathic cerebellar ataxia (Wessel, Langenberger, Nitschke, & Kompf, 1997). Other studies with the acetylcholine precursor choline also showed improvements in patients with Friedreich’s ataxia, idiopathic cerebellar degeneration, multiple sclerosis-linked ataxia, and ataxias associated with sporadic cerebellar degeneration and atypical spinocerebellar degeneration, although no improvements were observed in other ataxias (Livingstone, Mastaglia, Pennington, & Skilbeck, 1981) (Blattel, 1979; Legg, 1978; Philcox & Kies, 1979). Since choline may represent an endogenous ligand for α7 nAChRs, these reports may suggest that α7 nAChRs play a role (Alkondon, Pereira, Cortes, Maelicke, & Albuquerque, 1997).
More recent studies with the general nAChR agonist varenicline further supported a role for the nicotinic cholinergic system in patients with various types of ataxic neurodegenerative disorders. Case reports initially showed that varenicline, which was being used for smoking cessation, improved ataxia and imbalance in a man with Fragile X tremor/ataxia syndrome (Zesiewicz, Sullivan, Freeman, & Juncos, 2009). It also improved proprioception in the extremities in two patients with Friedreich’s ataxia (Zesiewicz, Sullivan, Gooch, & Lynch, 2009) and gait, balance and depth perception in a patient with spinocerebellar ataxia (Zesiewicz & Sullivan, 2008). A subsequent double-blind, placebo-controlled, randomized trial with 20 patients with spinocerebellar ataxia showed that 2 months of varenicline ameliorated axial symptoms and rapid alternating movements (Zesiewicz, et al., 2012), although poor tolerability and little therapeutic benefit was also reported in a mixed ataxia population (Connolly, Prashanth, Shah, Marras, & Lang, 2012).
In summary, both preclinical and clinical studies support the idea that drugs targeting nAChRs may improve components of ataxia.
5. Nicotine administration improves motor symptoms associated with Tourette’s syndrome
Over the last two decades, reports have indicated that the use of nicotine gum and the transdermal nicotine patch potentiated the therapeutic effects of neuroleptics in reducing the frequency and severity of tics in Tourette’s (Sanberg, et al., 1997). Tourette’s syndrome is a neurodevelopmental disorder characterized by sudden, rapid and brief motor and vocal tics, as well as a wide spectrum of other behavioral problems including obsessions, compulsions, impulsivity, distractibility, and hyperactivity (Roessner, et al., 2013; Termine, Selvini, Rossi, & Balottin, 2013; Thomas & Cavanna, 2013). Although the etiology of Tourette’s is currently unclear, hyperactivity of the brain dopaminergic system appears to be involved as dopamine blockers symptomatically improve the motor and vocal tics (Roessner, et al., 2013; Termine, et al., 2013; Thomas & Cavanna, 2013). However, currently available drugs are only partially effective and associated with side effects and thus new therapies are constantly being sought.
Initial open label studies with the nicotine gum showed a substantial decrease in tics and improvement of concentration/attention span in Tourette’s syndrome patients treated with haloperidol, although nicotine alone appeared to have little effect (McConville, et al., 1991; Sanberg, et al., 1989). However, side effects, such as nausea and bitter taste limited the usefulness of the nicotine gum. Subsequent open-label studies showed that the low dose transdermal nicotine patch led to varying reductions in tic severity with an average duration of effect lasting up to 4 weeks (Dursun & Kutcher, 1999; Dursun, Reveley, Bird, & Stirton, 1994; Silver, Shytle, Philipp, & Sanberg, 1996). Importantly, positive results were also observed in a subsequent double-blind placebo-controlled trial in which seventy Tourette’s patients on haloperidol were randomly assigned to either low transdermal nicotine or placebo patches for several weeks (Silver, Shytle, Philipp, et al., 2001). Transdermal nicotine was superior to placebo in reducing the symptoms of Tourette’s disorder for several weeks after nicotine discontinuation. Nicotine may thus have potential as an adjunct to neuroleptic therapy for Tourette’s syndrome, although side effects may limit its chronic use.
It had been noted that the benefits of the nicotine patch in Tourette’s outweighed those of the nicotine gum in terms of duration of the response, with the effects of the patch lasting much longer than those of the gum (Sanberg, Vindrola-Padros, & Shytle, 2012). These observations led to the hypothesis that desensitization of nAChRs may represent a mechanism underlying the beneficial effect of nicotine in Tourette’s. Desensitization of nAChRs on striatal dopamine terminals would subsequently result in a functional nAChR blockade with a consequent decrease in dopamine release and a dampening of striatal dopaminergic activity (Sanberg, et al., 2012; Shytle, Silver, & Sanberg, 2000). This possibility is supported by results suggesting that nicotine exerts its beneficial effects on other behaviors, such as dyskinesias via receptor desensitization (Bordia, et al., 2010).
This idea that nicotine may exert its effects via a functional blockade led to the hypothesis that a nAChR antagonist, such as mecamylamine, may prove useful in Tourette’s but be associated with fewer side effects that arise with nicotine treatment. Mecamylamine, which had originally been used for the treatment of hypertension, was subsequently tested in a retrospective, open-label study. A significant improvement was observed in motor and vocal tics, and also in mood and behavior disturbances of children, adolescents, and adults with Tourette’s with no significant peripheral parasympathetic effects (Silver, Shytle, & Sanberg, 2000). A subsequent double-blind placebo-controlled study to examine the efficacy of mecamylamine as a monotherapy was subsequently carried out; however, the results suggested it was not effective although it was tolerated (Silver, Shytle, Sheehan, et al., 2001).
In summary, clinical trial data indicate that nAChR drugs have potential to attenuate the occurrence of tics in Tourette’s syndrome, although no further studies appear to have been conducted since the work reported above.
6. Concluding remarks
Mounting evidence in experimental animal models indicate that nicotine and nAChR drugs improve motor deficits (summarized in Table 1) and may be useful for attenuating movement disorders in the clinic (summarized in Table 2). As well, nAChR drugs hold promise as neuroprotectants against neuronal degeneration as occurs in Parkinson’s disease (Picciotto & Zoli, 2008; Quik, Perez, et al., 2012). In fact, the Michael J Fox Foundation is currently funding a clinical trial to investigate the neuroprotective ability of transdermal nicotine in early Parkinson’s disease (ClinicalTrials.gov Identifier NCT01560754). Overall, these observations suggest that drugs interacting with nAChRs may be of therapeutic benefit in the management of neurodegenerative disorders.
Table 1.
nAChR drugs improve motor abnormalities in animal models of movement disorders
| Animal model | nAChR drug | Species used | Effect of nAChR drug | References |
|---|---|---|---|---|
| L-dopa-induced dyskinesias | Nicotine, varenicline, ABT-089, ABT-894, | NHPs | Up to 60% decline in L-dopa-induced dyskinesias | (Quik, Campos, Bordia, et al., 2013; Quik, et al., 2007; Quik, Mallela, Chin, et al., 2013; Zhang, et al., 2014; D. Zhang, et al., 2013) |
| L-dopa-induced dyskinesias | Nicotine, varenicline, A85380, sazetidine, TC-2696, TC-8831, TC10600, TI-10165 | Rodents | Up to 60% decline in L-dopa-induced dyskinesias | (Bordia, Campos, Huang, & Quik, 2008; L. Z. Huang, et al., 2011) |
| Tardive dyskinesia | Nicotine, varenicline | Rodents | Up to 90% decline in tardive dyskinesias | (Bordia, et al., 2013; Bordia, McIntosh, et al., 2012) |
| Ataxia | Nicotine, varenicline, PNU-282987 | Rodents | Up to a near complete reduction in ataxia | (Al-Rejaie & Dar, 2006; Taslim, et al., 2008; Taslim & Saeed Dar, 2011; Wecker, et al., 2013) |
| Tourette’s syndrome | Nicotine | Rodents | Reduced haloperidol- induced hypokinesia | (Elazar & Paz, 1999; Emerich, Zanol, Norman, McConville, & Sanberg, 1991; Sanberg, et al., 1989) |
Table 2.
Nicotine and varenicline improve motor abnormalities in clinical studies
| Disorder | nAChR drug | Study | Effect of nAChR drug | References |
|---|---|---|---|---|
| L-dopa-induced dyskinesias | Oral nicotine | Double-blind, placebo-controlled | Reduced some components of dyskinesias | http://www.neuraltus.com/pages/news_rel12_03_10.html |
| Ataxia | Oral varenicline | Case reports | Improve ataxia in Fragile X, Friedreich’s ataxia and spinocerebellar ataxia | (Zesiewicz & Sullivan, 2008; Zesiewicz, Sullivan, Freeman, et al., 2009; Zesiewicz, Sullivan, Gooch, et al., 2009) |
| Double-blind, placebo-controlled | Improved ataxia in spinocerebellar ataxia | (Zesiewicz, et al., 2012) but see (Connolly, et al., 2012) | ||
| Tourette’s syndrome | Nicotine gum or patch | Open-label | Decreased tics or tic severity | (Dursun & Kutcher, 1999; Dursun, et al., 1994; McConville, et al., 1991; Sanberg, et al., 1989; Silver, et al., 1996) |
| Nicotine patch | Double-blind, placebo-controlled | Decreased tic severity | (Silver, Shytle, Philipp, et al., 2001) |
Acknowledgments
Funding
This work was supported by grants NS59910 and NS 65851 from the National Institutes of Health.
Abbreviations
- LIDs
L-dopa-induced dyskinesias
- nAChRs
nicotinic acetylcholine receptors
- VCMs
vacuous chewing movements
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Al-Rejaie S, Dar MS. Behavioral interaction between nicotine and ethanol: possible modulation by mouse cerebellar glutamate. Alcohol Clin Exp Res. 2006;30:1223–1233. doi: 10.1111/j.1530-0277.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Malysz J, Gronlien JH, El Kouhen R, Hakerud M, Wetterstrand C, Briggs CA, Gopalakrishnan M. Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination. Biochem Pharmacol. 2009;78:844–851. doi: 10.1016/j.bcp.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, Pritchett YL, Feifel D, Collins MA, Saltarelli MD. Efficacy and safety of the novel alpha(4)beta (2) neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology (Berl) 2012;219:715–725. doi: 10.1007/s00213-011-2393-2. [DOI] [PubMed] [Google Scholar]
- Bain EE, Robieson W, Pritchett Y, Garimella T, Abi-Saab W, Apostol G, McGough JJ, Saltarelli MD. A randomized, double-blind, placebo-controlled phase 2 study of alpha4beta2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology. 2013;38:405–413. doi: 10.1038/npp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Tronci E, Pioli EY, Li Q, Porras G, Bjorklund A, Carta M. Study of the Antidyskinetic Effect of Eltoprazine in Animal Models of Levodopa-Induced Dyskinesia. Mov Disord. 2013 doi: 10.1002/mds.25366. [DOI] [PubMed] [Google Scholar]
- Blandini F, Armentero MT. New pharmacological avenues for the treatment of L-DOPA-induced dyskinesias in Parkinson’s disease: targeting glutamate and adenosine receptors. Expert Opin Investig Drugs. 2012;21:153–168. doi: 10.1517/13543784.2012.651457. [DOI] [PubMed] [Google Scholar]
- Blattel RA. Use of choline in the treatment of ataxia associated with multiple sclerosis. Can Med Assoc J. 1979;121:1568. [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Carroll FI, Quik M. Varenicline Markedly Decreases Antipsychotic-induced Tardive Dyskinesia in a Rodent Model. Soc Neurosci Abstr. 2013;33:32.07. [Google Scholar]
- Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at alpha6beta2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342:327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, McIntosh JM, Quik M. Nicotine reduces antipsychotic-induced orofacial dyskinesia in rats. J Pharmacol Exp Ther. 2012;340:612–619. doi: 10.1124/jpet.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotchie J, Jenner P. New approaches to therapy. Int Rev Neurobiol. 2011;98:123–150. doi: 10.1016/B978-0-12-381328-2.00005-5. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Changeux JP. Allosteric receptors: from electric organ to cognition. Annu Rev Pharmacol Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [DOI] [PubMed] [Google Scholar]
- Clarke PB. Dopaminergic mechanisms in the locomotor stimulant effects of nicotine. Biochem Pharmacol. 1990;40:1427–1432. doi: 10.1016/0006-2952(90)90436-o. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Connolly BS, Prashanth LK, Shah BB, Marras C, Lang AE. A randomized trial of varenicline (chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;79:2218. doi: 10.1212/WNL.0b013e318278a059. [DOI] [PubMed] [Google Scholar]
- Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161:414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21:151–156. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- Creed-Carson M, Oraha A, Nobrega JN. Effects of 5-HT(2A) and 5-HT(2C) receptor antagonists on acute and chronic dyskinetic effects induced by haloperidol in rats. Behav Brain Res. 2011;219:273–279. doi: 10.1016/j.bbr.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Creed MC, Hamani C, Bridgman A, Fletcher PJ, Nobrega JN. Contribution of decreased serotonin release to the antidyskinetic effects of deep brain stimulation in a rodent model of tardive dyskinesia: comparison of the subthalamic and entopeduncular nuclei. J Neurosci. 2012;32:9574–9581. doi: 10.1523/JNEUROSCI.1196-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, Lin NH, Li Y, Daanen JF, Buccafusco JJ, Prendergast MA, Jackson WJ, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]: II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther. 1997;283:247–258. [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Steiniger A, Klioueva A, Negron GE, Lormand L, Park JY, Bishop C. Effects of coincident 5-HT1A receptor stimulation and NMDA receptor antagonism on L-DOPA-induced dyskinesia and rotational behaviors in the hemi-parkinsonian rat. Psychopharmacology (Berl) 2008;199:99–108. doi: 10.1007/s00213-008-1135-6. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Kutcher S. Smoking, nicotine and psychiatric disorders: evidence for therapeutic role, controversies and implications for future research. Med Hypotheses. 1999;52:101–109. doi: 10.1054/mehy.1997.0623. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Reveley MA, Bird R, Stirton F. Longlasting improvement of Tourette’s syndrome with transdermal nicotine. Lancet. 1994;344:1577. doi: 10.1016/s0140-6736(94)90388-3. [DOI] [PubMed] [Google Scholar]
- Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26:1017–1032. doi: 10.1007/s40263-012-0016-z. [DOI] [PubMed] [Google Scholar]
- Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar Z, Paz M. Potentiation of haloperidol catalepsy by microinjections of nicotine into the striatum or pons in rats. Life Sci. 1999;64:1117–1125. doi: 10.1016/s0024-3205(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Zanol MD, Norman AB, McConville BJ, Sanberg PR. Nicotine potentiates haloperidol-induced catalepsy and locomotor hypoactivity. Pharmacol Biochem Behav. 1991;38:875–880. doi: 10.1016/0091-3057(91)90256-2. [DOI] [PubMed] [Google Scholar]
- Fox SH. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs. 2013;73:1405–1415. doi: 10.1007/s40265-013-0105-4. [DOI] [PubMed] [Google Scholar]
- Fox SH, Chuang R, Brotchie JM. Serotonin and Parkinson’s disease: On movement, mood, and madness. Mov Disord. 2009 doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Di Paolo T, Gomez-Mancilla B. Metabotropic glutamate receptors for Parkinson’s disease therapy. Parkinsons Dis. 2013;2013:196028. doi: 10.1155/2013/196028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershanik OS, Gomez-Arevalo GJ. Typical and atypical neuroleptics. Handb Clin Neurol. 2011;100:579–599. doi: 10.1016/B978-0-444-52014-2.00042-2. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabo P, Palau F. Mitochondrial pathophysiology in Friedreich’s ataxia. J Neurochem. 2013;126(Suppl 1):53–64. doi: 10.1111/jnc.12303. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Dovero S, Aubert I, Li Q, Bioulac BH, Bloch B, Gurevich EV, Gross CE, Bezard E. Levodopa-induced dyskinesia in MPTP-treated macaques is not dependent on the extent and pattern of nigrostrial lesioning. Eur J Neurosci. 2005;22:283–287. doi: 10.1111/j.1460-9568.2005.04196.x. [DOI] [PubMed] [Google Scholar]
- Hill MP, Ravenscroft P, Bezard E, Crossman AR, Brotchie JM, Michel A, Grimee R, Klitgaard H. Levetiracetam potentiates the antidyskinetic action of amantadine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson’s disease. J Pharmacol Exp Ther. 2004;310:386–394. doi: 10.1124/jpet.104.066191. [DOI] [PubMed] [Google Scholar]
- Huang L, Grady SR, Quik M. Nicotine Reduces L-Dopa-Induced Dyskinesias by Acting at {beta}2 Nicotinic Receptors. J Pharmacol Exp Ther. 2011;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Campos C, Ly J, Carroll FI, Quik M. Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in moderately lesioned parkinsonian rats. Neuropharmacology. 2011;60:861–868. doi: 10.1016/j.neuropharm.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, Yasuda RP, Wolfe BB, Perry DC, Rezvani AH, Levin ED, Blendy JA, Kellar KJ. Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. J Pharmacol Exp Ther. 2012;343:441–450. doi: 10.1124/jpet.112.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iderberg H, Francardo V, Pioli EY. Animal models of L-DOPA-induced dyskinesia: an update on the current options. Neuroscience. 2012;211:13–27. doi: 10.1016/j.neuroscience.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Iderberg H, Rylander D, Bimpisidis Z, Cenci MA. Modulating mGluR5 and 5-HT1A/1B receptors to treat l-DOPA-induced dyskinesia: Effects of combined treatment and possible mechanisms of action. Exp Neurol. 2013;250:116–124. doi: 10.1016/j.expneurol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Iravani MM, McCreary AC, Jenner P. Striatal plasticity in Parkinson’s disease and L-DOPA induced dyskinesia. Parkinsonism Relat Disord. 2012;18(Suppl 1):S123–125. doi: 10.1016/S1353-8020(11)70038-4. [DOI] [PubMed] [Google Scholar]
- Jenner P. Functional models of Parkinson’s disease: A valuable tool in the development of novel therapies. Ann Neurol. 2009;64:S16–S29. doi: 10.1002/ana.21489. [DOI] [PubMed] [Google Scholar]
- Ji J, Schrimpf MR, Sippy KB, Bunnelle WH, Li T, Anderson DJ, Faltynek C, Surowy CS, Dyhring T, Ahring PK, Meyer MD. Synthesis and structure-activity relationship studies of 3,6-diazabicyclo[3.2.0]heptanes as novel alpha4beta2 nicotinic acetylcholine receptor selective agonists. J Med Chem. 2007;50:5493–5508. doi: 10.1021/jm070755h. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Huot P, Fox SH, Koprich JB, Szeliga KT, James JW, Graef JD, Letchworth SR, Jordan KG, Hill MP, Brotchie JM. TC-8831, a nicotinic acetylcholine receptor agonist, reduces l-DOPA-induced dyskinesia in the MPTP macaque. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Kark RA, Blass JP, Spence MA. Physostigmine in familial ataxias. Neurology. 1977;27:70–72. doi: 10.1212/wnl.27.1.70. [DOI] [PubMed] [Google Scholar]
- Kark RA, Budelli MM, Wachsner R. Double-blind, triple-crossover trial of low doses of oral physostigmine in inherited ataxias. Neurology. 1981;31:288–292. doi: 10.1212/wnl.31.3.288. [DOI] [PubMed] [Google Scholar]
- Kobylecki C, Hill MP, Crossman AR, Ravenscroft P. Synergistic antidyskinetic effects of topiramate and amantadine in animal models of Parkinson’s disease. Mov Disord. 2011;26:2354–2363. doi: 10.1002/mds.23867. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Mazurkiewicz JE. Friedreich ataxia: neuropathology revised. J Neuropathol Exp Neurol. 2013;72:78–90. doi: 10.1097/NEN.0b013e31827e5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legg NJ. Oral choline in cerebellar ataxia. Br Med J. 1978;2:1403–1404. doi: 10.1136/bmj.2.6149.1403-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone IR, Mastaglia FL, Pennington RJ, Skilbeck C. Choline chloride in the treatment of cerebellar and spinocerebellar ataxia. J Neurol Sci. 1981;50:161–174. doi: 10.1016/0022-510x(81)90162-3. [DOI] [PubMed] [Google Scholar]
- Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J Pharmacol Exp Ther. 2011;339:125–131. doi: 10.1124/jpet.111.185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. J Manag Care Pharm. 2012;18:S1–20. doi: 10.18553/jmcp.2012.18.S5-B.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA. Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain. Biochem Pharmacol. 2009;78:795–802. doi: 10.1016/j.bcp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Zhang H, Neff NH, Hadjiconstantinou M. Desensitization of delta-opioid receptors in nucleus accumbens during nicotine withdrawal. Psychopharmacology (Berl) 2011;213:735–744. doi: 10.1007/s00213-010-2028-z. [DOI] [PubMed] [Google Scholar]
- McConville BJ, Fogelson MH, Norman AB, Klykylo WM, Manderscheid PZ, Parker KW, Sanberg PR. Nicotine potentiation of haloperidol in reducing tic frequency in Tourette’s disorder. Am J Psychiatry. 1991;148:793–794. doi: 10.1176/ajp.148.6.793. [DOI] [PubMed] [Google Scholar]
- Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, Weiner DM, Tison F, Bezard E. Priorities in Parkinson’s disease research. Nat Rev Drug Discov. 2011;10:377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- Menza MA, Grossman N, Van Horn M, Cody R, Forman N. Smoking and movement disorders in psychiatric patients. Biol Psychiatry. 1991;30:109–115. doi: 10.1016/0006-3223(91)90163-g. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Kulkarni SK. Effect of 5-HT1A and 5-HT2A/2C receptor modulation on neuroleptic-induced vacuous chewing movements. Eur J Pharmacol. 2001;428:81–86. doi: 10.1016/s0014-2999(01)01284-5. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Waller L, Rosengren A, Adlerberth A, Wilhelmsen L. Cigarette smoking is associated with abnormal involuntary movements in the general male population--a study of men born in 1933. Biol Psychiatry. 1997;41:717–723. doi: 10.1016/S0006-3223(96)00289-2. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Heikkila M, Linden IB, Jenner P. L-dopa induces dyskinesia in normal monkeys: behavioural and pharmacokinetic observations. Psychopharmacology (Berl) 2001;156:402–409. doi: 10.1007/s002130100733. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Lewis SW, Barnes TR, Jones PB. Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. Br J Psychiatry. 2012;200:387–392. doi: 10.1192/bjp.bp.111.101485. [DOI] [PubMed] [Google Scholar]
- Philcox DV, Kies B. Choline in hereditary ataxia. Br Med J. 1979;2:613. doi: 10.1136/bmj.2.6190.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Quik M, Bordia T, Okihara M, Fan H, Marks MJ, McIntosh JM, Whiteaker P. L-DOPA Treatment Modulates Nicotinic Receptors in Monkey Striatum. Mol Pharmacol. 2003;64:619–628. doi: 10.1124/mol.64.3.619. [DOI] [PubMed] [Google Scholar]
- Quik M, Campos C, Bordia T, Strachan JP, Zhang J, McIntosh JM, Letchworth S, Jordan K. alpha4beta2 nicotinic receptors play a role in the nAChR-mediated decline in l-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology. 2013;71:191–203. doi: 10.1016/j.neuropharm.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Campos C, Grady S. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias; studies with parkinsonian nicotinic receptor knockout mice. Biochemical Pharmacology. 2013a doi: 10.1016/j.bcp.2013.06.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Campos C, Grady SR. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol. 2013b;86:1153–1162. doi: 10.1016/j.bcp.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. Nicotine-mediated improvement in l-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis. 2013;50:30–41. doi: 10.1016/j.nbd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Mallela A, Ly J, Zhang D. Nicotine reduces established L-dopa-induced dyskinesias in a monkey model of Parkinson’s disease. Movement Disorders. 2013 doi: 10.1002/mds.25594. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Park KM, Hrachova M, Mallela A, Huang LZ, McIntosh JM, Grady SR. Role for alpha6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice. Neuropharmacology. 2012;63:450–459. doi: 10.1016/j.neuropharm.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27:947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, Kulak JM, McIntosh JM. Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. J Neurosci. 2001;21:5494–5500. doi: 10.1523/JNEUROSCI.21-15-05494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, McIntosh JM, Collins AC, Grady SR. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–1179. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson’s Disease. Pharmacol Rev. 2011;63:938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Timofeeva O, Sexton HG, DeCuir D, Xiao Y, Gordon CJ, Kellar KJ, Levin ED. Effects of sazetidine-A, a selective alpha4beta2* nicotinic receptor desensitizing agent, on body temperature regulation in mice and rats. Eur J Pharmacol. 2012;682:110–117. doi: 10.1016/j.ejphar.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Budelli MM, Kark RA, Blass JP, Spence MA. Action of physostigmine on inherited ataxias. Adv Neurol. 1978;21:195–202. [PubMed] [Google Scholar]
- Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Munchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology. 2013;68:143–149. doi: 10.1016/j.neuropharm.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha(4)beta(2) nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O’Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, Williams KE, de Vries M, Cremers T, Bertrand S, Bertrand D. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Arslanian A, Nothaft W, Duan WR, Best AE, Pritchett Y, Zhou Q, Stacey BR. Efficacy and safety of the alpha4beta2 neuronal nicotinic receptor agonist ABT-894 in patients with diabetic peripheral neuropathic pain. Pain. 2012;153:862–868. doi: 10.1016/j.pain.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Rub U, Schols L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf HW, Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Rylander D. The serotonin system: a potential target for anti-dyskinetic treatments and biomarker discovery. Parkinsonism Relat Disord. 2012;18(Suppl 1):S126–128. doi: 10.1016/S1353-8020(11)70039-6. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi P, Bedard PJ, Rouillard C. Opioids and motor complications in Parkinson’s disease. Trends Pharmacol Sci. 2006;27:512–517. doi: 10.1016/j.tips.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, McConville BJ, Fogelson HM, Manderscheid PZ, Parker KW, Blythe MM, Klykylo WM, Norman AB. Nicotine potentiates the effects of haloperidol in animals and in patients with Tourette syndrome. Biomed Pharmacother. 1989;43:19–23. doi: 10.1016/0753-3322(89)90186-8. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Silver AA, Shytle RD, Philipp MK, Cahill DW, Fogelson HM, McConville BJ. Nicotine for the treatment of Tourette’s syndrome. Pharmacol Ther. 1997;74:21–25. doi: 10.1016/s0163-7258(96)00199-4. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Vindrola-Padros C, Shytle RD. Translating laboratory discovery to the clinic: from nicotine and mecamylamine to Tourette’s, depression, and beyond. Physiol Behav. 2012;107:801–808. doi: 10.1016/j.physbeh.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci. 2009;30:41–47. doi: 10.1016/j.tips.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses. 2010;4:56–73. doi: 10.3371/CSRP.4.1.5. [DOI] [PubMed] [Google Scholar]
- Sgambato-Faure V, Cenci MA. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson’s disease. Prog Neurobiol. 2012;96:69–86. doi: 10.1016/j.pneurobio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sanberg PR. Comorbid bipolar disorder in Tourette’s syndrome responds to the nicotinic receptor antagonist mecamylamine (Inversine) Biol Psychiatry. 2000;48:1028–1031. doi: 10.1016/s0006-3223(00)00945-8. [DOI] [PubMed] [Google Scholar]
- Silver AA, Shytle RD, Philipp MK, Sanberg PR. Case study: long-term potentiation of neuroleptics with transdermal nicotine in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35:1631–1636. doi: 10.1097/00004583-199612000-00015. [DOI] [PubMed] [Google Scholar]
- Silver AA, Shytle RD, Philipp MK, Wilkinson BJ, McConville B, Sanberg PR. Transdermal nicotine and haloperidol in Tourette’s disorder: a double-blind placebo-controlled study. J Clin Psychiatry. 2001;62:707–714. doi: 10.4088/jcp.v62n0908. [DOI] [PubMed] [Google Scholar]
- Silver AA, Shytle RD, Sanberg PR. Mecamylamine in Tourette’s syndrome: a two-year retrospective case study. J Child Adolesc Psychopharmacol. 2000;10:59–68. doi: 10.1089/cap.2000.10.59. [DOI] [PubMed] [Google Scholar]
- Silver AA, Shytle RD, Sheehan KH, Sheehan DV, Ramos A, Sanberg PR. Multicenter, double-blind, placebo-controlled study of mecamylamine monotherapy for Tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 2001;40:1103–1110. doi: 10.1097/00004583-200109000-00020. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Behavioural pharmacology of nicotine: implications for multiple brain nicotinic receptors. Ciba Found Symp. 1990;152:3–16. doi: 10.1002/9780470513965.ch2. discussion 16–22. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Xue IC, Piattoni-Kaplan M, Molinari E, Campbell JE, McKenna DG, Gunn DE, Lin NH, Ryther KB, He Y, Holladay MW, Wonnacott S, Williams M, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties. J Pharmacol Exp Ther. 1997;283:235–246. [PubMed] [Google Scholar]
- Tarsy D, Lungu C, Baldessarini RJ. Epidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugs. Handb Clin Neurol. 2011;100:601–616. doi: 10.1016/B978-0-444-52014-2.00043-4. [DOI] [PubMed] [Google Scholar]
- Taslim N, Al-Rejaie S, Saeed Dar M. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: a functional interaction. Neuroscience. 2008;157:204–213. doi: 10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Taslim N, Saeed Dar M. The role of nicotinic acetylcholine receptor (nAChR) alpha7 subtype in the functional interaction between nicotine and ethanol in mouse cerebellum. Alcohol Clin Exp Res. 2011;35:540–549. doi: 10.1111/j.1530-0277.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- Termine C, Selvini C, Rossi G, Balottin U. Emerging treatment strategies in Tourette syndrome: what’s in the pipeline? Int Rev Neurobiol. 2013;112:445–480. doi: 10.1016/B978-0-12-411546-0.00015-9. [DOI] [PubMed] [Google Scholar]
- Thomas R, Cavanna AE. The pharmacology of Tourette syndrome. J Neural Transm. 2013;120:689–694. doi: 10.1007/s00702-013-0979-z. [DOI] [PubMed] [Google Scholar]
- Togasaki DM, Tan L, Protell P, Di Monte DA, Quik M, Langston JW. Levodopa induces dyskinesias in normal squirrel monkeys. Ann Neurol. 2001;50:254–257. doi: 10.1002/ana.1099. [DOI] [PubMed] [Google Scholar]
- Turrone P, Remington G, Kapur S, Nobrega JN. The relationship between dopamine D2 receptor occupancy and the vacuous chewing movement syndrome in rats. Psychopharmacology (Berl) 2003;165:166–171. doi: 10.1007/s00213-002-1259-z. [DOI] [PubMed] [Google Scholar]
- van de Warrenburg BP, van Gaalen J, Boesch S, Burgunder JM, Durr A, Giunti P, Klockgether T, Mariotti C, Pandolfo M, Riess O. EFNS/ENS Consensus on the diagnosis and management of chronic ataxias in adulthood. Eur J Neurol. 2014 doi: 10.1111/ene.12341. [DOI] [PubMed] [Google Scholar]
- Wecker L, Engberg ME, Philpot RM, Lambert CS, Kang CW, Antilla JC, Bickford PC, Hudson CE, Zesiewicz TA, Rowell PP. Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia. Neuropharmacology. 2013;73:75–86. doi: 10.1016/j.neuropharm.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel K, Langenberger K, Nitschke MF, Kompf D. Double-blind crossover study with physostigmine in patients with degenerative cerebellar diseases. Arch Neurol. 1997;54:397–400. doi: 10.1001/archneur.1997.00550160041013. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR, Guridi J, Obeso JA. Milestones in research on the pathophysiology of Parkinson’s disease. Mov Disord. 2011;26:1032–1041. doi: 10.1002/mds.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Woods SW, Morgenstern H, Saksa JR, Walsh BC, Sullivan MC, Money R, Hawkins KA, Gueorguieva RV, Glazer WM. Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: a prospective cohort study. J Clin Psychiatry. 2010;71:463–474. doi: 10.4088/JCP.07m03890yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa R, Lal S, Korpassy A, Ally J. Nicotine exposure and tardive dyskinesia. Biol Psychiatry. 1987;22:67–72. doi: 10.1016/0006-3223(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Greenstein PE, Sullivan KL, Wecker L, Miller A, Jahan I, Chen R, Perlman SL. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78:545–550. doi: 10.1212/WNL.0b013e318247cc7a. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Sullivan KL. Treatment of ataxia and imbalance with varenicline (chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14) Clin Neuropharmacol. 2008;31:363–365. doi: 10.1097/WNF.0b013e31818736a9. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Sullivan KL, Freeman A, Juncos JL. Treatment of imbalance with varenicline Chantix(R): report of a patient with fragile X tremor/ataxia syndrome. Acta Neurol Scand. 2009;119:135–138. doi: 10.1111/j.1600-0404.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Sullivan KL, Gooch CL, Lynch DR. Subjective improvement in proprioception in 2 patients with atypical Friedreich ataxia treated with varenicline (Chantix) J Clin Neuromuscul Dis. 2009;10:191–193. doi: 10.1097/CND.0b013e3181910074. [DOI] [PubMed] [Google Scholar]
- Zhang D, Bordia T, McGregor M, McIntosh JM, Decker MW, Quik M. ABT-089 and ABT-894 Reduce L-Dopa-Induced Dyskinesias in a Monkey Model of Parkinson’s Disease. Movement Disorders. 2014 doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Mallela A, Sohn D, Carroll FI, Bencherif M, Letchworth S, Quik M. Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson’s disease. J Pharmacol Exp Ther. 2013;347:225–234. doi: 10.1124/jpet.113.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Yu YQ, Sun S, Zhang X, Li W, Xiu MH, Chen da C, Yang FD, Zhu F, Kosten TA, Kosten TR. Smoking and tardive dyskinesia in male patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1765–1769. doi: 10.1016/j.pnpbp.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K. Aberrant Restoration of Spines and their Synapses in L-DOPA-Induced Dyskinesia: Involvement of Corticostriatal but Not Thalamostriatal Synapses. J Neurosci. 2013;33:11655–11667. doi: 10.1523/JNEUROSCI.0288-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic ML. Role of atypical antipsychotics in rapid cycling bipolar disorder: a review of the literature. Ann Clin Psychiatry. 2011;23:141–149. [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]