Abstract
Apurinic/apyrimidinic and oxidized abasic sites are chemically reactive DNA lesions that are produced by a variety of damaging agents. The effects of these molecules that lack a Watson-Crick base on polymerase enzymes are well documented. More recently, multiple consequences of the electrophilic nature of abasic lesions have been revealed. Members of this family of DNA lesions have been shown to inactivate repair enzymes and undergo spontaneous transformation into more deleterious forms of damage. Abasic site reactivity provides insight into the chemical basis for the cytotoxicity of DNA damaging agents that produce them and are valuable examples of how looking beneath the surface of seemingly simple molecules can reveal biologically relevant chemical complexity.
Introduction
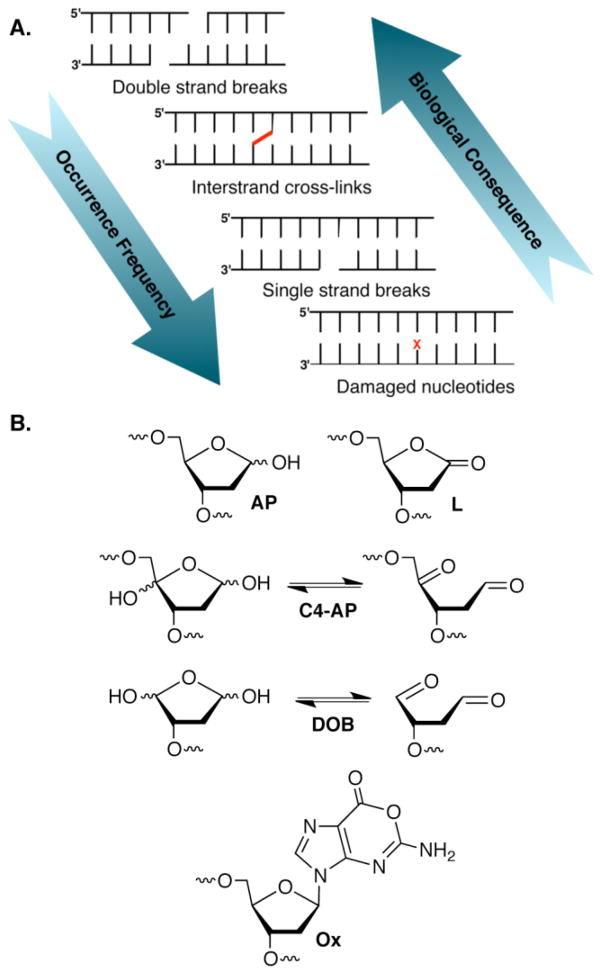
DNA is the target of many cytotoxic agents that alkylate or oxidize the biopolymer. Although double-strand breaks (dsbs) are typically considered the most deleterious form of DNA damage, they are the least frequently formed (Figure 1). The products of many DNA damaging agents are known, and in some cases even the detailed mechanisms for their formation have been elucidated [1,2]. Commonly formed lesions, such as strand breaks and abasic sites, reside lower in the biological hierarchy of DNA damage because cells have evolved to cope with them. Attention has largely been focused on two aspects of the biochemical consequences of DNA damage. The first concerns DNA replication and to a lesser extent transcription. The effects of numerous lesions on polymerase efficiency and fidelity have been determined in prokaryotic and eukaryotic cells [3–6]. In addition, the base excision and nucleotide excision repair pathways that remove a variety of lesions from damaged DNA, as well as the tailoring of the termini of cleaved DNA to prepare them as substrates for polymerase and/or ligase enzymes have been surveyed [7,8]. This extensive and redundant group of enzymes reduces the concern over simple lesions compared to less frequently formed dsbs and interstrand cross-links (ICLs). However, other aspects of the chemistry of some electrophilic DNA lesions have been uncovered during the past decade that suggest that their biochemical consequences may be more significant than previously thought. Several lesions that are commonly produced by oxidizing agents, including γ-radiolysis and antitumor antibiotics have been discovered to inactivate repair enzymes and undergo chemical transformations in DNA that create potentially more deleterious modifications. Characterization of these processes provides insight into the chemical basis of the cytotoxicity of the damaging agents that produce these lesions.
Figure 1.
DNA damage. A. There is an inverse relationship between the biological hierarchy of DNA damage and their formation frequency. (X = DNA lesion) B. Representative electrophilic DNA lesions.
Abasic sites yield DNA interstrand cross-links
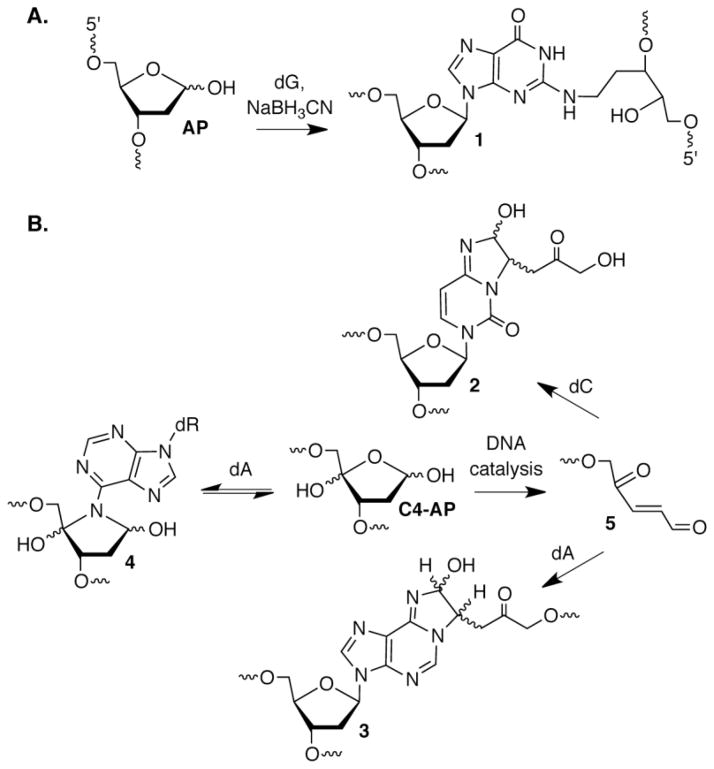
DNA interstrand cross-links (ICLs) prevent DNA dehybridization and consequently are absolute blocks to replication and transcription [9]. A variety of bis-alkylating agents produce ICLs, and the design of more effective and cell selective DNA cross-linking agents is an active area of research due to the biological potency of ICLs [10]. Bis-electrophiles produced from biomolecules by oxidative stress react with DNA, ultimately forming ICLs [11,12]. However, Gates was the first to unequivocally establish ICL formation in DNA that did not involve an exogenous bis-alkylating agent to serve as a bridge between the opposing strands [13,14]. AP forms ICLs selectively with the N2-amino group of the dG that is base paired with the 2′-deoxycytidine in 5′-dC-AP (1, Figure 2) sequences under mild reducing conditions (NaBH3CN). Reaction with the N2-amino group of dG is also consistent with preferential ICL formation in the 5′-dC-AP sequence. Examination of molecular models indicates that the guanine 2-amino group at this position is in closer proximity (~3.7 Å) than when it is either opposite AP (~5.3 Å) or present in the 5′-AP-C (~8.6 Å) sequence.
Figure 2.
DNA interstrand cross-link formation. A. Trapping of an ICL between AP and dG under reductive conditions. B. ICL formation from C4-AP.
Interstrand cross-links between AP and dG (1) have not yet been reported in cells. However, given that 10,000 – 50,000 AP sites are produced in one cell per day under normal conditions, ICL yields that are far less than 1% (an approximate lower limit of detection by phosphorimaging analysis) would give rise to detectable levels of cross-links in cells. A report on a related abasic lesion (C4-AP) by Ravanat provides encouragement that 1 (or its precursor) will be identified in cells [15]. C4-AP results from C4′-hydrogen atom abstraction in DNA [2]. The relatively modest carbon-hydrogen bond dissociation energy and accessibility of the C4′-hydrogen at the outer edge of the minor groove where many small molecule DNA damaging agents bind, combine to make C4-AP a commonly formed abasic lesion [16]. Following enzyme digestion of cellular DNA, Ravanat detected a diastereomeric mixture of 2 (Figure 2) by LC/MS. Treatment of human lymphocytes with bleomycin or ionizing radiation, agents known to generate C4-AP, produced 2 in a dose dependent manner.
It was not possible to unequivocally determine whether 2 that was detected in cellular DNA was derived from inter- or intrastrand cross-links. Examining C4-AP reactivity in synthetic duplexes where the lesion was incorporated at a defined site removed any ambiguity and uncovered additional chemistry [17,18]. These experiments confirmed that C4-AP yields interstrand cross-links containing 2 and revealed and that its yield was sequence dependent. In contrast to the reactivity of AP with dG, the preferred cross-linking sites did not strictly correlate with the distance between the C1-aldehyde of the lesion and the N4-amino group of cytosine. Depending upon the local sequence, cross-linking occurred preferentially with dC that was either opposite C4-AP or a 5′- or 3′-flanking dG. C4-AP also cross-linked with dA in a variety of sequence contexts, but in contrast to AP and common exogenous electrophiles, reaction was not observed with dG [17–19]. In addition to forming a cross-link with dA (3, Figure 2) whose structure was analogous to 2, C4-AP reacted with dA to produce an ICL (4, Figure 2) in which neither strand of the duplex was cleaved. (DOB forms an analogous cross-link with dA [20]. Although the formation of 3 showed similar flexibility as 2 with respect to changes in local sequence, 4 was only observed with a dA opposite thymidine in duplexes containing the 5′-C4-AP-T sequence.
In addition to forming in a very limited number of sequences, 4 proved to be unstable and reverted (t1/2 ~ 3.2 h) to C4-AP. In contrast, 3, which is formed ~10-times more slowly than 4, is chemically stable, as is 2. The sequence surrounding C4-AP also affected the ICL yield by catalyzing its formation. The nucleotide opposite C4-AP was particularly important in ICL catalysis even though it did not itself react [18]. For example, C4-AP was 5-fold more reactive when dA was opposite it than when the lesion was opposed by thymidine. Further evidence for catalysis by the opposing nucleotide was provided by experiments in which cross-linking was enhanced when C4-AP was opposed by thymidine upon adding adenine. Electron rich purines, such as 2,6-diaminopurine were even more effective at rescuing ICL formation. Exactly how the purine catalyzes cross-linking is not known, but other mechanistic studies indicated that the β elimination from C4-AP (5, Figure 2) was the rate-determining step [18].
Typically, ICLs are thought of as more deleterious forms of DNA damage than modified nucleotides. The self-catalyzed transformation of the C4-AP lesion into an ICL is a rare example in which DNA promotes a process to its own detriment [21,22]. The potential importance of the cross-linking is magnified by the action of bacterial (UvrABC) nucleotide excision repair (NER), which converts ~15% of the ICLs into double-strand breaks (DSBs) [23]. After the initial report, two other examples of ICL misrepair by UvrABC have been described and one might wonder how common this process is [24,25]. Regardless, the conversion of 2 into a DSB is the final step in a series of reactions that transform a commonly observed abasic lesion into the most deleterious form of DNA damage, a dsb (Figure 1A).
Oxidized abasic lesions irreversibly inhibit base excision repair
The primary repair pathway for damaged nucleotides in mammalian cells (Figure 3) begins with removal of the damaged nucleobase and 5′-incision of the resulting AP lesion by apurinic endonuclease 1 (Ape1), followed by removal of the remaining sugar fragment (dRP) by the lyase domain (dRPase) of DNA polymerase β (Pol β) [26,27]. The dRPase activity is a β-elimination reaction that proceeds via a Schiff-base (Figure 3B). The resulting single nucleotide gap is filled in by Pol β and rejoined by DNA ligase. One secondary pathway takes advantage that some BER glycosylases (e.g. E. coli endonuclease III, Nth) are bifunctional and induce β-elimination of an AP site following hydrolysis of the glycosidic bond [28].
Figure 3.
Base excision repair. A. Series of enzyme reactions starting from excision of a damaged nucleotide (X) by a glycosylase. B. Incision of an AP site by Ape1, followed by removal of dRP by Pol β via Schiff-base formation. C. 2-Deoxyribonolactone (L) inactivation of Nth, a bifunctional glycosylase. D. Pol β inactivation by DOB. E. Pol β inactivation by incised C4-AP (pC4-AP).
2-Deoxyribonolactone (L), an oxidized abasic site produced by several antitumor antibiotics, inactivates Nth and forms DNA-protein cross-links (DPCs) with the enzyme [29,30]. DPC formation requires Lys120, the nucleophile responsible for Schiff-base formation during AP excision. Attack on the lactone carbonyl by this nucleophile presumably yields the DPC due to formation of a stable amide (6, Figure 3). L did not inactivate other bifunctional glycosylases, but Fpg and Neil1 formed DNA-protein cross-links with the preformed butenolide β-elimination product [30]. In another example, Pol β is inactivated by L following its incision by Ape1 [31,32]. Along with the nitric oxide modification product of dG, oxanine (Ox), inactivation by L and its β-elimination product were the first examples of irreversible repair enzyme inhibition by DNA lesions [33]. However, inactivation of Pol β and Nth by L is inefficient and it is uncertain whether this activity is biologically relevant.
The inefficiency of BER inactivation by L may be attributed to the less electrophilic nature of the lactone’s carbonyl compared to that of the aldehyde in AP. This fundamental chemical difference may also contribute to why Ape1 incised C4-AP (pC4-AP) and DOB (KI ~ 13 nM, kInact ~ 4 × 10−4 s−1) inactivate Pol β so much more effectively than L [34–36]. Furthermore, DOB and pC4-AP contain 1,4-ketoaldehyde functional groups (Figure 1B), which readily yield cyclic products with primary amines, such as that present in lysine [37,38]. Indeed, it is the presence of the second carbonyl group in these lesions that alters the reactivity from that observed between AP and Pol β. The majority of Pol β inactivation events by DOB resulted from DPC formation but a minor pathway involved release of the DNA and concomitant modification of lysines (7) in the lyase active site (Figure 3). Similarly, pC4-AP inactivates Pol β (Figure 3) and produces modification 8. These lesions also inactivate DNA polymerase γ (Pol γ), which has been proposed to play a back-up role for Pol β in DNA repair [35,39–41]. The efficient inactivation of enzymes integrally involved in BER by lesions produced by potent antitumor antibiotics suggests that this chemistry may contribute to the agents’ cytotoxicity [2,42].
Unconventional sources of lyase activity on DNA lesions
As illustrated above, lysine residues often play key roles in excising DNA lesions. There is a very high concentration of lysine residues in nucleosomes, the monomeric units of chromatin that are composed of an octameric core of histone proteins that ~145 bp of DNA wrap ~1.6 turns around (Figure 5A) [43]. In addition to being highly positively charged, the histone proteins contain lysine-rich termini (“tails”) that protrude through the nucleosome core particle (NCP). While studying the effects bleomycin and neocarzinostatin have on DNA in chromatin Povirk observed that the histone proteins induce DNA strand scission at C4-AP and L lesions [44]. More recently, histone catalyzed strand scission has been reported for AP, L, and C4-AP lesions in NCPs [45–49]. These studies have taken advantage of protein expression and chemical synthesis to produce NCPs composed of DNA containing an abasic lesion at a defined site wrapped around an octameric core of wild type or mutant histone proteins.
Strand scission at AP (Figure 5B) sites is accelerated as much as 100-fold in nucleosome core particles, and is as much as 550-times faster for C4-AP compared to that in free DNA [45,47–49]. 2-Deoxyribonolactone (L) cleavage is accelerated more modestly (< 50-fold) [46]. Mechanistic studies revealed that DNA-protein cross-links (DPCs) are intermediates in AP and C4-AP cleavage, and that Schiff-base formation involving lysines is critical. The persistence of DPCs involving AP depended upon the position of AP in the NCP but was longer than one day in some instances [45,48]. DPCs are not required for cleavage at L, but the lysine rich histone tails are involved in catalyzing strand scission [46]. More detailed mechanistic investigations were carried out using NCPs in which the AP lesion was positioned ~1.5 turns from the dyad axis of the NCP (superhelical location (SHL) 1.5) (Figure 4). Experiments with histone H4 variants revealed that ~95% of the accelerated rate of strand scission (ssb) compared to AP in free DNA is accounted for by mutating the five lysines and a single histidine in the protein’s amino terminus [47,48]. The ratio of DPCs containing cleaved (DPCcl) versus uncleaved DNA (DPCun) varied significantly depending upon the lysine content of the histone H4 tail, indicating that the residues in this portion of the protein are also involved in the elimination step subsequent to Schiff-base formation.
Figure 4.
DNA lesion reactivity in nucleosome core particles (NCPs). A. X-ray crystal structure of a single-gyre of the NCP composed of α-satellite DNA (PDB: 1aoi). The numbers 0–7 indicate superhelical locations (SHL). B. AP reactivity in a NCP.
DNA cleavage acceleration at AP sites in NCPs sites is more complicated when two lesions are present on opposite strands 3 nucleotides apart in the vicinity of SHL 1.5 [45,47]. Clusters of 2 or more lesions are produced by ionizing radiation and are biologically important because they are repaired more slowly than isolated lesions [50,51]. Kinetic analysis revealed that following cleavage at one AP site, strand scission at the lesion on the opposite strand increased 10-15-fold compared to that in a NCP where a single AP was present [45]. Synthesis of a NCP containing one AP lesion and a suitably positioned strand break on the opposing strand revealed that a proximal strand break was sufficient to provide the additional strand scission acceleration at an AP site during dsb formation [47]. Although comparable studies on L reactivity in NCPs have not been reported, it is worth noting that this lesion is typically formed as part of a bistranded lesion in which the other component is a strand break [42]. Consequently, any additional acceleration of L cleavage rate in NCPs over that noted above would further substantiate that bistranded lesions containing them are de facto dsbs in cells.
Schiff-base formation is also the initial step for C4-AP reactivity in NCPs.[49] However, unlike AP sites DPCs are short-lived and, only those containing uncleaved DNA (DPCun) are detected. The lesion is rapidly removed (t1/2 as short as 14 min) in its entirety leaving behind DNA cleavage fragments containing phosphate termini. Literature precedent and MS analysis indicate that the lesion is transferred to the ε-amino group in histone tail lysines in the form of a lactam modification whose structure is analogous to that produced (8) when pC4-AP inactivates Pol β (Figure 3E) [36,37]. It is not yet known whether the modified histones are produced in cells and whether they will affect chromatin remodeling enzymes, as histone formylation does [52,53].
Overall, the lyase-like behavior exhibited by histone proteins within the NCP is similar to that discussed above regarding Pol β [26]. Recently, there have been a number other reports of proteins exhibiting unexpected lyase activity. For instance, Ku70 an accessory protein involved in nonhomologous end joining, possesses dRPase activity [54,55]. In addition, the human AlkB protein that oxidatively dealkylates DNA, possesses AP lyase activity [56,57]. As with the chemistry described above, the biological significance of these findings is not yet established.
Conclusions
AP and related oxidized abasic sites are some of the most commonly observed DNA lesions. Until recently, they were thought of as mutagenic lesions that were readily repairable. The research described herein illustrates that abasic site reactivity and biochemical effects are broad, extending to the formation of the most deleterious forms of DNA damage, interstrand cross-links and double strand breaks. Abasic site reactivity provides chemical insight into the mechanisms of action of the chemotherapeutics that produce them and presents new questions and research opportunities.
Highlights (for review).
Abasic lesions are transformed spontaneously and through enzyme catalyzed reactions into more deleterious forms of DNA damage.
Abasic lesions for interstrand DNA cross-links.
Oxidized abasic lesions irreversibly inactivate DNA polymerase β, a vital component of base excision repair.
Histones catalyze DNA strand scission from abasic lesions and undergo lysine modification.
Abasic site reactivity provides insight into the mechanism of action of DNA damaging agents that produce these lesions.
Acknowledgments
I am grateful to the National Institute of General Medical Sciences (GM-063028) for generous support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105:739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- 2.Pitié M, Pratviel G. Activation of DNA carbon hydrogen bonds by metal complexes. Chem Rev. 2010;110:1018–1059. doi: 10.1021/cr900247m. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg MM. The formamidopyrimidines: Purine lesions formed in competition with 8-oxopurines from oxidative stress. Acc Chem Res. 2012;45:588–597. doi: 10.1021/ar2002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaney JC, Essigmann JM. Assays for determining lesion bypass efficiency and mutagenicity of site-specific DNA lesions in vivo. Methods Enzymol. 2006;408:1–15. doi: 10.1016/S0076-6879(06)08001-3. [DOI] [PubMed] [Google Scholar]
- 5.Bjelland S, Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutation Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Woodgate R. What a difference a decade makes: Insights into translesion DNA synthesis. Proc Natl Acad Sci USA. 2007;104:15591–15598. doi: 10.1073/pnas.0704219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA repair and mutagenesis. ASM Press; Washington, D.C: 2006. [Google Scholar]
- 8.Weinfeld M, Mani RS, Abdou I, Aceytuno RD, Glover JNM. Tidying up loose ends: The role of polynucleotide kinase/phosphatase in DNA strand break repair. Trends Biochem Sci. 2011;36:262–271. doi: 10.1016/j.tibs.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guainazzi A, Scharer OD. Using synthetic DNA interstrand crosslinks to elucidate repair pathways and identify new therapeutic targets for cancer chemotherapy. Cell Mol Life Sci. 2010;67:3683–3697. doi: 10.1007/s00018-010-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuang Y, Balakrishnan K, Gandhi V, Peng X. Hydrogen peroxide inducible DNA cross-linking agents: Targeted anticancer prodrugs. J Am Chem Soc. 2011;133:19278–19281. doi: 10.1021/ja2073824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone MP, Cho Y-J, Huang H, Kim H-Y, Kozekov ID, Kozekova A, Wang H, Minko IG, Lloyd RS, Harris TM, Rizzo CJ. Interstrand DNA cross-links induced by α,β-unsaturated aldehydes derived from lipid peroxidation and environmental sources. Acc Chem Res. 2008;41:793–804. doi: 10.1021/ar700246x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozekov ID, Turesky RJ, Alas GR, Harris CM, Harris TM, Rizzo CJ. Formation of deoxyguanosine cross-links from calf thymus DNA treated with acrolein and 4-hydroxy-2-nonenal. Chem Res Toxicol. 2010;23:1701–1713. doi: 10.1021/tx100179g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta S, Chowdhury G, Gates KS. Interstrand cross-links generated by abasic sites in duplex DNA. J Am Chem Soc. 2007;129:1852–1853. doi: 10.1021/ja067294u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Johnson KM, Price NE, Wang J, Fekry MI, Dutta S, Seiner DR, Wang Y, Gates KS. On the formation and properties of interstrand DNA DNA cross-links forged by reaction of an abasic site with the opposing guanine residue of 5′-cap sequences in duplex DNA. J Am Chem Soc. 2013;135:1015–1025. doi: 10.1021/ja308119q. Using synthetic duplexes containing an AP site the authors demonstrate ICL formation with dG. The establish the required orientation of the reactants in the duplex and characterize the cross link. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Regulus P, Duroux B, Bayle P-A, Favier A, Cadet J, Ravanat J-L. Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion. Proc Nat Acad Sci USA. 2007;104:14032–14037. doi: 10.1073/pnas.0706044104. The authors establish adduct formation between dC and C4-AP using mass spectrometry. They establish endogenous levels of the adduct in cells and demonstrate that the product is formed in a dose dependent manner by modalities known to form C4-AP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Nat Acad Sci USA. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sczepanski JT, Jacobs AC, Greenberg MM. Self-promoted DNA interstrand cross-link formation by an abasic site. J Am Chem Soc. 2008;130:9646–9647. doi: 10.1021/ja8030642. [DOI] [PubMed] [Google Scholar]
- 18*.Sczepanski JT, Jacobs AC, Majumdar A, Greenberg MM. Scope and mechanism of interstrand cross-link formation by the C4′-oxidized abasic site. J Am Chem Soc. 2009;131:11132–11139. doi: 10.1021/ja903404v. Using synthetic duplexes containing independently prepared C4-AP, the authors establish that the lesion forms interestrand cross links with suitably positioned dA and dC. Mechanistic studies reveal that the local DNA sequence catalyzes rate limiting cleavage (β-elimination) of C4-AP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sczepanski JT, Hiemstra CN, Greenberg MM. Probing DNA interstrand cross-link formation by an oxidized abasic site using nonnative nucleotides. Bioorg Med Chem. 2011;19:5788–5793. doi: 10.1016/j.bmc.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan L, Greenberg MM. DNA interstrand cross-link formation by the 1,4-dioxobutane abasic lesion. J Am Chem Soc. 2009;131:15225–15231. doi: 10.1021/ja9061695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riggins JN, Pratt DA, Voehler M, Daniels JS, Marnett LJ. Kinetics and mechanism of the general-acid-catalyzed ring-closure of the malondialdehyde-DNA adduct, N2-(3-oxo-1-propenyl)deoxyguanosine (N2OpdG), to 3′-(2′-deoxy-β-d-erythro-pentofuranosyl)pyrimido [1,2−2]purin-10(3h)-one (M1dG) J Am Chem Soc. 2004;126:10571–10581. doi: 10.1021/ja040010q. [DOI] [PubMed] [Google Scholar]
- 22.Boger DL, Garbaccio RM. Shape-dependent catalysis: Insights into the source of catalysis for the CC-1065 and duocarmycin DNA alkylation reaction. Acc Chem Res. 1999;32:1043–1052. [Google Scholar]
- 23•.Sczepanski JT, Jacobs AC, Van Houten B, Greenberg MM. Double strand break formation during nucleotide excision repair of a DNA interstrand cross-link. Biochemistry. 2009;48:7565–7567. doi: 10.1021/bi901006b. The authors report the first example in which nucleotide excision repair proteins cleave the wrong strand of an interstrand cross-link, converting it into a double strand break. This observation marks the completion of the transformation of an oxidized abasic lesion into the most deleterious form of DNA damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng X, Ghosh AK, Van Houten B, Greenberg MM. Nucleotide excision repair of a DNA interstrand cross-link produces single- and double-strand breaks. Biochemistry. 2010;49:11–19. doi: 10.1021/bi901603h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng M-w, Zheng Y, Jasti VP, Champeil E, Tomasz M, Wang Y, Basu AK, Tang M-s. Repair of mitomycin C mono- and interstrand cross-linked DNA adducts by UvrABC: A new model. Nucleic Acids Res. 2010;38:6976–6984. doi: 10.1093/nar/gkq576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase β during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DMI, Barsky D. The major human abasic endonuclease: Formation, consequences and repair of abasic lesions in DNA. Mutat Res. 2001;485:283–307. doi: 10.1016/s0921-8777(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 28.Kow YW, Wallace SS. Mechanism of action of Escherichia coli endonuclease III. Biochemistry. 1987;26:8200–8206. doi: 10.1021/bi00399a027. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto M, Greenberg MM, Kow YW, Hwang J-T, Cunningham RP. The 2-deoxyribonolactone lesion produced in DNA by neocarzinostatin and other DNA damaging agents forms cross-links with the base-excision repair enzyme endonuclease iii. J Am Chem Soc. 2001;123:3161–3162. doi: 10.1021/ja003354z. [DOI] [PubMed] [Google Scholar]
- 30.Kroeger KM, Hashimoto M, Kow YW, Greenberg MM. Cross-linking of 2-deoxyribonolactone and its β-elimination product by base excision repair enzymes. Biochemistry. 2003;42:2449–2455. doi: 10.1021/bi027168c. [DOI] [PubMed] [Google Scholar]
- 31.DeMott MS, Beyret E, Wong D, Bales BC, Hwang J-T, Greenberg MM, Demple B. Covalent trapping of human DNA polymerase β by the oxidative DNA lesion 2-deoxyribonolactone. J Biol Chem. 2002;277:7637–7640. doi: 10.1074/jbc.C100577200. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y-j, DeMottt MS, Hwang JT, Greenberg MM, Demple B. Action of human apurinic endonuclease (Ape1) on C1′-oxidized deoxyribose damage in DNA. DNA Repair. 2003;2:175–185. doi: 10.1016/s1568-7864(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 33.Nakano T, Terato H, Asagoshi K, Masaoka A, Mukuta M, Ohyama Y, Suzuki T, Makino K, Ide H. DNA-protein cross-link formation mediated by oxanine. A novel genotoxic mechanism of nitric oxide-induced DNA damage. J Biol Chem. 2003;278:25264–25272. doi: 10.1074/jbc.M212847200. [DOI] [PubMed] [Google Scholar]
- 34•.Guan L, Greenberg MM. Irreversible inhibition of DNA polymerase β by an oxidized abasic lesion. J Am Chem Soc. 2010;132:5004–5005. doi: 10.1021/ja101372c. The authors report the first example of efficient inactivation of a DNA repair enzyme by a lesion that is produced by cytotoxic antitumor agents. This provides a chemical basis for the cytotoxicity of agents that produce the lesion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan L, Bebenek K, Kunkel TA, Greenberg MM. Inhibition of short patch and long patch base excision repair by an oxidized abasic site. Biochemistry. 2010;49:9904–9910. doi: 10.1021/bi101533a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs AC, Kreller CR, Greenberg MM. Long patch base excision repair compensates for DNA polymerase β inactivation by the C4′-oxidized abasic site. Biochemistry. 2011;50:136–143. doi: 10.1021/bi1017667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usui K, Aso M, Fukuda M, Suemune H. Photochemical generation of oligodeoxynucleotide containing a C4′-oxidized abasic site and its efficient amine modification: Dependence on structure and microenvironment. J Org Chem. 2008;73:241–248. doi: 10.1021/jo702080r. [DOI] [PubMed] [Google Scholar]
- 38.Aso M, Kondo M, Suemune H, Hecht SM. Chemistry of the bleomycin-induced alkali-labile DNA lesion. J Am Chem Soc. 1999;121:9023–9033. [Google Scholar]
- 39.Garcia-Diaz M, Bebenek K, Kunkel TA, Blanco L. Identification of an intrinsic 5′-deoxyribose-5-phosphate lyase activity in human DNA polymerase γ. J Biol Chem. 2001;276:34659–34663. doi: 10.1074/jbc.M106336200. [DOI] [PubMed] [Google Scholar]
- 40.Braithwaite EK, Prasad R, Shock DD, Hou EW, Beard WA, Wilson SH. DNA polymerase lambda mediates a back-up base excision repair activity in extracts of mouse embryonic fibroblasts. J Biol Chem. 2005;280:18469–18475. doi: 10.1074/jbc.M411864200. [DOI] [PubMed] [Google Scholar]
- 41.Stevens AJ, Guan L, Bebenek K, Kunkel TA, Greenberg MM. DNA polymerase γ inactivation by oxidized abasic sites. Biochemistry. 2013;52:975–983. doi: 10.1021/bi301592x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi Z, Goldberg IH. DNA-damaging enediyne compounds. In: Kool ET, editor. Comprehensive natural products chemistry. Vol. 7. Elsevier; Amsterdam: 1999. pp. 553–592. [Google Scholar]
- 43.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2. 8 å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 44**.Bennett RAO, Swerdlow PS, Povirk LF. Spontaneous cleavage of bleomycin-induced abasic sites in chromatin and their mutagenicity in mammalian shuttle vectors. Biochemistry. 1993;32:3188–3195. doi: 10.1021/bi00063a034. This is the first evidence that DNA lesions are less stable in nucleosome core particles than in free DNA and that histone proteins catalyze the formation of strand breaks from them. [DOI] [PubMed] [Google Scholar]
- 45*.Sczepanski JT, Wong RS, McKnight JN, Bowman GD, Greenberg MM. Rapid DNA-protein cross-linking and strand scission by an abasic site in a nucleosome core particle. Proc Natl Acad Sci U S A. 2010;107:22475–22480. doi: 10.1073/pnas.1012860108. This is the first report using synthetic nucleosome core particles containing an abasic lesion in which histone-catalyzed strand scission is quantified. The authors also report an additional acceleration of double strand cleavage from clustered lesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou C, Greenberg MM. Histone-catalyzed cleavage of nucleosomal DNA containing 2-deoxyribonolactone. J Am Chem Soc. 2012;134:8090–8093. doi: 10.1021/ja302993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou C, Sczepanski JT, Greenberg MM. Mechanistic studies on histone catalyzed cleavage of apyrimidinic/apurinic sites in nucleosome core particles. J Am Chem Soc. 2012;134:16734–16741. doi: 10.1021/ja306858m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sczepanski JT, Zhou C, Greenberg MM. Nucleosome core particle-catalyzed strand scission at abasic sites. Biochemistry. 2013;52:2157–2164. doi: 10.1021/bi3010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Zhou C, Sczepanski JT, Greenberg MM. Histone modification via rapid cleavage of C4′-oxidized abasic sites in nucleosome core particles. J Am Chem Soc. 2013;135:5274–5277. doi: 10.1021/ja400915w. The authors demonstrate that not only do histone proteins catalyze strand scission at C4-AP lesions that are present in nucleosome core particles, but that the lysine residues of histone proteins are modified in the process. Modification of amino acids that are often post-translationally modified may have signficant effects on genetic expression and provide additional sources of biological effects by DNA damaging agents that produce C4-AP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sage E, Harrison L. Clustered DNA lesion repair in eukaryotes: Relevance to mutagenesis and cell survival. Mutat Res. 2011;711:123–133. doi: 10.1016/j.mrfmmm.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Neill P, Wardman P. Radiation chemistry comes before radiation biology. Int J Radiat Biol. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- 52.Jiang T, Zhou X, Taghizadeh K, Dong M, Dedon PC. N-formylation of lysine in histone proteins as a secondary modification arising from oxidative DNA damage. Proc Nat Acad Sci USA. 2007;104:60–65. doi: 10.1073/pnas.0606775103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edrissi B, Taghizadeh K, Dedon PC. Quantitative analysis of histone modifications: Formaldehyde is a source of pathological N-6-formyllysine that is refractory to histone deacetylases. PLoS Genet. 2013;9:12. doi: 10.1371/journal.pgen.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts SA, Strande N, Burkhalter MD, Strom C, Havener JM, Hasty P, Ramsden DA. Ku is a 5 ′-dRp/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strande N, Roberts SA, Oh S, Hendrickson EA, Ramsden DA. Specificity of the dRp/AP lyase of Ku promotes nonhomologous end joining (NHEJ) fidelity at damaged ends. J Biol Chem. 2012;287:13686–13693. doi: 10.1074/jbc.M111.329730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller TA, Andrzejak MM, Hausinger RP. A covalent protein DNA 5′-product adduct is generated following AP lyase activity of human AlkBH1 (AlkB homologue 1) Biochem J. 2013;452:509–518. doi: 10.1042/BJ20121908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller TA, Meek K, Hausinger RP. Human AlkB homologue 1 (AbH1) exhibits DNA lyase activity at abasic sites. DNA Repair. 2010;9:58–65. doi: 10.1016/j.dnarep.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]