Abstract
Resveratrol has been found to have potent antioxidant, anti-inflammatory, and anticarcinogenic effects. The safety and efficacy of resveratrol supplementation in older adults are currently unknown. We conducted a double-blind, randomized, placebo-controlled trial to examine the safety and metabolic outcomes in 32 overweight, older adults (mean age, 73 ± 7 years). Participants were randomized into one of three treatment groups: (1) placebo, (2) moderate dose resveratrol (300 mg/day), and (3) high dose resveratrol (1000 mg/day). Both resveratrol and placebo were orally ingested in capsule form twice daily for 90 days. Blood chemistry values remained within the normal range, and there were no significant differences in the number of participants reporting adverse events across conditions. Compared to placebo, glucose levels were significantly lower at post-treatment among participants randomized to both resveratrol conditions, with and without adjustment for the corresponding baseline values (ps < 0.05). Glucose values of participants in the treatment groups, however, were not significantly different from baseline levels. These findings suggest that short-term resveratrol supplementation at doses of 300 mg/day and 1000 mg/day does not adversely affect blood chemistries and is well tolerated in overweight, older individuals. These findings support the study of resveratrol for improving cardio-metabolic health in older adults in larger clinical trials.
Keywords: Resveratrol, Safety, Metabolism, Glucose, Blood pressure
1. Introduction
Resveratrol (3,5,4′-trihydroxystilbene), a polyphenolic phytochemical that occurs naturally in peanuts and in the skin of red grapes, has been shown to have potent antioxidant and anti-inflammatory effects, as well as being capable of extending the lifespan of various species (Howitz et al., 2003 and Walle, 2011). Recent reviews of preclinical and clinical studies suggest that resveratrol improves cardio-metabolic functioning through a number of mechanisms (Li et al., 2012 and Vang et al., 2011). For example, resveratrol has been found to reduce diastolic and systolic blood pressure, as well as improve glycemic control in middle-aged individuals with elevated blood glucose levels (Bhatt et al., 2012, Crandall et al., 2012 and Timmers et al., 2012). Resveratrol has also been shown to decrease triglyceride levels and inflammatory markers in middle-aged adults with coronary artery disease (Militaru et al., 2013, Tome-Carneiro et al., 2012 and Tome-Carneiro et al., 2013). In addition to having cardio-protective and anti-inflammatory properties, accumulating evidence suggests resveratrol has significant neuro-protective effects (Gelinas and Martinoli, 2002 and Virgili and Contestabile, 2000). The therapeutic benefits of resveratrol are especially relevant to the health concerns facing older individuals, as the risks of chronic disease conditions and functional decline increases progressively with aging (Nocera et al., 2011).
Despite its promising therapeutic potential, few studies have evaluated the safety of long-term resveratrol supplementation in older adults. Two one-year, placebo-controlled studies found that a low dose of a resveratrol-containing grape product (grape phenolics plus 8 mg resveratrol) during the first six months, with a double dose provided during the second six-month period, was well tolerated in adult patients with stable coronary artery disease (Tome-Carneiro et al., 2012 and Tome-Carneiro et al., 2013). To our knowledge, only one study has evaluated the metabolic and safety outcomes of resveratrol supplementation in older individuals (age ≥ 65 years). This four-week study included 10 participants with impaired glucose tolerance and found that doses of 1 to 2 g per day of resveratrol resulted in improved insulin sensitivity and post-meal glucose stabilization with no increased incidence of adverse events compared to placebo (Crandall et al., 2012). Although the results of this study suggest that resveratrol is well tolerated in older adults, interpretation of the data was limited by the relatively small sample size and short study duration. Thus, the purpose of the present study was to evaluate the effects of short-term (90 days) resveratrol supplementation on metabolic and safety outcomes in a sample of generally healthy, overweight, older adults.
2. Materials and methods
2.1. Participants
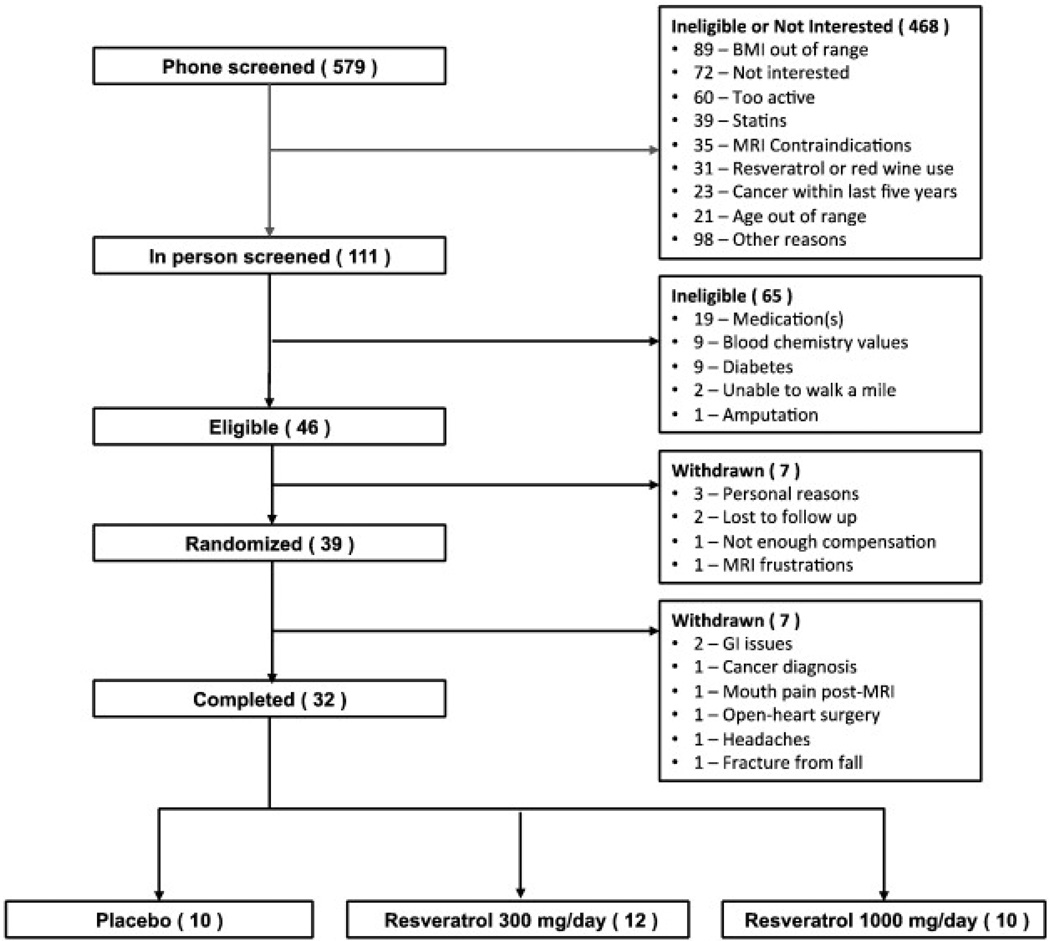
A total of 32 overweight adult men and women aged 65 years and older were recruited for this study. Participants were required to be non-smokers, sedentary (exercising less than 120 min per week), have a body mass index (BMI) between 25 and 34.9 kg/m2, and a self-reported ability to walk 1 mile. Fig. 1 displays participant flow through the study.
Fig. 1.
Consort diagram showing the participant flow during the trial.
2.2. Ethics
This study was approved by the University of Florida's Institutional Review Board (IRB project #238 — 2009). All participants provided written informed consent to participate in this study.
2.3. Screening procedures
Participants were initially screened at a clinic visit to determine eligibility. During this visit, participants provided blood samples to ensure that they were generally healthy and suitable to participate in the trial. Participants were required to obtain written consent from their primary care physicians stating they had no major medical complications that would limit their participation in the study or put them at risk for adverse events. Participants with active cancer, psychiatric illness (diagnosed depression or CES-D score < 20), dementing illness, a history of significant head injury, or contraindications to magnetic resonance imaging (MRI) were excluded from participating in the study. Participants taking anabolic medication, anti-cholinesterase inhibitors, or those supplementing with grape seed extract, ginkgo-biloba, or quercetin were also excluded. Additionally, consumption of red wine or purple grape juice more than once per week resulted in exclusion from the study. Table 1 lists all inclusion/exclusion criteria for this study.
Table 1.
Inclusion and exclusion criteria for the RIPE Trial.
| INCLUSION | EXCLUSION |
|---|---|
|
|
2.4. Study design and procedure
A double-blind, placebo-controlled design was utilized, in which two doses of resveratrol (300 mg/day and 1000 mg/day) were tested for 90 days in overweight men and women aged 65 and older. Eligible participants were invited to attend a baseline visit, at which time they completed psychological questionnaires, a multi-measure cognitive test battery, and a physical function battery. As part of the baseline assessment, participants also completed a cognitive assessment battery while undergoing an MRI evaluation on a separate day. The findings of outcomes related to cognitive and physical function are reported in a separate manuscript.
Once all baseline assessments were completed, participants were provided with a one-month supply of either resveratrol (300 mg/day or 1000 mg/day) or placebo. Participants in both the treatment and placebo conditions were instructed to consume one oral capsule twice daily, immediately following breakfast and dinner. All participants were closely monitored for safety and toxicity during the first ten days of the trial. During this initial evaluation period, participants returned to the clinic every three days, and blood chemistries (i.e., complete blood count and comprehensive metabolic profiles) and adverse events were evaluated at each visit. At monthly intervals, participants returned to the clinic and blood samples were collected to ensure that no adverse changes had occurred since the previous visit. Participants were asked to bring their study product to their monthly clinic visits, and adherence with the supplementation regimen was checked through pill counts at each clinic visit. After 90 days of taking either resveratrol or placebo, participants completed a post-treatment assessment visit, during which they completed the same assessment battery as they completed during their baseline assessment. Two follow-up safety evaluations occurred at 10 and 30 days following completion of the 90-day post-treatment assessment visit.
2.5. Study product
The study product was provided by Reserveage Organics and contained naturally-derived resveratrol from grapes and wild Japanese knotweed (Polygonum cuspidatum). Microcrystalline cellulose was used for the placebo. Prior to study initiation, the resveratrol content of the capsules was verified using high performance liquid chromatography (HPLC) analyses. These analyses indicated that the resveratrol content was within 7% of the stated milligram dose.
2.6. Outcomes
2.6.1. Safety outcomes
2.6.1.1. Blood chemistries
Blood chemistries (i.e., complete blood count and comprehensive metabolic profile) were assessed by Quest Diagnostic Clinical Laboratories, which is accredited by the College of American Pathologists, from blood samples obtained at each clinic visit.
2.6.1.2. Adverse events
Adverse events (AEs) were assessed at each clinic visit based on participant reports and clinical observations of symptoms throughout the study. During each visit, participants were asked to report any health-related problems or symptoms they were experiencing. A grading scale based on the latest National Cancer Institute's criteria for adverse events was used to quantify the severity of reported adverse effects. These sheets were reviewed by study staff before the participant was allowed to continue receiving the study product.
2.6.2. Anthropometric and metabolic outcomes
2.6.2.1. Body weight
Body weight was determined in a fasting state and following a morning void. Body mass index (BMI: kg/m2) was calculated with body weight and height measured using a stadiometer and standardized procedures.
2.6.2.2. Waist circumference
Waist circumference was measured at the narrowest part of the torso, between the xiphoid process and the umbilicus.
2.6.2.3. Blood pressure
Resting systolic and diastolic blood pressure was taken after participants spent 10 min seated in a quiet room, free of distractions. Blood pressure was obtained according to a standardized protocol (Chobanian et al., 2003) by the Study Registered Nurse. Blood pressure was taken from the brachial artery via auscultation while the participant was in a seated position. Three readings of blood pressure, spaced 1 min apart, were taken using a sphygmomanometer with appropriate cuff size. The first reading was discarded, and the last two readings were averaged. If large differences were observed between the second and third readings, an additional reading was taken and the median value for the three trials was used.
2.6.2.4. Blood glucose
Glucose levels were measured by Quest Diagnostic Clinical Laboratories, which is accredited by the College of American Pathologists.
2.7. Statistical analyses
Baseline health and demographic characteristics were summarized (by mean ± SE or proportion) and compared among the three treatment groups using the Kruskal–Wallis test (for continuous variables) or chi-square test (for categorical variables). The primary outcomes of interest were the change in safety and metabolic outcomes at Day 90 from baseline. Each outcome was summarized (by mean ± SE) and compared between treatment groups using the Wilcoxon rank sum test or two-sample t-test depending on the corresponding data distribution. The signed rank test or paired t-test was used for assessing the within treatment change in each safety and metabolic outcome at Day 90. In addition, an analysis of covariance (ANCOVA) model was used to assess the adjusted treatment effect on the change in each safety and metabolic outcome after controlling for the corresponding baseline measurement. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
3. Results
Participant flow during the trial is outlined in Fig. 1, and the descriptive characteristics of participants by treatment condition are summarized in Table 2. Among the 7 participants who withdrew from the study, there was no clear pattern of AEs related to group assignment (p > 0.10, chi-square test). Specifically, two participants withdrew from the placebo condition (reason for withdrawal: cancer diagnosis, headache associated with study product), two participants withdrew from the 300 mg/day condition (reason for withdrawal: open heart surgery, jaw pain following MRI), and three participants withdrew from the 1000 mg/day condition (reason for withdrawal: two due to gastrointestinal issues and one due to an injurious fall). Out of the thirty-nine participants who enrolled in the study, thirty-two completed the trial. Of the thirty-two participants who completed the study, ten received placebo, twelve received moderate dose resveratrol (300 mg/day), and ten received high dose resveratrol (1000 mg/day).
Table 2.
Participant demographic and health information.
| Resveratrol |
Placebo |
p-Value | ||
|---|---|---|---|---|
| 300 mg/d (n = 12) | 1000 mg/d (n= 10) | (n = 10) | ||
| Age (years) | 73.17 ± 2.08 | 73.60 ± 2.53 | 73.30 ± 2.06 | 0.97 |
| Caucasian, n (%) | 12 (100%) | 10 (100%) | 9 (90%) | 0.32 |
| Females, n (%) | 6 (50%) | 5 (50%) | 5 (50%) | 1.00 |
| BMI (kg/m2) | 29.84 ± 0.60 | 29.03 ± 1.00 | 29.74 ± 0.62 | 0.88 |
| Mini-Mental State Exam score | 27.67 ± 0.63 | 28.80 ± 0.29 | 27.70 ± 0.47 | 0.17 |
| Depression score (CES-D) | 7.33 ± 1.33 | 6.80 ± 2.02 | 6.60 ± 2.04 | 0.75 |
| Number of falls in the past year | 0.45 ± 0.21 | 0.80 ± 0.59 | 0.70 ± 0.33 | 0.88 |
| Excellent or good health, n (%) | 8 (67%) | 9 (90%) | 9 (90%) | 0.27 |
| Hypertension, n (%) | 5 (45%) | 2 (20%) | 7 (70%) | 0.08 |
Note. CES-D — Center for Epidemiologic Studies Depression.
3.1. Adherence
Study adherence rates were high across all conditions. The mean adherence level (percentage) for participants in all conditions was as follows: placebo (93%), 300 mg/day resveratrol condition (93%), and 1000 mg/day resveratrol condition (93%).
3.2. Safety outcomes
Blood chemistry values remained within normal ranges over time in all treatment groups, and there were few changes in blood chemistry markers over time in the treatment groups. Notable exceptions were that participants receiving moderate dose resveratrol (300 mg/day) had slightly lower hemoglobin (− 0.41 ± 0.17 g/dL, p = 0.04) and lower mean corpuscular hemoglobin concentration levels (− 0.66 ± 0.25 g/dL, p = 0.02) compared to baseline. Additionally, participants receiving high dose resveratrol (1000 mg/day) had higher alkaline phosphatase levels as compared to baseline (7.90 ± 2.94 g/dL, p = 0.03). After controlling for the corresponding baseline measurement, we found that: (1) participants receiving moderate dose (300 mg/day) resveratrol had greater reductions in albumin compared to participants in both the placebo (p = 0.03) and high dose (1000 mg/day) condition (p = 0.03); (2) participants receiving high dose resveratrol had greater increases in alkaline phosphatase levels compared to participants in the moderate dose condition (p = 0.02) and tended to differ from participants in the placebo condition (p = 0.06); (3) participants receiving high dose resveratrol had greater increases in aspartate aminotransferase levels compared to participants in the moderate dose condition (p = 0.04); (4) participants in both the moderate dose (300 mg/day) and high dose (1000 mg/day) resveratrol conditions had greater reductions in bilirubin levels compared to participants in the placebo condition (p = 0.01 and 0.04, respectively); and (5) participants in the moderate dose condition had greater reductions in hemoglobin (p = 0.02) and mean corpuscular hemoglobin concentration (p = 0.03) levels compared to participants receiving the placebo. Table 3 presents the changes in blood chemistry markers from baseline to the 90-day post-treatment assessment for participants in all conditions.
Table 3.
Participant blood chemistry profiles.
| Marker at baseline |
Change in marker at Day 90 from baseline |
P-value for Adjusted Comparisona |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A: 300 mg/d (n = 12) |
Group B: 1000 mg/d (n = 10) |
Group C: Placebo (n = 10) |
Group A: 300 mg/d (n = 12) |
Group B: 1000 mg/d (n = 10) |
Group C: Placebo (n = 10) |
A vs B | A vs C | B vs C | 3-Group comparison |
|
| Albumin (g/dL) | 4.34 ± 0.03 | 4.41 ± 0.05 | 4.38 ± 0.07 | −0.08 ± 0.04 | 0.04 ± 0.05 | 0.04 ± 0.07 | 0.028 | 0.028 | 0.943 | 0.038 |
| ALP (IU/L) | 74.17 ± 6.57 | 70.90 ± 6.58 | 79.50 ± 9.24 | −2.83 ± 3.07 | 7.90 ± 2.94b | −1.89 ± 3.56 | 0.025 | 0.806 | 0.060 | 0.056 |
| ALT (U/L) | 20.17 ± 2.51 | 22.60 ± 3.83 | 22.10 ± 3.46 | −0.75 ± 1.14 | −0.40 ± 2.45 | −0.22 ± 1.05 | 0.477 | 0.329 | 0.776 | 0.589 |
| AST (IU/L) | 22.42 ± 2.17 | 20.70 ± 1.54 | 22.50 ± 2.06 | −0.50 ± 0.93 | 3.00 ± 1.57 | 0.56 ± 1.20 | 0.041 | 0.418 | 0.236 | 0.119 |
| Bilirubin (mg/dL) | 0.53 ± 0.07 | 0.53 ± 0.10 | 0.56 ± 0.08 | −0.10 ± 0.06 | −0.05 ± 0.08 | 0.06 ± 0.04 | 0.415 | 0.006 | 0.045 | 0.018 |
| BUN (mg/dL) | 15.67 ± 0.98 | 17.70 ± 1.22 | 16.80 ± 1.10 | 0.25 ± 0.81 | −1.00 ± 1.42 | 2.22 ± 1.52 | 0.761 | 0.157 | 0.100 | 0.208 |
| Calcium (mg/dL) | 9.45 ± 0.12 | 9.62 ± 0.08 | 9.46 ± 0.11 | 0.11 ± 0.10 | 0.09 ± 0.13 | 0.16 ± 0.10 | 0.781 | 0.671 | 0.888 | 0.908 |
| CO2 (mEq/L) | 26.83 ± 0.56 | 25.90 ± 0.57 | 26.70 ± 0.70 | 0.92 ± 0.70 | 0.80 ± 0.88 | 1.78 ± 0.59 | 0.269 | 0.480 | 0.092 | 0.229 |
| Cloride (mEq/L) | 103.67 ± 0.40 | 104.70 ± 0.62 | 103.90 ± 1.11 | 0.42 ± 0.47 | −0.40 ± 0.52 | −1.11 ± 0.63 | 0.572 | 0.172 | 0.398 | 0.384 |
| Creatine (mg/dL) | 0.90 ± 0.05 | 0.85 ± 0.03 | 0.90 ± 0.06 | −0.01 ± 0.03 | −0.02 ± 0.01 | 0.01 ± 0.02 | 0.804 | 0.472 | 0.361 | 0.632 |
| Hematocrit (%) | 41.95 ± 0.91 | 46.80 ± 5.73 | 40.90 ± 0.82 | −0.44 ± 0.61 | −5.18 ± 5.67 | −2.44 ± 3.24 | 0.932 | 0.237 | 0.387 | 0.533 |
| Hemoglobin (g/L) | 13.95 ± 0.28 | 13.87 ± 0.42 | 13.70 ± 0.31 | −0.41 ± 0.17b | −0.07 ± 0.16 | 0.28 ± 0.24 | 0.234 | 0.020 | 0.266 | 0.064 |
| MCH (pg/cell) | 29.78 ± 0.60 | 30.49 ± 0.35 | 28.77 ± 0.52 | −0.17 ± 0.26 | −0.07 ± 0.55 | 0.36 ± 0.24 | 0.634 | 0.501 | 0.863 | 0.771 |
| MCHC (g/dL) | 33.28 ± 0.25 | 33.58 ± 0.33 | 33.52 ± 0.43 | −0.66 ± 0.25b | −0.39 ± 0.24 | 0.21 ± 0.59 | 0.322 | 0.031 | 0.255 | 0.092 |
| MCV(fL) | 89.48 ± 1.65 | 90.84 ± 1.17 | 85.79 ± 1.25 | 1.30 ± 1.01 | 0.89 ± 1.64 | 0.66 ± 1.81 | 0.917 | 0.250 | 0.259 | 0.434 |
| MPV(fL) | 9.10 ± 0.15 | 8.89 ± 0.32 | 9.09 ± 0.27 | 0.16 ± 0.21 | 0.57 ± 0.31 | 0.29 ± 0.15 | 0.269 | 0.675 | 0.495 | 0.535 |
| Potassium (mEq/L) | 4.24 ± 0.06 | 4.34 ± 0.05 | 4.32 ± 0.10 | 0.03 ± 0.13 | 0.08 ± 0.09 | −0.19 ± 0.13 | 0.471 | 0.390 | 0.133 | 0.316 |
| Sodium (mEq/L) | 139.92 ± 0.75 | 140.90 ± 0.48 | 140.60 ± 1.26 | 0.83 ± 0.55 | 0.30 ± 0.52 | −0.44 ± 0.71 | 0.819 | 0.477 | 0.610 | 0.766 |
| RBC count (cells/mcL) | 4.70 ± 0.11 | 4.55 ± 0.13 | 4.75 ± 0.09 | −0.11 ± 0.08 | −0.01 ± 0.09 | 0.05 ± 0.07 | 0.522 | 0.128 | 0.426 | 0.308 |
| Total protein (g/dL) | 6.56 ± 0.10 | 6.83 ± 0.08 | 6.92 ± 0.11 | −0.02 ± 0.07 | 0.04 ± 0.06 | 0.00 ± 0.08 | 0.336 | 0.503 | 0.803 | 0.613 |
| WBC count (cells/mcL) | 5.59 ± 0.47 | 5.68 ± 0.34 | 6.59 ± 0.61 | 0.25 ± 0.30 | 0.21 ± 0.26 | −0.42 ± 0.40 | 0.999 | 0.534 | 0.557 | 0.785 |
Note. ALP — alkaline phosphatase; ALT — alanine aminotransferase; AST — aspartate aminotransferase; BUN — blood urea nitrogen; CO2 — carbon dioxide; MCH — mean corpuscular hemoglobin; MCHC — mean corpuscular hemoglobin concentration; MCV — mean corpuscular volume; RBC — red blood cell; and WBC — white blood cell.
p-Value for group comparisons after adjusting for the corresponding baseline measurements.
Bold values denote statistical significance (p-value < 0.05).
The rates of adverse events were low across all groups, and there were no statistically significant differences in adverse events reported from participants in either treatment group compared to the placebo group. Adverse event reporting for each treatment group is described in Table 4.
Table 4.
Adverse event incidence across treatment conditions.
| Resveratrol |
Placebo (n = 10) |
p-Value | ||
|---|---|---|---|---|
| 300 mg/d (n = 12) |
1000 mg/d (n = 10) |
|||
| Diarrhea | 4 (33%) | 2 (20%) | 1 (10%) | 0.5 |
| Constipation | 2 (17%) | 1 (10%) | 0 (0%) | 0.76 |
| Muscle cramps/pain | 2 (17%) | 2 (20%) | 0 (0%) | 0.52 |
| Fatigue | 2 (17%) | 1 (10%) | 1 (10%) | 0.99 |
| Memory loss | 1 (8%) | 1 (10%) | 2 (20%) | 0.82 |
| Allergies/URI | 2 (17%) | 0 (0%) | 1 (10%) | 0.76 |
| Difficulty swallowing | 0 (0%) | 2 (20%) | 0 (0%) | 0.18 |
| Rash | 2 (17%) | 0 (0%) | 0 (0%) | 0.3 |
| Headache | 1 (8%) | 0 (0%) | 2 (20%) | 0.5 |
| Others | 1 (8%) | 3 (30%) | 3 (30%) | 0.37 |
Note. URI - upper respiratory infection.
3.3. Anthropometric and metabolic outcomes
There were significant differences in changes in blood glucose levels at post-treatment among participants in the moderate and high dose resveratrol groups compared to participants receiving the placebo (p = 0.02 and p = 0.01, respectively) with/without adjustment of the baseline glucose levels. Participants receiving placebo had significantly increased blood glucose levels relative to baseline levels over the course of the study (p = 0.02). In contrast, blood glucose levels remained stable among participants in both of the resveratrol groups. No significant changes in blood pressure, body weight, or waist circumference were observed in any group. Table 5 presents the changes in anthropometric and metabolic outcomes from baseline to the 90-day post-treatment assessment for each treatment group.
Table 5.
Changes in metabolic outcomes across treatment conditions.
| Marker at baseline |
Change in marker at Day 90 from baseline |
p-Value for adjusted comparisona |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A: 300 mg/d (n = 12) |
Group B: 1000 mg/d (n = 10) |
Group C: Placebo (n= 10) |
Group A: 300 mg/d (n = 12) |
Group B: 1000 mg/d (n = 10) |
Group C: Placebo (n = 10) |
A vs B | A vs C | B vs C | 3-Group comparison |
|
| Glucose (mg/dL) | 93.00 ± 2.11 | 98.80 ± 3.07 | 95.00 ± 3.77 | 1.67 ± 1.51 | −0.30 ± 1.33 | 8.11 ± 2.86b | 0.576 | 0.023 | 0.008 | 0.018 |
| Diastolic BP (mm Hg) | 71.50 ± 2.81 | 73.50 ± 3.71 | 77.15 ± 2.93 | −1.46 ± 1.28 | −0.06 ± 2.99 | 3.60 ± 4.14 | 0.668 | 0.146 | 0.322 | 0.330 |
| Systolic BP (mm Hg) | 125.54 ± 3.23 | 132.06 ± 5.53 | 136.10 ± 3.43 | 2.92 ± 3.88 | 3.11 ± 3.76 | 2.35 ± 4.42 | 0.845 | 0.867 | 0.980 | 0.977 |
| Body weight (kg) | 81.41 ± 2.53 | 85.26 ± 4.65 | 83.94 ± 3.55 | −0.73 ± 0.86 | 0.31 ± 0.81 | 0.76 ± 1.06 | 0.379 | 0.236 | 0.761 | 0.461 |
| Waist circumference (cm) | 42.58 ± 1.48 | 47.60 ± 5.78 | 46.80 ± 4.84 | −1.42 ± 0.86 | 0.00 ± 1.00 | 1.00 ± 0.84 | 0.337 | 0.085 | 0.445 | 0.219 |
p-Value for group comparisons after adjusting for the corresponding baseline measurements.
Bold values denote statistical significance (p-value < 0.05).
4. Discussion
This study examined the effects of resveratrol supplementation at both a 300 mg and 1000 mg daily dose over a twelve week period on safety and metabolic outcomes in an overweight, older adult population. Blood chemistries remained in the normal range throughout the trial. In general, resveratrol at both doses did not alter blood chemistries, and there was not an increased incidence of adverse events compared to placebo at either dose tested. Of note, gastrointestinal adverse events have been previously reported following resveratrol supplementation (2500–5000 g/day) (Brown et al., 2010), and in this study, two participants in the 1000 mg/day condition withdrew due to gastrointestinal adverse events. Overall, our findings suggest that resveratrol is well tolerated at doses of 300 mg/day and 1000 mg/day in overweight, older adults, a population at high risk of metabolic disease conditions.
Compared to placebo, glucose levels were significantly lower at post-treatment in participants randomized to both resveratrol conditions. This finding appears to be due to an unexpected increase in glucose values among participants in the placebo condition, however, as glucose values of participants in the treatment groups were not significantly different from baseline levels. In a recent study, resveratrol supplementation at a dose of 75 mg/day did not improve metabolic outcomes (Yoshino et al., 2012); however, it should be noted that the participants in this study were nonobese women with normal glucose tolerance. In contrast, a study by Timmers et al. (2011) found that resveratrol supplementation at a dose of 150 mg/day also reduced circulating glucose levels and systolic blood pressure in obese men after thirty days of supplementation (Timmers et al., 2011). Similarly, older adults with impaired glucose tolerance showed enhanced insulin sensitivity and decreased post-meal glucose levels after four weeks of resveratrol supplementation (1–2 g/day) (Crandall et al., 2012). Thus, findings from clinical trials to date have been mixed; resveratrol has beneficial effects on metabolic parameters in studies conducted on participants with metabolic impairments but does not appear to produce additional improvements in individuals with normal glucose tolerance. To our knowledge, this is the first study to report the effects of chronic resveratrol use on albumin, hemoglobin, and mean corpuscular hemoglobin concentration in humans. Participants in the moderate dose condition (300 mg/day) had greater reductions in hemoglobin and mean corpuscular hemoglobin concentration levels compared to baseline. Additionally, participants receiving the high dose resveratrol (1000 mg/day) had higher alkaline phosphatase levels compared to baseline. Our findings related to the effects of resveratrol on hemoglobin levels are in contrast to recent studies. For example, a study by Bhatt and colleagues reported that resveratrol supplementation (250 mg/day) over a three-month period increased hemoglobin levels among individuals with type 2 diabetes (age range, 30–70 years) (Bhatt et al., 2012). The reason for the inconsistency in findings between our study and previous studies is unclear but may be related to age differences of the participants or the dose tested. Additionally, significant reductions in albumin were observed among participants in the moderate dose (300 mg/day) condition compared to participants in both the placebo and high dose (1000 mg/day) condition, suggesting a potential dose effect.
An important finding was that participants in both the moderate and high dose resveratrol conditions had greater reductions in bilirubin levels compared to participants in the placebo condition. To our knowledge, this is the first study to report the effects of chronic administration of resveratrol for a 12-week period on bilirubin levels in overweight, older adults. Our findings are in contrast to two recent trials. The first of these was conducted by Chow and colleagues, which found no changes in bilirubin levels among overweight but healthy volunteers receiving a 1000 mg/day dose of resveratrol over a four-week time period. The second was conducted by Timmers and colleagues (Timmers et al., 2011), which tested the effects of a 150 mg/day dose of resveratrol in obese men over a 30-day period in a crossover design. A potential reason for the discrepancy in findings may be the selected participant population (Chow et al., 2010 and Timmers et al., 2011). Participants in both previous studies had a mean age of 40 years. In contrast, participants in the present study were 65 years or older and had a mean BMI in the obese range; thus, the participants included in the present study may have been a higher risk population than the participants in either the Chow et al. or Timmers et al. studies. Extreme elevations in bilirubin levels have recently been shown to be a causal risk factor for specific health conditions (e.g., gallstone disease) (Stender et al., 2013), and elevated bilirubin levels predict poor outcomes in hospitalized patients (Chintanaboina et al., 2013 and Patel et al., 2013). It is presently unclear, however, whether a reduction in bilirubin levels in the absence of liver disease is a desirable outcome. Recent cross-sectional studies have found that higher levels of serum bilirubin are inversely related to specific cardiovascular disease conditions (Novotny and Vitek, 2003) and greater functional independence in older adults (Kao et al., 2012). Thus, longitudinal studies are needed to further evaluate the implications of this finding in terms of disease risk.
A potentially important finding of the present study was that alkaline phosphatase levels were elevated in participants receiving high dose resveratrol compared to baseline, as well as participants receiving moderate dose resveratrol. The significance of this finding is unknown, as reports from the literature are currently inconclusive. For example, Mohaved A and colleagues failed to detect a difference in alkaline phosphatase levels after high dose resveratrol (1000 mg/day) treatment for 45 days in their cohort of adults with type 2 diabetes mellitus (Mohaved et al., 2013). In contrast, Poulsen and colleagues reported significant and borderline significant increases in bone-specific and total alkaline phosphatase levels, respectively, after resveratrol treatment (1500 mg/day) in their study of obese, non-diabetic men (Poulsen et al., 2014). It is worth noting, however, that the elevations in alkaline phosphatase levels observed in our study were still within the normal range, and thus we do not believe the elevated levels of alkaline phosphatase in the high dose group are a cause for concern at the present time.
Another noteworthy finding was that levels of the enzyme aspartate aminotransferase (AST) were increased among participants in the high dose resveratrol condition relative to participants in the moderate dose condition but not the placebo condition. To our knowledge, only one human trial has previously demonstrated increased levels of AST after resveratrol use (3000 mg/day for 8 weeks) (Chachay et al., 2014), and data from animal experiments are inconclusive. A study by Juan and colleagues reported increased levels of AST in resveratrol-treated rats compared to rats treated with a placebo (Juan et al., 2002). In contrast, a number of other studies have demonstrated reduced levels of AST after resveratrol treatment in animals with induced hepatotoxicity (Bishayee et al., 2010).
Unlike AST, the levels of alanine aminotransferase (ALT) did not differ between groups or compared to baseline in this study. This finding is in line with the above-mentioned study by Juan and colleagues, who reported no changes in ALT, but increases in AST levels, in resveratrol-treated rats (Juan et al., 2002). Conversely, numerous other animal studies (Bishayee et al., 2010), as well as the Timmers et al. study in healthy, obese men (Timmers et al., 2011), have reported decreased levels of ALT after resveratrol administration, whereas a recent study by Chachay et al. reported increased ALT levels in overweight or obese men with non-alcoholic fatty liver disease (Chachay et al., 2014). Some experts have suggested that ALT is a more reliable parameter to use for evaluation of hepatic toxicity than AST, since AST is widely distributed (Ringler and Dabich, 1979). Despite both AST and ALT levels remaining within the normal range for all participants in this study, we believe that future studies are needed to further explore the potential effects of long-term administration of varying doses of resveratrol on the levels of these enzymes, which may serve as indicators of disease risk.
The results of the present study should be interpreted in the context of its limitations. First, the sample size was relatively small and consisted primarily of Caucasian individuals. Related to this, the study was not adequately powered to detect differences between men and women in response to the intervention. Second, this study only informs about the effects of a short-term (i.e., 3-month) resveratrol intervention; thus, the long-term effects of resveratrol supplementation in this population are not known. An additional limitation of this study is that the participants were generally healthy, overweight, older adults; therefore, these findings may not be generalizable to older adults with chronic health conditions. Finally, the study product tested was a resveratrol-containing product, which was comprised of grape polyphenols and resveratrol, rather than pure resveratrol. Although the content of other polyphenols of the capsules was small compared to the resveratrol content, we cannot be absolutely sure that the effects observed were due to resveratrol and not other polyphenols in the blend or perhaps the synergy of the ingredients.
The present study also had a number of strengths. Few studies have tested the effects of resveratrol in an older adult population. Given that participants had a mean age of 73, this study provides preliminary information regarding how adults over the age of 65 may respond to two different doses of resveratrol. The testing of moderate and higher doses (i.e., 300 mg/day and 1000 mg/day) of resveratrol represents an important aspect of this study design as the optimal dose of resveratrol for specific populations is currently unknown. The study also included a comprehensive safety evaluation plan, which included frequent monitoring of adverse events and blood chemistry changes during the initial 10-day supplementation period; thus, the study design allowed for evaluation of the initial (i.e., 3–10 days) and short-term (i.e., 90-day) effects of resveratrol supplementation in this population. An additional strength of this study was the inclusion of equal numbers of men and women in each treatment condition, which provides preliminary support for the observed effects being consistent across both sexes.
5. Conclusion
In summary, our findings indicate that daily resveratrol supplementation at doses of 300 mg and 1000 mg for a period of 90 days is generally well tolerated in overweight, older adults, a population known to be at high risk of chronic disease conditions. Thus, initial findings from this study support the safety of resveratrol supplementation in this at risk population. Future studies are needed, however, to establish the long-term safety and efficacy of varying doses of resveratrol in overweight, older adults, as well as other populations at high risk for chronic disease conditions.
Highlights.
Doses of 300 & 1000mg/d of resveratrol are well tolerated in overweight older adults.
Doses of 300 & 1000mg/d of resveratrol did not produce changes in metabolic parameters.
Older adults show high levels of adherence to 90 days of resveratrol supplementation.
Studies are needed to confirm optimal dosage of resveratrol and long-term safety.
Acknowledgments
Support was provided by the University of Florida's McKnight Brain Institute, Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG028740), and Clinical and Translational Science Institute (NIH/NCRR UL1TR000064). Stephen Anton is supported by a K23 AT004251-01A2, an Early Career Investigator Award from the American Heart Association (09CRP2390173), and Thomas H. Maren Foundation. The product for this trial was provided by Reserveage Organics.
Footnotes
Registration: ClinicalTrials.gov; NCT01126229; http://clinicaltrials.gov/ct2/show/NCT01126229.
Conflict of interest
Dr. Anton serves as a scientific advisor and as a consultant for Reserveage Organics, the provider of the resveratrol product used in this trial. Dr. Anton has not received personal financial gain from sales of the resveratrol product used in this trial. All findings and views expressed in this paper are those of the authors and do not necessarily reflect the views of the company.
References
- Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Darvesh AS, Politis T, McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010;30:1103–1114. doi: 10.1111/j.1478-3231.2010.02295.x. [DOI] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O'Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M, Coombes JS, Thomas GP, Cowin GJ, Kirkpatrick CM, Prins JB, Hickman IJ. Resveratrol does not benefit patients with non-alcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014 doi: 10.1016/j.cgh.2014.02.024. pii: S1542-3565 (14), 00309-7. http://dx.doi.org/10.1016/j.cgh.2014.02.024 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Chintanaboina J, Haner MS, Sethi A, Patel N, Tanyous W, Lalos A, Pancholy S. Serum bilirubin as a prognostic marker in patients with acute decompensated heart failure. Korean J. Intern. Med. 2013;28:300–305. doi: 10.3904/kjim.2013.28.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug- and carcinogen- metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. (Phila) 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tol- erance. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas S, Martinoli MG. Neuroprotective effect of estradiol and phytoestrogens on MPP + -induced cytotoxicity in neuronal PC12 cells. J. Neurosci. Res. 2002;70:90–96. doi: 10.1002/jnr.10315. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- Kao TW, Chou CH, Wang CC, Chou CC, Hu J, Chen WL. Associations between serum total bilirubin levels and functional dependence in the elderly. Int. Med. J. 2012;42:1199–1207. doi: 10.1111/j.1445-5994.2011.02620.x. [DOI] [PubMed] [Google Scholar]
- Li H, Xia N, Forstermann U. Cardiovascular effects and molecular targets of res- veratrol. Nitric Oxide. 2012;26:102–110. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM, Scorei RI. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition. 2013;29:178–183. doi: 10.1016/j.nut.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Movahed A, Nabipour I, Louis XL, Thandapilly SJ, Yu L, Kalantarhormozi M, Rekabpour SJ, Netticadan T. Antihyperglycemic effects of short-term resver- atrol supplementation in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2013;2013:851267. doi: 10.1155/2013/851267. http://dx.doi.org/10.1155/2013/851267 [Epub 2013 Sep 1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera J, Buford TW, Manini TM, Naugle K, Leeuwenburgh C, Pahor M, Perri MG, Anton SD. The impactof behavioral intervention on obesity mediated declines in mobility function: implications for longevity. J. Aging Res. 2011;2011:392510. doi: 10.4061/2011/392510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp. Biol. Med. (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- Patel JJ, Taneja A, Niccum D, Kumar G, Jacobs E, Nanchal R. The association of serum bilirubin levels on the outcomes of severe sepsis. J. Intensive Care Med. 2013 doi: 10.1177/0885066613488739. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Ornstrup MJ, Harslof T, Jessen N, Langdahl BL, Richelsen B, Jorgensen JO, Pederson SB. Short-term resveratrol supplementation stimulates serum levels of bone-specific alkaline phosphatase in obese non- diabetic men. J. Funct. Foods. 2014;6:305–310. [Bone Abstracts (2013) 1 PP110 http://dx.doi.org/10.1530/boneabs.1.PP110]. [Bone Abstracts (2013) 1 PP110 http://dx.doi.org/10.1530/boneabs.1.PP110]. [Google Scholar]
- Ringler DH, Dabich L. Hematology and clinical biochemistry. In: Baker HJ, Lindsey JR, Weisbroth SH, editors. The Laboratory Rat. London: Academic Press; 1979. pp. 105–121. [Google Scholar]
- Stender S, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Extreme bilirubin levels as a causal risk factor for symptomatic gallstone disease. JAMA Intern. Med. 2013:1–7. doi: 10.1001/jamainternmed.2013.6465. [DOI] [PubMed] [Google Scholar]
- Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany NY) 2012;4:146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, vandeWeijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Ker sten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012;110:356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili M, Contestabile A. Partial neuroprotection of invivo excitotoxic brain dam- age by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci. Lett. 2000;281:123–126. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi FF, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]