Abstract
Polyomaviruses are small, double stranded DNA viruses that replicate in the nucleus of the infected cell. Since 2005, clear evidence for miRNAs has been presented for a subset of the members of this viral family, each of which express a single miRNA. All the miRNAs share in common the ability to regulate expression of the major viral regulatory protein, large T antigen. Growing evidence suggests that the major role of the miRNA is to control viral replication. In vitro studies suggesting an immmunomodulatory role for the miRNA have not been supported by in vivo infections. Very little is known about cellular targets of the viral miRNAs, however. Thus, much remains to be learned about these interesting non-coding RNAs.
Polyomaviruses are small, non-enveloped, double-stranded DNA viruses that are known to infect a variety of species from birds to humans [1]. Since their discovery over a half century ago, studies of the molecular biology of murine polyomavirus (MPyV) and SV40 have led to seminal discoveries about eukaryotic gene expression and DNA replication, as well as oncogenic transformation. Indeed, it is only relatively recently that interest in the polyomaviruses as human pathogens has moved to the forefront. Currently, there are four human polyomaviruses that have a clear association with disease [2]. These are BK polyomavirus (BKPyV), which causes kidney and urinary tract diseases in renal and bone marrow transplant patients, respectively; JC polyomavirus (JCPyV), which causes progressive multifocal leukoencephalopathy in AIDS patients and individuals being treated with some therapies aimed at suppressing T cell infiltration; Merkel cell polyomavirus (MCPyV), which causes a rare, aggressive skin cancer called Merkel cell carcinoma; and trichodysplasia spinulosa-associated polyomavirus, which causes the extremely rare condition from which it derives its name.
The circular polyomavirus genome is approximately 5.0–5.5 kb in length, and can be divided into three genetic regions (Figure 1). The early region encodes regulatory proteins, including the large and small T antigens (LT and ST). The late region is expressed from the complementary strand of the genome and encodes the viral capsid proteins and, in some cases, a poorly-understood protein called the agnoprotein. The third region is the regulatory region, sometimes called the non-coding control region or the origin region depending on the virus. This contains the cis-acting elements that are required for DNA replication and transcription of the early and late regions. The life cycle begins with binding of the virus to a cell surface receptor followed by endocytosis and trafficking to the ER, where disassembly begins. The viral genome, which is associated with histones to form a cellular chromatin-like structure, is eventually delivered into the nucleus. Historically, it has been thought that the first event when the virus enters the nucleus is transcription of the early region by the cellular RNA polymerase II, resulting in LT and ST expression. These two proteins are involved in viral genome replication in two ways. First, they play an indirect role in stimulating the cell to enter S phase. This activity is important because the virus uses the host cell DNA synthetic machinery to replicate its DNA. Second, LT binds to the origin of DNA replication and recruits the cellular DNA polymerase α/primase complex to initiate replication. Concomitant with the onset of DNA replication, the late region is transcribed. As new genomes and capsid proteins are produced, they are assembled into progeny virions that can leave the cell and infect new cells.
Figure 1.
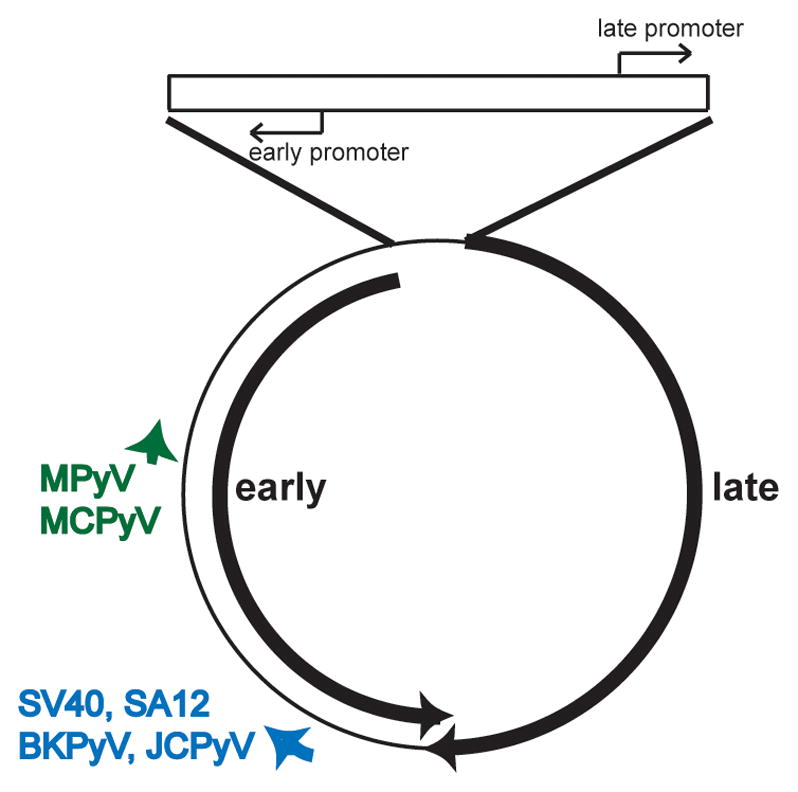
Map of the polyomavirus genome. The double stranded, circular DNA genome of the polyomaviruses described in the text is shown along with the regulatory region (top), and early and late primary RNA transcripts (black heavy arrows). The positions of the miRNAs (short heavy arrows) are also indicated. The miRNAs are expressed from the same strand as the late transcripts and are therefore perfectly complementary to the early mRNAs. As such, they target the early mRNAs for degradation. In BPCV, the miRNA maps to an additional non-coding region between the early and late transcripts
Polyomaviruses were one of the first organisms in which post-transcriptional regulation of eukaryotic gene expression was studied. In 1978, it was determined by Ford and Hsu that transcription of the SV40 late strand continued past the poly(A) site, indicating that the mRNA 3′ end was generated by a processing event [3]. That same year, Berk and Sharp demonstrated the presence of spliced early SV40 mRNAs [4]. The first hint that polyomaviruses encode a miRNA came from studies by Alwine on SV40 early gene expression, in which he discovered a short RNA molecule that was associated with early transcripts, which he named SV40-associated small (SAS) RNA [5,6]. A quarter of a century passed before this RNA was rediscovered by Sullivan and colleagues, who scanned the SV40 genome with a pre-miRNA prediction program [7]. Since that time, polyomavirus miRNAs have been reported in BKPyV, JCPyV, simian agent 12 (SA12), murine polyomavirus (MPyV), and MCPyV, as well as in an unusual polyomavirus-papillomavirus hybrid, bandicoot papillomatosis carcinomatosis virus (BPCV) [8–12].
The polyomavirus miRNA is encoded on the late strand and targets early region mRNAs. The genetic location of the miRNA gene and the target sequence differs among the viruses, however. For MPyV and MCPyV, the target is near the 5′ end of the LT open reading frame (ORF), while the other primate polyomavirus miRNAs target the 3′ end of the ORF. This difference in position of the miRNA target correlates with the evolutionary relationships of the viruses [13]. It is unusual for a miRNA target to be present in an ORF rather than in the 3′ untranslated region (UTR) of the mRNA, the usual site of action for miRNAs. Because the targets are exact complements to the miRNA, the early mRNAs are degraded in the presence of the miRNA [7,8]. In BPCV, the miRNA is encoded in a second non-coding region of the viral genome that maps between the 3′ ends of the early and late regions. Its target is in the 3′ UTR of the early mRNA, resulting in a more traditional miRNA-like mechanism of translation inhibition because it is not fully complementary to the target [10]. Overall, it appears that the main selective force on the polyomavirus miRNA during evolution has been to regulate early gene expression.
The biogenesis of the polyomavirus miRNA is not well-understood. In general, miRNAs are processed either from primary transcripts that are expressed as independent transcription units, or from the introns of RNA polymerase II primary transcripts [14]. Processing of the primary miRNA transcript ultimately results in the loading of one of the two arms of the precursor hairpin, called the 5p and 3p arms, into the RISC (RNA-induced silencing complex), which directs the miRNA to the target. This results in degradation of the target if there is perfect or near-perfect complementarity, or translational repression if there is less complementarity. The polyomavirus miRNAs are somewhat unique in that both the 5p and 3p arms are functional. In BKPyV, expression of the miRNA is controlled by sequences in the non-coding control region (NCCR) and correlates with late mRNA expression [15]. It is not known, however, whether miRNA transcription initiates from the late promoter. There is evidence from studies of the SAS RNA, however, which suggests that this is the case [16]. The BPCV miRNA appears to have its own promoter [10].
Until recently, the role of the polyomavirus miRNA remained elusive. When the SV40 miRNA was first discovered and found to regulate LT expression, it was assumed logically that mutating the miRNA would result in increased viral replication because of higher LT levels. Surprisingly, though, the mutant virus did not replicate better than wild type [7]. It was therefore proposed that perhaps SV40 used the miRNA to temper LT expression as a means of immune evasion. In support of this hypothesis, it was shown in the same report that cells infected with the miRNA mutant virus were more susceptible to killing by a LT-specific CTL clone than cells infected with wild type virus. The immune evasion hypothesis was challenged, however, by subsequent experiments examining a MPyV mutant that does not express its miRNA. In mice, this mutant exhibited the same infection and transformation phenotypes as wild type MPyV, and in particular the immune response was indistinguishable from that against the wild type virus [12].
Greater understanding of the function of the miRNA came from studies aimed at determining the differential replication abilities of two genetic forms of BKPyV, the archetype virus and a rearranged variant. The genetic differences occur in the NCCR of the virus. The archetype virus is the form of the virus that circulates through the population: upwards of 90% of all humans are infected with BKPyV [17]. It is periodically excreted into urine in both healthy individuals and immunosuppressed transplant patients in whom BKPyV causes disease. Rearranged variants are usually only isolated from the serum of renal transplant patients, and it is rare to find them in the urine. Experimentally, rearranged viruses have been studied in the laboratory because they can replicate in cells in culture whereas archetype virus cannot. Similar genetic variants are found in SV40, MPyV, and JCPyV. In studies examining which function of the BKPyV NCCR was responsible for the restriction to replication, it was found that expression of LT in culture was undetectable [18]. Because LT expression is controlled in part by the function of the viral miRNA, it was hypothesized that in the archetype virus, miRNA expression was dominant over LT mRNA expression. To test this, the miRNA was mutated in the context of the archetype BKPyV genome. The miRNA mutant virus was able to replicate much more efficiently than wild type archetype virus [15]. Interestingly, building the same mutation onto a rearranged viral genome had no effect on replication, similar to what had been reported for SV40 and MPyV [7,12]. Therefore, it was hypothesized that the polyomavirus miRNA controls replication of the archetype virus.
Similar results were subsequently obtained in a small animal model of SV40 infection, the Syrian golden hamster [19]. The authors constructed miRNA mutants in both an archetype and rearranged regulatory region genetic background. After intracardiac injection, both wild type and miRNA mutant SV40 were able to establish persistent infections, but viral loads were higher in animals infected with the miRNA mutants. However, there was no significant difference in clearance of the wild type and mutant viruses by the immune system. There was also no miRNA-dependent difference in tumor formation in the infected animals or in transformation of infected murine cells in culture.
Little is known about whether polyomavirus miRNAs regulate the expression of cellular targets, whether viral proteins influence cellular miRNA expression, or whether cellular miRNAs regulate polyomavirus replication. While the manuscript that identified the MCPyV miRNA also included a bioinformatic analysis of potential cellular targets [9], to date these have not been verified experimentally. There is one report in the literature that suggests that the BKPyV and JCPyV 3p miRNAs, which happen to be identical, target UL16-binding protein 3, a stress-induced ligand that leads to killing by natural killer cells [20]. This would again point to an immunomodulatory role for the viral miRNA. Another report demonstrated that the seed sequence of the SV40 5p miRNA is identical to the cellular hsa-miR423 5p molecule seed [21]. When the targets of hsa-miRNA423 were cloned into a fluorescent reporter system, expression of the reporter was down-regulated by the SV40 miRNA. However, the authors did not test what happens to the authentic cellular genes in the context of a viral infection, nor did they examine possible reciprocal effects of hsa-miR423 on SV40 early expression. A more global analysis of cellular gene expression in the context of infection with SV40 variants that express miRNAs whose sequence differs indicated that the majority of host targets of the variants were different, supporting the notion that the major selective force on the miRNA is likely its viral target [22]. It is possible, however, that the small number of shared cellular targets are also important.
With respect to viral regulation of cellular miRNAs, a comparison of MCPyV-positive and MCPyV-negative Merkel cell carcinomas indicated that expression of six cellular miRNAs was different: five were overexpressed in the virus-associated tumors and one was underexpressed [23]. The latter, miRNA203, appears to behave as a tumor suppressor in MCPyV-positive Merkel cell carcinomas but not in virus-negative tumors, indicating that virus infection is interfering with negative cell growth control in much the same way that LT inhibits p53 and pRb [24].
In summary, the study of the roles of miRNAs during polyomavirus infection is still in its infancy, with many more questions remaining to be answered. Among these are: (1) Does the miRNA play a role in persistent infection or oncogenesis in the natural host? (2) How is viral miRNA expression regulated? (3) Are there other cellular protein-coding genes or miRNAs that are regulated by the virus, and do these functions also impact the outcome of infection at either the cellular or organismal levels? (4) Do any cellular miRNAs regulate viral gene expression? It seems that a combination of animal studies with those viruses that can infect animals, analysis of human patient specimens, and in vitro cell culture approaches will be required to fully assess the intricacies of the polyomavirus miRNAs.
Highlights.
polyomaviruses encode a single miRNA
the miRNA regulates expression of the major viral regulatory protein, large T antigen
the viral miRNA restricts viral replication
Acknowledgments
I would like to thank Mengxi Jiang, Shauna Bennett, and Adam Lauring for critcal comments on the manuscript, and Janet Butel for sharing results prior to publication. This work was supported by grant number AI060584 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeCaprio JA, Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B, editors. Fields Virology. 6. Lippincott Williams & Wilkins; 2013. pp. 1633–1661. [Google Scholar]
- 2.Dalianis T, Hirsch HH. Human polyomaviruses in disease and cancer. Virology. 2013;437:63–72. doi: 10.1016/j.virol.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Ford JP, Hsu MT. Transcription pattern of in vivo-labeled late simian virus 40 RNA: equimolar transcription beyond the mRNA 3′ terminus. J Virol. 1978;28:795–801. doi: 10.1128/jvi.28.3.795-801.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk AJ, Sharp PA. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978;75:1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Alwine JC, Dhar R, Khoury G. A small RNA induced late in simian virus 40 infection can associate with early viral mRNAs. Proc Natl Acad Sci U S A. 1980;77:1379–1383. doi: 10.1073/pnas.77.3.1379. First evidence for a polyomavirus miRNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alwine JC, Khoury G. Simian virus 40-associated small RNA: mapping on the simian virus 40 genome and characterization of its synthesis. J Virol. 1980;36:701–708. doi: 10.1128/jvi.36.3.701-708.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–686. doi: 10.1038/nature03576. First conclusive evidence for a miRNA encoded by a polyomavirus. [DOI] [PubMed] [Google Scholar]
- 8.Seo GJ, Fink LH, O’Hara B, Atwood WJ, Sullivan CS. Evolutionarily conserved function of a viral microRNA. J Virol. 2008;82:9823–9828. doi: 10.1128/JVI.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Paulson KG, Murchison EP, Afanasiev OK, Alkan C, Leonard JH, Byrd DR, Hannon GJ, Nghiem P. Identification and validation of a novel mature microRNA encoded by the Merkel cell polyomavirus in human Merkel cell carcinomas. J Clin Virol. 2011;52:272–275. doi: 10.1016/j.jcv.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CJ, Kincaid RP, Seo GJ, Bennett MD, Sullivan CS. Insights into Polyomaviridae microRNA function derived from study of the bandicoot papillomatosis carcinomatosis viruses. J Virol. 2011;85:4487–4500. doi: 10.1128/JVI.02557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantalupo P, Doering A, Sullivan CS, Pal A, Peden KW, Lewis AM, Pipas JM. Complete nucleotide sequence of polyomavirus SA12. J Virol. 2005;79:13094–13104. doi: 10.1128/JVI.79.20.13094-13104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan CS, Sung CK, Pack CD, Grundhoff A, Lukacher AE, Benjamin TL, Ganem D. Murine Polyomavirus encodes a microRNA that cleaves early RNA transcripts but is not essential for experimental infection. Virology. 2009;387:157–167. doi: 10.1016/j.virol.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johne R, Buck CB, Allander T, Atwood WJ, Garcea RL, Imperiale MJ, Major EO, Ramqvist T, Norkin LC. Taxonomical developments in the family Polyomaviridae. Arch Virol. 2011;156:1627–1634. doi: 10.1007/s00705-011-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 15**.Broekema NM, Imperiale MJ. miRNA regulation of BK polyomavirus replication during early infection. Proc Natl Acad Sci U S A. 2013;110:8200–8205. doi: 10.1073/pnas.1301907110. First evidence for a role of a polyomavirus miRNA during replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alwine JC. Hybrid selection of small RNAs by using simian virus 40 DNA: evidence that the simian virus 40-associated small RNA is synthesized by specific cleavage from large viral transcripts. J Virol. 1982;43:987–996. doi: 10.1128/jvi.43.3.987-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broekema NM, Imperiale MJ. Efficient propagation of archetype BK and JC polyomaviruses. Virology. 2012;422:235–241. doi: 10.1016/j.virol.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Zhang S, Sroller V, Zanwar P, Chen CJ, Halvorson SJ, Ajami NJ, Hecksel CW, Swain JL, Wong C, Sullivan CS, et al. Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003912. Comprehensive analysis of the role of the SV40 miRNA in experimental animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauman Y, Nachmani D, Vitenshtein A, Tsukerman P, Drayman N, Stern-Ginossar N, Lankry D, Gruda R, Mandelboim O. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9:93–102. doi: 10.1016/j.chom.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 21.You X, Zhang Z, Fan J, Cui Z, Zhang XE. Functionally orthologous viral and cellular microRNAs studied by a novel dual-fluorescent reporter system. PLoS One. 2012;7:e36157. doi: 10.1371/journal.pone.0036157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CJ, Cox JE, Kincaid RP, Martinez A, Sullivan CS. Divergent MicroRNA targetomes of closely related circulating strains of a polyomavirus. J Virol. 2013;87:11135–11147. doi: 10.1128/JVI.01711-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie H, Lee L, Caramuta S, Hoog A, Browaldh N, Bjornhagen V, Larsson C, Lui WO. MicroRNA Expression Patterns Related to Merkel Cell Polyomavirus Infection in Human Merkel Cell Carcinoma. J Invest Dermatol. 2014;134:507–517. doi: 10.1038/jid.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abend JR, Jiang M, Imperiale MJ. BK virus and human cancer: innocent until proven guilty. Semin Cancer Biol. 2009;19:252–260. doi: 10.1016/j.semcancer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


