Abstract
Background
While allergic sensitization can be generated against various allergens, it is unknown how such a diversity of antigens is able to promote type 2 helper cell (Th2)-mediated inflammation leading to atopy. Our previous studies demonstrated that allergen-specific IgG immune complexes (ICs) and house dust mite (HDM) extract both induced dendritic cells (DCs) to drive Th2-mediated inflammation, but the mechanism by which these diverse stimuli produce similar responses are unknown.
Objective
To identify the DC signaling pathways utilized by Th2 stimuli to promote Th2-mediated inflammation.
Methods
C57BL/6, FcγRIII−/−, FcRγ−/−, and ST2−/− mice were sensitized and challenged with HDM, and inflammation was assessed by flow cytometry, histology, and cytokine production. Bone-marrow derived DCs (BMDCs) from these strains were utilized in signaling and adoptive transfer experiments.
Results
Our findings indicate that two distinct Th2 stimuli, ICs and HDM, both utilize FcRγ-associated receptors, FcγRIII and Dectin-2 respectively, to promote Th2-mediated lung inflammation. In this study we demonstrate that both ICs and HDM induce expression of IL-33, a critical mediator in asthma pathogenesis and the differentiation of Th2 cells, in DCs. Upregulation of IL-33 in DCs is dependent on FcRγ, toll-like receptor 4 (TLR4), and phosphoinositide 3 (PI3)-kinase. Exogenous IL-33 is sufficient to restore development of Th2 responses in FcRγ-deficient mice. Finally, adoptive transfer of allergen-pulsed FcRγ+/− BMDCs restores development of Th2-type inflammation in FcRγ-deficient mice, demonstrating the necessity of this signaling pathway in DCs for allergen-induced inflammation.
Conclusion
These data identify a mechanism whereby Th2 stimuli signal through FcRγ-associated receptors on DCs to upregulate IL-33 production and induce Th2-mediated allergic airway inflammation.
Keywords: Dendritic cells, immune complexes, house dust mite, FcRγ, Th2, allergic airway inflammation, lungs
INTRODUCTION
Atopic asthma is a chronic inflammatory disease of the lungs that causes recurrent narrowing of the airways resulting in wheezing, shortness of breath, and coughing (1). This disease presents with a variety of phenotypes but is most commonly associated with a Th2-type response (2). Development of a Th2-type response is dependent on respiratory DCs that produce specific cytokines, including IL-10 and IL-33, to promote skewing towards Th2 differentiation (3–5). Allergic responses can be triggered by a variety of structurally diverse allergens with varying biological functions including pollen, animal dander, fungal spores, and dust mites (6). These allergens can be characterized based on whether or not they exhibit enzymatic activity. It has been suggested that allergens be divided into Class I allergens that have enzymatic activity (i.e. dust mite and cockroach) and Class II allergens that do not have enzymatic activity (i.e. pet dander) (7). While allergic sensitization can be generated against various allergens, it is unknown how these diverse allergens can promote Th2-skewing and differentiation leading to atopy.
Protein allergens that lack enzymatic activity are often modeled in mice using the inert protein ovalbumin (OVA). Using this model allergen, we previously demonstrated that during secondary responses, OVA-specific IgG formed ICs with OVA instilled in the airways. These ICs signaled through FcγRIII on DCs, and in the presence of a TLR4 stimuli, upregulated IL-33 expression and promoted Th2-mediated lung inflammation (8). While ICs represented one mechanism by which homogenous protein allergens could promote Th2-mediated allergic responses, it was unclear whether this model was relevant for other complex allergens such as HDM that are comprised of many immunogenic proteins in association with glycans and endotoxin. This allergen contains a wide variety of components that may contribute to its allergenicity including cysteine proteases, chitinases, serine proteases, and others (9). We recently found that HDM induced IL-33 expression in BMDCs in the absence of specific IgG suggesting alternative pathways for the induction of Th2 responses (5). This study was designed to further clarify how diverse allergens are able to induce similar Th2-type responses in the lungs.
In this study, we determine that Th2 stimuli (i.e. ICs and HDM) signal through FcRγ-associated receptors on DCs to promote Th2 responses in the lungs by increasing expression of IL-33. FcRγ is a common signaling component of multiple receptors including FcγRIII and Dectin-2 that have been shown to bind ICs and HDM, respectively (10, 11). Using these two different Th2 stimuli, we demonstrate that ICs and HDM both induce expression of IL-33 in BMDCs in an FcRγ, TLR4, and PI3-kinase dependent manner. HDM-induced allergic airway inflammation is FcRγ dependent, and this inflammatory response was restored in FcRγ−/− mice upon administration of recombinant IL-33 during sensitization with HDM. Moreover, adoptive transfer of allergen-pulsed FcRγ+/− BMDCs is sufficient to restore development of Th2-type inflammation in FcRγ−/− mice. These data identify a mechanism that can be engaged by different Th2 stimuli to promote atopic asthma by activating DCs through FcRγ-associated receptors to induce IL-33 production and drive Th2 responses.
METHODS
Mice
C57BL/6 mice (WT) were purchased from Harlan Laboratories. B6.FcγRIII−/− and B6.FcRγ−/−mice were purchased from Jackson Laboratory. B6.ST2−/− mice were provided by Dr. A. McKenzie (Medical Research Laboratory, University of Cambridge, UK) (12). B6.Dectin-2−/−mice were generated by Dr. Y. Iwakura (The Institute of Medical Science, Tokyo, Japan) (13). B6.TLR4−/− were provided by Dr. C. Nagler (University of Chicago, Chicago, IL). All animal procedures and housing were approved by the University of Chicago Animal Resources Center. The studies conformed to the principles set forth by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
Reagents
House dust mite (XPB82D3A25, Greer Laboratories), Grade V chicken egg OVA (Sigma Aldrich), rabbit anti–chicken egg OVA sera (080M4812, Sigma Aldrich), Grade V BSA (Sigma Aldrich), rabbit anti–BSA sera (Sigma Aldrich), and recombinant mouse IL-33 (Biolegend) were used in murine experiments. OVA-ICs were made by mixing a 10:1 excess of anti-OVA:OVA at 37°C for 30 minutes. PI3-kinase inhibitor (Ly294002) or Syk inhibitor (piceatannol) (Sigma Aldrich) were used in cell culture experiments.
Sensitization and challenge
Mice were sensitized and challenged as described on day 0 (PBS, 100 µg of HDM, 500 ng of rIL-33, or both HDM and rIL-33) and day 7 (PBS or 100 µg of HDM) and then sacrificed on day 11. For Fig. 5, mice were sensitized by instilling 1×106 treated BMDCs i.t. into naïve mice. On day 7 all the mice were challenged i.t. with 100 µg of HDM and sacrificed on day 11. Using the passive transfer model developed in our previous study (8), 100 µL of α-OVA or α-BSA sera was injected intravenously into mice followed by a 100 µg OVA i.t. sensitization the next day; one week later, animals were challenged with three OVA i.t. administrations. Airway inflammation was assessed as described in the supplementary materials.
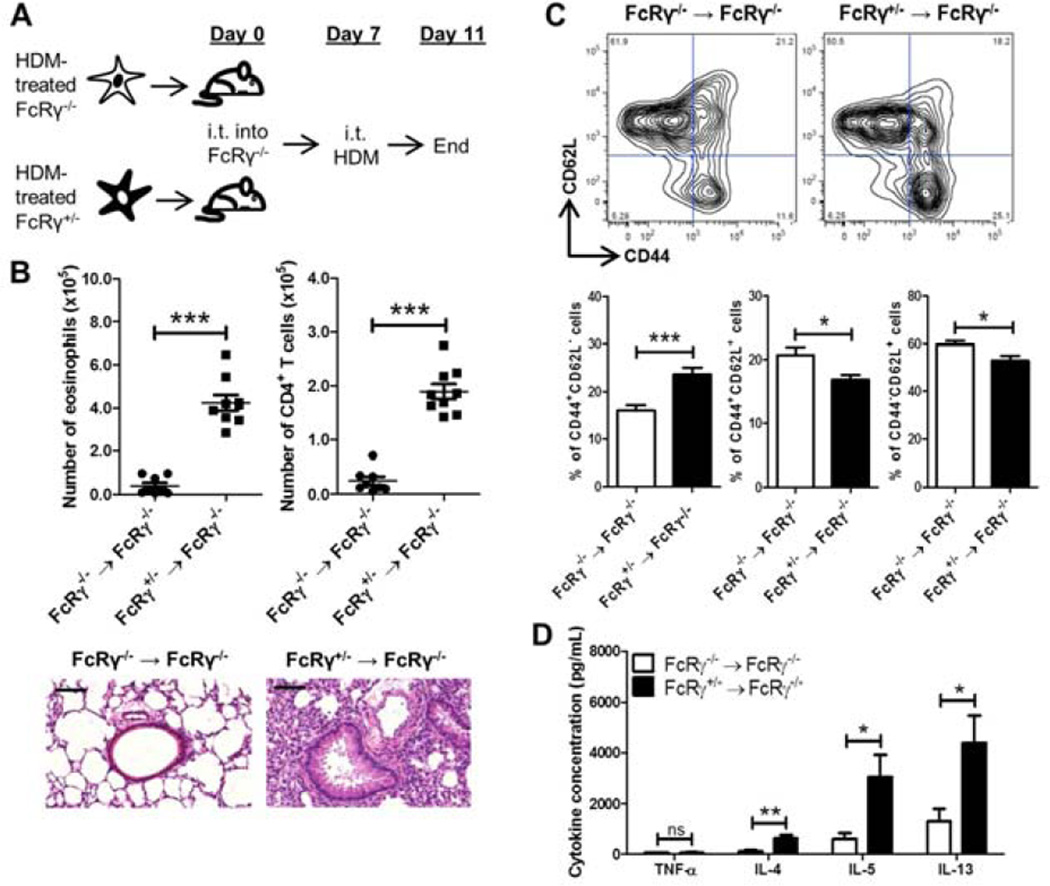
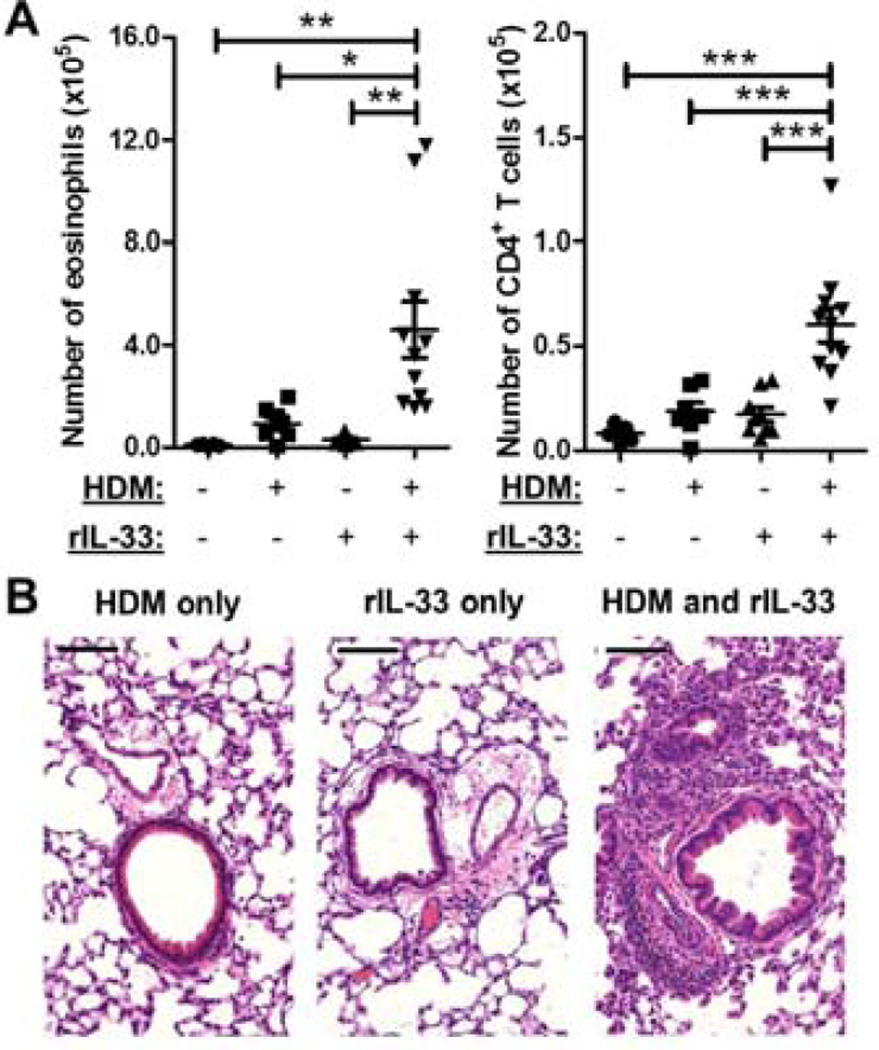
Figure 5. FcRγ+/− DCs are sufficient to reconstitute HDM responses in FcRγ−/− mice.
(A) FcRγ+/− or FcRγ−/− BMDCs were generated and treated overnight with HDM before being instilled i.t. into naïve FcRγ−/−mice. On day 7 the mice were challenged i.t. with HDM before being sacrificed on day 11. (B) Airway inflammation was assessed by determining the number of eosinophils (left panel), and CD4+ T cells (right panel) in the BAL. Representative H&E sections of lung tissue from the treated mice. Black bars = 100 µm. (C) Representative flow plots of draining lymph node cells gated on CD3+CD4+ cells used to determine the percentage of CD44+ CD62L−, CD44+ CD62L+ , and CD44− CD62L− cells. (D) Amount of cytokine in culture supernatants from HDM-restimulated mediastinal lymph node cells. Data represent the mean ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns = not significant). The data are combined from two independent experiments with a total of at least eight mice analyzed per group.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism software, and a P-value less than 0.05 was considered significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns = not significant). Experiments with two groups were analyzed using an unpaired Student’s two-tailed t test. Experiments with greater than two groups were analyzed with a one-way ANOVA and post-hoc Tukey test. Error bars represent the SEM.
Cytokine Analysis
For Fig. 4, BMDCs were stimulated overnight as described above. For Fig. 5D, single cell suspensions from the mediastinal lymph node were cultured with 25 µg/mL of HDM at 2×105 cells/well for 48 hours. The plates were put through a freeze-thaw cycle to release intracellular cytokines before supernatants were collected and analyzed by Multiplex bead array according to the manufacturer’s protocol (Millipore).
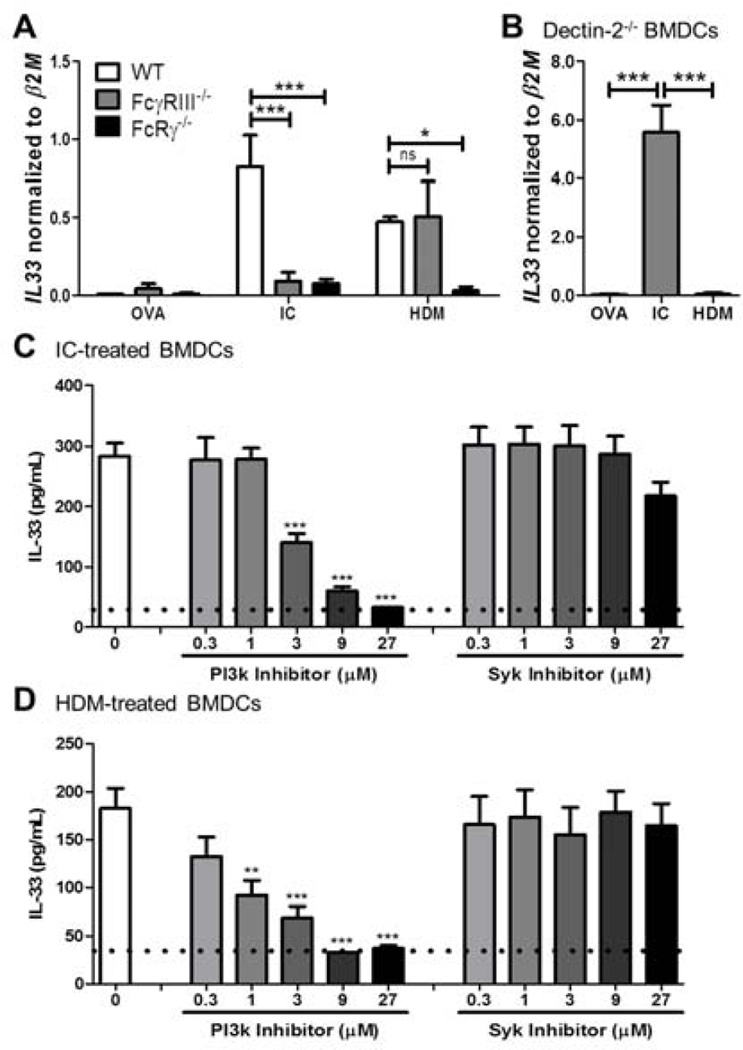
Figure 4. IL-33 upregulation in DCs is FcRγ and PI3-kinase dependent.
BMDCs were generated from (A) WT, FcγRIII−/−, and FcRγ−/− mice or (B) Dectin-2−/− mice and treated with OVA, ICs, or HDM overnight before assessing for IL-33 mRNA expression normalized to β2M mRNA expression. IL-33 protein expression was determined in WT BMDCs stimulated with (C) ICs or (D) HDM and treated with PI3-kinase inhibitor (Ly294002) or Syk inhibitor (piceatannol). Data represent the mean ± SEM from three independent culture sets (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns = not significant).
RESULTS
HDM-mediated Th2-type responses are dependent on FcRγ but not FcγRIII
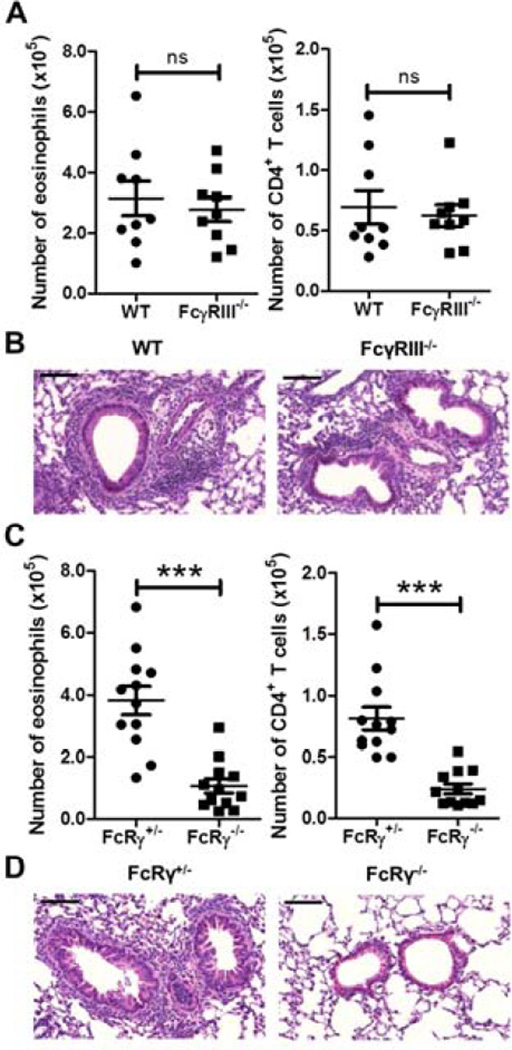
Although we had previously demonstrated that HDM treatment of BMDCs was able to upregulate IL-33 even in the absence of HDM-specific IgG (5), we hypothesized that IC formation during secondary responses could be playing a role in HDM-induced Th2 responses. We first demonstrated that an eosinophilic and CD4+ T cell response was only seen in WT mice that had received both an intratracheal (i.t.) sensitization and challenge with HDM a week apart; the mice were sacrificed four days after the last challenge (Fig. S1). No significant response was seen in mice that had received a HDM sensitization/PBS challenge, PBS sensitization/HDM challenge, or PBS sensitization/PBS challenge (Fig. S1). We then sensitized and challenged WT and FcγRIII−/− mice with HDM using the protocol described above. Interestingly, we found no difference in the number of eosinophils or CD4+ T cells in the bronchoalveolar lavage (BAL) between the two strains (Fig. 1A). Histological examination of the lungs showed that both strains had thickening of the airway epithelium and inflammatory cell infiltrates around the airways (Fig. 1B). These results suggested that HDM utilized an FcγRIII-independent pathway to promote Th2 inflammation in the lungs.
Figure 1. HDM-mediated inflammation is dependent on FcRγ but not FcγRIII.
WT or FcγRIII−/− (A and B) and FcRγ+/− or FcRγ−/− (C and D) mice were sensitized on day 0 and challenged on day 7 with HDM. Airway inflammation was assessed on day 11 by determining (A) and (C) the number of eosinophils (left panel), and CD4+ T cells (right panel) in the BAL by flow cytometry. (B) and (D) Representative H&E sections of lung tissue from treated mice. Black bars = 100 µm. Data represent the mean ± SEM (***, P < 0.001, ns = not significant). The data are combined from at least three independent experiments with a total of at least nine mice analyzed per group.
We had previously shown that glycans in HDM were recognized by the Dectin-2/FcRγ receptor complex, which led to increased Th2-type responses through the production of cysteinyl leukotrienes (cysLTs), TNF-α, IL-6, IL-10, and IL-23 (14, 15). Given that FcγRIII and Dectin-2 both signal through FcRγ, we hypothesized that FcRγ-containing receptors were utilized by Th2 stimuli to induce Th2 responses in vivo. It was previously shown that allergen-induced airway hyperresponsiveness and inflammation was FcRγ-dependent using an OVA sensitization and challenge model (16). Thus, it led us to question whether HDM-mediated responses were also FcRγ dependent. Heterozygous (FcRγ+/−) and knockout (FcRγ−/−) littermates were compared following sensitization and challenge with HDM. The numbers of both eosinophils and CD4+ T cells were significantly decreased in the BAL of FcRγ−/−mice demonstrating that this receptor component was important for the induction of Th2 responses to HDM (Fig. 1C). Further, there was decreased inflammation in the airways of the FcRγ−/−mice as evidenced by histology (Fig. 1D). These findings supported our hypothesis that FcRγ was part of a common signaling pathway that induced allergic airway inflammation.
IL-33 restores allergic airway inflammation in FcRγ−/− mice
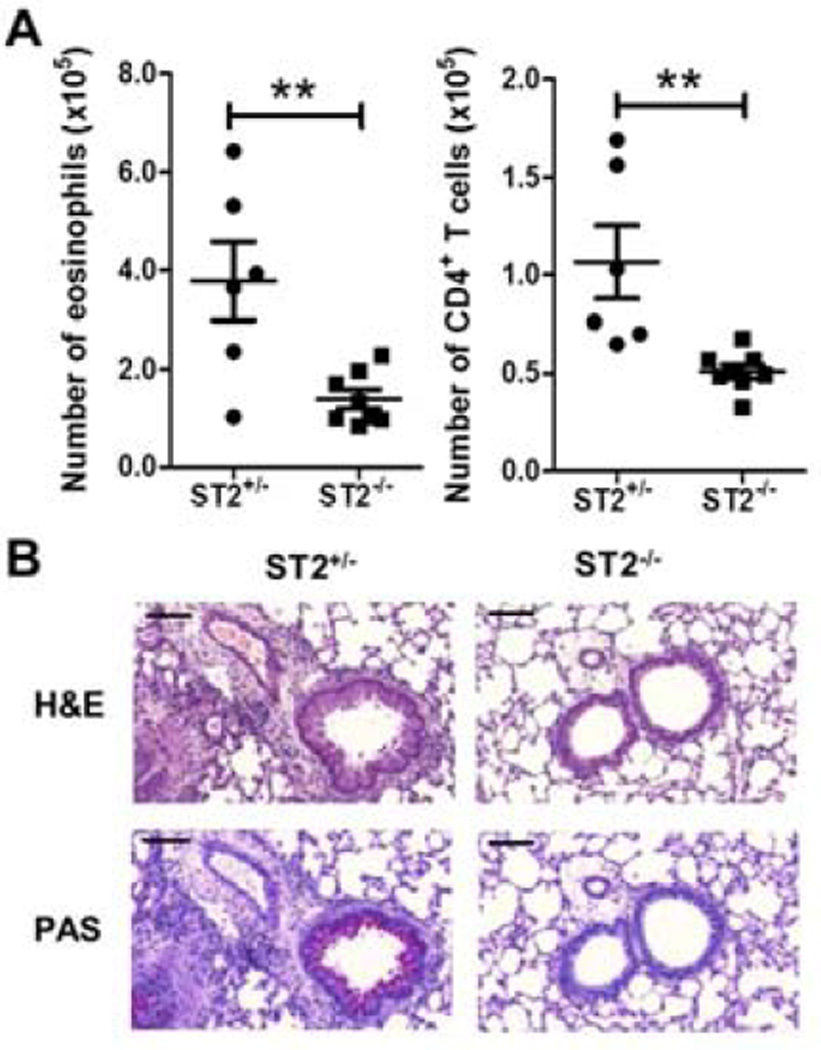
Our previous work had shown that HDM treatment of BMDCs induced expression of IL-33 suggesting that HDM allergic airway inflammation may be dependent on the ST2/IL-33 pathway (5). To determine if HDM sensitization required ST2, ST2+/− and ST2−/− littermates were sensitized and challenged with HDM. Compared to the ST2+/− mice, the ST2−/− mice had a significant reduction in the number of eosinophils and CD4+ T cells in the BAL as well as decreased lung inflammation and mucus production as shown by histology (Fig. 2A and 2B). Since HDM-induced responses were ST2 dependent, we examined whether administration of recombinant IL-33 (rIL-33) during sensitization with HDM was enough to restore allergic airway inflammation in FcRγ−/− mice. On day 0, FcRγ−/− mice received an i.t. of PBS alone, HDM alone, rIL-33 alone, or HDM plus rIL33 combined. On day 7, all mice were challenged with HDM. The FcRγ−/− mice sensitized with PBS, HDM, or IL-33 alone failed to develop airway or lung inflammation after HDM challenge (Fig. 3A and 3B). Strikingly, the FcRγ−/− mice that were sensitized with HDM in combination with rIL-33 had increased eosinophilia and CD4+ T cells in the BAL, and significant thickening of the airway epithelium and lymphocytic infiltration after challenge with HDM (Fig. 3A and 3B). Thus, the addition of IL-33 was sufficient to restore airway and lung inflammation in FcRγ−/− mice. These data suggested that signaling through FcRγ-containing receptors could lead to IL-33 production which was necessary for primary sensitization of mice to HDM.
Figure 2. HDM-mediated Th2-type responses are ST2 dependent.
ST2+/− or ST2−/− mice were sensitized and challenged with HDM. (A) Airway inflammation was assessed by determining the number of eosinophils (left panel), and CD4+ T cells (right panel) in the BAL. (B) Representative H&E and PAS sections of lung tissue from treated mice. Black bars = 100 µm. Data represent the mean ± SEM (**, P < 0.01). The data are combined from at least two independent experiments with a total of at least six mice analyzed per group.
Figure 3. Administration of IL-33 during the sensitization phase restores airway inflammation in FcRγ−/− mice.
FcRγ−/− mice were sensitized with PBS, HDM, rIL-33, or HDM and rIL-33 on day 0. On day 7, all mice received an i.t. challenge of HDM. Airway inflammation was assessed on Day 11 by determining (A) the number of eosinophils (left panel), and CD4+ T cells (right panel) in the BAL. (B) Representative H&E sections of lung tissue from treated mice. Black bars = 100 µm. Data represent the mean ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The data are combined from three independent experiments with a total of at least five mice analyzed per group.
HDM-induced IL-33 upregulation in DCs is FcRγ and Dectin-2 dependent
To investigate whether signaling through FcRγ was required for increased IL-33 expression and production in DCs, WT, FcγRIII−/−, and FcRγ−/− BMDCs were generated and treated overnight with OVA, ICs, or HDM. IC- or HDM-treatment of WT BMDCs upregulated MHC II and CD86 expression more than OVA suggesting that these stimuli induced greater activation of BMDCs (Fig. S2A). As we had shown in our earlier study, ICs induced IL-33 in an FcγRIII dependent manner (Fig. 4A)(8). HDM-induced upregulation of IL-33 was FcγRIII independent supporting our earlier findings that HDM-mediated Th2 inflammation did not require FcγRIII. Interestingly, similar to the induction by ICs, increased expression of IL-33 following treatment with HDM was dependent on FcRγ (Fig. 4A). We determined that after treatment with HDM, there was no significant difference in the expression of MHC II, CD86, or CD40 on WT and FcRγ−/− BMDCs (Fig. S2B). Furthermore, our previous work had suggested there may be crosstalk between FcRγ-associated receptors and TLR4, and indeed we found that IL-33 upregulation following HDM treatment was TLR4 dependent (Fig. S3). To determine if IL-33 upregulation was downstream of the Dectin-2/FcRγ receptor complex, Dectin-2−/− BMDCs were treated with OVA, ICs, or HDM. In the absence of Dectin-2, there was no significant increase in IL-33 mRNA expression following treatment with HDM (Fig. 4B). From these findings, we determined that IC signaling through FcγRIII or HDM signaling through Dectin-2 utilized the common signaling component FcRγ to promote IL-33 expression.
Since both ICs and HDM upregulated IL-33 in an FcRγ dependent manner, but used different co-receptors, we investigated whether they engaged the same downstream signaling molecules for IL-33 induction. Previously, we found that downstream of FcRγ signaling on BMDCs, cysLT upregulation was Syk dependent whereas IL-10 upregulation was PI3-kinase dependent (14, 16). In addition, work on mast cells had demonstrated that IL-33 upregulation upon FcεRI ligation was PI3-kinase dependent (17). To investigate which pathway led to IL-33 upregulation, WT BMDCs were treated with either ICs or HDM in the presence of a PI3-kinase inhibitor (Ly294002) or Syk inhibitor (piceatannol). Interestingly, compared to the control group that received no inhibitors, IC- and HDM-induced IL-33 production was PI3-kinase dependent. However, the Syk inhibitor failed to reduce the IC- and HDM-induced IL-33 production found in the control groups (Fig. 4C and 4D). Taken together, these data identified a common pathway through FcRγ and PI3-kinase which was utilized by different Th2 stimuli to induce production of IL-33 in DCs, which is distinct from the pathway utilized to produce cysLTs.
FcRγ-sufficient BMDCs reconstitute HDM-mediated inflammation in FcRγ-deficient mice
In our studies on IC-mediated Th2 inflammation, we demonstrated that T cells were necessary and sufficient for development of allergic airway inflammation (8). To determine if T cells were playing a role in our HDM allergen model, WT or Rag−/− were sensitized and challenged with HDM as described above. Notably, there was a significant decrease in the number of total cells and eosinophils in the BAL of Rag−/− mice (Fig. S4A). This inflammatory response was restored in Rag−/− mice that had received an adoptive transfer of nylon-wool nonadherent cells (Fig. S4B). These data demonstrate that T cells are necessary for HDM-mediated allergic airway inflammation.
Development of Th2 inflammation relies on stimulation from antigen presenting cells, primarily DCs, which are able to direct differentiation into specific T-cell lineages (3). Given that HDM-mediated responses were T cell dependent, we investigated whether the lack of inflammation in the FcRγ−/− mice was due to a deficient response from the DCs in the lungs. To address this question allergen-pulsed FcRγ−/− or FcRγ+/− BMDCs were adoptively transferred into naïve FcRγ−/− mice (Fig. 5A). FcRγ−/− and FcRγ+/− BMDCs were stimulated overnight with HDM before 1×106 BMDCs were transferred i.t. into naïve FcRγ−/− mice. On day 7, all the mice received an i.t. challenge of HDM before being sacrificed on day 11. The mice that received the FcRγ+/− BMDCs had significantly increased numbers of eosinophils and CD4+ T cells in the BAL as well as increased inflammation as seen on histology (Fig. 5B). Further analysis of the T cells demonstrated that there was a greater percentage of effector memory CD4+ T cells (CD44+CD62L−) and a lower percentage of naïve CD4+ T cells (CD44−CD62L+) in the mediastinal lymph nodes of mice that had received FcRγ+/− BMDCs (Fig. 5C). Moreover, the draining lymph node cells from mice that had received FcRγ+/− BMDCs produced significantly more IL-4, IL-5, and IL-13 upon restimulation with HDM (Fig. 5D). Together, these data demonstrated that signaling through FcRγ-associated receptors on DCs during sensitization was sufficient to restore effective priming of T cells in the draining lymph node, which in turn, led to the development of Th2-type inflammation.
DISCUSSION
The focus of this study was to understand how two structurally and biologically diverse allergens were able to induce similar Th2-type responses in the lungs. Utilizing ICs and HDM, we determined that they both signaled through FcRγ-associated receptors, TLR4, and PI3-kinase to upregulate IL-33 production in DCs. We further demonstrated that signaling through FcRγ-associated receptors on DCs was sufficient to restore Th2 responses in FcRγ-deficient mice. These data identify a mechanism that can be engaged by different Th2 stimuli to promote atopic asthma by activating DCs through FcRγ-associated receptors to induce IL-33 production and drive Th2 responses.
Our results demonstrate that hematopoietic cells expressing FcRγ-associated receptors are able to upregulate IL-33 suggesting that this pathway may contribute to the pathogenesis of Th2-mediated diseases. In humans, significant clinical correlations have been discovered between IL-33 and asthma. Several genome-wide association studies have identified genetic variations in the IL33 and IL1RL1 genes (encoding IL-33 and ST2, respectively) as being asthma susceptibility loci (18). These findings support clinical studies demonstrating increased levels of IL-33 in the serum and tissues of patients with asthma or allergies (19–22). However, it has not been as well characterized which human hematopoietic cells can act as a source of IL-33 during allergic airway inflammation. Our studies point to cells that express FcRγ-associated receptors, which in humans includes DCs, monocytes, macrophages, mast cells, NK cells, and basophils (23). An association between FcRγ and asthma was recently identified by Hinds et al in a genome-wide association study of self-reported asthma for rs2070902 in the FcRγ locus (FCER1G) (24). While further investigations need to be carried out to clarify if IL-33 upregulation in human cells is also downstream of FcRγ-associated receptors, taken together with our findings, these studies suggest that binding of allergens to FcRγ-associated receptors induce IL-33 production which may represent one mechanism for the promotion of allergic airway inflammation.
Interestingly, our study finds that HDM-mediated allergic airway inflammation does not require the presence of ICs, while our previous findings demonstrate that OVA does require ICs. We propose a model that classifies allergens based on their ability to bind directly or indirectly to FcRγ-associated receptors. Allergens like HDM that contain glycans may enhance the Th2 response through direct binding of its components to FcRγ-associated receptors such as Dectin-2. On the other hand, homogenous protein allergens like OVA cannot be directly recognized by an FcRγ-associated receptor, but in the presence of antigen-specific IgG generated during a primary response, it can form ICs that can ligate and activate FcγRIII. We have further demonstrated that the IC-mediated Th2 inflammation model works with other homogeneous proteins like bovine serum albumin (BSA). An eosinophilic and CD4+ T cell response in the lungs was only seen in mice that received antigen that was complementary to the serum administered highlighting the specificity of IC-mediated Th2 inflammation (Fig. S5). Our study demonstrated that the presence of allergen-specific IgG could lead to antigen-specific IC formation that would contribute to the development of allergic responses. In humans, circulating allergen-specific ICs have been detected in allergic individuals, but whether they contribute to development of Th2 responses in humans is still unclear (25–27). Translation of our findings into human studies may help to elucidate whether this dichotomy occurs in humans as well.
Interestingly, our study suggests that FcRγ-associated receptors and TLR4 act in concert together to augment allergic airway inflammation. In this study and in our previous work, we have demonstrated that HDM or IC treatment of BMDCs upregulated IL-33 in a manner that was dependent on both an FcRγ-associated receptor and TLR4 (8). This finding is consistent with other studies showing that activation of TLR4 led to the rapid upregulation of PI3-kinase, and that IgG-IC-elicited cytokines were dependent on TLR4 and FcγRIII association and signaling in peritoneal macrophages (28, 29). It is not clear whether TLR4 associates with other FcRγ-associated receptors, but our findings suggest a requirement for both signals to upregulate IL-33 upon HDM treatment.
In addition, we demonstrated that Dectin-2 mediated HDM-induced IL-33 production from BMDCs. Canonical signaling downstream of Dectin-2/FcRγ is thought to primarily involve activation of Syk, which has been shown to play a critical role in host defense against Candida Albicans (13), Schistosoma Mansoni (30), Microsporum Audouinii, and Trichophyton Rubrum (31). Although Dectin-2/FcRγ signaling is known to activate Syk and generate NF- κB dependent cytokines through the adaptor protein CARD9, here we demonstrate a novel Syk independent function (14). Overall, our findings identify a pathway downstream of Dectin-2, FcRγ, and TLR4 activation which promotes the production of IL-33 that can contribute to the development of Th2 responses.
DCs express a wide variety of receptors that associate and signal through FcRγ including FcγRI, FcγRIII, FcεRI, Dectin-2, activating immunoglobulin-like receptors (LILR, ILT, or LIR), DC activating receptor (DCAR), and paired immunoglobulin-like receptor A (PIR-A) (23, 32). More importantly, several studies have demonstrated or suggested that expression of these receptors on DCs can promote and exacerbate Th2-type responses and allergic airway inflammation including FcγRI (11, 33), FcγRIII (8), FcεRI (34), and Dectin-2 (35). It has been shown that these receptors can bind to other ligands associated with allergic lung disease including Candida albicans (36), Aspergillus fumigatus (14), and pollen starch granules (37). Thus, our findings may have broader implications for other allergens that utilize FcRγ-associated receptors to promote and exacerbate allergic responses.
Asthma is a heterogenous disease, and a more comprehensive view of how allergens are activating immune cells to promote Th2-type responses must be taken into account in order to develop more effective therapeutics. Several clinical trials have investigated the potential use of anti-cytokine therapy against IL-1, IL-4, IL-5, IL-9, IL-10, IL-12, IL-13, IL-18, and TNF-α; however, there has been limited success with these approaches (38, 39). Taken together with our previous studies, FcRγ signaling on DCs upregulates cysLTs, IL-6, IL-10, IL-23, TNF-α, and IL-33 (14, 16, 35). These results highlight the extensive cascade of inflammatory mediators that can be induced by ligation of receptors that signal through FcRγ, and it suggests that a more comprehensive strategy for inhibiting the entire cascade may be more effective than many of the anti-cytokine therapeutic strategies that were previously employed.
Material and Methods
Assessment of airway inflammation
For histology, the right middle lobe was removed from the mice after BAL and fixed by immersion into 10% formalin. Fixed sections were stained with hematoxylin and eosin (H&E) or Periodic acid-Schiff (PAS) by the University of Chicago Tissue Resource Center. BAL was performed by delivering sterile PBS into the airway and repeatedly aspirating the fluid for a total recovery of ~3 mL. For FACs analysis, 5×105 cells were resuspended in 100 µL of FACs buffer (PBS containing 0.1% sodium azide and 1% BSA) and blocked with 20 µL of 2.4G2 (anti-CD16/32) supernatant for 10 minutes at 25°C. The cells were then stained for 30 minutes at 4°C with fluorescently conjugated antibodies. Eosinophils were identified as SSChiCCR3+Grl− while CD4+ T cells were gated as SSC °CD3+CD4CD8−. Flow cytometric analysis was performed on an LSRFortessa (BD Biosciences), and the data were analyzed with FlowJo software (Tree Star Inc.).
| Specificity | Clone | Company | Dilution |
|---|---|---|---|
| CCR3 | 83101 | R&D Systems | 1 µg/mL |
| CD3 | 145-2C11 | eBiosciences | 1 µg/mL |
| CD4 | RM4-5 | eBiosciences | 125 ng/mL |
| CD8 | 53-6.7 | eBiosciences | 250 ng/mL |
| CD44 | IM7 | eBiosciences | 500 ng/mL |
| CD62L | MEL-14 | eBiosciences | 250 ng/mL |
| Ly6C/G(Grl) | 1A8 | BD Pharmigen | 1 µg/mL |
Quantitative PCR (qPCR) primer sequences
| Primer Sequence | |
|---|---|
| IL-33 Forward | 5’-GCTGCGTCTGTTGACACATT-3’ |
| IL-33 Reverse | 5’-CACCTGGTCTTGCTCTTGGT-3’ |
| β2M Forward | 5’- CATACGCCTGCAGAGTTAAGCA-3’ |
| β2M Reverse | 5’-GATCACATGTCCGATCCCAGTAG-3’ |
Nylon wool non-adherent T cell enrichment and transfer
Brachial, inguinal, cervical, and mesenteric lymph nodes were isolated from mice and made into single cell suspensions. Autoclaved nylon wool columns (Polysciences, Inc.) were equilibrated after which cell suspensions were loaded onto the column. Non-adherent cells were eluted, and 1.0×107 cells were administered i.v. into naive mice 2 days before being sensitized with HDM. They were then challenged with HDM a week later and sacrificed four days later.
Isolation of draining (mediastinal) lymph node cells
Draining (mediastinal) lymph nodes were made into single cell suspensions. The tissue was mechanically dissociated and passed through a filter to remove debris. Red blood cells were lysed with ammonium chloride-potassium lysing buffer after which the single cell suspension was stained for flow cytometry.
Isolation of lung cells
The lungs were perfused with 25 U/mL of heparin (Sigma Aldrich) in PBS and minced with scissors. Tissue dissociation was achieved by incubating the tissue for 30 minutes at 37°C in 5% complete media with 150 U/mL collagenase I (Invitrogen). The digest was passed through a filter to remove debris, and red blood cells were lysed with ammonium chloride-potassium lysing buffer after which the single cell suspension was stained for flow cytometry.
Production of bone marrow-dendritic cells (BMDCs)
BMDCs were generated as previously described (40). Bone marrow was flushed from the femurs and tibias of mice, and the cells were cultured in 10% complete DMEM supplemented with 20 ng/mL GM-CSF (Shenandoah Biotechnology). On day 3 and 6, the media was replenished with 20 ng/mL GM-CSF. On day 8, the suspension cells were harvested, and 5×l05 BMDCs were cultured in 24-well plates with OVA (percent viability: 91.9 ± 3.0), OVA-IC (percent viability: 93.1 ±3.1), or HDM (percent viability: 91.7 ± 2.1) at a concentration of 25 µg/mL.
Quantitative PCR (qPCR)
RNA was isolated from cells using an RNeasy Micro Kit according to the manufacturer’s protocol (Qiagen) and quantified by nanodrop (Thermo Scientific). cDNA synthesis was done using the Superscript III Reverse Transcriptase kit according to the manufacturer’s protocol (Invitrogen). cDNA samples were amplified with the Power Sybr Green PCR master mix (Applied Biosystems) and run on a ABI 7300 cycler (Bio-Rad). Primer sequences used are available in the supplementary materials.
Statistical analysis
All statistical analyses were performed with GraphPad Prism software, and a P-value less than 0.05 was considered significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns = not significant). Experiments with two groups were analyzed using an unpaired Student’s two-tailed t test. Experiments with greater than two groups were analyzed with a one-way ANOVA and post-hoc Tukey test. Error bars represent the SEM.
Supplementary Material
Key Message.
This study identifies a mechanism through which allergens are able to promote allergic airway inflammation by activating FcRγ-associated receptors in concert with TLR4 on DCs.
Ligation of FcRγ-associated receptors on DCs induces production of IL-33 that leads to the development of Th2 responses in vivo.
Acknowledgements
For lab support, we would like to thank D.C. Decker and J. Casaos. We would also like to thank A. McKenzie, Y. Iwakura, and C. Nagler for generously providing mice used in this study.
This work was supported by NIH R21AI094408 (A.I.S.), K08AI080948 (N.A.B.), R01HL120952 (N.A.B.), 5T32HL007605 (C.L.H.), 5T32HL007237 (J.W.W.), and a Naomi Ragins-Goldsmith Fellowship, University of Chicago (M.Y.T.). The University of Chicago Cancer Center Core Facilities utilized are supported by NIH P30CA14599.
Abbreviations
- DCs
dendritic cells
- BMDCs
bone-marrow derived dendritic cells
- BAL
bronchoalveolar lavage
- OVA
ovalbumin
- ICs
immune complexes
- HDM
house dust mite
- WT
wildtype
- cysLTs
cysteinyl leukotrienes
- i.t
intratracheal
- TLR
toll-like receptor
- Th
T helper cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
References
- 1.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012 May;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012 May;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010 Aug;11(8):647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008 Jan 21;205(1):13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills TA, Woodfolk JA. Allergens and their role in the allergic immune response. Immunol Rev. 2011 Jul;242(1):51–68. doi: 10.1111/j.1600-065X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- 7.Erwin EA, Platts-Mills TA. Allergens. Immunol Allergy Clin North Am. 2005 Feb;25(1):1–14. doi: 10.1016/j.iac.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, et al. IL-33-dependent induction of allergic lung inflammation by FcgammaRIII signaling. J Clin Invest. 2013 May 1;123(5):2287–2297. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends Mol Med. 2011 Oct;17(10):604–611. doi: 10.1016/j.molmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009 Nov;232(1):42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura K, Takeda K, Koya T, Miyahara N, Kodama T, Dakhama A, et al. Critical role of the Fc receptor gamma-chain on APCs in the development of allergen-induced airway hyperresponsiveness and inflammation. J Immunol. 2007 Jan 1;178(1):480–488. doi: 10.4049/jimmunol.178.1.480. [DOI] [PubMed] [Google Scholar]
- 12.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000 Mar 20;191(6):1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010 May 28;32(5):681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009 Jan 15;182(2):1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011 Mar 14;208(3):593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, et al. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007 Aug 6;204(8):1875–1889. doi: 10.1084/jem.20061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CL, Bryce PJ. Inducible IL-33 expression by mast cells is regulated by a calcium-dependent pathway. J Immunol. 2012 Oct 1;189(7):3421–3429. doi: 10.4049/jimmunol.1201224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grotenboer NS, Ketelaar ME, Koppelman GH, Nawijn MC. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J Allergy Clin Immunol. 2013 Mar;131(3):856–865. doi: 10.1016/j.jaci.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009 Oct 15;183(8):5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 20.Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol. 2013 Sep;132(3):676–685. e13. doi: 10.1016/j.jaci.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluck J, Rymarczyk B, Rogala B. Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Inflamm Res. 2012 Jun;61(6):547–550. doi: 10.1007/s00011-012-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013 Oct;50(8):803–809. doi: 10.3109/02770903.2013.816317. [DOI] [PubMed] [Google Scholar]
- 23.Williams JW, Tjota MY, Sperling AI. The contribution of allergen-specific IgG to the development of th2-mediated airway inflammation. J Allergy (Cairo) 2012;2012:236075. doi: 10.1155/2012/236075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinds DTC, Kiefer AK, Mountain JL, Eriksson N, Tung JY. A large scale genome wide association study of asthma in the 23andMe cohort; Boston, MA. American Society of Human Genetics Annual Meeting.2013. [Google Scholar]
- 25.Jarzab J, Gawlik R. Immune complexes IgE/IgG in airborne allergy: increase during pollen season. J Investig Allergol Clin Immunol. 2000 Jan-Feb;10(1):24–29. [PubMed] [Google Scholar]
- 26.Stevens WJ, Bridts CH. IgG-containing and IgE-containing circulating immune complexes in patients with asthma and rhinitis. J Allergy Clin Immunol. 1984 Feb;73(2):276–282. doi: 10.1016/s0091-6749(84)80020-2. [DOI] [PubMed] [Google Scholar]
- 27.Casas R, Djerf P, Haggstrom P, Ferrandiz R, Bjorksten B. Circulating cat allergen and immune complexes in cat-allergic children. Clin Exp Allergy. 1998 Oct;28(10):1258–1263. doi: 10.1046/j.1365-2222.1998.00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Rittirsch D, Flierl MA, Day DE, Nadeau BA, Zetoune FS, Sarma JV, et al. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009 Jun;5(6):e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009 Jun;85(6):966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010 Nov 23;107(47):20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006 Dec 15;281(50):38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 32.Kanazawa N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J Dermatol Sci. 2007 Feb;45(2):77–86. doi: 10.1016/j.jdermsci.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, et al. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol. 2010 Jan;125(1):247–256. e1–e8. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallmann E, Reininger B, Brandt S, Duschek N, Hoflehner E, Garner-Spitzer E, et al. High-affinity IgE receptors on dendritic cells exacerbate Th2-dependent inflammation. J Immunol. 2011 Jul 1;187(1):164–171. doi: 10.4049/jimmunol.1003392. [DOI] [PubMed] [Google Scholar]
- 35.Parsons MW, Li L, Wallace AM, Lee MJ, Katz HR, Fernandez JM, et al. Dectin-2 Regulates the Effector Phase of House Dust Mite-Elicited Pulmonary Inflammation Independently from Its Role in Sensitization. J Immunol. 2014 Jan;:22. doi: 10.4049/jimmunol.1301809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009 Aug 31;206(9):2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Currie AJ, Stewart GA, McWilliam AS. Alveolar macrophages bind and phagocytose allergen-containing pollen starch granules via C-type lectin and integrin receptors: implications for airway inflammatory disease. J Immunol. 2000 Apr 1;164(7):3878–3886. doi: 10.4049/jimmunol.164.7.3878. [DOI] [PubMed] [Google Scholar]
- 38.Desai D, Brightling C. Cytokine and anti-cytokine therapy in asthma: ready for the clinic. Clin Exp Immunol. 2009 Oct;158(1):10–19. doi: 10.1111/j.1365-2249.2009.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes PJ. Cytokine modulators as novel therapies for asthma. Annu Rev Pharmacol Toxicol. 2002;42:81–98. doi: 10.1146/annurev.pharmtox.42.111901.111143. [DOI] [PubMed] [Google Scholar]
- 40.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999 Feb 1;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.