Abstract
Balance tests are commonly used to screen for impairments that put older adults at risk for falls. The purpose of this study was to determine the attributes that were associated with balance performance as measured by the The Frailty and Injuries: Cooperative Studies of Intervention Techniques (FICSIT) balance test. This study was a cross-sectional secondary analysis of baseline data from a longitudinal cohort study, the Boston Rehabilitative Impairment Study of the Elderly (Boston RISE). Boston RISE was performed in an outpatient rehabilitation research center and evaluated Boston area primary care patients aged 65 to 96 (N=364) with self-reported difficulty or task-modification climbing a flight of stairs or walking ½ of a mile. The outcome measure was standing balance as measured by the FICSIT-4 balance assessment. Other measures included: self-efficacy, pain, depression, executive function, vision, sensory loss, reaction time, kyphosis, leg range of motion, trunk extensor muscle endurance, leg strength and leg velocity at peak power. Participants were 67% female, had an average age of 76.5 (± 7.0) years, an average of 4.1 (± 2.0) chronic conditions, and an average FICSIT-4 score of 6.7 (± 2.2) out of 9. After adjusting for age and gender, attributes significantly associated with balance performance were falls self-efficacy, trunk extensor muscle endurance, sensory loss, and leg velocity at peak power. FICSIT-4 balance performance is associated with a number of behavioral and physiologic attributes, many of which are amenable to rehabilitative treatment. Our findings support a consideration of balance as multidimensional activity as proposed by the current International Classification of Functioning, Disability, and Health (ICF) model.
Keywords: Balance, Body Systems, Activity, FICSIT, Boston Rehabilitative Study of the Elderly
1.1 INTRODUCTION
Balance is a skill that, when deficient, leads to falls and fall-related injuries such as hip fractures.1, 2 Clinical tests of balance are commonly used to screen older adults who are at risk for falls and hip fractures.3, 4 Conceptually, balance tests have been developed as a way to screen for the presence of balance impairments.5, 6 However, within the most current conceptual paradigms of physical function, the World Health Organization’s International Classification of Functioning, Disability, and Health (ICF), impairments are deficiencies in the function of a single body system or the anatomical parts of a single body structure. Activity is a higher order concept, and represents the execution of a task relating to the interaction of multiple body systems.7 Operationally, most clinical balance tests involve the performance of multiple tasks and thus conceptually, the maintenance of balance is better characterized as an activity.8, 9
A number of studies have sought to determine which impairments are most associated with clinical assessments of activities, including gait speed and general physical performance.6, 10–12 Reviews, such as Pasma et al., have called for further research to be done to determine attributes underlying balance while standing.13 There is ample evidence within separate investigations identifying body systems such as mental function [ie. self-efficacy]14, 15, sensory function [ie. peripheral sensation]16–18, and neuromuscular function [ie. strength19 and velocity]10, 20 as being relevant to balance performance. In our review of this literature, however, we identified inconsistencies in the categorization of static balance as performance of an activity, as it is categorized within the current ICF paradigm.21–23 Furthermore, no study has evaluated the relative contribution of these different attributes (mental function, behavioral factors, sensory function, and neuromuscular function).
Most static clinical balance tests such as the FICSIT-4 or the balance component of the Short Physical Performance Battery (SPPB) are quite suitable for clinical settings because they can be completed easily and quickly (i.e., <5 minutes). The FICSIT-4 was developed as part of the The Frailty and Injuries: Cooperative Studies of Intervention Techniques (FICSIT trials).24 It includes all three stances from the SPPB balance component as well as the more complex unipedal stance to evaluate individuals across a broader range of balance performance. While the FICSIT-4 might be considered a potentially useful clinical tool, it is not known which attributes determine performance. From a rehabilitative perspective, it would be important to understand the body system categories underlying performance in order to know which attributes should be targeted in rehabilitative care.
We hypothesized not only that attributes from multiple body systems identified by the ICF framework (mental functions, sensory, and neuromuscular) combine to determine balance performance as measured by the FICSIT-4, but that consequently the ICF framework has correctly categorized balance as an activity. Related to this goal, we sought to identify the attributes underlying balance performance in order to understand potential body systems to be targeted through rehabilitative care.
2.1 METHODS
We performed a secondary cross-sectional analysis of baseline assessment information from the Boston Rehabilitative Impairment Study of the Elderly (Boston RISE), a cohort study of older primary care patients (N=430) living in the greater Boston area. All methods and procedures were approved by the Spaulding Rehabilitation Hospital Institutional Review Board. A detailed description of the Boston RISE protocol is published elsewhere.25
Participants were adults aged ≥ 65 years receiving primary care at one of two large academic medical centers in Boston—Massachusetts General Hospital (MGH) or Brigham and Women’s Hospital (BWH). Initial eligibility was defined as self-reported difficulty or task modification either climbing a flight of stairs or walking a ½ mile. Exclusion criteria included inability to communicate in English, presence of a terminal disease, major surgery or myocardial infarction in the past 6 months, planned major surgery, or major medical problems interfering with safe and successful testing. A final eligibility screen excluded individuals with moderate to severe cognitive impairment as measured by a Mini-Mental State Exam (MMSE) score of less than 18, or presence of severe mobility limitations as measured by a score of less than 4 on the Short Physical Performance Battery (SPPB).25 The SPPB is a physical performance measure that is predictive of disability and mortality and has three components: standing balance, gait speed, and chair stands. Each section has a maximum score of 4 points, summing to the maximum score of 12, which represents lower body mobility performance. We included only those Boston RISE participants (N=364) that completed all of the measures utilized in this ancillary study at baseline.
2.2 Outcome measure
The FICSIT-4 balance test involves asking participants to maintain four progressively difficult foot positions for ten seconds each. For the first position (side by side stance), the participant stands with their feet together, side-by-side and touching. The second position (semi-tandem stance) involves the participant standing with the side of one heel touching the big toe of the other foot. For this position, the participant was able to pick whichever foot was more comfortable to place in front. In the third balance position (tandem stance), participants were asked to stand with one foot directly in front of the other, the heel of one foot touching the tip of the big toe of the other foot. Again, participants were allowed to choose which foot was placed in front. For the final position (the unipedal stance), participants were asked to stand on one foot for 10 seconds. 24 Within the unipedal stance position, if the participant did not successfully maintain the position for even 1 second, no further attempts were allowed. If the participant maintained the position for 2–9 seconds, a second attempt was allotted. If the second attempt also failed, the test was considered terminated. They could choose to stand on whichever foot they found most comfortable. Participants were instructed to avoid resting the non-weight bearing foot on the weight bearing leg.
For scoring the side by side, semi-tandem, and tandem positions, participants received 0 points for holding the position <1 second, 1 point for holding from 1 second to <10 seconds, and 2 points for completing 10 seconds in the position. For the unipedal stance, participants received 0 points for holding the position for <1 second, 1 point for holding from 1 second to <5 seconds, 2 points for holding from 5 second to <10 seconds, and 3 points for completing 10 seconds in the position. The sum of the 4 components yields the maximum FICSIT-4 score was 9 points.
2.3 Mental Functions
Executive function was assessed using the Trail Making Test (TMT).26 The TMT consists of two components: a task that is highly dependent on processing speed (Trails A), and a task that is more dependent on executive function, but is also influenced by processing speed (Trails B). To best isolate the unique contribution of executive function, we used the standard approach of calculating the difference between the Trails B and Trails A results.27, 28 Self-efficacy, or balance confidence, was measured using the Activities-Specific Balance Confidence Scale.29 Symptoms of depression were defined using a clinical cut-point of 5 or greater on the Patient Health Questionnaire (PHQ-9).30
2.4 Sensory Body Systems
Pain severity was assessed using the Pain Severity Subscale of the Brief Pain Inventory. This measure averages four pain ratings: the least pain, worst pain, and average pain in the previous week, and current pain.31 Sensory loss was assessed using the Semmes Weinstein Monofilament Test, using 4.17g and 5.07g monofilaments.32 For each foot, the assessor began by applying the 4.17g monofilament to the dorsum of the great toe four times at randomly varying intervals. Participants were instructed to say ―yes‖ each time they felt the monofilament. If they reported they felt at least three accurately, the test was completed for that side. Otherwise, the procedure was repeated using the 5.07g monofilament. Sensory loss was defined as feeling less than three touches with the 4.17 monofilament and less than three touches with the 5.07 monofilament on one or both sides. Visual acuity was measured using a Snellen chart with a vision score of 20/50 or worse being defined as vision impairment.33
2.5 Neuromuscular Body Systems
Reaction time was measured using methods and a device developed by Lord et al. 34 Participants were asked to click a computer mouse as fast as possible in response to illumination of a red light connected to the device. The participant was given five practice trials and then the next ten response times were collected. An electronic timer measured the time it took for the participant to click the mouse in response to the light stimulus. Kyphosis was measured using a flexicurve ruler. An index was derived by dividing the measured curve length in centimeters by the curve height in centimeters as previously described. 35 Rapid leg coordination was assessed in each leg using the heel-to-shin test. Participants were seated in a chair with their arms crossed over their chest. They were instructed to lift one foot off of the floor, tap the lateral aspect of the opposite leg four inches below the patella using their heel, and then return the foot to the floor. They were asked to repeat this movement as quickly as possible ten times in a row. Rapid leg coordination was reported as the fastest time to complete the ten repetitions regardless of side.
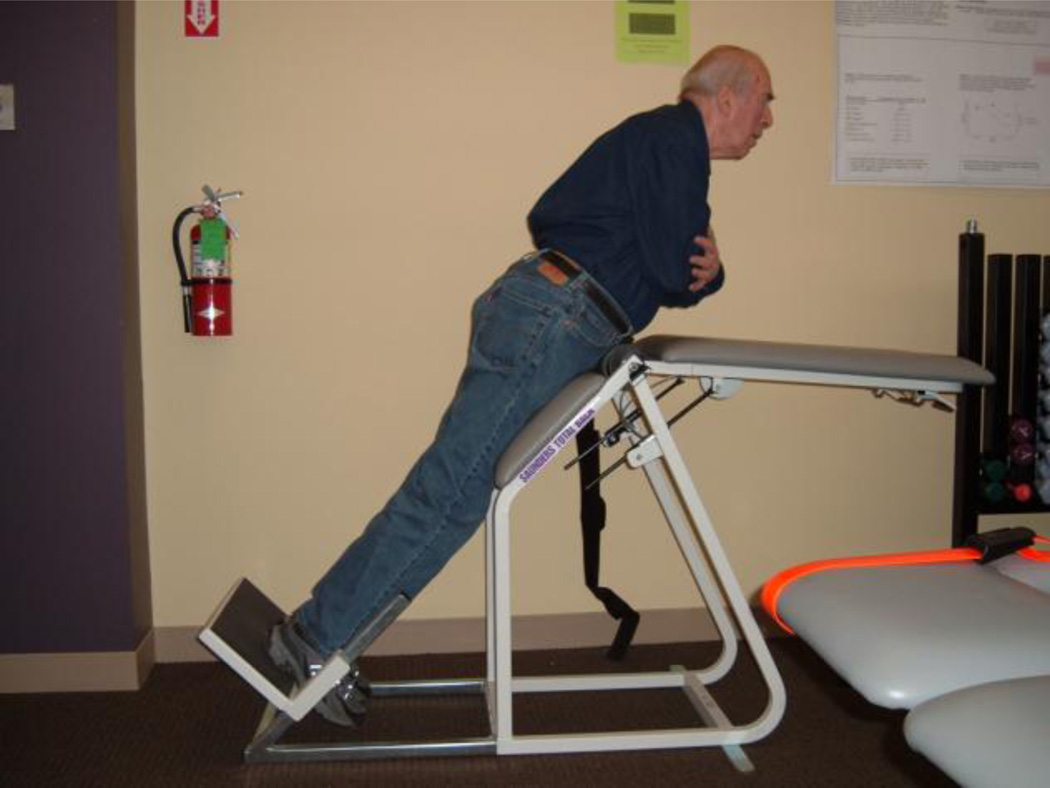
Trunk flexor muscle endurance was measured with the participant lying supine on an adjustable table positioned at 45 degrees from vertical with a foam pad placed between the participant’s back and the table. The participant’s legs were bent so that their feet were flat on the table and they were fixed into position. The participant was asked to cross their arms across their chest and to lift their upper body off of the table just enough so that the foam pad could be removed. They were asked to maintain that 45 degree position as long as possible with their entire back off of the table. The test was terminated when the participant could no longer maintain the unsupported position and their back touched the table. Time was recorded in seconds. Trunk extensor muscle endurance was measured using the commercially available exam table seen in Figure 1. The table was positioned at a 45-degree angle from the horizontal plane. The table is hinged so that the upper portion of the table could be fixed at a horizontal position. The table also had an adjustable footplate, which was adjusted to position the participant’s hips over the hinged portion of the table. The participant was in a prone position with their feet on the footplate, their thighs resting on the lower body segment of the table, and their elbows supporting their upper body on the horizontal upper body segment. The participant was then instructed to cross their arms over their chest and raise their upper body off of the table and into alignment with their lower body (at a 45 degree angle from the horizontal). The participant was asked to maintain this position for as long as possible with arms across their chest. The test was terminated when the participant was no longer able to maintain the unsupported position. Time was recorded in seconds.
Figure 1.
The commercially available exam table used for the trunk extension endurance assessment. A participant is seen appropriately performing the task.
Knee flexion and extension were measured with the participant lying flat on a table. For knee extension, a goniometer was centered over the lateral femoral epicondyle with arms directed towards the sagittal midpoint of the thigh and foreleg. The participant was asked to fully extend their leg, pressing the back of their knee against the table. For knee flexion, the goniometer placement was identical and the participant was asked to actively maximally flex their knee. Hip position was kept at 90 degrees during flexion. Ankle range of motion loss was defined as a dorsiflexion less than 90 degrees or plantarflexion less than 110 degrees on either foot.
Leg strength and leg velocity at peak power were measured using the Keiser A420 Pneumatic Leg Press (Keiser Co.; Fresno, CA) as previously described.10 The one repetition maximum (1RM) and maximum leg power were determined using the protocol described by Cuoco et al.36 Leg velocity was derived from maximum leg power. The Keiser A420 graphical output was inspected and the peak values for strength and power were determined for each leg. Leg velocity was derived by dividing the peak power by the force recorded at peak power (Velocity = power/force). Leg strength asymmetry was calculated as the quotient of the 1RM on the stronger side over the 1RM on the weaker side.
2.6 Adjustment Variables
Baseline adjustment variables considered for inclusion in our models were age, sex, and body mass index (BMI). Weight status was defined by standard cut points: overweight (BMI ≥25kg/m2 to <30kg/m2) and obese (BMI ≥30kg/m2).37 The number of active medical conditions were collected using the comorbidity questionnaire developed by Sangha et al.38
2.7 Statistical Analysis
All statistical analyses were performed with SAS software, version 9.2 (SAS Institute, Inc., Cary, NC). Descriptive statistics were calculated, including frequencies and percentages for categorical variables and mean and standard deviations for continuous variables. Descriptive characteristics were also calculated for the 66 Boston RISE participants that were excluded from the analysis to identify if they differed meaningfully from the participants included in the analyses. Leg strength was normalized by body weight in kilograms. Correlations were evaluated between all physiologic attributes and adjustment variables to evaluate for co-linearity (r >0.40). Multivariable linear regressions were constructed using a manual, backwards elimination process as advocated by Sun et al.39 Briefly, an initial model was run with all the included variables. The attribute with the largest p-value was removed and the remaining attributes were run in a new model. This process was repeated until all remaining variables were statistically significant. The final model was evaluated for inappropriately influential observations using the DFFITS and DFBETA influential statistical calculations in SAS.40 Individuals with potentially influential values were identified using a cut-off of 2 as suggested by Belsey et al.41 If their exclusion did not materially alter the model, they were included within the final model.
3.1 RESULTS
Baseline characteristics, and the mental, sensory, and neuromuscular body systems measured are reported in Table 1. Associations between individual attributes and the FICSIT-4 balance score are reported in Table 2. All attributes demonstrated significant associations with the FICSIT-4 with the exception of sex, weight status, PHQ-9, kyphosis, and leg strength asymmetry. Variables that described relatively large amounts of variance (>10%) in FICSIT-4 balance performance were age (R2=0.14), self-efficacy (R2=0.13), and trunk extensor muscle endurance (R2=0.18).
Table 1.
Baseline characteristics of the BostonRISE cohort (N=364)
| Category | Characteristic | Mean (SD) or Frequency (%) |
Range | |
|---|---|---|---|---|
| Demographics | Age (y) | 76.5 ± 7.0 | 65–94 | |
| Women | 246 (68%) | n/a | ||
| BMI (kg/m2) | 29.2 ± 5.8 | 18.4–55.7 | ||
| Race | ||||
| White | 297 (82%) | n/a | ||
| Black | 43 (12%) | n/a | ||
| Other | 24 (6%) | n/a | ||
| Attended college | 196 (54%) | n/a | ||
| Comorbidity | 4.0 ± 2.0 | 0–11 | ||
| Mental Functions | PHQ-9(≥5) | 18 (5%) | n/a | |
| ABC (0–100) | 76.9 ± 17 | 8–100 | ||
| <50 | 26 (7%) | n/a | ||
| 50–80 | 152 (42%) | n/a | ||
| >80 | 186 (51%) | n/a | ||
| Trails B time - Trails A time (s) | 74.3 ± 48.7 | −2.9–266.7 | ||
| Sensory Functions | BPI (1–10) | 2.5 ± 1.8 | 0–9.75 | |
| Sensory Loss | 109 (30%) | n/a | ||
| Visual Impairment | 18 (5%) | n/a | ||
| Neuromuscular | FICSIT-4 Balance (0–9) | 6.9 ± 2.1 | 1–9 | |
| Functions | Reaction Time (milliseconds) | 248.4 ± 51.2 | 172.1–648.4.4 | |
| Kyphosis Index (cm) | 10.4 ± 3.1 | 3.3–22.4 | ||
| Rapid Leg Coordination (s) | 11.1 ± 3.0 | 5.5–28.3 | ||
| Trunk Extension Endurance (s) | 95.4 ± 58.6 | 0–150 | ||
| Knee Flexion (degrees) | 125.5 ± 12.7 | 69.0–149.0 | ||
| Knee Extension (degrees) | 7.6 ± 7.8 | −5.0 – 25.0 | ||
| Loss of Ankle ROM | 99 (27%) | n/a | ||
| Leg Strength (N/kg) | 9.5 ± 2.5 | 3.1–20.3 | ||
| Leg Strength Asymmetry (ratio of stronger side to weaker side) | 1.2 ± 0.3 | 1–4.3 | ||
| Leg Velocity at Peak Power (m/s) | 1.0 ± 0.3 | 0.2–1.9 | ||
BPI = Brief Pain Inventory (Severity); PHQ-9 = Patient Health Questionnaire; ABC = Activities-Specific Balance Confidence Scale; ROM = Range of Motion
Table 2.
Bivariate linear regression models of associations between patient characteristics and balance performance (N=364)
| Category | Parameter | Estimate (SE) |
R2 | P-value |
|---|---|---|---|---|
| Demographics | Age | −0.114 (0.01) | 0.14 | <0.001 |
| Sex | −0.465 (0.24) | 0.01 | 0.049 | |
| Overweight | −0.012 (0.23) | 0.00 | 0.959 | |
| Obese | −0.027 (0.23) | 0.00 | 0.909 | |
| Attended College | 0.369 (0.22) | 0.01 | 0.098 | |
| Attributes | Comorbidity | −0.156 (0.06) | 0.02 | 0.005 |
| BPI (Severity) | −0.094 (0.06) | 0.01 | 0.123 | |
| PHQ-9 | −0.025 (0.51) | 0.00 | 0.962 | |
| ABC | 0.046 (0.01) | 0.13 | <0.001 | |
| Trails B – Trails A | −0.007 (0.00) | 0.03 | 0.005 | |
| Reaction Time | −0.007 (0.00) | 0.03 | <0.001 | |
| Kyphosis | −0.072 (0.04) | 0.01 | 0.044 | |
| Rapid Leg Coordination | −0.980 (0.04) | 0.03 | 0.003 | |
| Trunk Extension Endurance | 0.015 (0.00) | 0.18 | <0.001 | |
| Sensory Loss | −1.10 (0.24) | 0.06 | <0.001 | |
| Knee Flexion ROM | 0.025 (0.01) | 0.02 | 0.004 | |
| Knee Extension ROM | 0.013 (0.02) | 0.00 | 0.478 | |
| Loss of Ankle ROM | −1.105 (0.24) | 0.05 | <0.001 | |
| Leg Strength | 0.218 (0.04) | 0.07 | <0.001 | |
| Leg Strength Asymmetry | −0.420 (0.38) | 0.00 | 0.264 | |
| Leg Velocity at Peak Power | 1.926 (0.42) | 0.05 | <0.001 | |
| Visual Impairment | −1.489 (0.51) | 0.02 | 0.003 | |
BPI = Brief Pain Inventory (Severity); PHQ-9 = Patient Health Questionnaire; ABC = Activities-Specific Balance Confidence Scale; ROM = Range of Motion
Table 3 shows the factors that were associated with the balance score in the multivariable adjustment. This final set of characteristics and attributes described 37% of the variance in balance performance. After adjustment (adjustment variables-age and sex), falls self-efficacy, trunk extensor muscle endurance, sensory loss, and leg velocity were found to be significantly associated with static balance performance as measured by the FICSIT-4 balance scale.
Table 3.
Final Multivariate Linear Regression Model Predicting Static Balance as measured by the FICSIT-4 Balance Test (N=364; R2=0.3659)
| Predictor | Parameter Estimate (SE) |
p-value | Standardized Estimate |
|---|---|---|---|
| Age | −0.070 (0.01) | <0.001 | −0.23 |
| Sex | −0.764 (0.22) | <0.001 | −0.17 |
| ABC | 0.025 (0.01) | <0.001 | 0.20 |
| Trunk Extension Endurance | 0.011 (0.01) | <0.001 | 0.30 |
| Sensory Loss | −0.587 (0.20) | 0.005 | −0.13 |
| Leg Velocity | 1.051 (0.41) | 0.01 | 0.13 |
Final model was determined using a manual, backwards elimination.39; ABC = Activities-Specific Balance Confidence Scale.
A comparison was conducted between those excluded and those included in the final model. This analysis revealed that those excluded had significantly lower body mass indexes, a higher number of chronic conditions, and lower self-efficacy scores.
4.1 DISCUSSION
The major finding of our study is that multiple ICF body system attributes including mental (self-efficacy), sensory, and neuromuscular (trunk extensor muscle endurance and leg velocity) were independently associated with FICSIT-4 balance performance. Our study’s aim was not only to identify the body systems that were associated with balance performance, but also to confirm that this clinical test of balance (FICSIT-4) is best characterized as a measure of activity as opposed to a test representing a single body system according to the ICF disablement framework.5, 7 The current findings support the hypothesis that attributes from multiple body systems determine balance performance as measured by the FICSIT-4, and highlight the point that poor performance in maintaining a standing position is viewed correctly within the ICF framework as an activity restriction and not as a body system impairment.
The observed associations of body systems within this study are well justified mechanistically. Sensory function, measured as peripheral sensation and proprioceptive feedback from the lower extremities, contribute to one’s ability to maintain balance.16–18 A deficit in either of these feedback mechanisms could conceivably contribute to difficulty with static balance. The trunk muscles are used to maintain an upright position necessary for sitting, standing, ambulating, and performing activities of daily living. The association of trunk extensor muscle endurance with balance performance has been demonstrated within other studies. For example, in a report evaluating the Berg Balance Scale, changes in trunk extensor muscle endurance in response to rehabilitative exercises were predictive of clinically meaningful improvements in Berg Balance Performance.19 Additionally, the ability to generate a force with sufficient velocity to make rapid corrections in body position and prevent a fall can clinically account for the association between leg velocity and balance while standing.10, 20 Our findings using the FICSIT-4, which includes the three balance tasks from the SPPB, are consistent with previous findings supporting the association between leg velocity and SPPB performance.6, 42 Leg velocity and trunk muscle extensor endurance are categorized within the ICF as neuromuscular functions. We evaluated the mental function of self-efficacy with regard to balance and mobility tasks and quantified it using the Activities-Specific Balance Confidence Scale. Its association with balance performance has been previously reported in studies evaluating falls and the Timed Up and Go as outcomes.14, 15, 29
While these attributes have all been previously associated with other physical performance tests, to our knowledge, this is the first study to extensively evaluate a clinical standing balance test within the context of the ICF model. Other studies have examined attributes associated with balance and mobility tasks, but this investigation has a more extensive evaluation of clinically relevant attributes associated with performance and is the first to examine a broad range of factors in relation to the FICSIT-4 performance. Also, it is important to acknowledge that the FICSIT-4 balance test is very similar technically to the balance components of the Short Physical Performance Battery and the balance component of the Health ABC performance battery.43, 44 Thus, it is reasonable to consider that our findings will have relevance to the performance of the balance components of these tests as well.
The FICSIT-4 offers certain clinical advantages. It is relatively quick to perform (< 5 minutes) and requires only a small space, making it a potentially useful screening tool for balance limitations within primary care settings. Other clinical balance tests such as the Berg Balance Scale45, Performance Oriented Mobility Assessment46, or Dynamic Gait Index47 are advantageous in their ability to provide broad assessment, but they are less feasible clinically since they take much longer to perform. Our findings can also direct rehabilitative clinicians towards body systems that underlie balance performance (e.g., self-efficacy, trunk extensor muscle endurance, leg strength) and should be targeted within care. Although our investigation has focused on balance performance, it has not focused on falls. The relevance of FICSIT-4 performance and its underlying attributes to falls among primary care patients has yet to be comprehensively evaluated and thus our findings should be viewed in the appropriate context. Further research is needed to demonstrate whether a rehabilitation program targeting deficits in the balance-related attributes we have identified will lead to better balance and reduction in fall risk.
There are limitations to our study. We performed a secondary cross-sectional analysis of baseline data, so this study lacks a temporal basis needed for any causal relationship. Only a single static balance outcome measure was used and there may be other measures of balance that, if evaluated, would elicit different attributes. Also, there may be other factors that contribute to poor balance while standing that were not assessed in this investigation, such as vestibular impairments. The Boston RISE study included, the monofilament test, a measure of light touch, as a measure of sensory loss. We recognize that other sensory modalities not included within Boston RISE, such as ankle proprioception may be relevant to static balance.
However, this study has a number of strengths. Our baseline analysis of an extensive impairment-based assessment evaluated the association between a wide variety of body systems. The Boston RISE study design is a cohort study of primary care patients and thus provides clinical relevance to patients who may be referred for rehabilitative care.
5.1 CONCLUSION
In conclusion, this study was conceived using the ICF framework and identified multiple body systems relevant to static balance performance as measured by the FICSIT-4. Our findings suggest that self-efficacy, sensory loss, trunk extensor muscle endurance, and leg velocity are attributes that are relevant to FICSIT-4 performance. These results support the ICF categorization of balance problems as an activity limitation as opposed to a single body system impairment.
Highlights.
Balance performance is associated with multiple body systems.
Balance is a multidimensional activity as proposed by the current ICF model.
Balance associated body systems are addressed in various health care settings.
ACKNOWLEDGMENTS
Medical Student Training in Aging Research 1T35AG038027-02; American Federation for Aging Research (T35AG038027-01); NIH grants R01 AG032052 and K24 HD070966 (PI-Jonathan F. Bean): and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resource
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julia C. Thomas, Email: juthomas@wakehealth.edu.
Charles Odonkor, Email: charles.odonkor@yale.edu.
Laura Griffith, Email: lagriffith@partners.org.
Nicole Holt, Email: nholt1@partners.org.
Sanja Percac-Lima, Email: spercaclima@partners.org.
Suzanne Leveille, Email: suzanne.leveille@umb.edu.
Pensheng Ni, Email: psni@bu.edu.
Nancy K. Latham, Email: nlatham@bu.edu.
Alan M. Jette, Email: ajette@bu.edu.
Jonathan F. Bean, Email: jfbean@partners.org.
REFERENCES
- 1.Berg KO, Wooddauphinee SL, Williams JI. MEASURING BALANCE IN THE ELDERLY - VALIDATION OF AN INSTRUMENT. Canadian Journal of Public Health-Revue Canadienne De Sante Publique. 1992;83 [PubMed] [Google Scholar]
- 2.Speechley M, Tinetti M. FALLS AND INJURIES IN FRAIL AND VIGOROUS COMMUNITY ELDERLY PERSONS. Journal of the American Geriatrics Society. 1991;39 doi: 10.1111/j.1532-5415.1991.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 3.VanSwearingen JM, Brach JS. Making geriatric assessment work: Selecting useful measures. Physical Therapy. 2001;81 [PubMed] [Google Scholar]
- 4.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age and Ageing. 2006;35 doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 5.Jette AM. Disablement outcomes in geriatric rehabilitation. Medical Care. 1997;35:JS28–JS37. doi: 10.1097/00005650-199706001-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bean JF, Kiely DK, LaRose S, Leveille SG. Which Impairments Are Most Associated With High Mobility Performance in Older Adults? Implications for a Rehabilitation Prescription. Archives of Physical Medicine and Rehabilitation. 2008;89:2278–2284. doi: 10.1016/j.apmr.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Jette AM. Toward a common language for function, disability, and health. Physical Therapy. 2006;86 [PubMed] [Google Scholar]
- 8.Salter K, Jutai JW, Teasell R, Foley NC, Bitensky J, Bayley M. Issues for selection of outcome measures in stroke rehabilitation: ICF activity. Disability and Rehabilitation. 2005;27:315–340. doi: 10.1080/09638280400008545. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Whitney SL. Quantification of Static and Dynamic Balance While Maintaining and Changing Body Position. Topics in Geriatric Rehabilitation. 2012;28:17–26. [Google Scholar]
- 10.Mayson DJ, Kiely DK, LaRose SI, Bean JF. Leg Strength or Velocity of Movement. American Journal of Physical Medicine & Rehabilitation. 2008;87:969–976. doi: 10.1097/PHM.0b013e31818dfee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JB, Bremmer MA, Deeg DJH, Beekman ATF. Do Depressive Symptoms and Gait Speed Impairment Predict Each Other’s Incidence? A 16-Year Prospective Study in the Community. Journal of the American Geriatrics Society. 2012;60 doi: 10.1111/j.1532-5415.2012.04114.x. [DOI] [PubMed] [Google Scholar]
- 12.Rantanen T, Guralnik JM, Ferrucci L, et al. Coimpairments as predictors of severe walking disability in older women. Journal of the American Geriatrics Society. 2001;49 doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 13.Pasma JH, Engelhart D, Schouten AC, van der Kooij H, Maier AB, Meskers CG. Impaired standing balance: The clinical need for closing the loop. Neuroscience. 2014;267c:157–165. doi: 10.1016/j.neuroscience.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Myers AM, Powell LE, Maki BE, Holliday PJ, Brawley LR, Sherk W. Psychological indicators of balance confidence: Relationship to actual and perceived abilities. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 1996;51 doi: 10.1093/gerona/51a.1.m37. [DOI] [PubMed] [Google Scholar]
- 15.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and Evaluative Properties of the Activities-specific Balance Confidence (ABC) Scale. Journal of Gerontology. 1998;53A:M287–M294. doi: 10.1093/gerona/53a.4.m287. [DOI] [PubMed] [Google Scholar]
- 16.Meyer PF, Oddsson LIE, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Experimental Brain Research. 2004;156:505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- 17.Richardson JK, AshtonMiller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Archives of Physical Medicine and Rehabilitation. 1996;77:1152–1156. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 18.Strotmeyer ES, De Rekeneire N, Schwartz AV, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults - The Health, Aging, and Body Composition (Health ABC) Study. Diabetes Care. 2008;31:1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Increased Trunk Extension Endurance Is Associated With Meaningful Improvement in Balance Among Older Adults With Mobility Problems. Archives of Physical Medicine and Rehabilitation. 2011;92:1038–1043. doi: 10.1016/j.apmr.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bean JF, Kiely DK, LaRose SI, Goldstein R, Frontera WR, Leveille SG. Are Changes in Leg Power Responsible for Clinically Meaningful Improvements in Mobility in Older Adults? Journal of the American Geriatrics Society. 2010;58:2363–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai A, Goodman V, Kapadia N, Shay B, Szturm T. Relationship Between Dynamic Balance Measures and Functional Performance in Community-Dwelling Elderly People. Physical Therapy. 2010;90:748–760. doi: 10.2522/ptj.20090100. [DOI] [PubMed] [Google Scholar]
- 22.Pardasaney PK, Latham NK, Jette AM, et al. Sensitivity to Change and Responsiveness of Four Balance Measures for Community-Dwelling Older Adults. Physical Therapy. 2012;92:388–397. doi: 10.2522/ptj.20100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allet L, Kim H, Ashton-Miller J, De Mott T, Richardson JK. Frontal plane hip and ankle sensorimotor function, not age, predicts unipedal stance time. Muscle Nerve. 2012;45:578–585. doi: 10.1002/mus.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossiter-Fornoff JE, Wolf SL, Wolfson LI, Buchner DM, Group F. A Cross-sectional validation study of the FICSIT common database static balance measures. Frailty and Injuries: Cooperative Studies of Intervention Techniques. Journal of Gerontology. 1995;50A:M291–M297. doi: 10.1093/gerona/50a.6.m291. [DOI] [PubMed] [Google Scholar]
- 25.Holt NE, Percac-Lima S, Kurlinski LA, et al. The Boston Rehabilitative Impairment Study of the Elderly: A Description of Methods. Archives of Physical Medicine and Rehabilitation. 2013;94:347–355. doi: 10.1016/j.apmr.2012.08.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 7:e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39:222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry KA, Brach JS, Nebes RD, Studenski SA, VanSwearingen JM. Contributions of Cognitive Function to Straight- and Curved-Path Walking in Older Adults. Archives of Physical Medicine and Rehabilitation. 2012;93:802–807. doi: 10.1016/j.apmr.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powell LE, Myers AM. THE ACTIVITIES-SPECIFIC BALANCE CONFIDENCE (ABC) SCALE. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 1995;50:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:509–515. [Google Scholar]
- 31.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Annals Academy of Medicine Singapore. 1994;23 [PubMed] [Google Scholar]
- 32.Olaleye D, Perkins B, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathyn in the diabetes clinic. Diabetes Research and Clinical Practice. 2001;54:115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 33.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. Jama-Journal of the American Medical Association. 2006;295 doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 34.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 35.Milne JS, Williamson J. A longitudinal study of kyphosis in older people. Age Ageing. 1983;12:225–233. doi: 10.1093/ageing/12.3.225. [DOI] [PubMed] [Google Scholar]
- 36.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 37.LamonFava S, Wilson PWF, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women - The Framingham Offspring Study. Arteriosclerosis Thrombosis and Vascular Biology. 1996;16 doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 38.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A New Method to Assess Comorbidity for Clinical and Health Services Research. Arthritis & Rheumatism. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 39.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology. 1996;49:907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 40.SAS Institute Inc. SAS/STAT ® 9.2 User’s Guide. Second Edition. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- 41.A. BD KK, WR E. Regression diagnostics: Identifying influential data and sources of collinearity. New York: John Wiley & Sons; 1980. [Google Scholar]
- 42.Bean JF, Olveczky DD, Kiely DK, LaRose SI, Jette AM. Performance-Based Versus Patient-Reported Physical Function: What Are the Underlying Predictors. Physical Therapy. 2011;91:1804–1811. doi: 10.2522/ptj.20100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guralnik JM, Simonsick EM, Ferrucci L, et al. A SHORT PHYSICAL PERFORMANCE BATTERY ASSESSING LOWER-EXTREMITY FUNCTION - ASSOCIATION WITH SELF-REPORTED DISABILITY AND PREDICTION OF MORTALITY AND NURSING-HOME ADMISSION. Journals of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 44.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring Higher Level Physical Function in Well-Functioning Older Adults: Expanding Familiar Approaches in the Health ABC Study. Journal of Gerontology. 2001;56A:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 45.Berg K, Maki B, Williams JI, Holliday P, Wood-Dauphinee S. A comparison of clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med and Rehabil. 1992;73:1073–1083. [PubMed] [Google Scholar]
- 46.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 47.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 48.Son J, Ashton-Miller JA, Richardson JK. Frontal plane ankle proprioceptive thresholds and unipedal balance. Muscle Nerve. 2009;39:150–157. doi: 10.1002/mus.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]