Abstract
Background
Airway remodeling may explain lung function decline among asthmatic children. Extracellular matrix (ECM) deposition by human lung fibroblasts (HLFs) is implicated in airway remodeling. Airway epithelial cell (AEC) signaling may regulate HLF ECM expression.
Objectives
Determine whether AECs from asthmatic children differentially regulate HLF expression of ECM constituents.
Methods
Primary AECs were obtained from well-characterized atopic-asthmatic (N=10) and healthy children (N=10) intubated under anesthesia for an elective surgical procedure. AECs were differentiated at an air-liquid interface (ALI) for 3 weeks, then co-cultured with HLFs from a healthy child for 96 hours. Collagen I (COL1A1), collagen III (COL3A1), hyaluronan synthase 2 (HAS2), and fibronectin (FNDC) expression by HLFs and prostaglandin E2 synthase (PGE2S) expression by AECs was assessed by RT-PCR. TGFb1&2 concentrations in media were measured by ELISA.
Results
COL1A1 and COL3A1 expression by HLFs co-cultured with asthmatic AECs was greater than HLFs co-cultured with healthy AECs (2.2 fold, p<0.02; 10.8 fold, p<0.02). HAS2 expression by HLFs co-cultured with asthmatic AECs was 2.5-fold higher than by HLFs co-cultured with healthy AECs (p<0.002). FNDC expression by HLFs co-cultured with asthmatic AECs was significantly greater than by HLFs alone. TGFb2 activity was elevated in asthmatic AEC-HLF co-cultures (p<0.05) while PGES2 was down regulated in AEC-HLF co-cultures (2.2 fold, p<0.006).
Conclusions
HLFs co-cultured with asthmatic AECs showed differential expression of ECM constituents COL1A1 & COL3A1, and HAS2 compared to HLFs co-cultured with healthy AECs. These findings support a role for altered ECM production in asthmatic airway remodeling, possibly regulated by unbalanced AEC signaling.
Keywords: asthma, children, airway remodeling, epithelial cells, human lung fibroblasts, extracellular matrix, collagen I, collagen III, hyaluronic acid, fibronectin, TGFb2
INTRODUCTION
Childhood asthma is a significant global public health burden that affects the lives of millions of children worldwide, including an estimated 7.1 million American children.1, 2 In addition to the high cost of missed school attendance and health care utilization, poorly controlled asthma results in significant morbidity and even mortality.3 Furthermore, evidence from population based epidemiologic studies has shown that as a group lung function in asthmatic children declines between birth and school age. Indeed, the Tucson Children’s Respiratory Study has established that children who subsequently develop asthma demonstrate deficits in lung function by 6 years of age despite being born with normal lung function.4 Data from the Melbourne Asthma Study, the largest and longest running asthma cohort study to monitor lung function, confirmed that children with persistent asthma had decreased lung function, which did not significantly worsen after age 10 years, yet endured into adulthood and was not ultimately recovered.5 This pattern is not observed in children that were early transient wheezers or children that never wheezed.4 Unfortunately, significant phenotypic overlap among children that wheeze at an early age exists making both treatment and early diagnosis of persistent asthma challenging.6
Airway remodeling, with changes to underlying airway structure, is a possible explanation for epidemiologic data demonstrating declines in lung function among children with asthma. Pathologic examination of the airways of asthmatic children has revealed evidence of airway remodeling including goblet cell hyperplasia, matrix protein deposition in the basement membrane, angiogenesis, and both smooth muscle cell hypertrophy and hyperplasia.7, 8 Furthermore, thickening of the basement membrane is a common feature of both adult and pediatric asthmatic airway remodeling suggesting that ECM protein deposition in the epithelial basement membrane occurs in early childhood and persists into adulthood.7-10 Evidence gathered from the histologic study of airways from asthmatic patients demonstrates increased amounts of collagen subtypes, fibronectin, hyaluronan (HA), as well as several other ECM constituents.11, 12
While the components of the ECM have been thoroughly characterized, the active role of the ECM in airway remodeling and fibrosis is only beginning to be elucidated (reviewed by Wight and Potter-Perigo).13 One mechanism central to thickening of the basement membranes and promotion of airway fibrosis is the accumulation of fibrillar collagens by either increased production or decreased degradation.14 The primary source of the collagens found in the airways is thought to be derived from the resident fibroblasts, particularly those that have further differentiated into a myofibroblast phenotype, i.e. those who express alpha smooth muscle actin (α-SMA).15, 16 Further evidence would suggest that collagen production is augmented by fibroblast to myofibroblast transition and that this process is highly dependent on TGFb activity17 and modulated by HA. 19, 20 Separate studies have demonstrated that collagen production is elevated in asthmatic airways and may also be related to inherent increases in TGFb activity.18, 19
In recent years, there has been a growing appreciation for the role of crosstalk between mesenchymal cells, such as fibroblasts, and the airway epithelial cells (AEC) in the pathogenesis of asthma. Multiple lines of evidence now point to the AEC as playing an active role in the orchestration of inflammatory responses, wound repair, and host immunity.20-22 Furthermore, data supporting the concept that dysfunctional regulation of these responses may play a role in the pathogenesis of asthma is accumulating. 22 Evidence of this dysfunctional regulation has been observed in both in vivo animal model systems as well as bronchial biopsy specimens from humans.23-25
In order to further investigate the role of cellular signaling from AECs on the production of ECM, we employed a co-culture model using primary AECs from both healthy and asthmatic children and human lung fibroblasts (HLF) derived from a single healthy donor. We hypothesized that HLFs co-cultured with asthmatic AECs would exhibit greater expression of ECM components, specifically type I and type III collagen, HA, and fibronectin compared to those co-cultured with healthy AECs. Furthermore, we hypothesized that these changes are associated with increased TGFb signaling or conversely decreased inhibitory signaling such as prostaglandin E2 (PGE2) activity. The latter could in turn be a mechanism to explain the predisposition for ECM deposition in the subepithelium observed in asthmatic airways.
METHODS
Subjects
Atopic asthmatic and healthy non-atopic non-asthmatic children ages 6-18 years who were undergoing an elective surgical procedure requiring endotracheal intubation and general anesthesia were recruited for this study. A detailed medical history was obtained at enrollment to ensure that participants met the following inclusion and exclusion criteria. Children with asthma had at least a 1 year history of physician-diagnosed asthma, physician documented wheezing in the 12 months prior to study enrollment, used a short-acting beta-agonist (SABA, i.e. albuterol) ≥ twice a month or were taking a daily inhaled corticosteroid or leukotriene receptor antagonist, and were born at ≥ 36 weeks gestation. Healthy subjects were born at ≥ 36 weeks gestation, had no history of asthma, reactive airway disease, chronic daily cough, or physician-diagnosed obstructive lung disease, and no history of prior treatment with a systemic or inhaled corticosteroid, SABA, or oxygen. Children with asthma had one or more of the following atopic features: history of positive skin prick test, positive radioallergosorbent testing (RAST) for a common aeroallergen (discussed below), elevated serum IgE (>100 IU/mL), history of physician-diagnosed allergic rhinitis, history of physician-diagnosed atopic dermatitis. Healthy subjects lacked a history of any of the above atopic features and were excluded if they had any other atopic comorbidity.
From each subject, a blood sample was drawn and used to measure total serum IgE and RAST allergen-specific IgE to dust mites (D. farinae and D. pteronyssinus), cat epithelium, dog epithelium, alternaria tenuis, aspergillus fumigatus, and timothy grass. The fraction of exhaled nitric oxide (FENO) was measured according to American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines 26 using a NIOX MINO nitric oxide analyzer (Aerocrine®, Sweden). This device measures eNO precisely only above a cut-off of 5 ppb and can thus only be used for clinical purposes. Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and forced expiratory flow between 25% and 75% of FVC (FEF25-75) were measured according to ATS guidelines using a VMAX® series 2130 spirometer (VIASYS Healthcare, Hong Kong). Spirometry was repeated 15 minutes following administration of 2 puffs of albuterol in children with asthma.
Written consent was obtained from parents of subjects and assent was obtained for children ≥ age 7 years. The Seattle Children’s Hospital Institutional Review Board approved this study.
Epithelial Cell Isolation, Proliferation, and Differentiation
Immediately after the endotracheal tube was secured three bronchial epithelial cell samples were obtained from subjects while under general anesthesia using 4mm Harrell® unsheathed bronchoscope cytology brushs (CONMED® Corporation, Utica, NY). As described by Lane et al.,27 the unprotected brush was inserted through an endotracheal tube, advanced until resistance was felt, and rubbed against the airway surface for 2 seconds. Cells were seeded onto T-25 cell culture flasks pre-coated with type I collagen and proliferated under submerged culture conditions. Using passage 2 cells, epithelial cells were differentiated at an air-liquid interface (ALI) using methods previously described by our lab (Please refer to the online supplement for further details).28
AEC-Fibroblast Co-Cultures
HLFs from a healthy child were obtained from a commercial vender (Lonza, Walkersville, MD) and used for each experiment (≤ passage 5). HLF cultures were established using a Fibroblast Cell Media Bulletkit™ (FGM) per Lonza recommendations. HLF were seeded at a density of ~2,500 cells/cm2 in 12-well Collagen I BD BioCoat™ plates (Becton Dickinson, Bedford, MA) and incubated for 7 days to achieve a confluent monolayer prior to initiation of AEC-HLF co-cultures. Media changes of FGM occurred at 48 hour intervals. At 48 hours prior to experimental Day 0, the HLF media was replaced with co-culture media (1:1 FGM and PneumaCult ALI Maintenance Media). ALI transwells were placed in co-culture with the HLF cells at experimental Day 0. The co-culture media in the basolateral chamber was changed every 24 hours and stored at −80°C for subsequent analysis. The media and cells were collected for studies 96 hours following initiation of co-culture experiments.
RNA Extraction and Real-Time PCR
Total RNA was isolated from HLF cells co-cultured with AEC’s grown at an ALI. Three wells from each experimental condition were harvested and pooled to isolate RNA using the RNAqueous kit for total RNA purification from Ambion®-Applied Biosystems (Austin, TX). RNA concentration and integrity were determined using the Agilent® 2100 Bioanalyzer system and Agilent® RNA 6000 Nano Chips (Agilent® Technologies, Foster City, CA). RNA samples (1μg) with a RNA integrity number (RIN) ≥8 were reverse transcribed with MMLV reverse transcriptase with a combination of random hexamers and oligo-dTs using the SuperScript® VILO cDNA Synthesis Kit (Life Technologies, Grand Island, NY). Samples were diluted up to a final volume of 100μl (10ng/μl). Semi-quantitative real-time qPCR was performed using validated TaqMan® probes (Life Technologies, Grand Island, NY) for human Collagen I (COL1A1), Collagen III (COL3A1), Fibronectin (FNDC), Prostaglandin E2 Synthase (PGE2S), Prostaglandin-Endoperoxide Synthase 2 (PTGS2/COX-2), and Hyaluronic Acid Synthase II (HAS2). Although there are 3 hyaluronan synthase (HAS) enzyme isoforms (HAS1, HAS2, and HAS3), HAS2 is the major isoform expressed by human lung fibroblasts, and has previously been reported to be differentially expressed by fibroblasts from asthmatic subjects.29 Assays were performed using the TaqMan® Fast Advanced Master Mix reagents and accompanying protocol and the Applied Biosystems StepOnePlus™ Real-Time PCR System with StepOne Software v2.2.2 (Life Technologies, Grand Island, NY).
Immunohistochemistry (IHC)
Sterilized 12mm round glass coverslips were coated with type I collagen and placed in the bottom of one of the replicate chambers of the 12 well plates prior to seeding the HLFs. Following 96hrs of co-culture, the coverslips were carefully removed and placed in a separate 12 well plate for IHC. Coverslips containing cells were then washed 3 times in room temperature PBS to remove residual media and were then fixed with 50:50 methanol and acetone at 20°C for 10 minutes. Coverslips were then washed with PBS and blocked with 10% FBS for 30 minutes. Following this, coverslips were washed again with PBS and then incubated with primary antibodies to collagen III (1:1000 ab6310, Abcam, Cambridge, MA) and HA (1:500 ab53842, Abcam) for 1 hour at room temperature. Additional PBS washes were performed prior to incubation with appropriate secondary antibodies for 1 hour at room temperature (1:1000 A11016, 1:1000 A21202, Life Technologies, Grand Island, NY). Three final PBS washes preceded mounting the coverslips using ProLong® Gold antifade reagent with Dapi (Life Technologies, Grand Island, NY). Images were acquired using an automated Leica DM6000B fluorescent microscope (Leica Microsystems, Wetzlar, Germany) in 7 × 7 grids at 400x and stitched together using LASAF software to provide higher resolution images of larger areas of tissue. Given that coverslips required coating with type I collagen to support HLF cultures, we did not perform IHC studies for collagen I due to concern for background staining.
ELISA Analyses
For each condition, sampled basolateral medium from triplicate transwells was pooled. Measurement of protein levels of activated TGFb1 and activated TGFb2 in sampled basolateral conditioned media was completed using Duoset® ELISA Development Kits (R&D Systems®, Minneapolis, MN) according to manufacturer recommendations. All measurements were completed in duplicate. Samples in which concentrations were below the assay detection level were assigned a value of one half the lower limit of detection for analysis.
Statistical Analysis
Protein levels are presented as means +/− standard deviation (SD) when all groups (e.g. healthy, asthmatic, HLF alone) were normally distributed, and as medians with interquartile range if one or more groups were not normally distributed. The Kolmogorov-Smirnov test was used to determine if data were normally distributed. One-way ANOVA, or the Kruskal-Wallis test if data in one or more groups was non-normally distributed, were used to compare the distributions of protein levels in co-culture media across the three groups. Post hoc comparisons between pairs of groups (e.g. between HLF and asthmatic co-cultures) were made using Dunn’s multiple comparisons test, with a significance level set at p<0.05. For age, gender, lung function parameters, FENO, and IgE levels, the paired t-test or the Wilcoxon signed rank test for non-normally distributed data, was used for comparisons between asthmatic and healthy subjects. Statistical analyses of clinical data and protein levels in ALI cultures were performed using Prism® 6.0 software (GraphPad Software Inc., San Diego, CA.). The relative expression of COL1A1, COL3A1, HAS2, FNDC, PGE2S, and PTGS2/COX-2 was standardized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a non-regulated reference gene. Analyses of real-time qPCR results were performed using GenEx version 5.0.1 (MultiD Analyses AB, Göteborg, Sweden) based on methods described by Pfaffl.30 Statistical significance was set at p<0.05.
RESULTS
Bronchial epithelial brushings were obtained from 10 healthy and 10 atopic asthmatic subjects and were used for the co-culture studies. Characteristic data from the subjects, summary of laboratory findings, and lung function testing obtained during the follow-up visit are summarized in Table 1. Subjects were of comparable age (healthy 10.3 +/− 3.6 vs. asthmatic 11.7 +/− 3.4; p=0.4); however, there were more females in our healthy group compared to the asthmatic group. The majority of our asthmatic subjects were using daily inhaled corticosteroids. Most of the asthmatic subjects had positive RAST testing to a specific aeroallergen (90%). Compared to healthy subjects the asthmatic subjects had a significantly greater serum IgE (916 IU/mL in asthmatic subjects vs 21.3 IU/mL in healthy subjects; p<0.0005). The FEV1/FVC ratio was significantly lower in asthmatic as compared to healthy subjects (89% +/− 4% vs 83% +/− 5%, respectively; p=0.04). Asthmatic and healthy subjects had comparable FENO levels (14.9 +/− 8.8 vs 9.3 +/− 4.6; p=0.2).
Table 1. Subject Characteristics.
| Healthy Controls | Asthmatics | P value | |
|---|---|---|---|
| N=10 | N=10 | ||
| Age yrs. (mean +/− SD) | 10.3 (3.6) | 11.7 (3.4) | 0.4 |
| Female Gender (%) | 60% | 20% | 0.07 |
| Current use of inhaled steroids (yes;%) |
9 (90%) | ||
| History of Eczema (yes;%) | 4 (40%) | ||
| History of Allergic Rhinitis (yes;%) | 9 (90%) | ||
| Positive RAST (yes;%) | 9 (90%) | ||
| IgE IU/mL (median +/− IQR) | 20.5 (12 - 27) | 242 (81 - 242) |
0.004 |
| FVC % predicted (mean +/− SD) | 101 (13.2) | 100.7 (11.5) | 0.9 |
| FEV1/FVC Ratio (mean +/− SD) | 0.89 (0.04) | 0.83 (0.05) | 0.04 |
| FEV1 % predicted (mean +/− SD) | 99.8 (14.5) | 96.6 (12.1) | 0.6 |
| FEF25-75 % predicted (mean +/− SD) | 99 (20.4) | 88 (18.5) | 0.2 |
| FENO ppb (mean +/− SD) | 9.3 (4.6) | 14.9 (8.8) | 0.2 |
RAST = radioallergosorbent testing; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEF25-75 = forced expiratory flow between 25% and 75% of expiration; SD = standard deviation; IQR = interquartile range
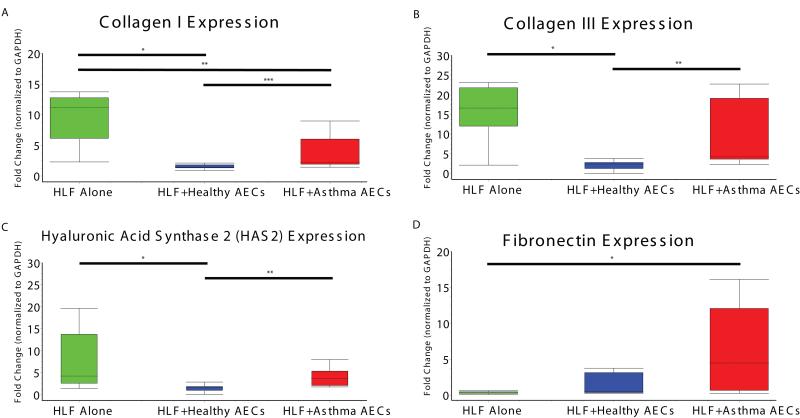
Expression of collagen I mRNA production as assessed by RT-PCR from HLF cells alone (non-co-cultured time control) was 8.3 fold greater compared to HLF co-cultured with healthy AECs (Figure 1A, 95% CI: 5.7 – 10.8; p=0.002). Collagen I expression by HLF cells alone was 6.6 fold greater than by HLFs co-cultured with asthmatic AECs (95% CI: 3.1 – 10.1; p=0.002). Direct comparison of collagen I expression by HLF co-cultured with healthy AECs and HLF co-cultured with asthmatic AECs revealed 2.2 fold greater gene expression by asthmatic co-cultures (95% CI: 0.4 – 4.3; p=0.02), consistent with a lesser degree of attenuation than by healthy co-cultures. We observed similar findings for collagen III, with expression by HLF cells alone 15.6 fold higher than by HLFs co-cultured with healthy AECs (Figure 1B; 95% CI: 7.5 – 23.8; p=0.002). Comparison of collagen III expression by HLFs co-cultured with asthmatic AECs to HLFs co-cultured with healthy AECs demonstrated 10.8 fold greater gene expression by asthmatic co-cultures (95% CI: 3.2 – 24.9; p=0.02). No significant difference in expression between HLF cultured alone and HLFs co-cultured with asthmatic AECs was observed. Staining of HLFs for collagen III protein accumulation in each group was performed in parallel to RNA expression studies. Representative images are shown in Figure 2. Additionally, we examined HAS2 expression from HLF time controls as well as HLFs co-cultured with both healthy and asthmatic AECs. Expression of HAS2 by HLFs alone was 6.3 fold greater than by HLFs co-cultured with healthy AECs (Figure 1C; 95% CI: 1.8-10.8; p=0.005). When compared to HLFs co-cultured with asthmatic AECs, a trend towards greater expression by HLFs alone was observed, but failed to reach statistical significance (3.8 fold, 95%CI: −0.6 – 8.2; p=0.07). Compared to HLFs co-cultured with healthy AECs, HLFs co-cultured with asthmatic AECs expressed 2.5 fold greater HAS2 mRNA (95% CI: 1.2 – 3.8; p=0.002). HA accumulation was assessed by IHC staining of HLFs treated in parallel with those that were used for RNA extraction. Representative images are compared in Figure 3. Expression of fibronectin by HLFs co-cultured with asthmatic AECs was 5-fold greater than HLF alone (95% CI: 2 – 16; p=0.05); however, no significant difference between HLFs co-cultured with asthmatic AECs and HLFs co-cultured with healthy AECs was detected.
Fig 1.
Panel A: Expression of Collagen I (COL1A1) mRNA by HLFs alone compared to co-culture with healthy AECs or asthmatic AECs. Panel B: Expression of Collagen III (COL3A1) mRNA by HLFs alone compared to co-culture with healthy AECs or asthmatic AECs. Panel C: Expression of Hyaluronic Acid Synthase 2 (HAS2) mRNA by HLFs alone compared to co-culture with healthy AECs or asthmatic AECs. Panel D: Expression of Fibronectin (FNDC) by HLFs alone compared to co-culture with healthy AECs or asthmatic AECs.
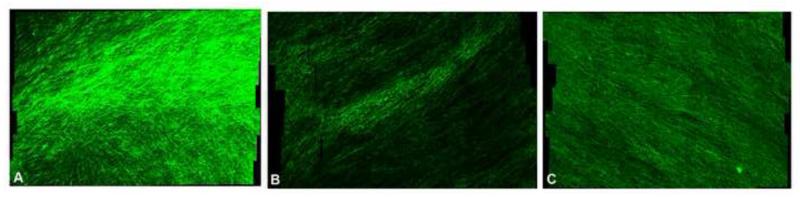
Fig 2.
Collagen III expression assessed by immunohistochemistry between HLFs alone (Panel A), HLFs+Healthy AECs (Panel B), and HLFs+Asthmatic AECs (Panel C).
Fig 3.
Hyaluronic Acid (HA) expression assessed by immunohistochemistry between HLFs alone (Panel A), HLFs+Healthy AECs (Panel B), and HLFs+Asthmatic AECs (Panel C).
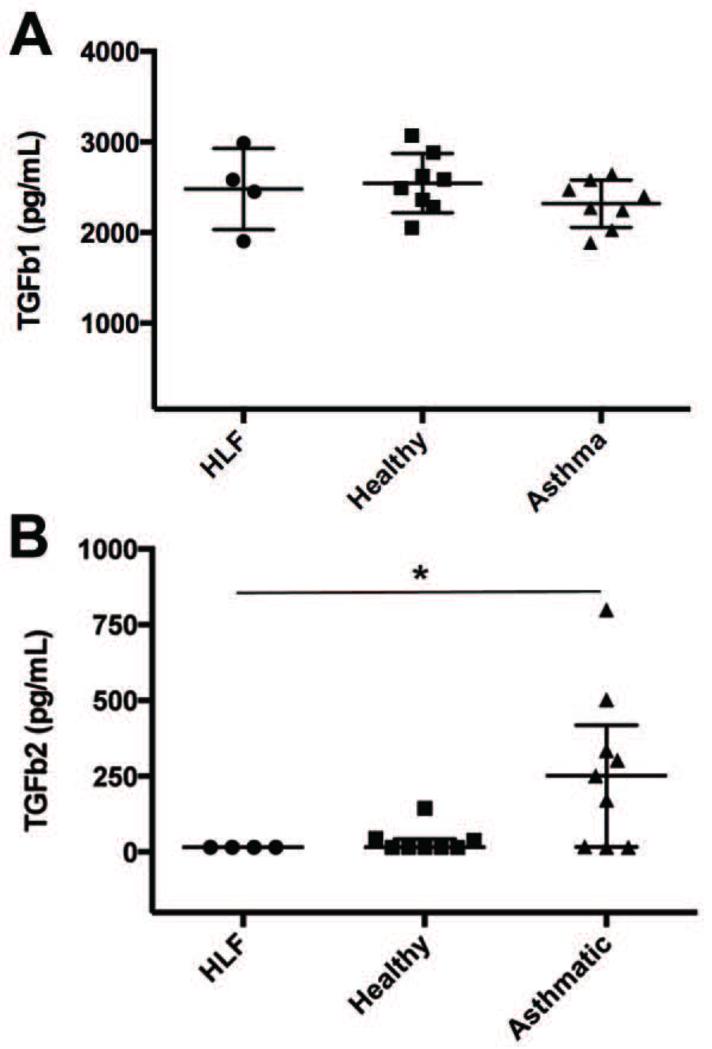
In order to examine potential mediators of the altered gene expression, we performed ELISAs for TGFb1 and TGFb2 activity in media samples obtained at the 96hr in co-culture time point. Mean TGFb1 concentrations for HLF alone (n=4), HLF co-cultured with healthy AECs (n=8), and HLF co-cultured with asthmatic AECs (n=8) are depicted in Figure 4A. No significant differences were observed among the groups. Similar values were also present at the 48hr time point (data not shown). In contrast, a separate ELISA performed for TGFb2 activity using a second aliquot of the same samples revealed significantly greater detectable levels of TGFb2 present in the culture media at the 96hr co-culture time point in asthmatic AEC co-cultures as compared to HLF alone and HLF co-cultured with healthy AECs (Figure 4B; p=0.01).
Fig 4.
Concentrations of TGFb1 measured in culture media collected at 96 hours from HLFs alone compared to healthy AEC+HLF co-cultures, and asthmatic AEC-HLF co-cultures (Panel A; lines at means with SD). Concentrations of TGFb2 measured in culture media collected at the 96 hour time point from HLF alone, HLF+healthy AEC, and HLF+asthmatic AEC co-cultures (Panel B; lines at medians with IQR).
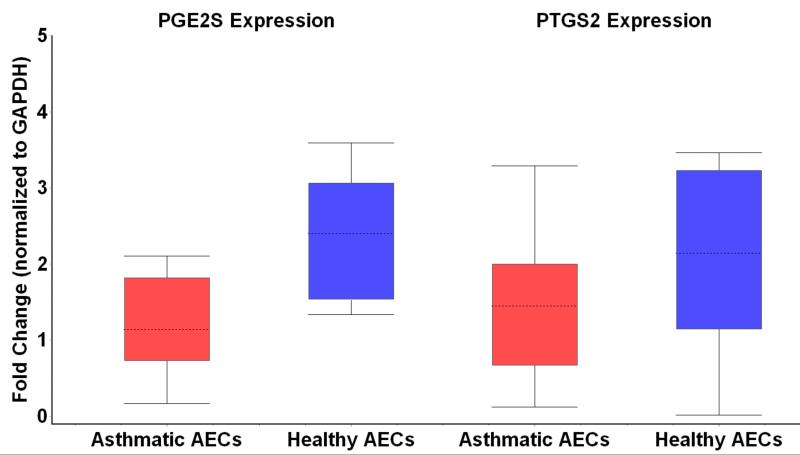
Given the interesting findings of suppressed ECM production in the healthy AEC-HLF co-cultures, we sought to examine the possibility that factors inhibiting fibroblast expression of ECM may be differentially expressed by asthmatic AECs. To study this, we examined mRNA expression of PGE2S as well as expression of PTGS2/COX-2 in asthmatic and healthy AECs that were co-cultured with HLFs (Figure 5). We found that PGE2S mRNA expression by healthy AECs was 2.2 fold greater than by asthmatic AECs (95% CI 1.3 – 3.8 fold; p=0.006). Conversely, PTGS2/COX-2 expression by healthy AECs was not significantly different than by asthmatic AECs (95% CI-1.8 – 11 fold; p=0.18).
Fig 5.
Prostaglandin E2 synthase (PGE2S) mRNA expression is depicted on the left side of the figure in either asthmatic airway epithelial cells (AEC) or healthy AEC. The right side of the figure demonstrates mRNA expression of prostaglandin-endoperoxide synthase 2 (PTGS2/COX-2) in asthmatic or healthy AEC, respectively.
Discussion
This is the first study to examine the regulation of ECM component expression by HLFs imposed by co-culture with differentiated primary human AECs from well characterized healthy and asthmatic children. Herein we report that co-culture of HLFs with both healthy and asthmatic AECs regulates the expression of ECM components, specifically collagen I, collagen III and HA. However, HLFs co-cultured with asthmatic AECs displayed decreased regulation compared to healthy AEC co-cultures. In addition, we found that TGFb2, but not TGFb1, is more highly expressed in asthmatic AEC co-cultures while expression PGE2S was downregulated in these culture systems. Furthermore, HLFs co-cultured with asthmatic AECs had greater expression of fibronectin than by HLFs alone, suggesting perhaps a positive AEC-derived stimulus as opposed to inhibitory regulation. Taken together these data point to a dynamic system that is influenced by the interaction of the AECs and HLFs and suggests that an altered balance of pro-remodeling and anti-remodeling signals from the asthmatic AECs may regulate HLF expression of ECM constituents.
One of the more striking findings of this study was that co-culture with healthy AECs markedly attenuated expression of ECM components compared to HLF time controls suggesting that tonic regulation of HLF phenotype via crosstalk with AECs must be occurring. In a previous study, Lama and colleagues demonstrated that murine fibroblasts grown alone in cell culture exhibited increased proliferation compared to those grown in co-culture with murine AECs. That study further linked the down regulation of cells grown in co-culture to the COX-2 pathway and the production of the anti-proliferative signaling of PGE2 using cells derived from transgenic mice.31 In line with this, our study demonstrates greater expression of the PGE2 synthase in the healthy AEC-HLF co-cultures, which correlated with attenuated production of ECM components. In our study, PTGS2/COX2 was not significantly different between the groups; however, it is important to note that production of PGE2 is a complex pathway with multiple areas of potential regulation. In a separate study, Hostettler et al. reported that incubation of cultured HLF cells with conditioned media obtained from AEC cell lines resulted in an approximately 50% reduction in fibroblast proliferation.32 This effect was negated by pre-incubation with indomethacin, a PGE2 inhibitor. Interestingly, in the same series of experiments the authors demonstrated that adding a TGFb neutralizing antibody to the conditioned media also blocked the inhibitory effect and concluded that TGFb was likely involved in the induction of the PGE2 axis. Similar findings were also reported using conditioned media from bovine AECs in another study.33 The findings of the latter two studies highlight the interdependence of the PGE2 and TGFb pathways; however, accumulating evidence would suggest that the activity of TGFb is likely far more complex than these studies might suggest. One important concept that these studies support is that there is crosstalk between the AECs and fibroblast cells. Other studies have highlighted the effects of fibroblasts on AEC proliferation34, 35; however, these effects were not directly examined in the present study.
The concept that the TGFb family of signaling molecules exhibits pro-remodeling effects on fibroblasts is becoming widely accepted.36-38 Correlation of increased TGFb expression and increased subepithelial fibrosis has been reported in adult subjects with severe eosinophilic asthma.39, 40 A more recent study by Brown and colleagues demonstrated increased levels of TGFb1 in brochoalveolar lavage fluid obtained from asthmatic children, which was associated with markers of increased oxidative stress and evidence of airway obstruction documented by PFTs.41 In addition to its other pro-fibrotic effects, TGFb has been shown to enhance the production of ECM constitutes, including collagens.42, 43 TGFb may also exert remodeling effects by augmenting the expression of tissue inhibitors of metalloproteinases (TIMPs), which in turn disrupt the ability of matrix metalloproteinases (MMPs) to turnover secreted ECM components, such as HA.44, 45 The latter is essential for appropriate wound repair following epithelial damage.
It is important to note that multiple isoforms of TGFb exist including TGFb1 (most abundant isoform, characteristically associated with endothelial, hematopoietic and connective tissue cells), TGFb2 (primarily synthesized by AECs and neuronal cells), and TGFb3 (principally secreted by mesenchymal cells).46 Both TGFb1 and TGFb2 have been shown to enhance fibroblast activity in the context of inflammatory remodeling in respiratory epithelium.47 In the latter study both isoforms were capable of activating fibroblasts in a concentration dependent fashion. In reality it is likely that both TGFb1 and TGFb2 are simultaneously active in orchestrating fibrosis in vivo with contributions from the epithelium (TGFb2), eosinophils (TGFb1), and macrophages (TGFb1).48, 49 Bronchial fibroblasts have been shown to respond to both isoforms and intriguingly fibroblasts derived from asthmatic donors displayed enhanced activity to both TGFb1 and TGFb2 suggesting the possibility that greater responses to TGFb could be accounted for by augmentation of both ends of the signaling axis.36 Additionally, our lab has previously reported increased secretion of TGFb2 from AECs obtained from asthmatic children.28 Data from the present study are in line with previously published findings; however, it is important to note that the relative amount of TGFb2 present in this system is lower than the overall levels of TGFb1 detected. Radaev and colleagues have recently reported that TGFb2 has significantly greater affinity for the TGFb receptor I (TBRI) and additionally may simultaneously engage TBRI and TGFb receptor II (TBRII) whereas TGFb1 sequentially engages these receptor complexes and thus has augmented activation kinetics. These variations in receptor subtype engagement by TGFb1 and TGFb2 may contribute to variable downstream signaling and have significant functional consequences.50
Chakir and colleagues have previously reported a link to the expression of TGFb and the deposition of both collagen I and III in bronchial biopsies.51 Notably, the expression of both collagen I and III in that study were significantly greater in the subjects with more severe asthma. This effect was not modified following treatment with corticosteroids suggesting that remodeling that had already occurred was not reversible. Earlier work by Minshall et al. also confirmed the presence of greater amounts of collagen I and III in asthmatic airways, which correlated with asthma severity.52 Similar studies have demonstrated that proteoglycan deposition in the ECM is increased in moderate to severe asthma and correlates with asthma severity.53 In addition, HA has also been implicated in playing a significant role in the increased deposition of ECM in asthmatics.54 Beyond its role as a scaffold for collagen deposition following airway inflammation,55 HA has also been implicated in the persistence of eosinophils and enhanced production of TGFb in asthmatic subjects.56 The latter finding suggests not only a significant role for HA in airway remolding, but also modulation of a variety of inflammatory and signaling mediators. Our findings of enhanced production of collagens and HA by HLFs co-cultured with asthmatic AECs compared to HLFs co-cultured with healthy AECs are in line with these previous reports and provide a novel model system in which relevant signaling pathways can be further investigated.
There are some inherent limitations to this study design. Our population of asthmatic subjects exhibited mild airflow obstruction, consistent with milder phenotypes of asthma independent of treatment. Alternatively, this could be related to good adherence to controller medications as our subjects reported that 90% of the cohort was presently taking daily ICS. Given that the cells used in the co-cultures were multiple passages beyond the initial sample collection it is unlikely that any medications being taken at the time of recruitment would still have an effect on the cells; however, we cannot completely exclude that possibility. If present, this effect would bias toward the null hypothesis and likely make differences between the groups less apparent. Despite a relatively mild asthma phenotype our subjects had significantly lower FEV1/FVC ratios than the healthy subjects, indicating the presence of airflow obstruction. Another limitation of this study is that gene expression was only assessed at a single time point.Given the findings of this study, future investigation of additional signaling pathways and the balance between pro-fibrotic and anti-fibrotic signaling pathways, as well as assessment of gene expression at multiple time points, is warranted. Once a more clear understanding of the likely multiple pathways that contribute to this crosstalk are achieved, future studies specifically targeting either or both the pro- and anti-fibrotic signaling may lead to a better understanding of how to ameliorate the increased deposition of ECM components observed in asthmatic airways.
While this is an in vitro model of cellular function, similar in vivo studies in healthy and asthmatic children would not be ethical or feasible. In addition, there are many inherent strengths to this approach. We have used a common healthy donor HLF cell line across the experiments, which not only limits the biologic variability of the HLF, but also helps to isolate differences seen between the healthy and asthmatic groups to activity of the AECs. Furthermore, our model allows for multi-faceted characterization of several outcome measures including gene expression, bound and excreted protein production, and histological examination. Additionally, we are able to clinically characterize our population based on history, laboratory testing, and lung function.
In conclusion, we have demonstrated that HLF cells from a common healthy donor behave differently when co-cultured with either AECs obtained from a healthy donor or AECs obtained from an asthmatic donor. Furthermore, we have shown differential expression of important ECM components including type I and type III collagens as well as HA and fibronectin depending on whether the HLF were co-cultured with healthy derived AECs or asthmatic derived AECs. These findings are clinically relevant given the role that ECM deposition plays in airway remodeling and may have further reaching implications given that we are just beginning to understand the active role that the ECM plays in airway remodeling and fibrosis13. In addition, we have shown that levels of TGFb2 are elevated in asthmatic co-cultures despite finding no differences in the levels of TGFb1 compared to healthy AEC-HLF co-cultures. The later finding is consistent with previous work published by our group28 and may be an important signaling mechanism driving augmented ECM deposition observed in asthmatic airways. In addition, we have shown in our model systerm that PGE2S is down regulated in asthmatic AEC-HLF co-cultures, which is in line with previous reports from other groups.31, 32 Taken together these findings are consistent with the paradigm that signaling from the AEC is an important regulatory mechanism in the functional coordination of airway cellular subsets22 and provide further evidence that intrinsic dysregulation of the epithelial-mesenchymal trophic unit may underlie asthma pathogenesis and/or airway remodeling.57
Supplementary Material
Acknowledgments
Supported by: Firland Foundation 201208 (PI: SR); American Lung Association Senior Research Training Fellowship RT-268263-N (PI: SR); NIH R01AI068731 (PI: SZ); NIH PO1 HL098067 (PI: SZ: Subproject TNW)
Abbreviations
- AEC
Airway epithelial cells
- ALI
air-liquid interface
- ATS
American Thoracic Society
- COL1A1
Collagen I
- COL3A1
Collagen III
- COX-2
cyclooxygenase 2
- ECM
Extracellular matrix
- ERS
European Respiratory Society
- FENO
Fraction of exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FEF25-75
forced expiratory flow between 25% and 75% of FVC
- FGM
fibroblast growth media
- FNDC
Fibronectin
- FVC
forced vital capacity
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HA
hyaluronan
- HAS2
hyaluronan synthase 2
- HLF
human lung fibroblast
- IHC
immunohistochemistry
- MMPs
matrix metalloproteinases
- PGE2
prostaglandin E2
- PGE2S
prostaglandin E2 synthase
- PTGS2
prostaglandin-endoperoxide synthase 2
- RAST
radioallergosorbent testing
- RIN
RNA integrity number
- SABA
short-acting beta-agonist
- TBRI
transforming growth factor beta receptor subunit I
- TBRII
transforming growth factor beta receptor subunit II
- TIMPs
tissue inhibitors of metalloproteinases
- TGFb1
transforming growth factor beta 1
- TGFb2
transforming growth factor beta 2
- TGFb3
transforming growth factor beta 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications
Extracellular matrix components expressed by lung fibroblasts are less regulated by airway epithelial cells from asthmatic children as compared to cells from healthy children, which may explain airway remodeling observed in asthma.
REFERENCES
- 1.Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, et al. International consensus on (ICON) pediatric asthma. Allergy. 2012;67:976–97. doi: 10.1111/j.1398-9995.2012.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unit ALAEaS, Association. AL. American Lung Association, Epidemiology and Statistic Unit, Research and Program Services . Estimated prevalence and incidence of lung disease by Lung Association territory [electronic resource] American Lung Association; New York, NY: 2006. [Google Scholar]
- 3.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2007. Vital Health Stat. 2009;10:1–80. [PubMed] [Google Scholar]
- 4.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, The Group Health Medical Associates Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 5.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964-1999. J Allergy Clin Immunol. 2002;109:189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 6.Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, Custovic A, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008;32:1096–110. doi: 10.1183/09031936.00002108. [DOI] [PubMed] [Google Scholar]
- 7.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 8.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–81. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 9.Malmstrom K, Pelkonen AS, Malmberg LP, Sarna S, Lindahl H, Kajosaari M, et al. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66:157–62. doi: 10.1136/thx.2010.139246. [DOI] [PubMed] [Google Scholar]
- 10.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–64. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med. 1997;156:951–8. doi: 10.1164/ajrccm.156.3.9610084. [DOI] [PubMed] [Google Scholar]
- 12.Roberts CR, Burke AK. Remodelling of the extracellular matrix in asthma: proteoglycan synthesis and degradation. Can Respir J. 1998;5:48–50. [PubMed] [Google Scholar]
- 13.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–5. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]
- 16.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–9S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 17.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–63. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PR, Burgess JK, Ge Q, Poniris M, Boustany S, Twigg SM, et al. Connective tissue growth factor induces extracellular matrix in asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2006;173:32–41. doi: 10.1164/rccm.200406-703OC. [DOI] [PubMed] [Google Scholar]
- 19.Goulet S, Bihl MP, Gambazzi F, Tamm M, Roth M. Opposite effect of corticosteroids and long-acting beta(2)-agonists on serum- and TGF-beta(1)-induced extracellular matrix deposition by primary human lung fibroblasts. J Cell Physiol. 2007;210:167–76. doi: 10.1002/jcp.20836. [DOI] [PubMed] [Google Scholar]
- 20.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–46. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 21.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–31. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–9. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 23.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, et al. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol. 2005;32:99–107. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 24.Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–13. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy. 2004;34:567–75. doi: 10.1111/j.1365-2222.2004.1917.x. [DOI] [PubMed] [Google Scholar]
- 26.American Thoracic S. European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 27.Lane C, Burgess S, Kicic A, Knight D, Stick S. The use of non-bronchoscopic brushings to study the paediatric airway. Respir Res. 2005;6:53. doi: 10.1186/1465-9921-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012;129:990–7. doi: 10.1016/j.jaci.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, et al. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–11. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27:752–8. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- 32.Hostettler KE, Roth M, Burgess JK, Gencay MM, Gambazzi F, Black JL, et al. Airway epithelium-derived transforming growth factor-beta is a regulator of fibroblast proliferation in both fibrotic and normal subjects. Clin Exp Allergy. 2008;38:1309–17. doi: 10.1111/j.1365-2222.2008.03017.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura Y, Tate L, Ertl RF, Kawamoto M, Mio T, Adachi Y, et al. Bronchial epithelial cells regulate fibroblast proliferation. Am J Physiol. 1995;269:L377–87. doi: 10.1152/ajplung.1995.269.3.L377. [DOI] [PubMed] [Google Scholar]
- 34.Semlali A, Jacques E, Rouabhia M, Milot J, Laviolette M, Chakir J. Regulation of epithelial cell proliferation by bronchial fibroblasts obtained from mild asthmatic subjects. Allergy. 2010;65:1438–45. doi: 10.1111/j.1398-9995.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 35.Skibinski G, Elborn JS, Ennis M. Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L69–76. doi: 10.1152/ajplung.00299.2006. [DOI] [PubMed] [Google Scholar]
- 36.Michalik M, Pierzchalska M, Legutko A, Ura M, Ostaszewska A, Soja J, et al. Asthmatic bronchial fibroblasts demonstrate enhanced potential to differentiate into myofibroblasts in culture. Med Sci Monit. 2009;15:BR194–201. [PubMed] [Google Scholar]
- 37.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–33. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 38.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121:560–70. doi: 10.1016/j.jaci.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, et al. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997;156:642–7. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 40.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1996;15:404–9. doi: 10.1165/ajrcmb.15.3.8810646. [DOI] [PubMed] [Google Scholar]
- 41.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-beta1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol. 2012;129:388–96. doi: 10.1016/j.jaci.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghu G, Masta S, Meyers D, Narayanan AS. Collagen synthesis by normal and fibrotic human lung fibroblasts and the effect of transforming growth factor-beta. Am Rev Respir Dis. 1989;140:95–100. doi: 10.1164/ajrccm/140.1.95. [DOI] [PubMed] [Google Scholar]
- 43.Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994;158:169–79. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- 44.Qing J, Zhang Y, Derynck R. Structural and functional characterization of the transforming growth factor-beta -induced Smad3/c-Jun transcriptional cooperativity. J Biol Chem. 2000;275:38802–12. doi: 10.1074/jbc.M004731200. [DOI] [PubMed] [Google Scholar]
- 45.Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, et al. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276:19945–53. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- 46.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 47.Serpero L, Petecchia L, Sabatini F, Giuliani M, Silvestri M, Di Blasi P, et al. The effect of transforming growth factor (TGF)-beta1 and (TGF)-beta2 on nasal polyp fibroblast activities involved upper airway remodeling: modulation by fluticasone propionate. Immunol Lett. 2006;105:61–7. doi: 10.1016/j.imlet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Eisma RJ, Allen JS, Lafreniere D, Leonard G, Kreutzer DL. Eosinophil expression of transforming growth factor-beta and its receptors in nasal polyposis: role of the cytokines in this disease process. Am J Otolaryngol. 1997;18:405–11. doi: 10.1016/s0196-0709(97)90062-4. [DOI] [PubMed] [Google Scholar]
- 49.Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci U S A. 1999;96:9660–5. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radaev S, Zou Z, Huang T, Lafer EM, Hinck AP, Sun PD. Ternary complex of transforming growth factor-beta1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J Biol Chem. 2010;285:14806–14. doi: 10.1074/jbc.M109.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: Effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. Journal of Allergy and Clinical Immunology. 2003;111:1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 52.Minshall E, Chakir J, Laviolette M, Molet S, Zhu Z, Olivenstein R, et al. IL-11 expression is increased in severe asthma: association with epithelial cells and eosinophils. J Allergy Clin Immunol. 2000;105:232–8. doi: 10.1016/s0091-6749(00)90070-8. [DOI] [PubMed] [Google Scholar]
- 53.Huang J, Olivenstein R, Taha R, Hamid Q, Ludwig M. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. Am J Respir Crit Care Med. 1999;160:725–9. doi: 10.1164/ajrccm.160.2.9809040. [DOI] [PubMed] [Google Scholar]
- 54.Roberts CR. Is asthma a fibrotic disease? Chest. 1995;107:111S–7S. doi: 10.1378/chest.107.3_supplement.111s. [DOI] [PubMed] [Google Scholar]
- 55.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30:126–34. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohkawara Y, Tamura G, Iwasaki T, Tanaka A, Kikuchi T, Shirato K. Activation and transforming growth factor-beta production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol. 2000;23:444–51. doi: 10.1165/ajrcmb.23.4.3875. [DOI] [PubMed] [Google Scholar]
- 57.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007;7:63–8. doi: 10.1097/ACI.0b013e328013d61b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.