Abstract
Background
CCR10 and CCL27 are the most skin-specific chemokine receptor/ligand pair implicated in skin allergy and inflammatory diseases including atopic dermatitis and psoriasis. This pair is thought to regulate migration and/or maintenance of skin T cells and suggested as therapeutic targets for treatment of skin diseases. However, the functional importance of CCR10/CCL27 in vivo remains elusive.
Objective
We sought to determine expression and function of CCR10 in different subsets of skin T cells under both homeostatic and inflammatory conditions to gain a mechanistic insight into potential roles of CCR10 during skin inflammation.
Methods
Using heterozygous and homozygous CCR10-knockout/EGFP-knockin mice, we assessed expression of CCR10 on regulatory and effector T cells of healthy and inflamed skin induced by chemicals, pathogens and auto-reactive T cells. In addition, we assessed the effect of CCR10-knockout on the maintenance and functions of different T cells and inflammatory status in the skin during different phases of the immune response.
Results
CCR10 expression is preferentially induced on memory-like skin-resident T cells and their progenitors for their maintenance in homeostatic skin but not expressed on most skin-infiltrating effector T cells during inflammation. In CCR10-knockout mice, the imbalanced presence and dysregulated function of resident regulatory and effector T cells result in over-reactive and prolonged innate and memory responses in the skin, leading to increased clearance of Leishmamia infection in the skin.
Conclusion
CCR10 is a critical regulator of skin immune homeostasis.
Keywords: Chemokine receptor CCR10, skin-resident T cells, regulatory T cells, migration, maintenance, immune homeostasis, allergy, dermatitis, inflammation, skin infection, Leishmania
INTRODUCTION
As the external surface of a body, skin is under frequent assaults from environmental agents. To maintain its integrity and function, immune cells in the skin are tightly regulated to tolerate harmless antigens but respond to dangerous assaults. Skin-resident T cells are unique populations of immune cells with memory cell-like properties (1). Among them, resident CD4+ regulatory T (Treg) cells are critical to maintain immune homeostasis of healthy skin (2, 3), while resident memory effector T (Teff) cells can provide faster immune responses to skin infection than those of the circulation (4–6). The antigen encounter in the skin might also induce memory Treg cells to counterbalance the Teff response (7). Dysregulation of Treg and Teff cells in the skin is associated with allergy and other skin inflammatory diseases.
CCR10 and its ligand CCL27 is the most skin-specific chemokine/receptor pair (8). With CCL27 constitutively expressed in healthy skin and further upregulated in inflamed skin in psoriasis and dermatitis patients and animal models, CCR10/CCL27 have been implicated in migration of skin T cells under both healthy and disease conditions (9–12). In humans, all blood CCR10+ T cells display memory cell markers, co-express the skin-homing molecule cutaneous lymphocyte antigen (CLA) and respond to chemoattraction of CCL27, suggesting a role of CCR10/CCL27 in recruitment of memory T cells into the skin (9, 12, 13). However, immunohistochemical staining in one early study found only scattered CCR10+ cells in healthy skin of humans (12). Instead, most T cells of inflamed skin of psoriatic and dermatitic patients express CCR10. Since CCL27 was also upregulated in inflamed skin, it was suggested that CCR10/CCL27 are involved in migration of T cells during the skin inflammation (12). However, another study found only a small percentage of CCR10+ T cells isolated from allergen and bacterial chancroid-induced inflamed human skin, suggesting that CCR10 is unlikely critical for migration of most T cells during the skin inflammation (13). Recently it was reported that CCR10 is co-expressed on half of human blood CCR8+ T cells, a population with the skin-homing potential, but T cells migrating out of the healthy human skin do not express CCR10; however, non-migrating skin-resident T cells were not assessed (14). The CCR10+ CD4+ T cell subset of human blood is enriched with IL-22-producing cells that also preferentially express CCR6, another chemokine receptor associated with skin localization (15, 16). The role of CCR10/CCL27 as homeostatic or inflammatory regulators of skin T cells in humans remains unclear.
Animal studies have provided complex and sometimes, seemingly, contradicting results on functions of CCR10/CCL27. Using a mouse model of DNFB (2,4-dinitro-1-fluorobenzene)-induced contact hypersensitivity (CHS), one study found that the antibody neutralization of CCL27 reduced T cell recruitment and inflammation in the skin, suggesting a pivotal role of the CCL27/CCR10 axis in T cell-mediated skin inflammation (12). Similarly, in the keratin-14 promoter-driven IL-4 transgenic mouse model of atopic dermatitis, subcutaneous injection of anti-CCL27 antibodies reduced inflammation (17). However, anti-CCL27 antibody treatment did not affect recruitment of transferred CD4+ T cells into the DNFB-inflamed skin or allergen-induced CHS responses in other studies (18, 19). Recently, it was reported that CCR10-sufficent and -knockout CD4+ T cells migrate similarly to the cognate-antigen-stimulated skin, demonstrating directly that CCR10 is not critical for T cell infiltration into inflamed skin (20) and the role of CCR10 in regulation of skin T cells in vivo remains unknown.
We recently generated CCR10-knockout (KO)/EGFP-knockin (KI) mice in which the CCR10 coding region was replaced with a DNA sequence coding for enhanced green fluorescent protein (EGFP) (21, 22). Using heterozygous and homozygous CCR10-KO/EGFP-KI (CCR10+/− and CCR10−/−) mice, we assessed expression of CCR10 and its roles in different phases of T cell responses during the skin inflammation. Here, we report the first definite evidence that CCR10 is a critical regulator of skin immune homeostasis through regulating the balanced presence and function of resident Treg and Teff cells.
METHODS
Mouse models and human bio-samples
CCR10-KO/EGFP-KI mice were generated in our laboratory (21). Rag1−/−, Scurfy and wild type (WT) CD45.1+ congenic C57BL6 mice were from The Jackson Laboratory (Bar Harbor, ME). CD45.1+CD45.2+ wild type C57BL6, CD45.1+CD45.2+ or CD45.1+CD45.2− CCR10+/−, CD45.1+CD45.2+ Rag1−/− mice were generated by proper crossing. Scurfy mice were also crossed to CCR10-KO/EGFP-KI mice to introduce a CCR10-KO/EGFP-KI allele for the EGFP reporter of CCR10 expression. All animal experiments were approved by The Pennsylvania State University Institutional Animal Care and Use Committee. The human healthy skin was from people undergoing the plastic surgery. Use of the bio-samples of humans was approved by the institutional review board of Anhui Medical University.
Chemical reagents and induction of skin inflammation
1-Fluoro-2,4-dinitrobenzene (DNFB), Phorbol 12-myristate 13-acetate (TPA) and Fluorescein 5(6)-isothiocyanate (FITC) and chicken ovalbumin (OVA) were purchased from Sigma-Aldrich (St. Louis, MO). Cholera toxin was purchased from List Biological (Campbell, CA).
To induce classic contact hypersensitive (CHS) responses, mouse abdomen was shaved and sensitized with 100μl 0.5% DNFB in 4:1 acetone/olive oil at day 0 and 1. At day 5, the baseline ear thicknesses of both right and left ears were measured by a micrometer gauge. Immediately following the ear measurement, each side of the ear was topically applied with 10μl of 0.2% DNFB solution or control solvents (20μl total). Ear thickness was measured at various days after the chemical challenge on the ear. The change in the ear thickness (ΔT) was calculated by subtracting the ear thickness before the chemical treatment from the ear thickness after the chemical application. The memory CHS response was induced similarly as the classic CHS response except that ears were challenged with DNFB one month after the DNFB sensitization.
For DNFB, FITC or TPA-induced innate skin inflammation, each side of an ear was applied with 10μl of the chemicals (0.5% DNFB in 4:1 acetone/olive oil, 0.5% FITC in 1:1 acetone/dibutylpthalate, or 100μg/ml TPA in acetone) once. The ear thickness was measured at various days after the application.
The OVA-induced skin inflammation was performed as reported (23), except that total OVA proteins instead of peptides were epicutaneously applied to the mouse skin.
Skin cell isolation
Skin cells were prepared similarly as previous described (21). Briefly, mouse hair was removed from the skin by hair clipper and Nair (Church & Dwight, Princeton, NJ). Mouse skin was excised, trimmed of subcutaneous fat and minced, following by 2-hour digestion with 4mg/ml Collagenase Type I (Worthington, Lakewood, NJ), 2mg/ml Collagenase Type IV (Worthington, Lakewood, NJ), 2mg/ml hyaluronidase type I-s (Sigma-Aldrich, St. Louis, MO) and 4% BSA (Sigma-Aldrich, St. Louis, MO) in DMEM. Thirty minutes before the end of digestion, 0.0001% DNase (Sigma-Aldrich, St. Louis, MO) was added into the digest buffer. Mononucleocytes were enriched from the cell preparations using Percoll gradients (40%/80%). The similarly isolated human skin cells were allowed to recover in the culture medium overnight before flow cytometric analysis.
Bone marrow cell reconstitution
Cell sorter-purified EGFP− BM cells of CCR10+/− (CD45.1+CD45.2−) and CCD10−/− (CD45.1− CD45.2+) mice were 1:1 mixed and injected intravenously into lethally irradiated (950 Rad) WT C57BL6 or CCR10+/− (CD45.1+D45.2+) mice (total 106 cells per mouse). The recipients were analyzed 7 to 8 wk after the transfer.
Skin Leishmania major infection
Leishmania major (L. major) NIH Friedlin V1 strain (MHOM/IL/80/FN) was grown in M199 medium supplemented with 25 mM HEPES and 20% FCS until a stationary phase. Mice were injected subcutaneously with 1×106 stationary-phase promastigotes in the ear dermis. Infected ears were collected at various time points and total genomic DNA was extracted with DNeasy Tissue Kit (Qiagen, Valencia, CA). Tissue L. major genomic DNA levels were quantified by qPCR with primers (JW11: CCTATTTTACACCAACCCCCAGT; JW12: GGGTAGGGGCGTTCTGCGAAA) specific to L. major 16S rDNA and normalized on levels of mouse genomic β-actin as previously described (24).
In vivo T cell transfer
EGFP− CD3+ T cells were sorter-purified from splenocytes of CCR10+/− or CCR10−/− mice. 4×105 splenic EGFP− CD3+ T cells of CCR10+/− or CCR10−/− mice were intraperitoneally injected into Rag1−/− mice separately, which were then tested for the DNFB-induced classic or memory CHS responses after the cell transfer. For adoptive transfer of OVA-specific T cells, about 0.5×106 purified splenic transgenic T cells of CCR10+/− or CCR10−/− OTI or OT-II mice were intraperitoneally injected into WT mice with different CD45 polymorphism (indicated in the relevant figure), which were then epicutaneously challenged with OVA proteins (23).
Naïve T cell transfer-induced skin inflammation
EGFP− naive T cells (CD3+CD4+CD25-CD45RBhigh, CD45.1+) and Treg cells (CD3+CD4+CD25+CD45RB−, CD45.2+) were sorted from splenocytes of CCR10+/− mice. 4×105 naive T cells alone, or 2×105 naive along with 2×105 Treg cells, were intravenously injected into Rag1−/− mice (CD45.1+CD45.2+). The recipients were analyzed 7 to 8 wk after the transfer.
Real-time RT-PCR analysis of cytokine transcripts
Total RNA of mouse ears were isolated and reverse-transcribed to cDNA, which were then subject to the Sybr green real-time PCR with following primers. TNF-α Forward: TTCTATGGCCCAGACCC, Reverse: GGCACCACTAGTTGGTTGTC; IL-1β: Forward: TCTCGCAGCAGCACATCA, Reverse: CACACCAGCAGGTTATCATCAT. IL-10 Forward: ACCAAAGCCACAAAGCAGCC, Reverse: CCGACTGGGAAGTGGGTGC; β-actin Forward: CCCATCTACGAGGGCTAT, Reverse: TGTCACGCACGATTTCC. Relative levels of transcripts were calculated with the delta delta CT method.
Antibodies and flow cytometry
Flow cytometry, antibodies and analysis strategies are detailed in the supplementary methods. Cell gating is indicated in the figures.
In vitro T cell stimulation
Skin EGFP+CD4+CD25− T cells purified from CCR+/− and CCR10−/− mice were cultured in the presence of IL-2 and coated anti-CD28/anti-TCRβ antibodies for 2 days. Supernatants were collected and analyzed by the mouse IL-17A ELISA Ready-SET-Go!® Set (eBioscience, San Diego, CA).
Statistical analyses
Data are expressed as means ± standard errors (SEM) and analyzed by two-tailed student T test or Fisher test to determine statistical significance for two-group comparison. ANOVA with Tukey adjustment was used for multiple group comparisons. P < 0.05 is considered significant.
RESULTS
Over-reactive innate immune response to allergen stimulation in skin of CCR10−/− mice
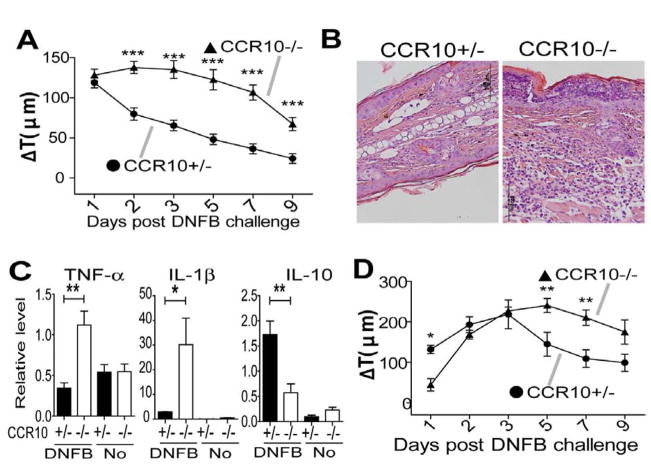
To assess roles of CCR10 in the skin inflammation, we tested CCR10−/− mice in several models. In a DNFB-induced CHS assay, CCR10−/− and CCR10+/− mice had similar ear thickness increases (Fig. 1A), indicating that CCR10 is not critical for the T-cell mediated CHS response and consistent with the report that CCR10-KO does not affect the T cell migration (20). Surprisingly, CCR10−/− mice had significantly larger ear thickness increases than CCR10+/− mice early after the one-time DNFB application (Fig. 1B), suggesting an enhanced innate response. Supporting this notion, the treated ears of CCR10−/− mice exhibited much higher levels of gene expression of TNF-α and lower IL-10 than CCR10+/− controls (Fig. 1C). CCR10−/− mice also had an enhanced innate response to one-time treatment of another irritant/allergen FITC (Fig. 1D–E). On the other hand, compared to CCR10−/− mice, CCR10+/− mice had marginally enhanced innate responses to topical application of TPA, a strong mitogen that activates both keratinocytes and immune cells (Fig. 1F and Fig. E1). Considering the one-time DNFB or FITC treatments are weaker stimulators than TPA or repeated DNFB treatments of the CHS assay, these results reveal that CCR10−/− mice have a reduced activation threshold to the weak stimulation but still mount full-scale responses once the threshold is overcome by strong stimulators, suggesting a defect in immune regulatory system in the skin.
Figure 1.
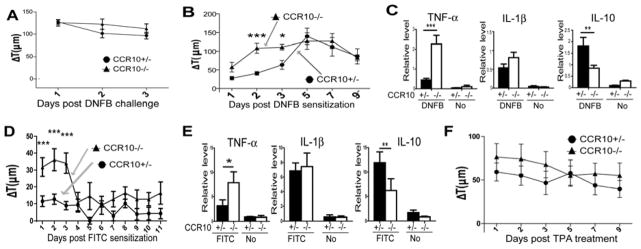
Over-reactive innate response to stimulation in skin of CCR10−/− mice. (A) Ear thickness changes (ΔT) of CCR10−/− and CCR10+/− mice in a DNFB-induced CHS assay. N≥6. (B) Ear thickness changes of CCR10−/− and CCR10+/− mice after the one-time topical application of DNFB on the ear. N≥10. *P<0.05; **P<0.005; ***P<0.001 and NS: no significant difference (applied to all figures). (C) qRT-PCR analysis of RNA isolated from treated ears 3 days after the one-time DNFB application for TNF-α, IL-1β and IL-10. N=5 each. “No” indicates untreated skin samples (n=3). The values are relative levels normalized on β-actin. (D) Ear thickness changes of CCR10−/− and CCR10+/− mice after the one-time topical application of FITC (0.5%). N=21 for CCR10+/− mice and N=19 for CCR10−/− mice. (E) qRT-PCR analysis of RNA isolated from the treated skin 3 days after the one-time FITC-application for TNF–α, IL-1β and IL-10 as in (C). N=4 each. (F) Ear thickness changes of CCR10−/− and CCR10+/− mice after the one-time topical application of TPA. N≥10 for each genotype.
Imbalanced maintenance of skin-resident Treg and Teff cells in CCR10−/− mice
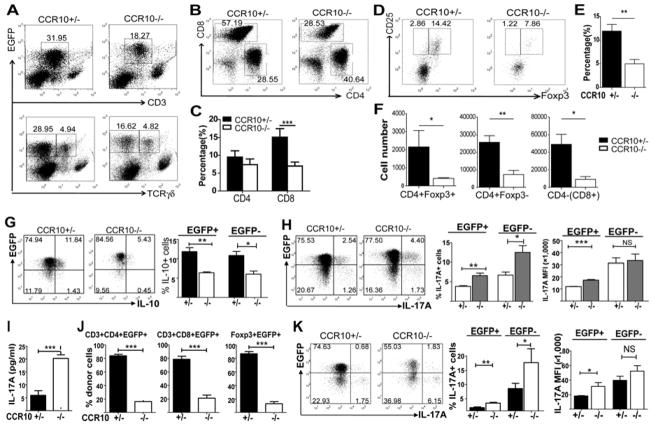
We next analyzed how CCR10-KO affected skin-resident T cells, including Treg cells that are critical in the immune regulation. Based on the EGFP reporter for CCR10 in CCR10+/− mice (21, 22), the majority of skin-resident αβT cells highly expressed CCR10 (EGFP) (Fig. 2A and Fig. E2) and memory T cell markers (KLRG1−CD62L− CD44highCD127+ for CD8+ and KLRG1±CD62L−CD44highCD127+ for CD4+ cells) (Fig. E3) (21, 25). Compared to CCR10+/− mice, CCR10−/− mice had fewer skin αβT (CD3+γδTCR−) cells (Fig. 2A), of which the CD8+ subset was impaired more profoundly than the CD4+ subset (Fig. 2B–C). The CCR10low CD3high population of T cells were Vγ3+ γδT cells that were increased in the dermis of CCR10−/− mice due to dysregulated localization (Fig. 2A), as we previously reported (21). Notably, CCR10−/− mice had significantly fewer Foxp3+ Treg cells within the skin CD4+ population than CCR10+/− mice (Fig. 2D–E). When total cell yields are considered, fewer Treg, CD4+ and CD8+(CD4−) Teff cells were isolated from skin of CCR10−/− mice than CCR10+/− mice (Fig. 2F). In contrast to the skin, very few EGFP+ T cells were found in internal lymphoid organs such as spleens and their numbers were even higher in CCR10−/− than in CCR10+/− mice (Fig. E4), suggesting that defective maintenance of T cells in the skin results in their abnormal accumulation in the internal sites.
Figure 2.
Imbalanced maintenance and dysregulated functions of Treg and Teff cells in the skin of CCR10−/− mice. (A) Flow cytometric (FACS) analysis of skin lymphocyte preparations of CCR10−/− and CCR10+/− mice for EGFP+ total CD3+ T cells (top) and γδT cells (bottom). The number next to each gate is the percentage (%) of the gated cells of total events in the histograph. The CD3highCCR10low cells are Vγ3+ γδT cells (21). (B) FACS analysis of gated skin EGFP+CD3+ T cells of CCR10−/− and CCR10+/− mice for CD4+ and CD8+ subsets. (C) Average percentages of EGFP+ CD8+ and CD4+ cells of skin lymphocyte preparations in CCR10+/− and CCR10−/− mice, calculated by multiplying % of total EGFP+ T cells (A) and % of CD4+ and CD8+ cells of the total EGFP+ T cells (B). (D) FACS analysis of gated skin EGFP+CD4+ T cells of CCR10−/− and CCR10+/− mice for Foxp3+ Treg cells. (E) Average percentages of Foxp3+CD25+ Treg cells of skin CD4+ T cells in CCR10+/− and CCR10−/− mice, calculated from the FACS analysis in (D). N≥10. (F) Average numbers of EGFP+ CD4+Foxp3+, CD4+ and CD4−(CD8+) T cells isolated from skin of CCR10+/− vs. CCR10−/− mice. N≥10. (G) FACS analysis of skin CD4+ T cells of CCR10−/− and CCR10+/− mice for the IL-10+ subset. Average percentages of IL-10+ cells of the EGFP+ or EGFP− CD4+ cells were shown on the left. N=4 each. (H) FACS analysis of skin CD4+ T cells of CCR10−/− and CCR10+/− mice for the IL-17A+ subset. The bar graphs show average percentages of IL-17A+ cells of EGFP+ or EGFP− CD4+ cells (middle) and their MFI for the IL-17A staining (right). N=6 each. (I) Levels of IL-17A production by purified CCR10+/− or CCR10−/− skin EGFP+CD4+CD25− T cells stimulated with IL-2 and coated anti-CD28/anti-TCRβ antibodies in culture. N=4–5. (J) Relative contribution of transferred CCR10−/− vs. CCR10+/− BM cells to the indicated skin T cell subsets in irradiated WT recipient mice. N=5 each. (K) FACS analysis of skin CD4+ T cells of CCR10−/− vs. CCR10+/− BM donor origins in recipient mice for the IL-17A+ cells, presented as in (H). N=5 each.
Impaired immune homeostasis in the skin of CCR10−/− mice
The imbalanced presence of resident Treg vs. Teff cells could impair skin immune homeostasis in CCR10−/− mice. Indeed, the skin CD4+ T cell subset of CCR10−/− mice contained a significantly lower percentage of IL-10+ and higher percentage of IL-17A+ (Th17) cells than CCR10+/− controls (Fig. 2G–H). EGFP+ Th17 cells of CCR10−/− mice also had markedly higher mean fluorescent intensity (MFI) for IL-17A staining than EGFP+ Th17 cells of CCR10+/− mice (Fig. 2H), suggesting that CCR10 regulates function of the resident CCR10+ Th17 cells as well as their maintenance. Supporting this conclusion, purified EGFP+ skin CD4+ Teff cells of CCR10−/− mice secreted much higher levels of IL-17A in culture than the CCR10+/− controls (Fig. 2I). Notably, EGFP(CCR10)− skin T cells of CCR10+/− mice contained higher percentage of Th17 cells with higher IL-17A production than EGFP(CCR10)+ skin T cells of the same mice (Fig. 2H), also supporting the role of CCR10 in suppression of Th17 functions.
To determine whether CCR10 is directly involved in regulating skin Treg and Teff subsets, we performed competitive bone marrow (BM) co-transfer experiments in which similar numbers of CCR10−/− and CCR10+/− BM cells were injected into irradiated WT mice. EGFP+ CD8+, CD4+ and Foxp3+ T cell subsets of the CCR10−/− donor were all greatly under-represented in the skin compared to corresponding populations of the CCR10+/− donor (Fig. 2J, Fig. E5A). In addition, EGFP− CD8+, CD4+ and Treg cell subsets of the CCR10−/− donor were also under-represented (to lesser extent than the EGFP+ subsets) in the skin compared to corresponding populations of the CCR10+/− donor (Fig. E5B). Since EGFP− cells do not express CCR10, the effect of CCR10-KO on these cells is likely secondary to the effect of CCR10-KO on EGFP+ cells. Furthermore, EGFP+ skin CD4+ T cells of the CCR10−/− donor still had higher percentages of Th17 cells with higher IL17 MFI staining than those of the CCR10+/− donor (Fig. 2K), demonstrating an intrinsic regulatory role of CCR10 in maintenance and function of the skin-resident T cells.
Treg cells-regulated immune homeostasis is critical for maintenance of CCR10+ memory-like resident T cells in the skin
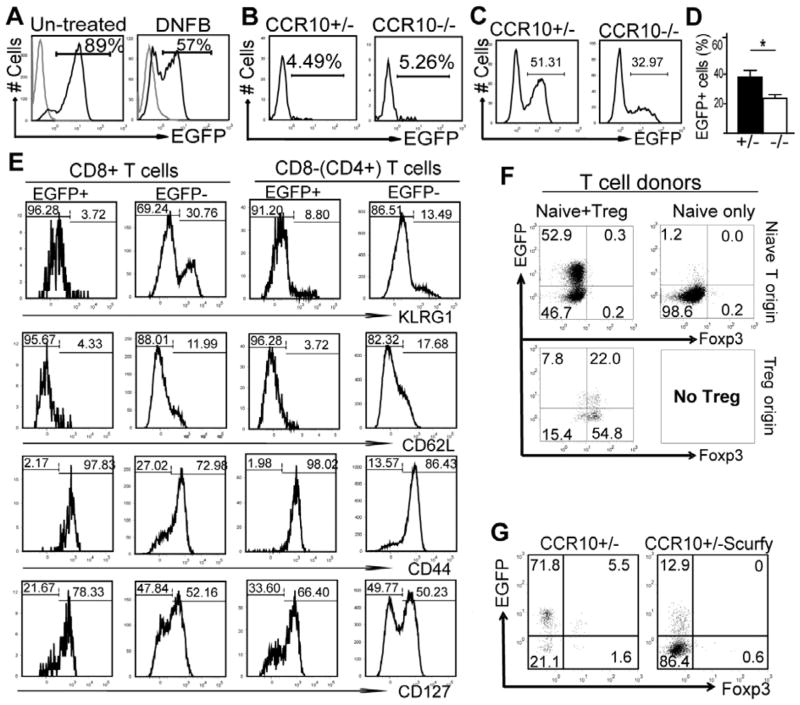
While the imbalanced presence and dysregulated function of skin-resident Treg vs. Teff cells are consistent with the over-reactive innate response in CCR10−/− mice, the normal CHS response (Fig. 1A) suggests that CCR10 is not critical for migration of infiltrating effector T cells during the inflammation. We noted that DNFB-inflamed skin of the CHS assay had significantly lower percentages of CCR10+ T cells than untreated skin (Fig. 3A), which suggest that CCR10 is not expressed on many new infiltrating T cells and might explain the different effects of CCR10-KO on skin-resident T cells under steady conditions vs. infiltrating T cells during inflammation. Consistent with this, most Treg cells of TPA-treated, inflamed ear skin did not express CCR10 (Fig. E6). However, these assays could not distinguish infiltrating T cells from resident T cells that potentially downregulated CCR10. To dissect this further, we transferred EGFP− splenic T cells of CCR10+/− or CCR10−/− mice into Rag1−/− mice and analyzed the skin-infiltrating donor T cells different times after induction of CHS. At the peak of inflammation (1 day after the DNFB challenge), only few skin-infiltrating T cells were EGFP+ (Fig. 3B). In marked contrast, 1 month after the DNFB challenge when inflammation was resolved, about half of skin T cells of CCR10+/− donor were EGFP+, and the percentage of EGFP+ skin T cells of CCR10−/− donor was significantly lower than that of CCR10+/− donor (Fig. 3C–D). Since the skin EGFP+ T cells of the donor in Rag1−/− recipients had preferentially displayed memory phenotypes early following challenge, even during the active phase of inflammation (Fig. 3E), these results suggest that CCR10 is preferentially imprinted on memory T cell progenitors for their maintenance in the skin after resolution of inflammation but not on effector T cells for their skin infiltration during inflammation.
Figure 3.
Treg cell-regulated immune homeostasis is critical for maintenance of CCR10+ memory-like resident T cells in the skin. (A) FACS analysis of T cells of untreated or inflamed skin of CCR10+/− mice for CCR10(EGFP) expression. The inflamed skin was of mice 1 day after DNFB-induced CHS response. The gray lines are of WT cells as negative controls for EGFP. N=2. (B–D) FACS analysis for EGFP on skin T cells in Rag1−/− mice transferred with CCR10+/− or CCR10−/− EGFP− splenic T cells 1 day (B) or 1 month (C, D) after the DNFB-induced CHS. Average percentages of EGFP+ skin T cells of CCR10+/− vs. CCR10−/− donors 1 month after the CHS induction are shown in (D). N=10 each. (E) Representative FACS analysis for molecules associated with memory on skin EGFP+ vs. EGFP− T cells of the CCR10+/− donor T cell origins in Rag1−/− mice 1 day after DNFB-induced CHS. N= 4. (F) FACS analysis of EGFP expression on Teff (top) and Treg (bottom) cells of the skin of Rag1−/− mice 7–8 weeks after they were transferred with EGFP− naïve splenic CD4+ Teff cells only (right) or a mixture of EGFP− naïve splenic CD4+ Teff and Treg cells (left). N=4. (G) FACS analysis for EGFP on skin T cells of one-month old CCR10+/− Scurfy and control mice. N=5.
We tested this notion further using two chronic inflammation models. First, we transferred Treg-depleted splenic naïve CD4+ T cells into Rag1−/− mice, which induces inflammation in the skin (and other tissues) due to absence of Treg cells (26, 27). Nearly no donor T cells expressed EGFP in the skin of recipients two months after transfer (Fig. 3F, right), while in Rag1−/− mice receiving splenic naïve CD4+ T and Treg cells, high percentages of skin T cells of both donor origins were EGFP+ (Fig. 3F, left), indicating that Treg-regulated immune homeostasis is critical for establishment and maintenance of CCR10+ T cells in skin. Consistent with this idea, only a few skin T cells were EGFP+ in Foxp3-defienct Scurfy mice, which lack Treg cells and develop the skin inflammation soon after the birth (Fig. 3G). Together, these results reveal an important role of CCR10 in a positive feedback circuit of immune homeostatic regulation in the skin where CCR10 maintains balanced presence and function of resident Treg vs. Teff cells, which in turn help to establish a homeostatic environment important for maintenance of the CCR10+ resident T cells.
CCR10 is critical for localization of CCR10+ T cells into the homeostatic skin
A drawback with the DNFB/FITC model is difficulty to follow antigen-specific T cells. Therefore, we crossed CCR10−/− mice to OT-I or OT-II mice that carry CD8+ or CD4+ T cells expressing transgenic αβTCRs specific for OVA antigens respectively. Purified naïve splenic EGFP− CCR10+/− and CCR10−/− OT-I (or OT-II) T cells were transferred into WT mice, followed by epicutaneous immunization with OVA on the ear (twice with a week interval) (23). One week after the second immunization, the inflamed ear and un-inflamed torso skin were analyzed for the OVA-specific T cells.
In the inflamed ear skin, newly infiltrating, OVA-specific CD8+ OT-I T cells accounted for the majority of total T cells (Fig. 4A). However, only a small portion of this subset was EGFP+ and CCR10-KO did not affect the percentage of EGFP+ OT-I T cells in the ear (Fig. 4A&C). In striking contrast, much higher percentages of infiltrating OT-I T cells in the untreated (and therefore un-inflamed) torso skin were EGFP+ and CCR10-KO significantly reduced the EGFP+ OT-I cells in the untreated skin (Fig. 4B&C), revealing that CCR10 is critical for localization of the CCR10+ CD8+ T cells into un-inflamed but not inflamed skin even in the same mouse. Similarly, CCR10 is also required for localization of CCR10+ CD4+ OT-II T cells into untreated skin but dispensable for their localization into inflamed skin stimulated by the OVA immunization (Fig. E7).
Figure 4.
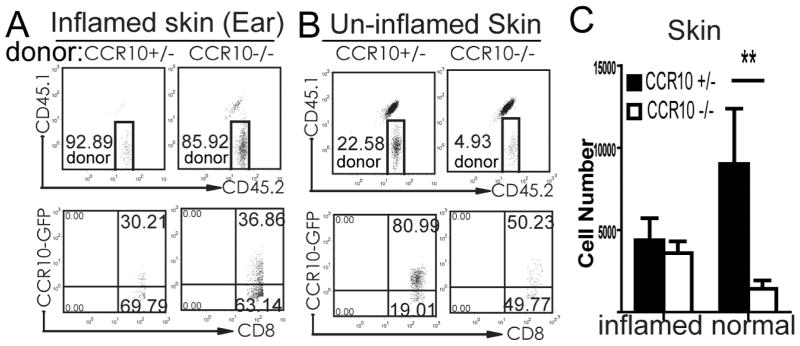
CCR10 is critical in localization of CCR10+ T cells into the homeostatic skin. (A–B) Flow cytometry of gated CD3+CD8+ T cells isolated from OVA-treated inflamed ear skin (A) and untreated torso skin (B) for the donor-derived OT-I T cells (CD45.1−CD45.2+) (top) and expression of EGFP of the donor OT-I T cells (bottom). (C) Average numbers of EGFP+ CCR10+/− and CCR10−/− OT-1 T cells isolated from the inflamed ear skin and un-affected torso skin based on the analyses of panel B. N=5–6.
Defective resolution of immune memory responses in skin of CCR10−/− mice
Skin-resident memory T cells play important roles in memory responses (1, 4–6). We tested CCR10−/− mice using a memory CHS assay, in which mice were challenged with DNFB one month (instead of five days in a classic CHS assay) after the initial DNFB sensitization. Compared to CCR10+/− mice, CCR10−/− mice had strikingly prolonged inflammation in the skin, associated with enhanced TNF-α and IL-1β and reduced IL-10 expression (Fig. 5A–C), suggesting that the imbalanced maintenance and function of the resident Treg vs. Teff cells also causes defective resolution of memory responses. Further supporting this notion, memory CHS responses in Rag1−/− mice transferred with splenic CCR10−/− T cells, which had defective maintenance of the donor-derived EGFP+ memory-like T cells in the skin after resolution of inflammation in classic CHS (Fig. 3C–D), were also prolonged compared to the recipients of CCR10+/− T cells (Fig. 5D). Notably, while there was no difference in the early memory response between immunized CCR10+/− and CCR10−/− mice the first day after re-application of DNFB (Fig. 5A), Rag1−/− mice receiving CCR10−/− T cells had slower memory responses than Rag1−/− mice receiving CCR10+/− T cells (Fig. 5D, Day 1). The different effects of CCR10-KO on the early response between the two models are likely because in direct comparison of CCR10−/− and CCR10+/− mice, the memory response could be affected by CCR10-regulated αβT as well as other cells while in Rag1−/− mice transferred with splenic T cells, some of the CCR10-regulated cells such as the epidermis-resident γδT cells are not reconstituted (21). The epidermis-resident γδT cells are known to impact the skin immune response (28).
Figure 5.
Defective resolution of immune memory responses in the skin of CCR10−/− mice. (A) Ear thickness changes of CCR10−/− and CCR10+/− mice in a DNFB-induced memory CHS assay. N≥6. (B) Representative H&E staining of ear sections 3 days after the DNFB-induced memory CHS response (X400). N=4. (C) qRT-PCR analysis of TNF-α, IL-1β and IL-10 transcripts in the treated skin 3 days after the DNFB-induced memory CHS. N=4. (D) Ear thickness changes of Rag1−/− mice transferred with EGFP− CCR10−/− and CCR10+/− splenic T cells in the DNFB-induced memory CHS. N≥6.
Enhanced immune response to and accelerated clearance of Leishmania major infection in the skin of CCR10−/− mice
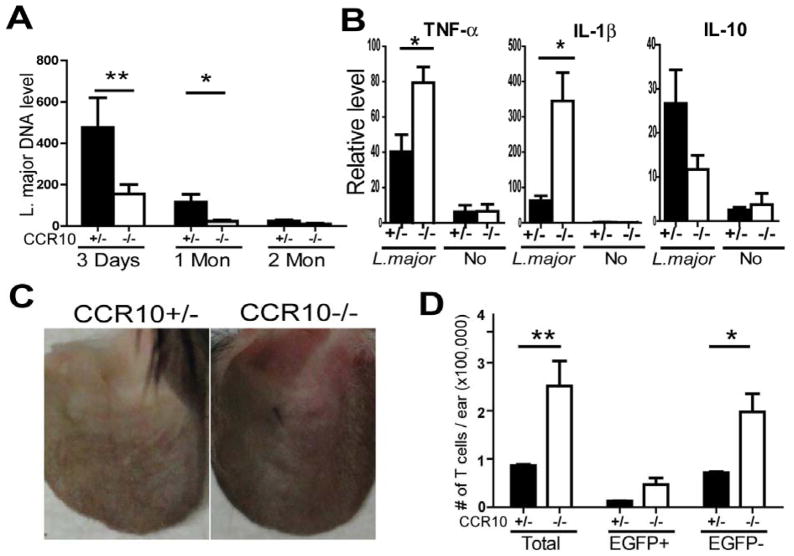
Our finding of the important role of CCR10 in regulating the skin-resident Treg and Teff cells to prevent over-active immune responses predicts that targeting CCR10 might enhance immune responses against skin-specific infections and other diseases. As a test, we infected CCR10−/− mice with L. major, a parasitic pathogen that could manipulate Treg cells to evade immune attack for its long-term survival in skin (29). Indeed, CCR10−/− mice cleared the parasite much faster than CCR10+/− mice (Fig. 6A). Consistent with an enhanced immune response, CCR10−/− mice had higher TNF-α and IL-1β production than CCR10+/− mice early post the infection (Fig. 6B), as in the case of chemical challenge (Fig. 1). In addition, 1 month post infection, significantly higher percentages of CCR10−/− mice developed visible inflammation at infection sites than CCR10+/− mice did [76% (13/17) vs. 38% (8/21), P=0.025](Fig. 6C), and the infected ears of CCR10−/− mice had many more T cells, most of which were EGFP−, than the CCR10+/− controls (Fig. 6D; Fig. E8), demonstrating that absence of CCR10 increased the effector T cell response for more efficient clearance of the infection in the skin.
Figure 6.
Accelerated clearance of L. major infection in skin of CCR10−/− mice. (A) Levels of Leishmania-specific 16S rDNA in the skin of CCR10−/− and CCR10+/− mice at different time-points after infection, determined by qPCR and normalized on mouse β-actin. N=11, 10 and 4 of each genotype for 3 days, 1 and 2 months post the infection respectively. (B) qRT-PCR analysis of TNF-α, IL-1β and IL-10 transcripts in the infected ears of CCR10−/− vs. CCR10+/− on Day 3 post the L. major infection. N=5 each. (C) Representative ear lesions at infection sites in CCR10−/− and CCR10+/− mice 1 month after the L. major injection. (D) Numbers of total, EGFP+ and EGFP− T cells in infected ears of CCR10−/− and CCR10+/− mice 1 month post the L. major infection. N=3 each.
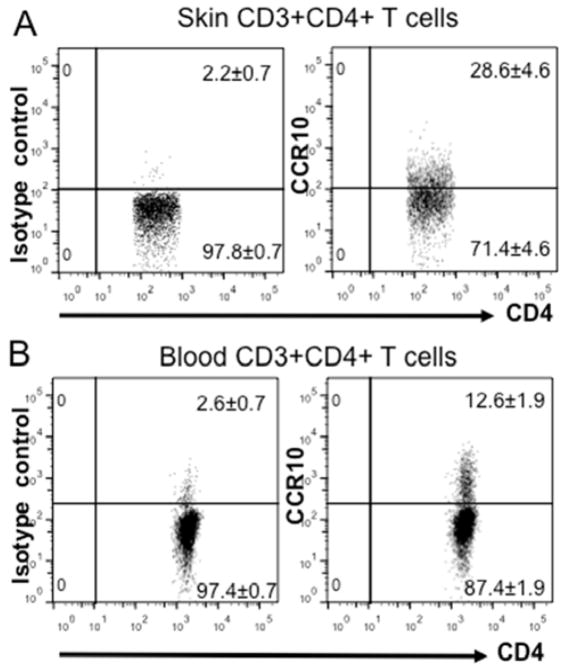
Significant percentages of T cells in healthy human skin express CCR10
In humans, all blood CCR10+ T cells display memory cell markers (9, 13). Therefore, CCR10/CCL27 might promote preferential maintenance of the CCR10+ memory-like T cells in the healthy skin. However, direct evidence of the enrichment of CCR10+ T cells in the skin is lacking. Therefore, we analyzed T cells isolated from human healthy skin for their expression of CCR10. Compared to T cells of blood, significantly higher percentages of T cells isolated from the normal human skin expressed CCR10 (Fig. 7), correlating with the different expression of CCR10 on circulating and skin-resident T cells in mice (Fig. 2 and Fig. E4). The similar expression pattern of CCR10 in the skin T cells of mice and humans suggests that CCR10 might function in the similar fashion in both human and mouse skin.
Figure 7.
Preferential expression of CCR10 by T cells of the healthy human skin. (A and B) Flow cytometric analysis of gated CD3+CD4+ T cells isolated from the healthy skin (A) and blood (B) of humans for the CCR10 expression. The number in each gate is the average percentage of cells in the gate of total events, expressed as means ± standard errors. N=3 individual samples for each analysis. The isotype control staining for the CCR10 staining was shown in the left panels.
DISCUSSION
CCR10/CCL27 are implicated in various skin allergy and inflammatory diseases but the in vivo function of the pair has been elusive, hindering our effort in understanding their involvement in the disease development (12). In this report, we provided the first definite evidence that CCR10 is critical in the regulation of immune homeostasis and resolution of inflammation, an advance from the current focus on the role of CCR10 as a homing molecule for infiltrating T cells to promote the immune response in the skin. Mechanistically, CCR10 could potentially function in several aspects to regulate the skin T cells (illustrated in Fig. E9). First, CCR10 is critical in migration of CCR10+ memory (or memory-like) T cell precursors into the skin under homeostatic conditions while it is dispensable for infiltration of T cells into the inflamed skin. In addition, CCR10 expressed on the skin-resident Treg and Teff cells is important for their balanced maintenance in the skin under homeostatic conditions, potentially by regulating their retention, proliferation and survival in the skin. Furthermore, our studies also suggest that CCR10 might signal to regulate functions of the CCR10+ skin-resident T cells such as their production of inflammatory and regulatory cytokines. In absence of CCR10, the dysreguated maintenance and functions of resident Teff and Treg cells in the skin could result in the over-active immune response and impaired resolution of skin inflammation. In light of our finding of CCR10 as a critical regulator of skin immune homeostasis in mice, the roles of the CCR10/CCL27 axis in skin allergy and inflammatory diseases in patients need to be carefully investigated before targeting the axis for treatment. It is possible that the upregulated expression of CCL27 in inflamed skin is secondary to the inflammation for a purpose of regulation. Therefore, developing a strategy to increase the number and activity of CCR10+ resident regulatory T cells might help to restore immune homeostasis and treat inflammatory diseases of the skin.
Considering both resident Treg and Teff cells are reduced in the skin of CCR10−/− mice, the over-active immune response to the skin stimulation is likely due to impaired presence and dysregulated function of both populations. Consistent with this, our study suggests that CCR10 has an intrinsic regulatory role in controlling function of Teff cells (Th17), which is in addition to potential regulatory effect of Treg cells on these effector T cells. Although mechanisms underlying the intrinsic regulatory functions of CCR10 in the Teff and Treg cells are not clear, our finding is reminiscent of functions of some other T cell regulatory receptors such as PD-1 and CTLA-4 that inhibit Teff cell functions and enhances Treg cell functions (30, 31). Considering the importance of balanced production of inflammatory and regulatory cytokines in immune homeostasis and inflammation, it would be important to elucidate the mechanism of CCR10 in regulating T cell activation and inflammatory cytokine production. In addition, addressing the origin of CCR10+ memory (or memory-like) Teff and Treg cells is another important question to assess.
CCR10-KO affects the presence of CD8+ Teff and Treg subsets in the skin to a larger extent than CD4+ Teff cells, suggesting that the different resident T cell subsets are intrinsically different in their requirements of CCR10 for maintenance. While the underlying mechanism is not clear, it seems to be associated with the different mobility and expression of skin retention molecules of the different T cells subsets (2, 32, 33). Of note, the skin-resident CD8+ memory T cells do not move while the CD4+ cells are highly mobile (2, 32, 33). While mobility of skin-resident Treg cells is well studied, they express the skin adhesion molecules CD103 as the resident CD8+ cells do while the resident CD4+ Teff cells do not (2, 32, 33).
While a predominantly net effect of CCR10-KO on skin immune responses is overreactive and prolonged inflammation, CCR10 might be important for an efficient effector memory T cell response. Rag1−/− mice reconstituted with CCR10−/− T cells had a slower and prolonged memory response compared to those reconstituted with CCR10+/− T cells (Fig. 5D), suggesting that both effector and resolution phases of the memory response are impaired. Likely, the CCR10-dependent efficient and balanced maintenance of resident Teff and Treg memory T cells in the skin allows a rapid effector T response to a challenge as well as proper resolution of the effector response after clearance of the challenge. Such efficient effector and regulatory responses are important for preservation of the function of skin while dealing with harmful environmental challenges. Dysregulation of either phase of the response could result in skin disease.
Supplementary Material
Key messages.
CCR10 is critical for skin immune homeostasis to control allergic responses.
CCR10 regulates balanced maintenance and function of skin-resident regulatory and effector T cells.
CCR10 is not expressed on infiltrating effector T cell for their migration during the skin inflammation.
Acknowledgments
Supported with a NIH grant and institutional funds from Penn State University (to N.X.).
Abbreviations
- CCR10
Chemokine (C-C motif) receptor 10
- CCL27
chemokine (C-C motif) ligand 27
- Treg
Regulatory T
- Teff
effector T
- CCR8
Chemokine (C-C motif) receptor 8
- CCR6
Chemokine (C-C motif) receptor 6
- DNFB
2,4-dinitro-1-fluorobenzene
- CHS
contact hypersensitive
- TPA
Phorbol 12-myristate 13-acetate
- FITC
Fluorescein 5(6)-isothiocyanate
- EGFP
Enhanced green fluorescent protein
- BM
bone marrow
- KO
Knockout
- KI
Knockin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 5.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2012;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 11.Jarmin DI, Rits M, Bota D, Gerard NP, Graham GJ, Clark-Lewis I, Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J Immunol. 2000;164:3460–3464. doi: 10.4049/jimmunol.164.7.3460. [DOI] [PubMed] [Google Scholar]
- 12.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 13.Soler D, Humphreys TL, Spinola SM, Campbell JJ. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- 14.McCully ML, Ladell K, Hakobyan S, Mansel RE, Price DA, Moser B. Epidermis instructs skin homing receptor expression in human T cells. Blood. 2012;120:4591–4598. doi: 10.1182/blood-2012-05-433037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 16.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Lin SX, Agha-Majzoub R, Overbergh L, Mathieu C, Chan LS. CCL27 is a critical factor for the development of atopic dermatitis in the keratin-14 IL-4 transgenic mouse model. Int Immunol. 2006;18:1233–1242. doi: 10.1093/intimm/dxl054. [DOI] [PubMed] [Google Scholar]
- 18.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirshahpanah P, Li YY, Burkhardt N, Asadullah K, Zollner TM. CCR4 and CCR10 ligands play additive roles in mouse contact hypersensitivity. Exp Dermatol. 2008;17:30–34. doi: 10.1111/j.1600-0625.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 20.Tubo NJ, McLachlan JB, Campbell JJ. Chemokine receptor requirements for epidermal T-cell trafficking. Am J Pathol. 2011;178:2496–2503. doi: 10.1016/j.ajpath.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 Is Important for the Development of Skin-Specific {gamma}{delta}T Cells by Regulating Their Migration and Location. J Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S, Yang K, Yang J, Li M, Xiong N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc Natl Acad Sci U S A. 2011;108:E1035–1044. doi: 10.1073/pnas.1100156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell JJ, O’Connell DJ, Wurbel MA. Cutting Edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolas L, Sidjanski S, Colle JH, Milon G. Leishmania major reaches distant cutaneous sites where it persists transiently while persisting durably in the primary dermal site and its draining lymph node: a study with laboratory mice. Infect Immun. 2000;68:6561–6566. doi: 10.1128/iai.68.12.6561-6566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Hong K, Chu A, Ludviksson BR, Berg EL, Ehrhardt RO. IL-12, independently of IFN-gamma, plays a crucial role in the pathogenesis of a murine psoriasis-like skin disorder. J Immunol. 1999;162:7480–7491. [PubMed] [Google Scholar]
- 27.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 30.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 33.Sharma R, Sung SS, Abaya CE, Ju AC, Fu SM, Ju ST. IL-2 regulates CD103 expression on CD4+ T cells in Scurfy mice that display both CD103-dependent and independent inflammation. J Immunol. 2009;183:1065–1073. doi: 10.4049/jimmunol.0804354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.