Abstract
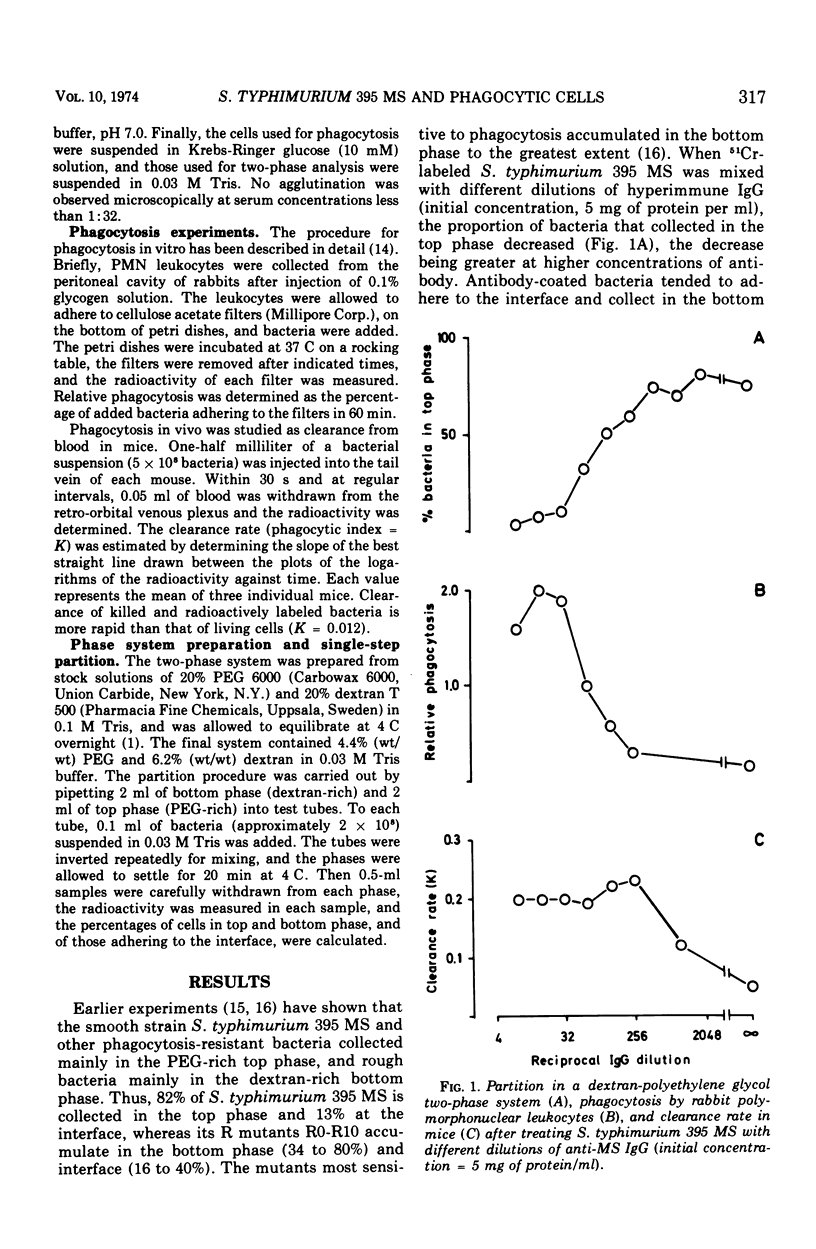
Partition in an aqueous, two-polymer phase system containing dextran and polyethylene glycol was employed to investigate the physicochemical changes inflicted by the presence of immunoglobulin G (IgG) antibodies on the cell surface of a smooth strain of Salmonella typhimurium. Adding increasing amounts of anti-Salmonella IgG to the bacteria decreased the affinity for the polyethylene glycol-rich top phase, with a concomitant increase in in vivo clearance and in vitro phagocytosis by rabbit polymorphonuclear cells. Similarly, S → R mutations in the same S. typhimurium strain decrease the affinity for the top phase and increase the liability to phagocytosis. The limiting antibody concentration to demonstrate increase of in vitro phagocytosis was approximately the same as that to produce a significant effect in the phase system, whereas lower concentrations were needed to increase the in vivo clearance. The results show that adsorption of IgG antibodies to bacteria brings about physicochemical changes of the cell surface which seem to promote the phagocytosis by polymorphonuclear cells and uptake in the reticuloendothelial system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edebo L., Normann B. Killing or protection of Salmonella typhimurium mutants by mammalian sera. Prog Immunobiol Stand. 1970;4:575–582. [PubMed] [Google Scholar]
- Gerisch G., Lüderitz O., Ruschmann E. Antikörper fördern die Phagozytose von Bakterien durch Amöben. Z Naturforsch B. 1967 Jan;22(1):109–109. [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Henson P. M. The adherence of leucocytes and platelets induced by fixed IgG antibody or complement. Immunology. 1969 Jan;16(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., De Stefano M. J. Antibody and plant agglutinins stimulate phagocytosis of erythrocytes by Acanthamoeba. Nature. 1971 Dec 17;234(5329):414–415. doi: 10.1038/234414a0. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Phagocytosis: the engulfment stage. Semin Hematol. 1968 Apr;5(2):134–155. [PubMed] [Google Scholar]
- Rowley D., Turner K. J. Number of molecules of antibody required to promote phagocytosis of one bacterium. Nature. 1966 Apr 30;210(5035):496–498. doi: 10.1038/210496a0. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Magnusson K. E., Tagesson C., Cunningham R., Edebo L. Characterization of mutants of Salmonella typhimurium by counter-current distribution in an aqueous two-polymer phase system. Infect Immun. 1973 Apr;7(4):573–577. doi: 10.1128/iai.7.4.573-577.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Edebo M. Partition of Salmonella typhimurium in a two-polymer acqueous phase system in relation to liability to phagocytosis. Infect Immun. 1973 Jul;8(1):36–41. doi: 10.1128/iai.8.1.36-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Weissmann G., Brand A., Franklin E. C. Interaction of immunoglobulins with liposomes. J Clin Invest. 1974 Feb;53(2):536–543. doi: 10.1172/JCI107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J., Gillman C. F., Good R. J. The influence of the shape of phagocytes on their adhesiveness. Immunol Commun. 1972;1(6):627–636. doi: 10.3109/08820137209022969. [DOI] [PubMed] [Google Scholar]



