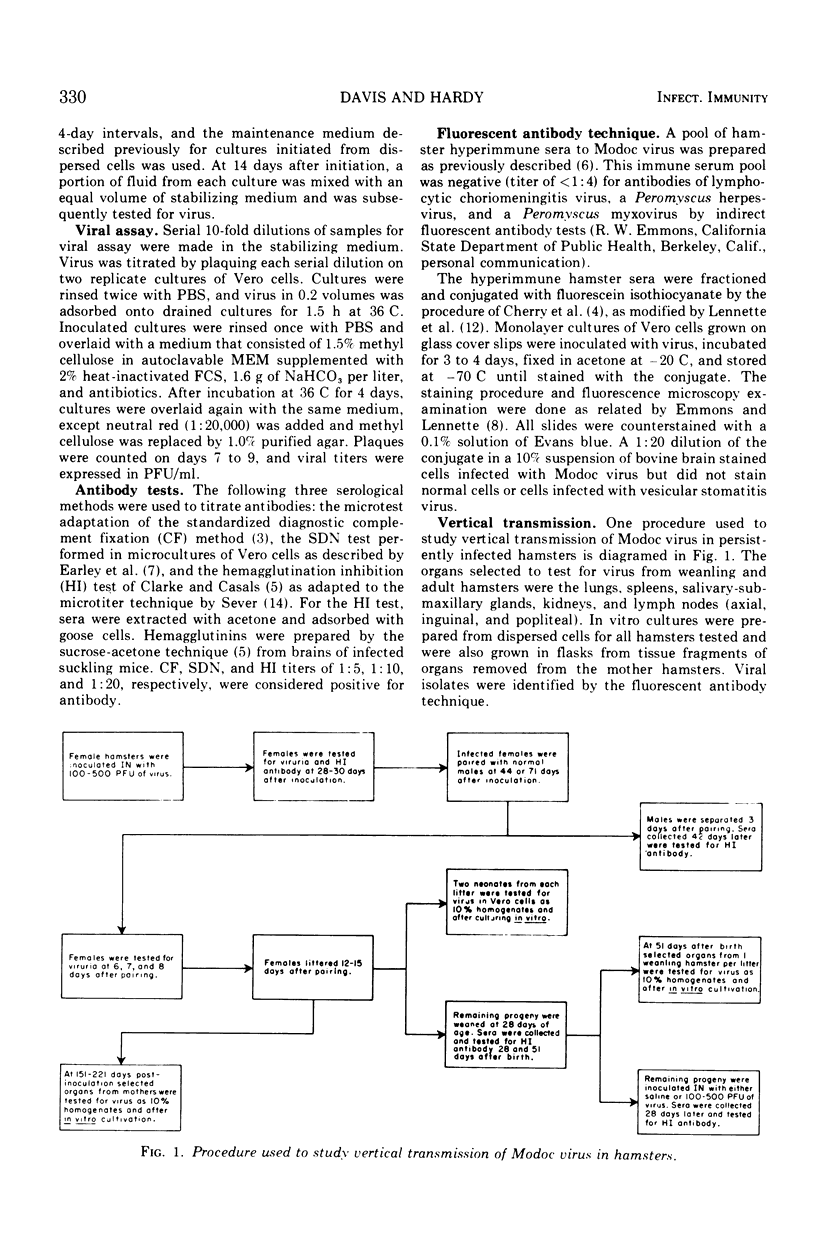
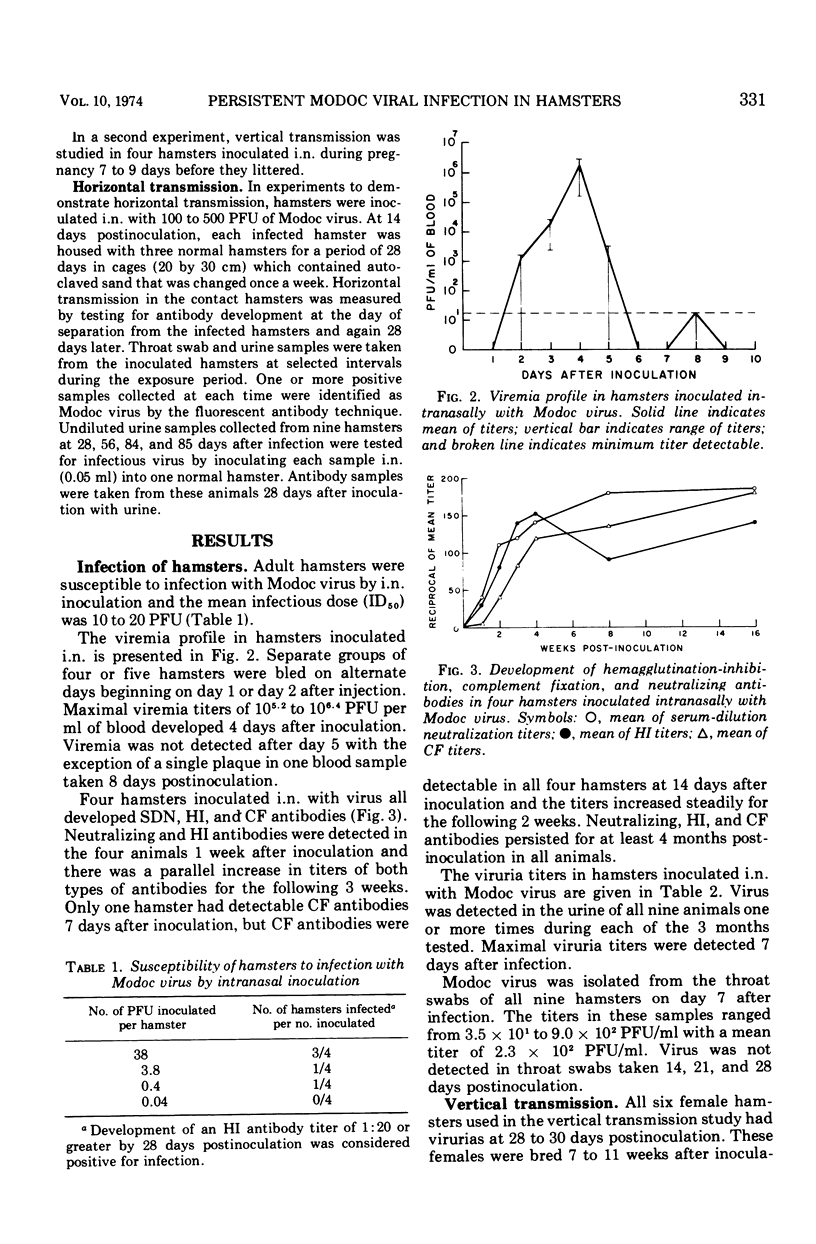
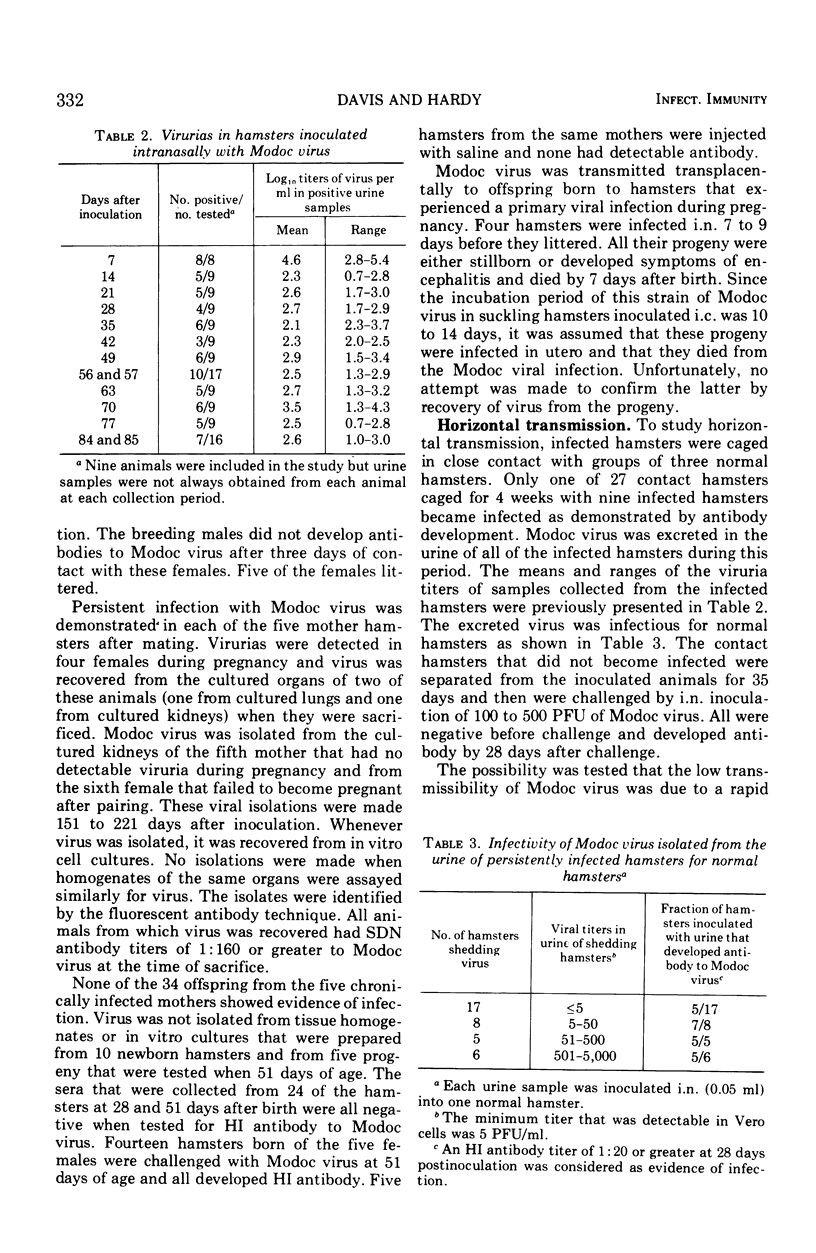
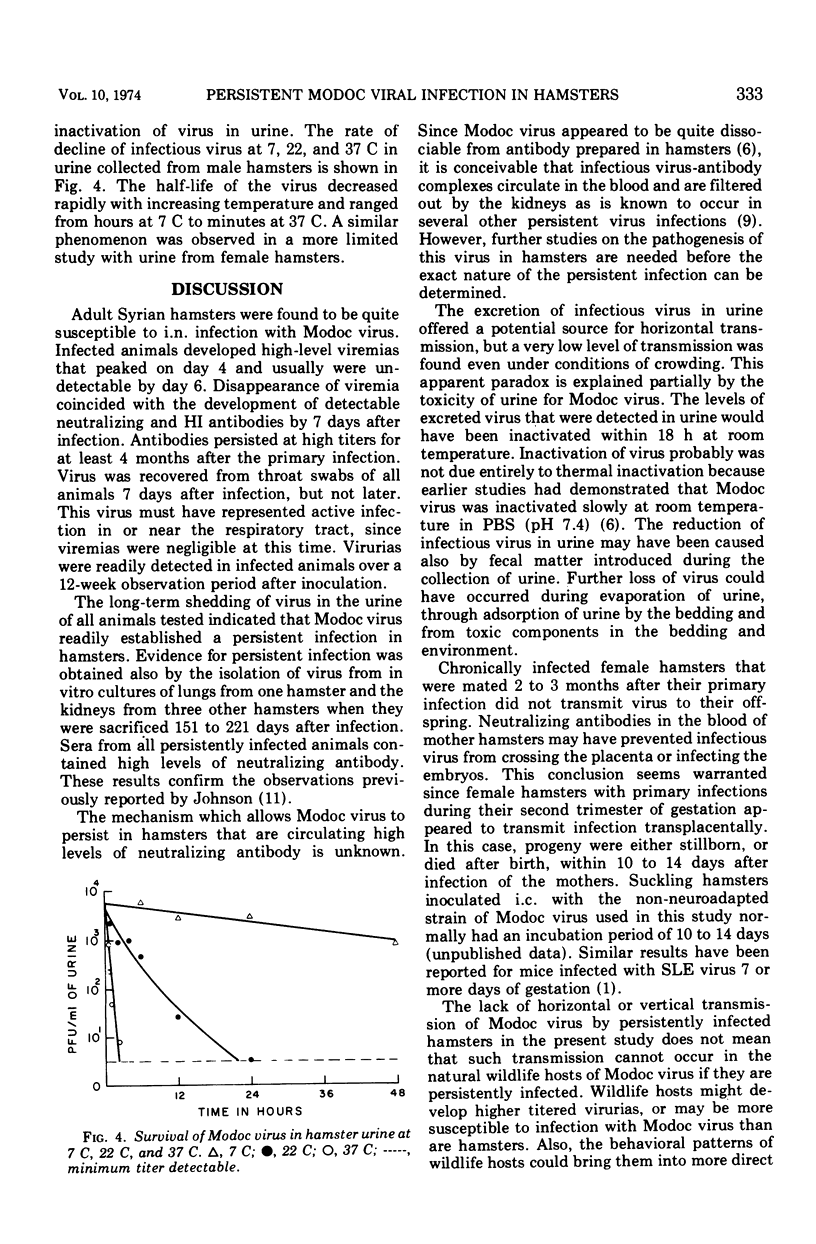
Abstract
Adult Syrian hamsters were readily infected by intranasal inoculation with Modoc virus. Viremias were detected 2 to 6 days after infection and peak viremia titers (106.2 plaque-forming units/ml of blood) occurred 4 days postinoculation. All infected animals developed neutralizing and hemagglutination-inhibiting antibodies by 7 days, and complement-fixing antibodies by 14 days postinoculation. High titers of antibodies persisted for at least 4 months. Modoc virus was recovered from throat swabs at 7 days postinoculation, but not at 14 days or later. Urine samples were positive for virus throughout a 12-week observation period. Isolation of virus from lungs and kidneys of one and three animals, respectively, at 151 to 221 days after inoculation confirmed chronic infection. Viral isolations were made only when organs were cultivated in vitro and were unsuccessful by tests on 10% homogenates of the organs. Horizontal viral transmission of virus by infected hamsters that were viruric was demonstrated in only 1 of 27 normal hamsters that were cocaged for 4 weeks under crowded conditions. General failure to obtain horizontal viral transmission may relate to rapid inactivation of virus in excreted urine. Vertical viral transmission was not demonstrated from five chronically infected female hamsters to their 34 offspring. However, if primary infection occurred during pregnancy, the progeny were either stillborn or died shortly after birth, and thus appeared to represent transplacental viral transmission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. A., Hanson R. P. Experimental transplacental transmission of st. Louis encephalitis virus in mice. Infect Immun. 1970 Sep;2(3):320–325. doi: 10.1128/iai.2.3.320-325.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASALS J. Antigenic relationship between Powassan and Russian spring-summer encephalitis viruses. Can Med Assoc J. 1960 Feb 13;82:355–358. [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Davis J. W., Hardy J. L. In vitro studies with Modoc virus in Vero cells: plaque assay and kinetics of growth, neutralization, and thermal inactivation. Appl Microbiol. 1973 Sep;26(3):344–348. doi: 10.1128/am.26.3.344-348.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley E., Peralta P. H., Johnson K. M. A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med. 1967 Jul;125(3):741–747. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- Emmons R. W., Lennette E. H. Immunofluorescent staining in the laboratory diagnosis of Colorado tick fever. J Lab Clin Med. 1966 Dec;68(6):923–929. [PubMed] [Google Scholar]
- Johnson H. N. Ecological implications of antigenically related mammalian viruses for which arthropod vectors are unknown and avian associated soft tick viruses. Jpn J Med Sci Biol. 1967 Dec;20 (Suppl):160–166. [PubMed] [Google Scholar]
- Johnson H. N. Long-term persistence of Modoc virus in hamster-kidney cells. In vivo and in vitro demonstration. Am J Trop Med Hyg. 1970 May;19(3):537–539. doi: 10.4269/ajtmh.1970.19.537. [DOI] [PubMed] [Google Scholar]
- LENNETTE E. H., WOODIE J. D., NAKAMURA K., MAGOFFIN R. L. THE DIAGNOSIS OF RABIES BY FLUORESCENT ANTIBODY METHOD (FRA) EMPLOYING IMMUNE HAMSTER SERUM. Health Lab Sci. 1965 Jan;2:24–34. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]



