Abstract
Background
The treatment of cancer patients with mistletoe extract is said to prolong their survival and, above all, improve their quality of life. We studied whether the quality of life of patients with advanced pancreatic cancer could be improved by mistletoe extract.
Method
An open, single-center, group-sequential, randomized phase III trial (ISRCTN70760582) was conducted. From January 2009 to December 2010, 220 patients with locally advanced or metastatic pancreatic cancer who were receiving no further treatment for pancreatic cancer other than best supportive care were included in this trial. They were stratified by prognosis and randomly allocated either to a group that received mistletoe treatment or to one that did not. Mistletoe extract was given in escalating doses by subcutaneous injection three times a week. The planned interim evaluation of data from 220 patients indicated that mistletoe treatment was associated with longer overall survival, and the trial was terminated prematurely. After termination of the study, the results with respect to quality of life (assessed with the QLO-C30 scales of the European Organisation for Research and Treatment of Cancer) and trends in body weight were evaluated.
Results
Data on quality of life and body weight were obtained from 96 patients treated with mistletoe and 72 control patients. Those treated with mistletoe did better on all 6 functional scales and on 7 of 9 symptom scales, including pain (95% confidence interval [CI] -29 to –17), fatigue (95% CI –36.1 to –25.0), appetite loss (95% CI -51 to -36.7), and insomnia (95% CI –45.8 to –28.6). This is reflected by the trend in body weight during the trial.
Conclusion
In patients with locally advanced or metastatic pancreatic carcinoma, mistletoe treatment significantly improves the quality of life in comparison to best supportive care alone. Mistletoe is an effective second-line treatment for this disease.
For patients with pancreatic carcinoma who cannot tolerate first-line treatment as specified in the S3 guideline recommendations (1) or who decline to undergo such treatment, best supportive care is often the only available therapeutic option (2– 5). Second-line treatment for these patients must be very well tolerated and should not only prolong survival, but also improve the quality of life (6).
Prolongation of survival and improvement of the quality of life are the two goals of mistletoe treatment. Mistletoe is well tolerated, even in high doses (7), but a Cochrane Review found inadequate evidence to document its efficacy (8). In a critical evaluation of this review, however, it was pointed out that the review ignored 14 previously published studies providing grade I and II evidence on survival, tumor behavior, and quality of life (Kienle, G. S. and Kiene, H. Comment on “Mistletoe therapy in oncology” [Cochrane-Review 2008]; Web/URL: www.ifaemm.de/Abstract/PDFs/GK08_2.pdf). A later retrospective study of mistletoe treatment for pancreatic carcinoma yielded further evidence of its efficacy (9).
In this prospective, randomized trial, we investigated the efficacy of mistletoe monotherapy on the survival and quality of life (QoL) of patients with locally advanced or metastatic pancreatic carcinoma.
Such trials, if conducted in countries where mistletoe extracts are approved or registered, are bound to meet with difficulties in recruitment and compliance (10), because the physicians and patients already have a clear preference. In Serbia, however, mistletoe extracts are unknown and unavailable, and we were able to carry out this trial there without any problem with respect to recruitment.
The analysis of overall survival (the primary endpoint of the trial), undesired events, and disease-related symptoms has been published elsewhere (11).
Methods
This randomized, prospective, open phase III trial was conducted in accordance with the Declaration of Helsinki and with the approval of the central ethics committee of the First Surgical Clinic of the Clinical Centers of Serbia (CCS) and the of Serbian approval authority, in the department of hepatobiliary diseases of the CCS in Belgrade. All patients were fully informed by the trial physician and gave their written consent to participation. This trial is registered in the Current Controlled Trials database with the registration number ISRCTN70760582.
The group-sequential design of the trial included a planned initial interim assessment of overall survival and drug safety after the inclusion of the first 220 patients, to be carried out by an independent committee of assessors (Independent Data Monitoring Committee, IDMC). The IDMC recommended the early termination of the trial and publication of its findings because of the demonstrated efficacy of mistletoe treatment.
Patients
Potential participants in the trial from across Serbia were identified in the weekly oncological consultations of the CCS. Patients with inoperable locally advanced or metastatic pancreatic carcinoma were referred to the trial director for assessment of their eligibility if they were not treated with any form of chemotherapy (gemcitabine or other) because chemotherapy was not medically recommended or was declined by the patient, or if any of the following criteria were met:
Eastern Cooperative Oncology Group (ECOG) performance status > 2
bilirubin > 50 µmoL/L
transaminases > 100 U/L
leukocyte count > 10.0 × 109
The decision of the CCS consultation service was considered final.
The inclusion criteria were as follows:
locally advanced or metastatic pancreatic carcinoma (Union for International Cancer Control [UICC] stage III/IV)
age ≥ 18 years
written consent
any type of prior treatment was allowed
unsuitability for, or unwillingness to undergo, any other type of cancer treatment
leukocyte count ≥ 3000/mm³
platelet count ≥ 100 000/mm³
The main exclusion criteria were the following:
life expectancy < 4 weeks
weight loss of ≥ 20% in the past 6 weeks
brain metastases
Patients who met all inclusion criteria and none of the exclusion criteria were included in the trial.
During the trial, all patients received best supportive care (BSC), which was delivered by the trial physicians. The nature of BSC was determined in the trial center; it consisted of the symptomatic treatment of pain, nausea, vomiting, and dyspepsia and was individually adapted at each of the patient's visits (in Months 1, 2, 3, 6, 9, and 12).
In addition, patients in the mistletoe-extract group were treated three times per week, every week for the duration of the trial (up to one year), with subcutaneous injections of 1 ml of mistletoe extract. The injections were given by the patients themselves or by family members, or else by personnel in local treatment centers. The mistletoe extract used was identical to Iscador Qu, a commercially available extract of Viscum album (L.) quercus (manufacturer: Weleda AG, Arlesheim, Switzerland). Iscador is approved for subcutaneous administration for the adjuvant and palliative treatment of cancer, alone or in combination with conventional treatment, in Germany, Switzerland, Austria, Sweden, and Georgia, and it is a registered homeopathic drug in Italy, France, and the United Kingdom (12). In this trial, it was given initially in two injections of 0.01 mg mistletoe extract each, followed by two of 0.1 mg, five of 1 mg, five of 2 mg, eight of 5 mg, and finally by the constant target dose of 10 mg per injection over the remaining duration of the trial.
Hypotheses, calculation of case numbers, stratification, and randomization
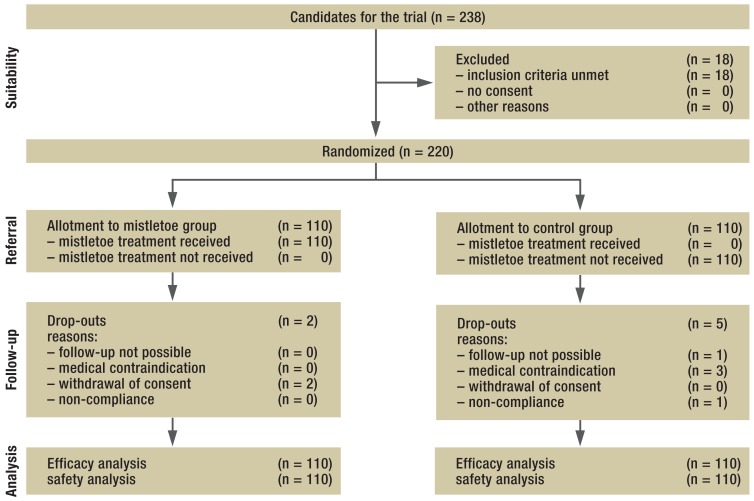
The primary endpoint of the trial was overall survival; quality of life was a secondary endpoint. An improvement in both endpoints with mistletoe, compared to control treatment, was expected a priori. On the basis of earlier trial data on mistletoe treatment of pancreatic carcinoma (9, 13), it was estimated that this trial would need to include 214 patients per group, or 173 patients per group without drop-outs, to demonstrate a survival advantage for mistletoe treatment (11). Patients were randomly allocated to the two groups (mistletoe and control) in a 1:1 ratio after stratification according to their prognosis, which was rated as either good or poor. A poor prognosis was assigned if at least two of the following criteria were met: UICC = IV, age > 65 years, ECOG ≥ 2 (eFigure). Separate randomization lists with variable block sizes (4, 6, and 8) were generated for each prognosis group, and the opaque, sealed envelopes containing randomization assignments were stored at the study headquarters.
eFigure.
The distribution of patients with locally advanced or metastatic pancreatic carcinoma who received mistletoe treatment or belonged to the untreated control group
Statistical methods
The multiple dimensions of quality of life were assessed with the basis questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30), Version 3.0, in its validated Serbian version. Patients filled out the questionnaire themselves, or with the aid of the trial nurse, upon enrollment in the trial and before each visit in Months 1, 2, 3, 6, 9, and 12. The questionnaires were not evaluated until the end of the trial. The EORTC QLQ-C30 has 15 scales: five functional scales, nine symptom scales, and a scale for the patient's global quality of health. The scale values were calculated as specified in the EORTC-QLQ-C30 manual (14), and the results were tabulated in accordance with the guidelines (15). All patients who made at least one post-baseline visit were included in the analysis (the evaluable population). Values that were missing because a patient had died were not replaced.
The risk of dying before the first post-baseline visit because of certain demographic, clinical, or quality-of-life characteristics, with resulting non-inclusion in the quality-of-life analysis (non-evaluable population), was studied with the aid of logistic regression models that were based on each parameter to be tested and its interaction with the trial treatment as factors. The EORTC-QLQ-C30 values of the quality-of-life population at baseline were compared between the two treatment groups with a t-test.
The primary statistical evaluation of the EORTC-QLQ-C30 scales was performed with a mixed linear model in which the difference between the quality-of-life score at each post-baseline visit and its baseline value served as the dependent variable. The independent variables were the timepoint of the visit 2 to 7 and the arm of the trial (mistletoe treatment vs. control); the patients were modeled as random factors, and the dependence of individual times of evaluation in a single patient was reflected in a variance-component covariance structure. This model yields valid test values and estimates, as long as missing values can be assumed to be missing at random. The patients in the two study groups, however, differed in overall survival (the median survival of patients receiving mistletoe treatment was 4.8 months, while that of control patients was 2.7 months; hazard ratio 0.49, p < 0.0001 [11]). Therefore, for sensitivity analysis, an interaction term between the arm of the trial and the timepoint of the visit was additionally incorporated into the model. Moreover, the non-parametric Van Elteren test (16) was used to analyze four values from the temporal course of each quality-of-life variable: the mean, median, worst, and temporally last post-baseline value of each patient, stratified by the timepoint of his or her last regular visit (and thus, approximately, his or her duration of survival). The resulting 15 significance values for the various quality-of-life scales were adjusted for multiple testing with a Bonferroni-Holm correction (17). The clinical relevance of group differences on the various quality-of-life scales was characterized as described by Osoba et al. (18) as small (a difference of 5–10 points), moderate (11–20 points), or large (> 20 points).
The test physicians examined the patients at each visit and documented the severity of typical symptoms of cancer, including weight loss according to CTCAE 3.0 (Common Terminology Criteria for Adverse Events) and undesired events according to Good Clinical Practice (GCP). Toleration of the trial medication (mistletoe extract) was assessed with the aid of a patient diary, which also enabled compliance checking. Body weight was evaluated according to the quality-of-life scales. The analysis of further safety variables has been described elsewhere (11).
All statistical tests were two-tailed; p-values less than or equal to 0.05 were considered significant. Statistical analyses were performed with SAS Versions 9.3 and 9.4.
Results
Patients and treatment
Of the 238 patients screened, 220 were enrolled in the trial from January 2009 to December 2010 (eFigure). 25 had been given a diagnosis of locally advanced or metastatic pancreatic cancer on the basis of imaging studies; for the remaining 195, the diagnosis had been made at surgery. 43 had histologically confirmed adenocarcinoma.
All patients were given best supportive care. The 110 patients in the mistletoe treatment group received a median of 61.5 documented subcutaneous injections (minimum 3, maximum 156 per patient). No deviations from the planned dose-increasing scheme were necessary. Patients in the control group were not treated with mistletoe. Although the decisions of the oncology consultation service were intended to be final, three patients in the control group began chemotherapy during the trial and were removed from the trial as stipulated by the drug-approving authority.
Quality of life and body weight
At the outset of the trial, the two groups had similar body weight and similar characteristics on all EORTC-QLQ-C30 scales, except for a few scales on which the values of the mistletoe group were worse (Tables 1 and (2). Fewer questionnaires were filled out at later visits because patients died in the intervening time (Table 4). 14 patients in the mistletoe group and 38 in the control group did not come for a single follow-up visit. Significant risk factors for this were:
Table 1. Demographic and clinical characteristics of patients with locally advanced or metastatic pancreas carcinoma who were or were not able to attend at least one follow-up visit (the evaluable and non-evaluable populations, respectively), at baseline.
| Non-evaluable population | Evaluable population | ||||
|---|---|---|---|---|---|
| Patient characteristics | Mistletoe treatment (14 pts.) | Control(38 pts.) | Mistletoe treatment (96 pts.) | Control (72 pts.) | |
| Sex | male | 7 (50.0%) | 26 (68.4%) | 58 (60.4%) | 37 (51.4%) |
| female | 7 (50.0%) | 12 (31.6%) | 38 (39.6%) | 35 (48.6%) | |
| Age (years) | ≤ 65 | 7 (50.0%) | 22 (57.9%) | 64 (66.7%) | 34 (47.2%) |
| > 65 | 7 (50.0%) | 16 (42.1%) | 32 (33.3%) | 38 (52.8%) | |
| Ethnicity | Caucasian | 14 (100%) | 38 (100%) | 96 (100%) | 72 (100%) |
| ECOG* | 0–1 | 3 (21.4%) | 14 (36.8%) | 53 (55.2%) | 42 (58.3%) |
| 2–4 | 11 (78.6%) | 24 (63.2%) | 43 (44.8%) | 30 (41.7%) | |
| UICC stage* | III | 4 (28.6%) | 17 (44.7%) | 53 (55.2%) | 47 (65.3%) |
| IV | 10 (71.4%) | 21 (55.3%) | 43 (44.8%) | 25 (34.7%) | |
| Prognostic group | unfavorable | 10 (71.4%) | 22 (57.9%) | 45 (46.9%) | 34 (47.2%) |
| favorable | 4 (28.6%) | 16 (42.1%) | 51 (53.1%) | 38 (52.8%) | |
| Operation* | no | 2 (14.3%) | 6 (15.8%) | 4 (4.2%) | 3 (4.2%) |
| yes | 12 (85.7%) | 32 (84.2%) | 92 (95.8%) | 69 (95.8%) | |
| Affected part of the pancreas | head | 7 (50.0%) | 18 (47.4%) | 51 (53.1%) | 38 (52.8%) |
| body | 1 (7.1%) | 4 (10.5%) | 11 (11.5%) | 8 (11.1%) | |
| tail | 2 (14.3%) | 1 (2.6%) | 5 (5.2%) | 2 (2.8%) | |
| head and body | 1 (7.1%) | 6 (15.8%) | 17 (17.7%) | 12 (16.7%) | |
| body and tail | 3 (21.4%) | 9 (23.7%) | 11 (11.5%) | 12 (16.7%) | |
| head. body. and tail | 0 (0%) | 0 (0%) | 1 (1.0%) | 0 (0%) | |
| TNM (T) | 3 | 2 (14.3%) | 1 (2.6%) | 0 (0%) | 0 (0%) |
| 4 | 12 (85.7%) | 37 (97.4%) | 95 (99.0%) | 72 (100%) | |
| X | 0 (0%) | 0 (0%) | 1 (1.0%) | 0 (0%) | |
| TNM (N) | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.4%) |
| 1 | 2 (14.3%) | 2 (5.3%) | 12 (12.5%) | 11 (15.3%) | |
| X | 12 (85.7%) | 36 (94.7%) | 84 (87.5%) | 60 (83.3%) | |
| TNM (M)* | 0 | 4 (28.6%) | 17 (44.7%) | 53 (55.2%) | 47 (65.3%) |
| 1 | 10 (71.4%) | 21 (55.3%) | 43 (44.8%) | 25 (34.7%) | |
| Hepatic metastases** | no | 6 (42.9%) | 19 (50.0%) | 59 (61.5%) | 55 (76.4%) |
| yes | 8 (57.1%) | 19 (50.0%) | 37 (38.5%) | 17 (23.6%) | |
A significant effect of the variable in question on inclusion in the QoL population is indicated by *(p < 0.05) or **(p>0.01) (logistic regression with an interaction factor between the variable in question and treatment group). No significant interactions were found between the variables and the treatment groups.
*ECOG. Eastern Cooperative Oncology Group (performance scale); UICC. Union for International Cancer Control; TNM. TNM classification of the UICC.
Table 2. Baseline values (mean ± standard deviation) of the EORTC-QLQ-C30 scale values of patients with locally advanced or metastatic pancreatic cancer who were or were not able to attend at least one follow-up visit (i.e., respectively, the evaluable population, with 96 patients treated with mistletoe and 72 control patients, and the non-evaluable population, with 14 patients treated with mistletoe and 38 control patients).*.
| EORTC scale | Group | Non-evaluable population | Evaluablepopulation | Factor for inclusion in the evaluable population | Evaluable population, mistletoe therapy vs. control | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | p-value | ||
| Global quality of health | Mistletoe treatment | 29.2 | ± 7.1 | 32.0 | ±11.6 | 0.0385 | 0.1531 |
| Control | 29.4 | ± 11.6 | 34.8 | ± 13.2 | |||
| Role functioning | Mistletoe treatment | 56.0 | ± 19.2 | 63.0 | ± 18.0 | 0.7597 | 0.8847 |
| Control | 62.3 | ± 20.4 | 63.4 | ± 17.8 | |||
| Social functioning | Mistletoe treatment | 58.3 | ± 22.4 | 61.5 | ± 21.0 | 0.6133 | 0.2540 |
| Control | 67.1 | ± 22.1 | 65.0 | ± 19.4 | |||
| Cognitive functioning | Mistletoe treatment | 71.4 | ± 22.1 | 73.1 | ± 21.4 | 0.8804 | 0.6199 |
| Control | 75.4 | ± 23.2 | 74.8 | ± 21.8 | |||
| Physical functioning | Mistletoe treatment | 71.9 | ± 13.4 | 76.7 | ± 12.8 | 0.1265 | 0.7715 |
| Control | 71.1 | ± 16.1 | 76.0 | ± 15.3 | |||
| Emotional functioning | Mistletoe treatment | 71.4 | ± 21.1 | 75.1 | ± 19.7 | 0.6903 | 0.1408 |
| Control | 80.8 | ± 18.8 | 79.4 | ± 17.9 | |||
| Pain | Mistletoe treatment | 52.4 | ± 18.3 | 48.1 | ± 17.9 | 0.3786 | 0.9120 |
| Control | 51.3 | ± 17.9 | 48.4 | ± 15.9 | |||
| Fatigue | Mistletoe treatment | 50.0 | ± 13.6 | 48.0 | ± 15.7 | 0.5534 | 0.5467 |
| Control | 48.5 | ± 18.9 | 46.6 | ± 14.8 | |||
| Appetite loss | Mistletoe treatment | 42.9 | ± 24.2 | 37.2 | ± 21.6 | 0.3954 | 0.3066 |
| Control | 37.7 | ± 27.0 | 33.8 | ± 20.6 | |||
| Financial problems | Mistletoe treatment | 40.5 | ± 23.3 | 39.2 | ± 22.7 | 0.9385 | 0.0375 |
| Control | 31.6 | ± 26.8 | 31.9 | ± 22.0 | |||
| Insomnia | Mistletoe treatment | 35.7 | ± 27.6 | 30.6 | ± 25.0 | 0.3413 | 0.5266 |
| Control | 23.7 | ± 26.7 | 28.2 | ± 22.1 | |||
| Nausea/vomiting | Mistletoe treatment | 15.5 | ± 13.8 | 13.4 | ± 14.2 | 0.5058 | 0.5229 |
| Control | 12.7 | ± 17.5 | 14.8 | ± 14.7 | |||
| Diarrhea | Mistletoe treatment | 0.0 | ± 0.0 | 2.4 | ± 8.7 | 0.6498 | 0.0517 |
| Control | 0.9 | ± 5.4 | 0.5 | ± 3.9 | |||
| Constipation | Mistletoe treatment | 0.0 | ± 0.0 | 0.7 | ± 4.8 | 0.9883 | 0.1584 |
| Control | 0.0 | ± 0.0 | 0.0 | ± 0.0 | |||
| Dyspnea | Mistletoe treatment | 0.0 | ± 0.0 | 0.0 | ± 0.0 | – | – |
| Control | 0.0 | ± 0.0 | 0.9 | ± 7.9 | |||
*The function scales are arranged in increasing order of baseline values, and the symptom scales in decreasing order. A significant influence of any particular variable on inclusion in the QoL population was determined with the aid of logistic regression with the variable in question and the interaction between this variable and the treatment groups (factor for inclusion in the evaluable population). The two treatment groups within the evaluable population were compared with a t-test.
Effects that could not be estimated because a factor was too weak are designated with a dash.
EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30;
SD, standard deviation.
Table 4. The number of questionnaires filled out and returned by the 220 patients with locally advanced or metastatic pancreatic cancer who received mistletoe therapy or were in the untreated control group.
| Visit no. | Timepoint(month) | Planned timepoint (day) | Actual time-point of visit (Day) | Not returned because of death | Drop-outs* | Number of questionnaires expected | Number of questionnaires received |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| 1 | 0 | 0 | 0–0 | 0 | 0 | 110 | 110 (100%) |
| 2 | 1 | 31 | 19–69 | 7 | 0 | 103 | 72 (70%) |
| 3 | 2 | 61 | 50–12 | 48 | 0 | 62 | 54 (87%) |
| 4 | 3 | 92 | 84–131 | 69 | 0 | 41 | 32 (78%) |
| 5 | 6 | 183 | 164–203 | 94 | 0 | 16 | 11 (69%) |
| 6 | 9 | 275 | 260–279 | 103 | 2 | 5 | 3 (60%) |
| 7 | 12 | 365 | – | 105 | 3 | 2 | 0 (0%) |
| Mistletoe treatment | |||||||
| 1 | 0 | 0 | 0–0 | 0 | 0 | 110 | 110 (100%) |
| 2 | 1 | 31 | 21–56 | 2 | 1 | 107 | 96 (90%) |
| 3 | 2 | 61 | 49–89 | 26 | 1 | 83 | 76 (92%) |
| 4 | 3 | 92 | 78–143 | 39 | 1 | 70 | 62 (89%) |
| 5 | 6 | 183 | 169–238 | 67 | 2 | 41 | 34 (83%) |
| 6 | 9 | 275 | 240–328 | 81 | 2 | 27 | 23 (83%) |
| 7 | 12 | 365 | 363–425 | 91 | 0 | 19 | 17 (89%) |
*3 patients dropped out because of a medical contraindication, and 2 dropped out by withdrawing their consent to participation.
an ECOG score from 2 to 4 (odds ratio [OR] 2.85, 95% confidence interval [CI] 1.45–5.62)
not having undergone surgery (OR 4.05, 95% CI 1.33–12.32)
UICC stage IV (OR 2.51, 95% CI 1.29–4.88), due in most cases to hepatic metastases (OR 2.77, 95% CI 1.41–5.43)
low values on the EORTC-QLQ-C30 scale “global quality of health” (OR for a difference of 10 points, 1.393; 95% CI 1.042–1.861).
There were no significant interactions between these or other risk factors and the treatment groups (Tables 1 and (2).
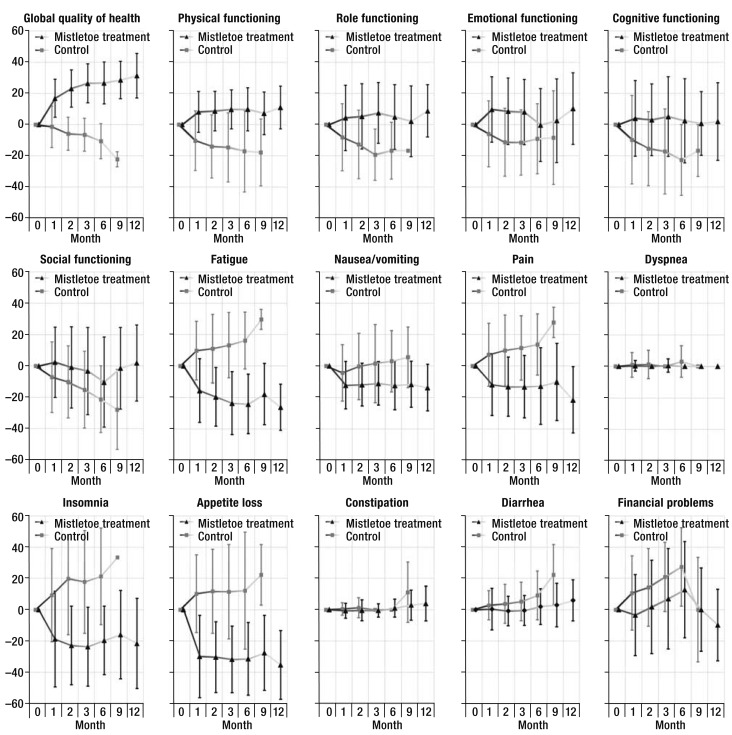
The principal analysis of all 15 scales of the EORTC QLQ-C30 revealed a significant difference between the two treatment groups on 13 of the scales; the constipation and dyspnea scales were the exceptions. The clinical relevance of the difference between groups was large for 6 of the 13 scales with significant differences, and moderate for 5. The inclusion of an interaction term between visits and trial treatment generally increased the statistical significance of intergroup differences, with two exceptions (the scales relating to social function and financial problems). The results of the principal analysis were confirmed in non-parametric, stratified sensitivity analysis for all scales except the constipation scale (Table 3, eTable 1). All 15 scales of the EORTC QLQ-C30 are represented in Figure 2.
Table 3. Estimated values (Mean and 95% confidence interval) for differences between the mistletoe treatment and control groups of patients with locally advanced or metastatic pancreatic cancer with respect to mean differences from the baseline in EORTC-QLQ-C30 scale variables and relative body weight.
| EORTC scale | Mixed model | |||
|---|---|---|---|---|
| Mean[95% CI] | p-value for treatment without interaction | p-valuefor treatment with interaction | p-value for interaction “visit × treatment” | |
| Global quality of health | 26.1 [22.7; 29.6] |
< 0.001 | < 0.001 | < 0.001 |
| Role functioning | 17.8 [11.9; 23.6] |
< 0.001 | < 0.001 | < 0.001 |
| Social functioning | 11.4 [4.72;18.16] |
0.004 | < 0.001 | 0.506 |
| Cognitive functioning | 18.7 [11.8; 25.6] |
< 0.001 | < 0.001 | 0.016 |
| Physical functioning | 22.3 [17.6; 27.1] |
< 0.001 | < 0.001 | < 0.001 |
| Emotional functioning | 19.5 [13.6; 25.4] |
< 0.001 | < 0.001 | 0.045 |
| Pain | –23.0 [–29.0; –17.0] |
< 0.001 | < 0.001 | < 0.001 |
| Fatigue | –30.6 [–36.1; –25.0] |
< 0.001 | < 0.001 | < 0.001 |
| Appetite loss | –43.9 [–51.0; –36;7] |
< 0.001 | < 0.001 | 0.031 |
| Financial problems | –15.6 [–23.1; –8.2] |
< 0.001 | < 0.001 | 0.626 |
| Insomnia | –37.2 [–45.8; –28.6] |
< 0.001 | < 0.001 | < 0.001 |
| Nausea/vomiting | –10.9 [–16.0; –5.9] |
< 0.001 | < 0.001 | < 0.001 |
| Diarrhea | –4.5 [–7.3; –1.7] |
0.005 | < 0.001 | 0.028 |
| Constipation | –1.3 [–2.8; 0.1] |
0.140 | 0.026 | 0.121 |
| Shortness of breath | –0.8 [–2.5; 0.8] |
0.320 | 0.339 | 0.040 |
| Body weight[% change relative to baseline] | 8.56 [7.0; 10.0] |
< 0.001 | < 0.001 | < 0.001 |
The function scales are arranged in increasing order of baseline values, and the symptom scales in decreasing order. The main analysis is the mixed model, without any interaction between trial treatment and visits; sensitivity analyses are the mixed model with interaction, stratified by the timepoint of the last regular follow-up visit. For each individual analysis, the p-values of the quality-of-life scales were adjusted for multiple testing with the Bonferroni-Holm correction.
EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; CI, confidence interval.
eTable. Stratified Van Elteren tests relating to Table 3 in the article: differences between the mistletoe and control groups in mean changes from baseline values of EORTC-QLQ-C30 scale variables and body weight, in patients with locally advanced or metastatic pancreatic carcinoma*.
| EORTC Scale | Stratified Van Elteren tests | |||
|---|---|---|---|---|
| p-value for comparison of means | p-value for comparison of medians | p-value for comparison of worst values | p-value for comparison of last values | |
| Global quality of health | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Role functioning | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Social functioning | 0.019 | 0.004 | 0.040 | 0.001 |
| Cognitive functioning | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Physical functioning | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Emotional functioning | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Pain | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Fatigue | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Appetite loss | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Financial problems | 0.019 | 0.027 | 0.040 | 0.005 |
| Insomnia | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Nausea/vomiting | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Diarrhea | 0.243 | 0.289 | 0.325 | 0.004 |
| Constipation | 1.000 | 0.379 | 1.000 | 1.000 |
| Dyspnea | 1.000 | 0.425 | 1.000 | 0.005 |
| Body weight [% change relative to baseline] | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
*The functional scales are arranged in increasing order of baseline values, and the disease manifestation scales in decreasing order. These sensitivity analyses are non-parametric analyses of 4 variables related to the course of the EORTC-QLQ-C30 scales (mean, median, worst value, last measured value), stratified according to the timepoint of the last regularly scheduled follow-up visit. For each individual analysis, the p-values of the quality-of-life scales were adjusted for multiple testing with the Bonferroni-Holm correction.
EORTC QLQ-C30; European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30.
Figure 2.
Changes on the 15 quality-of-life scales of the EORTC-QLQ-C30 questionnaire relative to baseline (mean ± standard deviation) in patients with locally advanced or metastatic pancreatic cancer who underwent at least one follow-up examination. 96 patients were treated with mistletoe, and 72 control patients were not. The increasingly pale connecting lines are meant to represent the diminishing number of patients, as seen in Table 4. The related data tables, along with a further representation stratified by time of death with corresponding data tables, can be found in the eSupplement, as can the raw data for the Figures.
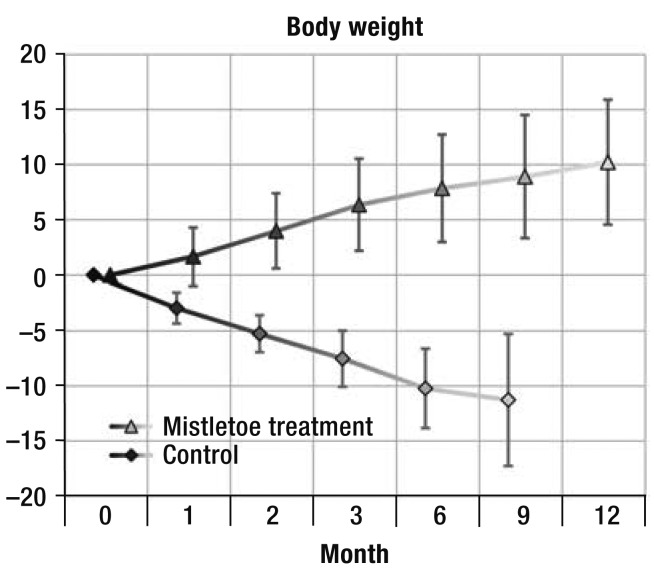
Five patients in the mistletoe treatment group (4.5%) and 51 in the control group (46.4%) lost weight during the study. Averaged over all follow-up visits, the patients in the mistletoe group gained 5.3% of their initial weight, while the patients in the control group lost 3.2% (p < 0.001). This difference, too, was confirmed in all sensitivity analyses (Figure 1, Table 3, eTable 1).
Figure 1.
Changes in body weight relative to baseline (in percent; mean ± standard deviation) in patients with locally advanced or metastatic pancreatic cancer who underwent at least one follow-up examination (96 patients were treated with mistletoe, and 72 control patients were not). The percent changes from baseline were consistently different between the two groups in the mixed model both with and without interactions as well as in non-parametric, stratified analyses.
Discussion
The patients with locally advanced or metastatic pancreatic carcinoma who were treated with mistletoe had a better quality of life and longer overall survival than the patients in the control group. No side effects of mistletoe were observed, and the patients in the mistletoe treatment group had less frequent and less severe disease-related symptoms (11). On the basis of the planned survival and safety analysis of 220 patients, the IDMC recommended early termination of the trial, as required by the protocol.
The patients' initial clinical condition was worst on the EORTC-QLQ-C30 scales concerning global quality of health, physical function, pain, fatigue, appetite loss, insomnia and nausea/vomiting. The fact that it was precisely the values on these scales (which are of the highest relevance to patients) that improved to the greatest extent in the mistletoe treatment group could be thought to reflect regression to the mean; the patients in the control group, however, did not manifest this effect, instead experiencing further worsening on all of these scales.The lack of sensitivity of the EORTC-QLQ-C30 diarrhea and constipation scales in patients with pancreatic carcinoma is already known (19), and the dyspnea scale seems to be irrelevant to this patient group. The course of values on the appetite loss scale tracked with patients' body weight in both groups showed that the control patients generally had a progressive loss of appetite along with progressively declining weight, while the patients treated with mistletoe no longer complained of appetite loss and tended to gain weight.
The trial was intentionally not blinded, because mistletoe treatment is supposed to be initiated with dose escalation until it produces local cutaneous reactions of a certain size, temporary flu-like manifestations, and a mild elevation of temperature. According to the treatment recommendations, these manifestations indicate that the dose is optimal. The simultaneous occurrence of these manifestations and their dynamics cannot be achieved with the use of a placebo drug. According to the guidelines of the United States Food and Drug Administration, an open design is an acceptable option for trials that are intended to study overall survival as a primary endpoint (20). On the other hand, the patients' subjective reporting of their quality of life should be interpreted with a degree of caution, as it may be subject to distorting effects of various types, including differential compliance (attrition bias) and other treatment-related differences (performance bias) between the two groups. To keep such distortions to a minimum, all patients were offered a centralized, unchanging regimen of best supportive care, optimized with respect to local conditions, at the trial headquarters. The very low drop-out rate in both groups seems to indicate that this measure was effective. As the trial physicians did not intervene in any way other than to administer medications and examine the patients at their regularly scheduled follow-up visits, one would not expect any major degree of performance bias to be caused by the variable frequency of physician contact. Indeed, the complete lack of any expectation of success from mistletoe treatment on the part of the Serbian physicians and patients may well have counteracted any possible distorting effects that would have favored a positive outcome. Moreover, a Cochrane review (21) of 202 controlled trials involving a total of 16 566 patients showed that, in trials with continuously distributed or binary endpoints, such as the quality-of-life data in this trial, the findings concerning efficacy did not differ significantly with the use of a blinded (placebo) versus unblinded (open) control group.
The frequent lack of histologic confirmation of diagnoses in this trial was largely due to the prevailing opinion in the participating Serbian centers that direct tumor biopsy creates the risk of a pancreatic fistula. The Serbian physicians consider tumor histology unnecessary in principle if the tumor is in a locally inoperable stage (infiltration of the mesenteric artery and vein, the mesenteric root, the retroperitoneal space, and the major blood vessels). Imaging studies showing cancerous involvement of the body and tail of the pancreas, along with metastases in the liver or in the peritoneum, are considered adequate to establish the diagnosis.
In 2009, there were 450 men and 357 women in Serbia with the primary diagnosis of pancreatic carcinoma (22); for a subgroup of these patients, no treatment other than best supportive care was considered to be indicated. In the trial enrollment center, 238 such patients were sent by the Serbian oncological consultation service to be screened for the trial. The recruited patients can be considered representative of the entire group of patients in this class.
In very advanced stages of cancer, their physicians, the patients themselves, and (in some cases) relatives who represent the patients' best interests must consider whether it is still reasonable to administer the conventional treatments intended to prolong life in view of their low likelihood of success. Although second-line life-prolonging treatments are now available in Germany, patients are often given no more than best supportive care, because these treatments can have marked side effects. The findings of the present study suggest that mistletoe treatment can be given in such situations, as it has practically no side effects, improves the quality of life, and prolongs survival. It would also be reasonable for future clinical trials to study the effect of mistletoe in combination with conventional treatment.
Key Messages.
Patients with advanced pancreatic carcinoma must weigh the side effects of conventional treatments against their low probability of success.
Mistletoe treatment was found to improve the global quality of health, as evaluated by the EORTC QLQ-C30.
The mean intergroup difference in the global quality of health was 26.1 points (95% confidence interval 22.7 to 29.6]), with further differences of varying sizes with respect to appetite loss (-43.9 [-51.0 to –36.7]), fatigue (-30.6 [-36.1 to –25.0]), pain (-23.0 [-29.0 to –17.0]), and nausea (-10.9 [-16.9 to –5.9]). All of these differences were statistically significant, with p < 0.001.
On average, the patients in the mistletoe group gained 5.3% of their initial weight, while the patients in the control group lost 3.2% (intergroup difference 8.5% [7.0% to 10.0%], p < 0.001). This result was in keeping with the improvement in appetite loss reported by the patients who were treated with mistletoe.
In patients with advanced pancreatic carcinoma, mistletoe treatment prolongs survival and improves the quality of life.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
The authors thank the trial participants and Dr. D. Basarić (assistant physician), K. Stokuća (trial nurse), and the nurses of the CCS; R. Beutke and S. Weippert (data management); and the physicians who referred the patients and sent documentation of their diagnoses and operative reports, who were: Prof. Dr. S. Knežević, Prof. Dr. S. Ostojić, Prof. Dr. M. Petrović, Doc. Dr. D. Radenković, Prof. Dr. M. Kerkez, Doc. Dr. S. Matić, Dr. P. Bulajić, Dr. Z. Ðorðević, Dr. I. Pavlović, Dr. D. Knežević, Dr. N. Grubor, Dr. M. Jagodić, Dr. I. Pejović, Dr. Z. Ražnatović, Dr. M. Jovanović, Dr. N. Zarić, Dr. D. Jezdić, Dr. D. Veličković, Dr. G. Barišić, Dr. A. Antić, and Dr. V. Dugalić from the various divisions of the surgical department of the CCS; Prof. Dr. Ž. Laušević, Dr. M. Gvozdenović, Dr. G. Kaljević, Dr. P. Savić, and Dr. V. Resanović from the various divisions of the emergency department of the CCS; Prof. Dr. D. Bilanović, Dr. B. Tošković and Dr. V. Kovčin of KBC Bežanijska Kosa; Dr. A. Filipović, Dr. V. Cijan, and Dr. Z. Bokun of KBC Zvezdara as well as Dr. R. Marković of CC Kragujevac, Dr. D. Dabić of ZC Čačak, and Dr. B. Jovanović of ZC Požarevac.
Footnotes
Conflict of interest statement
The trial described here was financially supported by the Swiss Cancer Research Association (Verein für Krebsforschung e. V. (VfK), Schweiz) as its sole sponsor. The VfK had no influence on the planning and course of the trial or on the evaluation and publication of its findings. The VfK receives licence fees for the preparation of the active substance for the commercially available mistletoe drug Iscador from Weleda AG, the company that obtained approval for the drug. The VfK is a non-profit organization and is required to use its revenues for cancer research. Weleda AG was neither a sponsor of this trial as defined by the German Pharmaceuticals Law nor a financer of the trial. Weleda AG did, however, produce the trial drug as a separate lot and was paid for doing so by the VfK. Wilfried Tröger, Marcus Reif, and Agnes Schumann are also involved in the performance of other studies for the VfK. All of the authors declare that no conflict of interest exists.
References
- 1.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Exokrines Pankreaskarzinom, Langversion 1.0, 2013. AWMF Registernummer: 032-010OL. 2013 [Google Scholar]
- 2.Bayraktar S, Bayraktar UD, Rocha-Lima CM. Recent developments in palliative chemotherapy for locally advanced and metastatic pancreas cancer. World J Gastroenterol. 2010;16:673–682. doi: 10.3748/wjg.v16.i6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeck S, Bruns CJ, Sargent M, Schafer C, Seufferlein T, Jauch KW, et al. Current oncological treatment of patients with pancreatic cancer in Germany: results from a national survey on behalf of the Arbeitsgemeinschaft Internistische Onkologie and the Chirurgische Arbeitsgemeinschaft Onkologie of the Germany Cancer Society. Oncology. 2009;77:40–48. doi: 10.1159/000226110. [DOI] [PubMed] [Google Scholar]
- 4.Cascinu S, Falconi M, Valentini V, Jelic S. Pancreatic cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v55–v58. doi: 10.1093/annonc/mdq165. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus Gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Walker EJ, Ko AH. Beyond first-line chemotherapy for advanced pancreatic cancer: An expanding array of therapeutic options? World J Gastroenterol. 2014;20:2224–2236. doi: 10.3748/wjg.v20.i9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L in animals and humans—systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011;11 doi: 10.1186/1472-6882-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD003297.pub2. CD003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthes H, Friedel WE, Bock PR, Zanker KS. Molecular mistletoe therapy: friend or foe in established anti-tumor protocols? A multicenter, controlled, retrospective pharmaco-epidemiological study in pancreas cancer. Curr Mol Med. 2010;10:430–439. doi: 10.2174/156652410791317057. [DOI] [PubMed] [Google Scholar]
- 10.Rostock M, Huber R. Randomized and double-blind studies - demands and reality as demonstrated by two examples of mistletoe research. Forsch Komplementärmed Klass Naturheilkd. 2004;11:18–22. doi: 10.1159/000080571. [DOI] [PubMed] [Google Scholar]
- 11.Tröger W, Galun D, Reif M, Schumann A, Stankovic N, Milicevic M. Viscum album [L] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur J Cancer. 2013;49:3788–3797. doi: 10.1016/j.ejca.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Kienle GS, Kiene H. Review article: Influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142–157. doi: 10.1177/1534735410369673. [DOI] [PubMed] [Google Scholar]
- 13.Schaefermeyer G, Schaefermeyer H. Treatment of pancreatic cancer with Viscum album (Iscador): a retrospective study of 292 patients 1986-1996. Complement Ther Med. 1998;6:172–177. [Google Scholar]
- 14.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 Scoring Manual 3rd ed. Brussels: European Organization for Research and Treatment of Cancer. EORTC Quality of Life Group. 2001 [Google Scholar]
- 15.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. J Am Med Assoc. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 16.van Elteren PH. On the combination of independent two-sample tests of Wilcoxon. Bulletin of the International Statistical Institute. 1960;37:351–361. [Google Scholar]
- 17.Horn M, Vollandt R. Fischer-Verlag. Stuttgart: 1995. Multiple Tests und Auswahlverfahren. [Google Scholar]
- 18.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimmons D, Johnson CD, George S, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/s0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 20.FDA. Guidance for Industry. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071590.pdf. Last accessed on 9 September 2013. [Google Scholar]
- 21.Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;20(1) doi: 10.1002/14651858.CD003974.pub3. CD003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihajlovic J, Pechlivanoglou P, Miladinov-Mikov M, Zivkovic S, Postma MJ. Cancer incidence and mortality in Serbia 1999-2009. BMC Cancer. 2013;13 doi: 10.1186/1471-2407-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]