Abstract
Amacrine cells constitute a diverse class of interneurons that contribute to visual signal processing in the inner retina, but surprisingly, little is known about the physiology of most amacrine cell subtypes. Here, we have taken advantage of the sparse expression of vesicular glutamate transporter 3 (VGLUT3) in the mammalian retina to target the expression of yellow fluorescent protein (YFP) to a unique population of amacrine cells using a new transgenic mouse line. Electrophysiological recordings made from YFP-positive (VGLUT3+) amacrine cells provide the first functional data regarding the active membrane properties and synaptic connections of this recently identified cell type. We found that VGLUT3+ amacrine cells receive direct synaptic input from bipolar cells via both N-methyl-d-aspartate receptors (NMDARs) and non-NMDARs. Voltage-gated sodium channels amplified these excitatory inputs but repetitive spiking was never observed. VGLUT3+ amacrine cells responded transiently to both light increments (ON response) and decrements (OFF response); ON responses consisted exclusively of inhibitory inputs, while OFF responses comprised both excitatory and inhibitory components, although the inhibitory conductance was larger in amplitude and longer in time course. The physiological properties and anatomical features of the VGLUT3+ amacrine cells suggest that this bistratified interneuron may play a role in disinhibitory signaling and/or crossover inhibition between parallel pathways in the retina.
Keywords: Interneurons, Transgenic Cre mice, Synaptic physiology
Introduction
Amacrine cells make up the majority of inhibitory interneurons in the retina and exhibit remarkable morphological and molecular diversity, yet they are the least understood cell type in the retina. At least 24 distinct subtypes have been identified in mammals (MacNeil & Masland, 1998; MacNeil et al., 1999), yet we know a great deal about the functional features of only 3–4 amacrine cell subtypes. This deficit is largely attributable to the difficulty in reliably targeting particular amacrine cells for physiological study: most amacrine cell bodies are densely packed in the inner nuclear layer of the retina, along with those of bipolar cells, Müller cells, and horizontal cells, making them difficult to identify consistently with conventional light microscopy. Emerging molecular markers for specific amacrine cell subtypes are now allowing for genetic and physiological targeting and are revealing new information about the characteristics of individual subtypes within this cell class.
Recent immunohistochemistry studies have shown that a sparse population of amacrine cells expresses the vesicular glutamate transporter 3 (VGLUT3) (Haverkamp & Wassle, 2004; Johnson et al., 2004; Gong et al., 2006; Stella et al., 2008). VGLUT3+ amacrine cells exhibit immunoreactivity for both glutamate and glycine but lack other neurotransmitter markers often associated with amacrine cells (i.e., GABA, acetylcholine, and/or dopamine). The regular mosaic arrangement of their cell bodies suggests that VGLUT3+ amacrine cells constitute a single subtype (Haverkamp & Wassle, 2004). Here, we have taken advantage of this molecular specificity to genetically label these neurons with yellow fluorescent protein (YFP) and examine their physiological and morphological characteristics using patch-clamp electrophysiology and fluorescent imaging techniques. Consistent with previous immunohistochemistry studies (Haverkamp & Wassle, 2004), we found that individual VGLUT3+ amacrine cells have bushy midsized (~100 µm) dendritic arbors that broadly span sublaminas 2 and 3 of the mouse inner retina. Patch-clamp recordings from VGLUT3+ amacrine cells revealed information about synaptic signaling and active membrane properties. Light-evoked (full-field stimulation) responses from VGLUT3+ amacrine cells indicated that they receive synaptic input in response to both ON and OFF pathway stimulation, and that ON responses are exclusively inhibitory, while the OFF response comprises both excitatory and inhibitory components. Active membrane properties were attributed to Nav and Kv channels, rapidly-inactivating large conductance calcium-activated potassium (BK) channels, and L- and T-type Cav channels. Nav channels amplified postsynaptic potentials in VGLUT3+ amacrine cells, but regenerative action potentials were rarely observed. Together, the basic morphological features and physiological properties of VGLUT3+ amacrine cells suggest that they play a unique role in visual processing in the retina.
Materials and methods
Construction of VGLUT3 Cre BAC transgenic mice
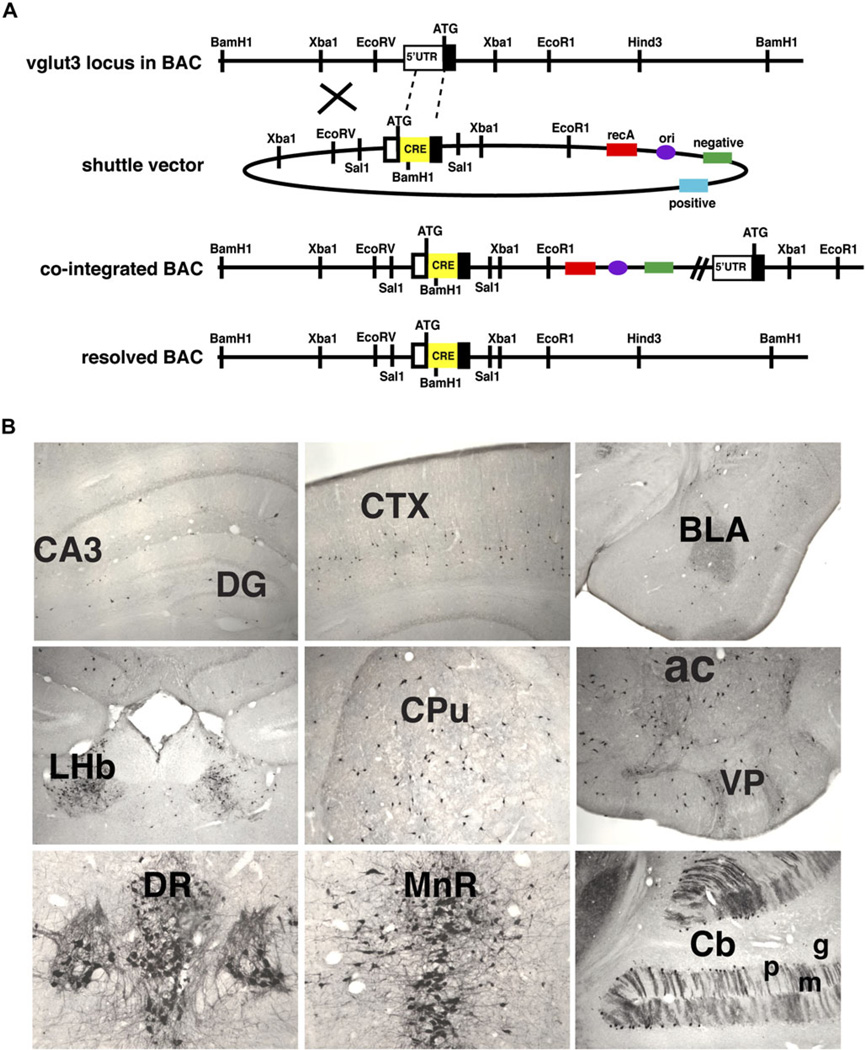
The VGLUT3 Cre bacterial artificial chromosome (BAC) transgene was constructed using the method of Yang et al. (1997). The Cre recombinase complementary deoxyribonucleic acid was followed by an SV40 polyadenylation signal that was amplified by polymerase chain reaction (PCR) from the vector IRES2-EGFP (BD Biosciences, San Jose, CA). We used a version of Cre recombinase with the codons optimized for expression in mammalian cells (GenBank: AY056050.1). Cre recombinase cDNA and SV40 polyadenyation signal were inserted into the pSV1.RecA shuttle vector flanked by vglut3 genomic sequences amplified from a C57Bl/6 BAC (Fig. 1A). The left arm (~0.8 kb) was amplified by PCR from the vglut3 5’ untranslated region and the right arm (~1.0 kb) from the beginning of the first exon into the first intron. Homologous recombination in bacteria between the modified shuttle vector and the vglut3 locus within the BAC first yielded a cointegrate, which we identified by Southern blotting with a probe from the left arm. The cointegrate resolved appropriately in a second round of recombination, to yield the BAC transgene, which we again identified by Southern blotting with the left-arm probe. The VGLUT3 Cre BAC was purified and microinjected into the pronuclei of fertilized Friend virus B-type mouse oocytes, which were then implanted into pseudopregnant females. The VGLUT3 Cre transgene was detected by PCR of tail genomic DNA using forward (catcagaaacctggactctg) and reverse (aggctccagaaacagtctaacg) primers to Cre recombinase and the vglut3 locus, respectively. From four founders, two expressed the Cre recombinase as determined by crossing the F1 offspring of each founder to the Rosa26Sor+/fsYFP reporter and immunostaining for YFP. One of the lines was used for these studies.
Fig. 1.
VGLUT3 Cre BAC transgenic mice. (A) Sequences coding for Cre recombinase (Cre) and the SV40 polyadenylation signal were inserted by homologous recombination in bacteria into exon 1 of a BAC that contains the entire vglut3 gene locus (top). The cointegrated intermediate and properly resolved products are shown below the shuttle vector. Proper insertion of Cre was confirmed by Southern blotting BamH1-digested DNA with the 5′ homology arm. (B) Cre is expressed by neurons known to express VGLUT3, including interneurons in the hippocampus, cortex, and striatum as well as neurons in the habenula and raphe nuclei and Purkinje cells in the cerebellum. Cornu ammonis region 3 (CA3), dentate gyrus (DG), cortex (CTX), caudate putamen (CPu), nucleus accumbens (ac), ventral pallidum (VP), basolateral amygdala (BLA), lateral habenula (LHb), dorsal raphe (DR), median raphe (MnR), cerebellum (Cb), Purkinje cell layer (p), molecular cell layer (m), and granule cell layer (g).
Histology
Adult mice were anesthetized with sodium pentobarbatol, transcardially perfused with 4% paraformaldehyde, and the brain and eyes removed. Brains were postfixed overnight and cryoprotected in 30% sucrose. Eyes were postfixed for 2 h and then rinsed in 0.1 mM phosphate buffer (PB). Cornea was cut away, lens removed, and eye cup cryoprotected overnight in 30% sucrose. Sections were cut on a cryostat (30 µm brain and 16 µM eyecup). Brain slices were cut as free floating and stored at 4°C. Retina slices were directly mounted onto permafrost plus slides and then stored at –20°C. Brain slices were incubated for 1 h at room temperature in phosphate-buffered saline (PBS) with 10% normal donkey serum (NDS) and 1% Triton X-100 (blocking buffer) and then incubated with a rabbit polyclonal antibody to EGFP (Invitrogen, Carlsbad, CA) at 1:2000 in blocking buffer overnight. Sections were washed and then incubated with horseradish peroxidase-conjugated secondary antibodies. Immunoperoxidase staining was performed using diaminobenzidine as previously described. Slices were dehydrated, mounted onto slides with Permount (Fisher Scientific; Pittsburgh, PA), and examined by light microscopy. Retina slices were blocked in PBS plus 5% NDS and 0.25% Triton X-100. The sections were then incubated with a rabbit polyclonal antibody to EGFP (Invitrogen) at 1:1000 and a guinea pig anti-VGLUT3 antibody at 1:5000 (R. Edwards Lab) in blocking buffer for 1–3 days at 4°C. Sections were washed in the same buffer and then incubated with fluorescein isothiocyanate or Cy3-conjugated secondary antibodies at room temperature for 2 h. Brain sections were mounted on slides with Prolong Antifade (Invitrogen) and examined by confocal microscopy (LSM510; Zeiss, Thornwood, NY).
Electrophysiology
Experiments were conducted at room temperature (22–25°C; unless otherwise noted) using retinal slices (210 µm thick) prepared from homozygous VGLUT3 Cre/YFP mice (P17–21), as previously described (Singer & Diamond, 2003; Chavez et al., 2006). For experiments involving light stimulation, tissue preparations were conducted under dim red illumination and stored in light-tight containers (to minimize rundown of the cone-driven light response); for all other experiments, tissue preparations were conducted and stored under ambient room lighting (light adapted). Mouse retinas were isolated in artificial cerebrospinal fluid (ACSF) containing (in millimolar): 119 NaCl, 26 NaHCO3, 1.25 Na2HPO4, 2.5 KCl, 2.5 CaCl2, 1.5 MgSO4, 10 glucose, 2 Na-pyruvate, 4 Na-lactate (continually bubbled with 95% O2/5% CO2). For experiments probing the excitatory synaptic mechanisms (i.e., Figs. 4 and 5), unless otherwise noted, ACSF was supplemented with the group III metabotropic glutamate receptor (mGluR) agonist L-AP4 (10 µM) to mimic dark conditions and strychnine (1 µM), SR95531 (10 µM), and methyl-(1,2,3,6-tetrahydropyridin-4-yl)phosphinic acid (TPMPA, 50 µM) to block glycine-, GABAA-, and GABACRs, respectively. For all nonsynaptic experiments (i.e., assessment of active membrane properties), ACSF was supplemented with L-AP4 (10 µM) and the 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid receptor (AMPAR) antagonist, 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f] quinoxaline −7 sulfonamide (NBQX) (10 µM). Drugs were purchased from Sigma or Tocris (St. Louis, MO) with the exception of tetrodotoxin (TTX) (Alamone Labs, Jerusalem, Israel). Fluorescent dyes were purchased from Molecular Probes (Eugene, OR). VGLUT3+ amacrine cells were visually identified for physiological recordings in retinal slices using either a modified 2PLSM (λ = 930 nm; Zeiss) or standard epifluorescence. When using two-photon laser-scanning microscopy (2PLSM) emissions from the YFP and Alexa 594 were spectrally separated into two distinct collection channels, using a combination of a 565 nm dichroic mirror and 500–550 nm and 570–640 nm band-pass filters (Chroma, Bellows Falls, VT). This allowed for near-independent control of the signals collected from the patch pipette and the cells of interest making high quality recordings relatively easy to achieve. Alternatively, short exposures (typically <30 s) of full-field epifluorescence were used to quickly identify YFP-labeled somas that could then be targeted for electrophysiological recording using infrared differential interference contrast (IR-DIC) video microscopy. Unless otherwise noted, whole-cell voltage-clamp recordings were made from VGLUT3+ amacrine cells using pipettes (~5–6 MΩ) containing (in millimolar): 100 Cs methanesulfonate, 20 tetraethylammonium-Cl, 10 HEPES, 10 EGTA, 10 Na phosphocreatine, 4 MgATP, 0.4 Na-GTP, and 0.04 Alexa 594 hydrazide (pH 7.4). Potassium-based internal for current-clamp recordings contained (in millimolar): 100 K methanesulfonate, 20 KCl, 10 HEPES, 2 EGTA, 10 Na phosphocreatine, 4 MgATP, 0.4 Na-GTP, and 0.04 Alexa 594 hydrazide (pH 7.4). The access resistance for all recordings presented in this study was ≤30 MΩ and remained uncompensated. Input resistance for VGLUT3+ amacrine cells was 1075 ± 477 MΩ (n = 10) when using the potassium-based internal and after correcting for access resistance. Recordings were made using an Axopatch 1D or Axoclamp 700B amplifier (Axon Instruments, Foster City, CA) controlled through an A/D board (Instrutech, Port Washingon, NY) by custom software written in Igor Pro (Wavemetrics, Lake Oswego, OR). All responses were collected at 20 s intervals, low-pass filtered at 5 kHz, and digitized at 10–50 kHz. Voltage steps were leak subtracted using a P/4 subtraction protocol. Isolated excitatory synaptic responses (Figs. 4 and 5) were elicited by electrical stimulation of bipolar cells in the outer plexiform layer (OPL) (~0.5–2 µA for 100–300 µs; FHC, Bowdoin, ME).
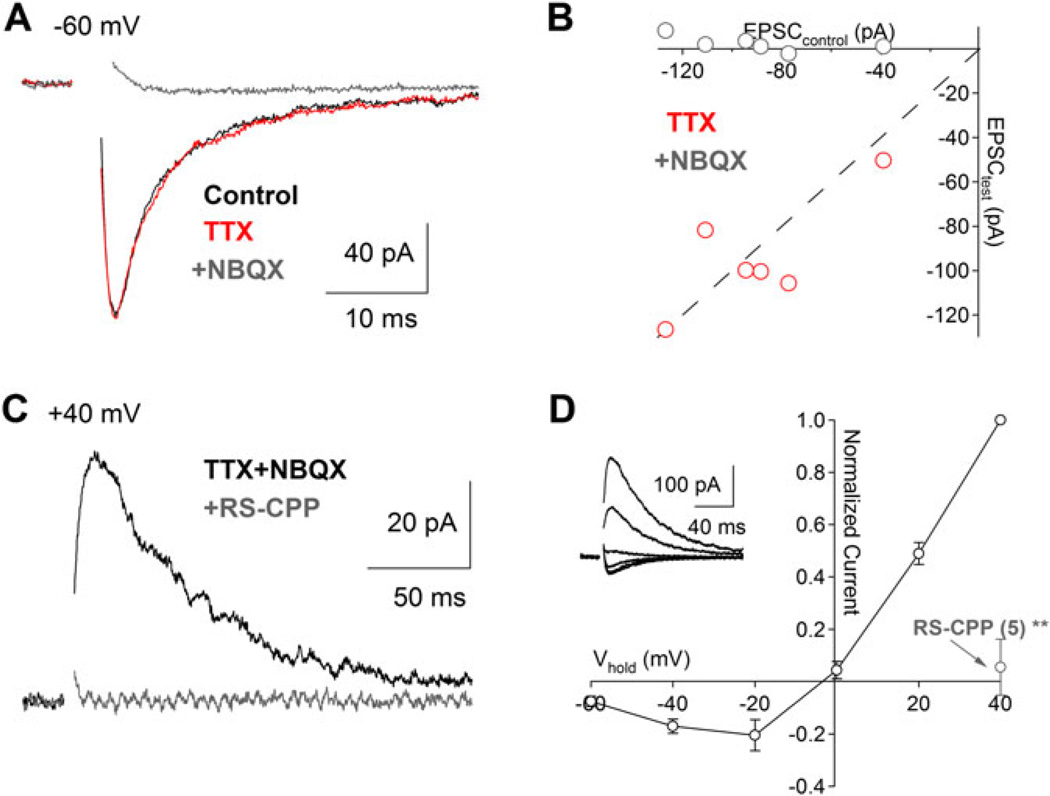
Fig. 4.
Excitatory synaptic input from bipolar cells to VGLUT3+ amacrine cells. (A) Glutamate release from presynaptic bipolar cells in the absence of inhibition (see Materials and methods section). Electrically evoked EPSCs (Vhold = −60 mV, inhibition blocked) were insensitive to TTX (1 µM, red trace) but were blocked by application of the AMPA-type receptor antagonist NBQX (10 µM, gray trace). (B) Scatter plot summarizing the drug effects for experiments in panel (A) (n = 6). (C) and (D) In the presence of NBQX (Vhold = +40 mV), a slower EPSC was blocked by the NMDAR antagonist RS-CPP (10 µM, gray trace). (D) EPSCs in the presence of NBQX exhibit a j-shaped current–voltage relationship characteristic of an NMDAR-mediated conductance.
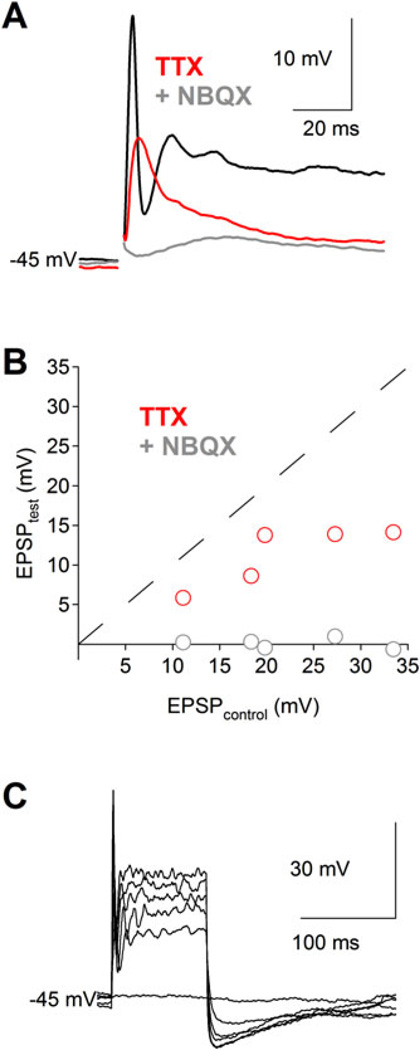
Fig. 5.
Nav channels amplify EPSPs and sometimes produce single action potentials in VGLUT3+ amacrine cells. (A) Electrically evoked EPSPs (inhibition blocked) elicit Nav channel-mediated action potentials in some VGLUT3+ amacrine cells. EPSPs were reduced by bath application of TTX (1 µM, red trace) and abolished by subsequent inclusion of NBQX (10 µM, gray trace). (B) Scatter plot summarizing the drug effects for experiments in panel (A) (n = 5). (C) In the absence of synaptic activity, direct depolarization of a VGLUT3+ amacrine cell with a somatic whole-cell electrode (0–500 pA steps in 100 pA increments for 100 ms) elicits at most a single action potential.
For light stimulation, full-field light stimulation was provided by a 470-nm LED (Thor Labs, Newton, NJ) directed through the epifluorescence port of the microscope, band-pass filtered between 450–490 nm, and reflected into the objective by a 510-nm dichroic mirror (Zeiss) to provide full-field illumination over the objective (water immersion 1.0 NA 40X; Zeiss) aperture (0.45 mm diameter). Photon flux at the slice was measured to be approximately 9.6 × 1018 photons/cm2/s using a DR-2000 radiometer (Gamma Scientific, San Diego, CA). To calculate changes in synaptic conductance evoked by light stimulation, we adapted methods similar to previous reports (Borg-Graham, 2001; Taylor & Vaney, 2002; Oesch & Taylor, 2010). We illuminated the slice for 1 s while holding the cell at a series of command voltages between −90 and 10 mV by increments of 20 mV and recorded current traces as described above, with the exception that these experiments were performed at 35–37°C and 10 mM QX-314 was added to the internal solution to block Nav channels intracellularly. Offline, we subtracted the leak current after the voltage step prior to the application of light to isolate the light-evoked current and then measured the light-evoked I–V relationship every 10 ms spanning the duration of the light stimulus. We fit each I–V with a line between −90 and 10 mV to extract the slope (gT) and x-intercept (Vr) for each I–V measurement. If we assume that light-evoked currents (Ie) arise from excitatory and inhibitory synaptic inputs and obey Ohm’s law, so that Ie=ge(t)(V – Ve) and Ii=gi(t)(V – Vi), where the inhibitory, gi(t) and excitatory, ge(t) conductances are functions of time. The total light-evoked synaptic current is IT = gT(t)(V – Vt(t)), where gT = ge + gi. The observed synaptic reversal potential Vr(t) is a weighted sum of Ve and Vi such that, Vr(t) = (ge(t)/gT(t))Ve + (gi(t)/gT(t))Vi. Then, the excitatory and inhibitory synaptic conductances can be calculated from gT(t) and Vr(t) according to the equations: ge(t) = gT(t){Vr(t) – Vi)/(Ve – Vi) and gi(t) = gT(t)(Vr(t) – Ve)/(Vi – Ve). Based on the ionic concentrations used in our internal and external recording solutions, we used a value of 0 mV for Ve and −48 mV for Vi.
Electrophysiology data were analyzed using Igor Pro and Excel (Microsoft). Paired two-tailed t-tests were used to compare data sets and significance was determined as P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***). Unless otherwise indicated, data are presented as mean ± S.D. and illustrated traces are averages of 5–10 responses.
Results
YFP labels VGLUT3+ amacrine cells
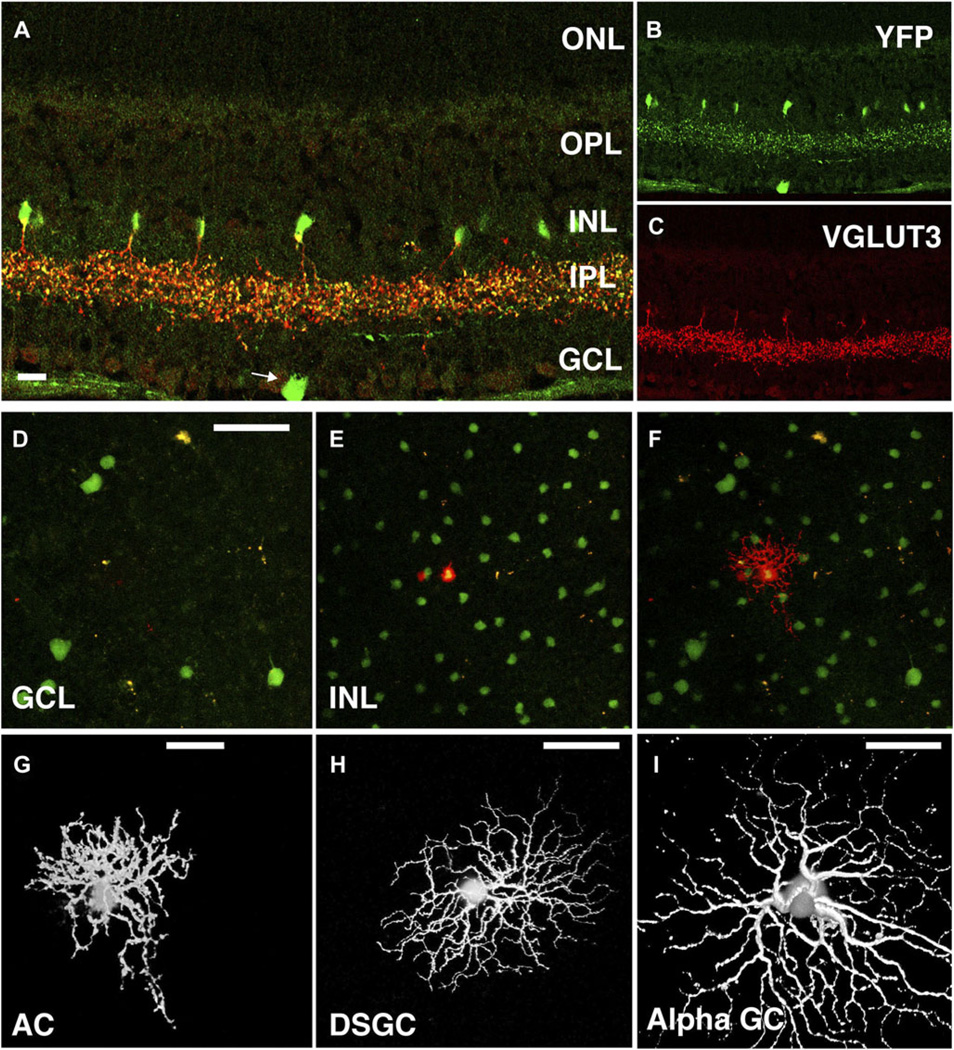
To study the physiological role of VGLUT3+ amacrine cells in the retina, we generated BAC transgenic mice that express Cre recombinase under the control of Vglut3 regulatory elements (VGLUT3 Cre mice; Fig. 1A). We crossed these mice with Cre-inducible floxed-stop YFP reporter mice (Rosa26Sor +/fsYEP ) to generate mice that express YFP in cells that normally express VGLUT3. Consistent with the faithful expression pattern of BAC transgenic mice, expression of YFP was observed in populations of neurons known to express the transporter, including interneurons in the hippocampus, layers 2/3 and 6 of the cortex and the striatum, pyramidal neurons in layer 5 of the cortex, and neurons in the lateral habenula as well as the dorsal and medial raphe (Fig. 1B; Fremeau et al., 2002; Gras et al., 2002; Schafer et al., 2002). Because recombination of the reporter locus by Cre results in the permanent expression of YFP, some cell populations, such as the Purkinje cells of the cerebellum (Fig. 1B), which express VGLUT3 only transiently during development (Gras et al., 2005), continue to express YFP in the adult. Additionally, in most neurons, VGLUTs traffic efficiently to nerve terminals and do not generally reside in cell bodies, whereas YFP fills the entire cell. Thus, new cell populations of VGLUT3+ neurons, not previously identified as immunopositive for the transporter, are detectable in these Cre reporter mice. Indeed, in the retina, YFP appeared to be expressed by a small number of ganglion cells (GCs) (Fig. 2A-2C), suggesting that these cells also express VGLUT3. Importantly, YFP was expressed by the VGLUT3+ amacrine cells (Fig. 2A–2C), allowing us to identify these cells for morphological and physiological analyses.
Fig. 2.
VGLUT3 Cre BAC transgenic mice crossed to Rosa26YFP reporter mice label both amacrine cells and GCs. (A–C) Immunostaining of a retinal slice shows YFP (green, B) expression in VGLUT3+ (red, C) amacrine cells as well as in a GC (arrow). Scale bar is 20 µM. (D–F) YFP was expressed in an irregular heterogeneous population of neurons in the ganglion cell layer (GCL; D) and a homogeneous population of interneurons in the inner nuclear layer (INL; E). Fluorescent cells were targeted for microelectrode recordings via a two-photon microscope (λ = 930 nm; F). Scale bar in (D) is 50 µM applies to panels (D–F). (G) Fluorescent cells in the INL constitute a single population of VGLUT3+ amacrine cells with small bushy dendritic arbors of various irregular shapes (~70 µM diameter). Scale bar: 20 µM. (H) and (I) VGLUT3+ GCs were identified as putative bistratified direction-selective ganglion cells (DSGC; H) and ON alpha GCs (I). Scale bar: 50 µM.
Single cell morphology
YFP-positive cells were targeted using a standard two-photon microscope (λ = 930 nm). Sharp electrodes were used to fill individual YFP+ cells with Alexa 594 prior to imaging, an approach that allowed for excellent spectral separation of the fluorescence from filled cells and the entire YFP+ amacrine cell population (see Materials and methods section; Fig. 2D–2F). Injected cells exhibited midsized dendritic arbors (~ 100 µM) with many bead-like processes in sublaminas 2 and 3 of the inner plexiform layer (IPL), presumably the sites of synaptic contact. VGLUT3+ amacrine cell morphology was less regular than other commonly studied amacrine cells (i.e., A17, AII, and starburst) in several respects. First, the dendritic arbors of the VGLUT3+ amacrine cells often displayed radial asymmetries: two-dimensional projections of arbors in whole-mount retinas often appeared “teardrop” shaped. (Fig. 2G and Fig. S1). Second, stretches of dendrites were not tightly constrained to individual lamina of the IPL; instead, processes located in the OFF sublamina were highly interconnected with those in the ON sublamina (Fig. 3B and Fig. S1). This particular observation was not apparent from YFP labeling of the entire VGLUT3+ population or previous studies using immunohistochemistry. Third, dendritic diameter was highly irregular within individual strata with no obvious dependence on radial distance (whole mount: Fig. 2G, vertical slice: Fig. 3B and Fig. S1); thicker sections and varicosities were often connected by thinner sections of inconsistent length. This was also true of the cell body, which typically connected to the cell’s substantial dendritic arbor via several thin sections. In addition to the amacrine cells, we also injected Alexa 594 into YFP+ cells in the GC layer (Fig. 2H and 2I). Intracellular dye injections indicated that the labeled cells were similar in morphology to two widely studied GC subtypes: ON alpha-like GCs and bistratified GCs with morphology matching that of direction-selective GCs.
Fig. 3.
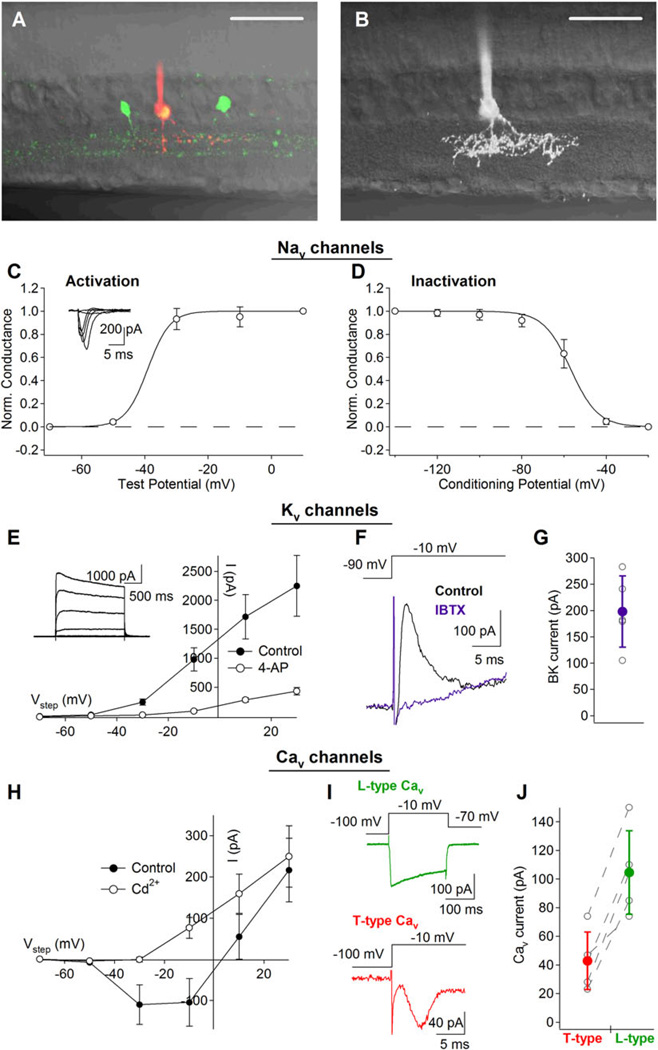
Biophysical properties of active membrane (Nav, Kv, and Cav) conductances in VGLUT3+ amacrine cells. (A) Two-photon targeting of VGLUT3+ amacrine cells in retinal slices allowed for easier access to cell bodies for whole-cell recordings and a greater appreciation for the cell’s dendritic stratification. Scale bar: 50 µM. (B) Three-dimensional projection of a single VGLUT3+ amacrine cell superimposed upon a transmitted DIC image of the retinal slice. Scale bar: 50 µm. (C) and (D) Kinetic properties of 1 µM TTX-sensitive Nav currents. (C) Nav activation measurements were achieved by stepping the command potential to a series of test potentials (−70 to +10 mV in 20 mV increments; 200 ms steps) after a 200-ms conditioning prestep (to –100 mV; for partial relieve of channel inactivation). (D) Nav inactivation measurements (100 ms steps to −10 mV preceded by a 200-ms condition step to a range of potentials: −140 to −20 mV in 20 mV increments) suggest that many channels may be unavailable at typical resting membrane potentials. (E) Kv currents isolated from VGLUT3+ amacrine cells were active at potentials positive to −50 mV and were significantly reduced by application of 4 mM 4-AP. (F) In the presence of 4 mM 4-AP, a fast and transient outward current remained. This current was completely blocked by 100 nM Iberiotoxin indicating that it was mediated by rapidly inactivating BK channels. (G) Individual and average BK (i.e., Iberiotoxin-sensitive) current amplitudes (n= 5). (H) Cav currents were activated at potentials positive to −50 mV as indicated the block of inward current by 100 µM Cd2+ in the family of voltage steps (−70 to +30 mV in 20 mV increments; 200 ms). (I) Pharmacological analysis of the Cav current revealed that it was mediated primarily by L-type (top) and T-type (bottom) Cav channels. (J) Individual and average L-(Isradipine-sensitive) and T-(Mibefridil-sensitive) type currents (n= 5).
Active membrane properties
Spatial and temporal signaling within dendrites depends not only on morphology but also on active membrane properties. To measure the primary active membrane conductances in VGLUT3+ amacrine cells, whole-cell patch-clamp recordings were made from YFP+ amacrine cells in an acute transverse retinal slice preparation (Fig. 3A and 3B). Voltage-gated sodium (Nav) currents, elicited by a series of depolarizing steps in membrane potential (−70 to +10 mV in 20 mV increments; 200 ms steps; with 100 µM Cd2+ extracellularly to block Cav currents and Cs-based internal solution to block Kv currents), were preceded by a 200-ms hyperpolarizing prestep (to −100 mV) to partially relieve channel inactivation (Fig. 3C). Results from an inactivation protocol (100 ms steps to − 10 mV preceded by a 200-ms condition step to a range of potentials: −140 to −20 mV in 20 mV increments) indicated that the midpoint of Nav inactivation was approximately −57 mV, and that the maximum Nav current was 467 ± 285 pA (steps from −140 to − 10mV; n = 5; Fig. 3D). These currents were completely blocked by bath application of TTX (1 µM; data not shown) confirming that they were mediated by Nav channels. Voltage-gated potassium (Kv) currents were characterized using similar protocols but with a potassium-based intracellular solution and the addition of TTX (1 µM) to the extracellular solution to block Nav currents. Under these conditions, we observed large outward currents that were primarily blocked by the A-type Kv channel blocker, 4-aminopyridine (4-AP, 4 µM; Fig. 3E). Inactivation protocols indicated that only 30% of the 4-AP-sensitive current displayed voltage-dependent inactivation (Fig. S2), suggesting that 4-AP also blocked other Kv channels with more sustained kinetics. In the continued presence of 4-AP, voltage steps from −90 to −10 mV elicited transient outward currents that were abolished by iberiotoxin (100 nM), a specific antagonist for BK channels (Fig. 3F and 3G). BK currents decayed within the first few milliseconds of a longer voltage step, indicating rapid inactivation that is characteristic of BK channels containing auxiliary β2 subunits (Wallner et al., 1999; Xia et al., 1999; Grimes et al., 2009). With Nav and Kv channels blocked (with TTX and Cs-based internal, respectively), VGLUT3+ amacrine cells also exhibited inward currents that were blocked by Cd2+ (100 µM), indicating the presence of Cav channels (Fig. 3H). Bath application of the T-type Cav channel antagonist mibefridil (10 µM) blocked a transient portion of the inward Cav current (elicited by voltage steps from −100 to −10 mV for 200 ms; Fig. 3I). Subsequent application of isradipine (10 µM) removed a large slowly inactivating portion of the Cav current, indicating that it was mediated by L-type Cav channels (Fig. 3I and 3J).
Synaptic inputs from bipolar cells
Anatomical evidence suggests that VGLUT3+ amacrine cells receive excitatory input from type 3 and/or type 5 cone bipolar cells (CBCs; Haverkamp & Wassle, 2004). To examine the functional properties of these excitatory synaptic inputs, a stimulating electrode placed in the OPL was used to briefly depolarize the dendrites of CBCs. In the presence of inhibitory blockers (see Materials and methods section), this stimulus elicited robust excitatory postsynaptic currents (EPSCs) in voltage-clamped VGLUT3+ amacrine cells (Fig. 4). When the voltage-clamped VGLUT3+ amacrine cells were held at −60 mV, EPSCs decayed rapidly (τ ~5 ms) and were completely blocked by bath application of NBQX (10 µM), indicating that they were mediated by AMPA-type glutamate receptors (Fig. 4A and 4B). Application of TTX (1 µM) did not affect these EPSCs (109 ± 22% of control; P = 0.57; n = 6), demonstrating that Nav channels do not enhance glutamate release from presynaptic CBCs. Although EPSCs were completely blocked by NBQX when the VGLUT3+ amacrine cell was held at −60 mV, a slower, NBQX-insensitive component emerged at more depolarized potentials (Fig. 4C) and exhibited a j-shaped current–voltage relationship characteristic of N-methyl-d-aspartate receptors (NMDARs), which are blocked in a voltage-dependent fashion by extracellular Mg2+ (Fig. 4D). Accordingly, the NBQX-insensitive component of the EPSC was blocked completely by the NMDAR antagonist (RS)-3-(2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid (RS-CPP, 10 µM; to 5 ± 10% of control; P = 0.009; n = 5; Fig. 4C and 4D), indicating that VGLUT3+ amacrine cells express both AMPARs and NMDARs postsynaptically to CBC inputs.
Transmitter release from GABAergic and glycinergic amacrine cells often relies upon activation of presynaptic Nav channels (Protti et al., 1997; Cook et al., 1998; Bieda & Copenhagen, 1999; Chavez & Diamond, 2008; Chavez et al., 2010). To test for the contribution of Nav channels to excitatory postsynaptic potential (EPSP) amplification and action potential initiation in VGLUT3+ amacrine cells, we used a stimulating electrode to drive synchronous glutamate release from bipolar cells while recording EPSPs under whole-cell current clamp (see Materials and methods section). In the presence of inhibitory synaptic blockers, this stimulus protocol elicited large EPSPs (>10 mV) that were strongly suppressed by bath application of TTX (1 µM; to 52 ± 10% of control; P = 0.014; n = 5; Fig. 5A and 5B), indicating that Nav channels in VGLUT3+ amacrine cells amplify postsynaptic responses to synaptic input. Subsequent application of NBQX abolished the remaining response (Fig. 5B), indicating that NMDARs alone are insufficient to drive a discernible synaptic response. While electrically evoked EPSPs sometimes exhibited transient spike-like components (black trace; Fig. 5A), repetitive spiking was never observed. In fact, large current injections (up to 500 pA for 100 ms) through the somatic recording electrode never produced more than a single spike (n = 5; Fig. 5C). These data indicate that, while the biophysical properties and expression of Nav channels in VGLUT3+ amacrine cells amplify EPSPs, they do not enable the cell to fire repetitive action potentials under any of the conditions tested.
Light-evoked responses
Previous morphological studies showed that VGLUT3+ amacrine cells receive synaptic contacts in both the ON and OFF sublaminae of the IPL (Haverkamp & Wassle, 2004; Johnson et al., 2004), but it is unclear how these intermixed inhibitory and excitatory synapses respond to light increments and decrements. We first recorded light-evoked synaptic inputs to current-clamped VGLUT3+ amacrine cells in response to full-field illumination (1 s step to 9.6 × 10 photons/µm2/s from darkness; see Materials and methods section); however, at typical resting potentials (–44 ± 8 mV, n = 19), voltage responses were largely absent and frequently hyperpolarizing, suggesting that a significant portion of light-evoked synaptic input to these interneurons may arise from other inhibitory amacrine cells instead of being driven by direct excitatory input from ON or OFF bipolar cells. To isolate the excitatory and inhibitory components of light-evoked synaptic input, we recorded synaptic currents from voltage-clamped VGLUT3+ amacrine cells in response to full-field illumination, while incrementally varying the holding potential from −90 to +10 mV in 20 mV increments (Fig. 6A). Transient synaptic currents were observed at the onset and offset of light stimulation. The rapid decay of these responses indicates a lack of sustained synaptic input during constant illumination (Fig. 6A). By measuring the current-voltage (I–V) relationship of light-evoked EPSCs, we found that the synaptic reversal potential (Erev) at the peak of the ON response was −47.6 ± 3.6 mV (n = 6). Given the similarity of this value and the calculated chloride Erev for our internal and external recording solutions (–48.4 mV at 35°C), the ON response appeared to consist exclusively of inhibitory synaptic input. In contrast, I–V analysis of the rising phase of the OFF response indicated a more depolarized Erev of −25.1 mV ± 9.1 (n = 6). This suggested that the OFF response consists of mixed excitation (Erev = 0 mV) and inhibition. When we measured the I–V relationship at a point just after the peak of the OFF response, we found a slightly more negative synaptic Erev of −36.0 ± 6.9 mV (n = 6), indicating that inhibition outlasts excitation during the OFF response. Fig. 6B shows one example of the I–V analysis and corresponds to the EPSCs in Fig. 6A. To extract the time course of the excitatory and inhibitory conductances from synaptic currents, we measured the leak-subtracted I–V relationship before, during, and after the light stimulus (see Materials and methods section). According to Ohm’s Law, the slope of the I–V relationship represents the total synaptically evoked conductance (Fig. 6C, black trace). If we assume that excitatory and inhibitory synaptic inputs have reversal potentials of 0 and −48 mV, respectively, and that excitatory and inhibitory synaptic currents sum linearly, then, we can divide the total conductance into excitatory and inhibitory conductances based on the reversal potential of the total conductance (see Materials and methods section). Fig. 6C shows the total, excitatory, and inhibitory conductances calculated for the same cell shown in Fig. 6A and 6B. Accordingly, the ON response appears entirely as an inhibitory conductance, whereas the OFF response comprises both excitatory and inhibitory components with distinct time courses. OFF excitation was more transient than OFF inhibition and peaked 41.7 ± 23.2 ms sooner than the inhibitory conductance (n = 6). By the time OFF inhibition reached its peak, the excitatory conductance had decayed to roughly 50% of its maximum conductance. While the absolute amplitudes of excitatory and inhibitory conductance varied from cell to cell and are likely altered by imperfect space clamp, this analysis revealed trends that were consistent across all cells (n = 6): synaptic excitation was absent during the ON response and was smaller and briefer than inhibition during the OFF response.
Fig. 6.
VGLUT3+ amacrine cells receive both ON and OFF synaptic input. (A) Representative light-evoked EPSCs recorded from a voltage-clamped VGLUT3+ amacrine cell at a range of holding potentials (–90 to +10 mV; 20 mV increments). Vertical dotted lines and numbers indicate time points used for the I–V plots in (B). (B) I–V relationships for the currents shown in (A) at different time points during the light responses. (C) Plots of the total conductance (black trace), excitatory conductance (green trace), and inhibitory conductance (red trace) measured currents shown in (A). (D) and (E) Average (solid line) and s.d. of excitatory (shaded area, D) and inhibitory (E) conductance measured from light-evoked currents in six cells.
Discussion
The IPL of the mammalian retina is a dense mesh of neuronal processes that are highly interconnected via both electrical and chemical synapses. Amacrine cells contribute to complex computations, such as directional selectivity (Borg-Graham, 2001; Euler et al., 2002; Vaney & Taylor, 2002), looming detection (Munch et al., 2009), and local reciprocal feedback (Hartveit, 1999; Chavez et al., 2006; Grimes et al., 2010), as they also contribute to center-surround inhibition (Bloomfield & Xin, 2000; Ichinose & Lukasiewicz, 2005). Only starburst, AII, and A17 amacrine cells have been studied in great physiological detail, but they only account for ~10% of the known amacrine cell subtypes (and <20% of the total amacrine cell population; MacNeil & Masland, 1998). Identification of cell-specific markers that can be used to genetically label-specific amacrine cell subtypes (e.g., Siegert et al., 2009) will be critical for gaining insight into the physiology of these elusive subpopulations.
Implications of VGLUT3+ amacrine cell morphology and biophysical mechanisms
In this study, we have genetically labeled VGLUT3 expressing neurons in mice. We used this model to assess the morphology and basic membrane properties of VGLUT3+ amacrine cells and characterize their synaptic inputs and basic light responsivity. As with all neurons, the physiology of VGLUT3+ amacrine cells is a product of their distinct morphology, synaptic mechanisms, and active membrane properties. Of particular morphological interest is the observation that individual dendrites of VGLUT3+ amacrine cells typically are not tightly confined to any one strata of the IPL but instead occupy both the ON and OFF sublaminae (Fig. 3B and Fig. S1). We interpret this particular anatomical feature to indicate that VGLUT3+ amacrine cells may mediate crossover signaling between the ON and OFF visual channels. Another interesting observation is that the somata were typically connected to the dendritic arbor via only a few thin neuritic segments. These thin segments are likely of high electrical resistance, suggesting that the somata of VGLUT3+ amacrine cells may be electrotonically isolated and contribute only minimally to the electrical signal processing occurring locally within the dendritic arbors.
The physiological recordings presented here identify several of the primary mechanisms that shape the VGLUT3+ amacrine cell’s functional response. VGLUT3+ amacrine cells receive excitatory synaptic input primarily from OFF CBCs (Fig. 6) via both AMPAR and NMDAR (Fig. 4). Additionally, isolated glutamatergic inputs were significantly amplified postsynaptically by Nav channels (Fig. 5). This interaction could help to spread synaptically evoked depolarizations to more distal locations within the dendrites (i.e., laterally or between sublamina) and/or enhance the coupling between input and output synapses. Our results with TTX are consistent with previous immunohistochemical work showing significant labeling of VGLUT3+ amacrine cells with pan-specific Nav antibodies (Johnson et al., 2004). L-type Cav channels provide a mechanism, by which tonic membrane depolarization can trigger sustained Ca2+ influx and, possibly, tonic neurotransmitter release, while T-type Cav channels and rapidly inactivating BK channels may transiently influence local Ca2+ signaling (~1–10 ms; Fig. 3). For example, rapidly inactivating BK currents, similar to those observed here, have been observed in other amacrine cells and been shown to regulate synaptic output by rapidly suppressing Cav channel activation (Grimes et al., 2009). In general, VGLUT3+ amacrine cells possess the membrane conductances necessary to produce both local and global Ca2+ responses to synaptic input.
Light-evoked synaptic inputs and functional implications
We observed that light-evoked synaptic inputs to VGLUT3+ amacrine cells were dominated by inhibition, suggesting that they receive synaptic input primarily from other amacrine cells. Similar circuit motifs have been observed in the retina, including serial inhibition to bipolar cells (Eggers & Lukasiewicz, 2010a,b) and GCs (Zhang et al., 1997); in these cases, however, the output amacrine cell at the end of the circuit was inhibitory and either GABAergic or glycinergic. Although VGLUT3+ amacrine cells contain glycine, they do not appear to express a vesicular glycine transporter (Johnson et al., 2004) and so may be purely excitatory. Another common type of amacrine-to-amacrine cell inhibition that is widely observed is “crossover inhibition,” so called because an ON amacrine cell receives inhibitory input from the OFF pathway or vice versa (Hsueh et al., 2008; Molnar et al., 2009). Although we observed ON–OFF inhibitory input to VGLUT3+ amacrine cells, this type of arrangement appears to be a rare amacrine cell feature (Hsueh et al., 2008), and it is not clear how crossover inhibition would apply to an amacrine cell that makes excitatory output. Care should be taken in interpreting these results, as the light-evoked responses of VGLUT3+ amacrine cells could be highly specific to particular aspects of visual stimuli, and thus, the results presented here only represent the cell’s response to full-field stimulation. Further experiments will be needed to determine which aspects of the visual scene are best encoded by the retinal circuitries containing these particular interneurons.
Because we still lack critical information regarding the nature and targets of VGLUT3+ amacrine cell output, we can only speculate about its function but several of the present results may be informative. First, our recordings indicate that VGLUT3+ amacrine cells rest at relatively depolarized membrane potentials (–44 ± 8 mV), raising the possibility that they may tonically release neurotransmitter in response to steady calcium influx through L-type calcium channels (Fig. 3H–3J). Second, because inhibition is only transiently evoked during ON and OFF inputs, it is possible that transmitter release may be suppressed particularly during changes in illumination. This would effectively create a low-pass filter for gating transmitter output. Third, OFF excitation precedes OFF inhibition by ~40 ms (Fig. 6), providing a brief time window during which OFF input may transiently gate an increase in transmitter release with high temporal fidelity, possibly employing T-type calcium channels. This arrangement would create a high-pass filter selectively for OFF information. Finally, because of the compact dendritic architecture and the lack of segregation between the ON and OFF sublaminae, this cell may act as a substrate for cross-channel (ON ↔ OFF) communication. Although this phenomenon is widely observed, to date, only the AII amacrine cell has been shown to mediate this type of signaling (Pang et al., 2003; Manookin et al., 2008; Murphy & Rieke, 2008; van Wyk et al., 2009).
VGLUT3+ amacrine cell output remains elusive
A recent anatomical study has suggested that VGLUT3+ amacrine cells make synaptic contacts onto retinal GCs; VGLUT3 labeling was found in vesicles presynaptic to GCs, and VGLUT3+ processes were found in close opposition to mGluR4 (Johnson et al., 2004). We made several attempts to record direct functional connections between VGLUT3+ amacrine cells and GCs using dual patch-clamp recordings but were unable to elicit discernible responses in the postsynaptic recording electrode regardless of postsynaptic holding potential (data not shown). Similar attempts were made between VGLUT3+ amacrine cells and other amacrine cells, but again, only negative results were obtained. In the future, it will be interesting to see if VGLUT3+ amacrine cells release glutamate, glycine, or both from conventional synapses, or if a more complex signaling arrangement exists, such as a nonsynaptic modulation by glutamate during development (Stella et al., 2008).
Supplementary Material
Acknowledgments
We thank Juliette Johnson for critically reading the manuscript and Hua Tian for breeding and genotyping the mice used in this study. W.N.G., N.O., and J.S.D. were supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. R.P.S. and R.H.E. were supported by National Institute of Mental Health.
References
- Bieda MC, Copenhagen DR. Sodium action potentials are not required for light-evoked release of GABA or glycine from retinal amacrine cells. Journal of Neurophysiology. 1999;81:3092–3095. doi: 10.1152/jn.1999.81.6.3092. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. The Journal of Physiology. 2000;523(Pt 3):771–783. doi: 10.1111/j.1469-7793.2000.t01-1-00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nature Neuroscience. 2001;4:176–183. doi: 10.1038/84007. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Diamond JS. Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina. The Journal of Neuroscience. 2008;28:7919–7928. doi: 10.1523/JNEUROSCI.0784-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. The Journal of Neuroscience. 2010;30:2330–2339. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, McReynolds JS. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. The Journal of Neuroscience. 1998;18:2301–2308. doi: 10.1523/JNEUROSCI.18-06-02301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. Journal of Neurophysiology. 2010a;103:25–37. doi: 10.1152/jn.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Visual Neuroscience. 2010b;28:95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Jellali A, Mutterer J, Sahel JA, Rendon A, Picaud S. Distribution of vesicular glutamate transporters in rat and human retina. Brain Research. 2006;1082:73–85. doi: 10.1016/j.brainres.2006.01.111. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. The Journal of Neuroscience. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Vinatier J, Amilhon B, Guerci A, Christov C, Ravassard P, Giros B, El Mestikawy S. Developmen-tally regulated expression of VGLUT3 during early post-natal life. Neuropharmacology. 2005;49:901–911. doi: 10.1016/j.neuropharm.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE, Diamond JS. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nature Neuroscience. 2009;12:585–592. doi: 10.1038/nn.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: More than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–885. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. Journal of Neurophysiology. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. The Journal of Comparative Neurology. 2004;468:251–263. doi: 10.1002/cne.10962. [DOI] [PubMed] [Google Scholar]
- Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. Journal of Neurophysiology. 2008;100:2077–2088. doi: 10.1152/jn.90417.2008. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. The Journal of Physiology. 2005;565:517–535. doi: 10.1113/jphysiol.2005.083436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Sherry DM, Liu X, Fremeau RT, Jr, Seal RP, Edwards RH, Copenhagen DR. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. The Journal of Comparative Neurology. 2004;477:386–398. doi: 10.1002/cne.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: Matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. The Journal of Comparative Neurology. 1999;413:305–326. [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: Implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. The Journal of Neuroscience. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: Circuitry that compensates for nonlinear rectifying synaptic transmission. Journal of Computational Neuroscience. 2009;27:569–590. doi: 10.1007/s10827-009-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nature Neuroscience. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nature Neuroscience. 2008;11:318–326. doi: 10.1038/nn2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch NW, Taylor WR. Tetrodotoxin-resistant sodium channels contribute to directional responses in starburst amacrine cells. PLoS One. 2010;5:e12447. doi: 10.1371/journal.pone.0012447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. The Journal of Neuroscience. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Gerschenfeld HM, Llano I. GABAergic and glycinergic IPSCs in ganglion cells of rat retinal slices. The Journal of Neuroscience. 1997;17:6075–6085. doi: 10.1523/JNEUROSCI.17-16-06075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. The Journal of Biological Chemistry. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Siegert S, Scherf BG, Del Punta K, Didkovsky N, Heintz N, Roska B. Genetic address book for retinal cell types. Nature Neuroscience. 2009;12:1197–1204. doi: 10.1038/nn.2370. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. The Journal of Neuroscience. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella SL, Jr, Li S, Sabatini A, Vila A, Brecha NC. Comparison of the ontogeny of the vesicular glutamate transporter 3 (VGLUT3) with VGLUT1 and VGLUT2 in the rat retina. Brain Research. 2008;1215:20–29. doi: 10.1016/j.brainres.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. The Journal of Neuroscience. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Taylor WR. Direction selectivity in the retina. Current Opinion in Neurobiology. 2002;12:405–410. doi: 10.1016/s0959-4388(02)00337-9. [DOI] [PubMed] [Google Scholar]
- van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Visual Neuroscience. 2009;26:297–308. doi: 10.1017/S0952523809990137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. The Journal of Neuroscience. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nature Biotechnology. 1997;15:859–865. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Visual Neuroscience. 1997;14:553–563. doi: 10.1017/s0952523800012219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.