Abstract
Introduction
Hypertension affects more than a quarter of the global adult population. Studies conducted worldwide suggest an overall small, yet useful, role of omega-3 PUFAs in reducing blood pressure in hypertensive patients. However there is no substantial data in this regard from population based in Middle East and Asia.
Objectives
To determine the effects of (omega-3) PUFA supplementation on the blood pressure of hypertensive patient.
To identify if male and female hypertensive patients respond differently to PUFA.
To identify if response of hypertensive patients to PUFA varies with the duration of hypertension and co-existence of diabetes/dyslipidemia.
Materials and methods
This observational study was conducted among hypertensive patients visiting OPD of the Gulf Medical College Hospital, Ajman, UAE, during the period Jan–Dec 2012. A total of 100 hypertensive patients on treatment with their antihypertensive medications, 50 of whom were taking n-3 PUFA supplementation, were followed up for a period of 3 months. Comparisons were drawn between the BP recordings at the time of enrollment in the study and their follow up values 3 months after enrollment.
Results
There was a statistically significant reduction in both the systolic and diastolic blood pressures after 3 months of PUFA therapy. The BP lowering effect of PUFA was more in males. A statistically significant reduction in BP was noted in non-diabetic patients and patients with long standing hypertension.
Conclusion
Findings of the study suggest that omega-3 PUFA dietary supplements augment the benefits of pharmacotherapy in hypertension.
Keywords: PUFA, Hypertension, Supplements
1. Introduction
Hypertension is a global health problem. Its worldwide prevalence in adults ages ≥25 years was about 40% in 2008. Despite the modest drop in prevalence during the last two decades, the number of individuals with uncontrolled hypertension increased by about 400 million due to natural population growth.1 Such prevalence is expected to increase by atleast 24% and 80% in developed countries and developing countries respectively.2 In 2007 a study by Pathan et al, stated that hypertension, even when severe, is commonly underdiagnosed and undertreated in the UAE; thus preventive strategies, better diagnosis and proper treatment compliance should be emphasized to reduce incidence of CVD in this population.3,4
Certain dietary interventions such as, reduced salt intake and moderation of alcohol consumption in habitual alcohol consumers, as well as, certain life style modifications such as body weight optimization and well-planned exercise have been shown to augment antihypertensive medication therapy in controlling hypertension. Diet that includes two to three servings of oily fish per week has been often recommended by several health organizations.5,6 Such recommendations stem from the understanding that the intake of long chain omega-3 (n-3) fatty acids – namely eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – which are highly available in fatty fish and fish oils, possess cardiovascular protective effects that include but are not limited to blood pressure reduction.7 Omega-3 fatty acids are a group of biologically occurring Polyunsaturated Fatty Acids (PUFAs). DHA and EPA are two long chain omega-3 fatty acids that function as precursors to eicosanoids – prostaglandins, thromboxanes, and leukotrienes – which are assumed to have anti-inflammatory, antithrombotic, antiarrhythmic, and vasodilatory properties. DHA and DPA are biosynthesized (in-vivo) from a short chain fatty acid called alpha-linoleic acid (ALA). The rate of this biosynthesis mechanism, however, is extremely low in humans; which renders dietary supplementation the sole source of DHA and EPA for humans.8,9
The purpose of our study is to verify whether there is an effective role for omega-3 PUFA dietary supplements in the treatment plans of hypertensive patients seen regularly at the outpatient Department of Gulf Medical College Hospital (GMCH) in Ajman, U.A.E and to examine if there exists unique effects of omega-3 PUFAs on BP in a certain gender or ethnicity of patients, and in patients with a certain co-morbidity, within our population of patients. It is worth noting that advising hypertensive patients to consume omega-3 PUFA as dietary supplements to augment their pharmacotherapy regimens is a strategy that is utilized by some medical practitioners at GMC. However, such dietary supplements are not covered by most of our patients’ health insurance plans and thus patients who opt to utilize those dietary supplements pay for them on an out of pocket basis. The main driving force behind conducting our study is to investigate whether we should continue to advice our hypertensive patients at GMC to regularly consume omega-3 PUFA dietary supplements. We searched the ProQuest, Cochrane, PubMed, and Google Scholar databases utilizing and meshing keywords such as PUFA, Omega-3 fatty acids, fish oil, dietary supplements, blood pressure, and hypertension. We found out that many small size randomized controlled trails (RCTs) were conducted over time in different parts of the world to objectively investigate the relationship between consuming omega-3 PUFAs and blood pressure. In general, the results of such studies did not have rigorous external validities so they could not be extrapolated to various populations. Moreover, false negative findings were likely in those randomized controlled trials whenever sample sizes were not large enough or if blood pressure measuring techniques were sub-optimal.10 We found, as well, that Meta analyses and population based cross sectional were consequently conducted to establish more clinically meaningful relationships between omega-3 PUFAs’ consumption and blood pressure.
There are also however evidence in contrary to the cardio protective effects of PUFA. Recent randomized controlled studies, however, have made conclusions that the so talked about benefits of PUFA are controversial and also suggest possible harm with fish oil supplementation to patients diagnosed with cardiovascular disease.11
Due to conflicting results in specific populations’ strata, we could not establish strong recommendations regarding the role of PUFAs dietary supplement preparations in the treatment plans of hypertensive patients regularly seen at GMC, nor could we determine whether such approach is more useful to a specific gender, age-group, or patients with particular co-morbidity such as Diabetes Mellitus. We decided to conduct a prospective observational study that has an intervention group and a comparison group to look at the effect of omega-3 PUFA regular intake on the level of hypertension control in a group of hypertensive patients who are recurrently seen at the outpatient department in GMC.
2. Materials and methods
2.1. Study settings and population
This study was conducted among hypertensive patients visiting OPD of Internal Medicine Department at the GMCH & RC during the period June 2011–Jan 2013. Patients diagnosed with hypertension on treatment and prescribed PUFA were included in the study. Patients using hormone replacement therapy, and diagnosed with cardiac or c/c renal disease or complications of hypertension were excluded from the study. Based on evidence from available literature we expected a mean difference of 5 mm Hg and a SD of 8 and hence the estimated sample size was calculated to be 50 in each arm.
2.2. Study design and data collection
This observational study was approved by the Ethics Committee of the University and therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The study participants were briefed about the purpose and objectives of the study before taking consent from them. As a part of patient education, all hypertensive patients attending the OPD of Internal medicine are being educated by their attending physician about the health benefits of PUFA as a nutritional supplement and prescribed 1 gm fish oil (Omacor tablets) daily. The researchers screened all the hypertensive patients attending the medicine OPD based on their inclusion and exclusion criteria and enrolled them for the study. A pre-designed, validated questionnaire was filled by one of the researchers after interviewing the patient. The questionnaire recorded the socioeconomic variables; details of hypertension-age of onset, duration; history of dyslipidemia/diabetes and treatment history – drugs, dosages and duration. Participants sat quiet for atleast 5 min and three blood pressure readings were taken using with a mercury manometer at 2 min intervals and the average value was recorded in the questionnaire. After a follow up of 1 month the participants who consumed PUFA supplement were categorized into PUFA group and the others into control group. All the patients were followed up for 2 more months. Enrollment of patients into the study continued till the sample size of 50 was attained in each group and each of the participants in both groups was followed up for 2 more months after being allocated into the groups (Fig. 1). Comparisons were drawn between the BP recordings at the time of enrollment in the study and their follow up values 3 months after enrollment.
Fig. 1.
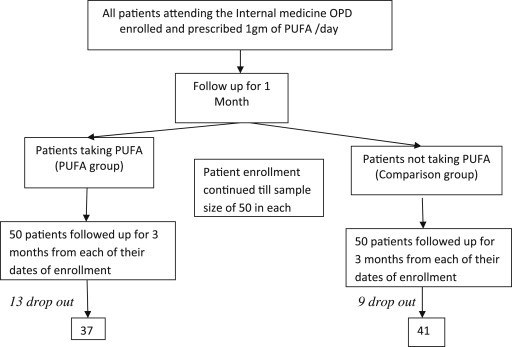
Patient enrollment and follow up.
2.3. Data analysis
Data was fed into Excel spreadsheet and transferred to PASW 19 version software for statistical analysis. Only subjects who completed the study and had both baseline and final measurements were included in the analysis. Chi-square test was used to determine the association between the variables. Paired and unpaired t-tests were used to compare the variables within the group and between the groups both prior to and after the intervention. The significance level was considered as p < 0.05.
3. Results
3.1. Socio demographic characteristics
The participants of this study were of the mean age of 46.4 years and the mean duration of hypertension was 5 years. Majority of them were males (73%). Analysis of demographic data revealed that 66% participants were of Arab origin while the remainder of the populations was Asian.
Table 1 shows the baseline characteristics of the PUFA group and control group. The two groups are found to be matched with regards to their mean age and mean duration of hypertension and their blood pressure values. There was a significant difference in the gender distribution among both the groups. The participants in the PUFA group were predominantly Arabs (94.6%) while Asians accounted for 58.5% of the control group. Prevalence of diabetes was 27% in PUFA group and 31% in control group. 89% of the patients in PUFA were on lipid lowering drugs between the two groups. The BP values of patients with diabetes and patients on lipid lowering drugs were also comparable at the time of enrollment (Table 2).
Table 1.
Gender, ethnicity and co-morbidity of comparison group and PUFA group.
| Variables | Groups | PUFA group (n = 37) n (%) | Comparison group (n = 41) n (%) | p |
|---|---|---|---|---|
| Gender | Male | 22 (59.5) | 35 (85.4%) | 0.01 |
| Female | 15 (40.5%) | 6 (14.6%) | ||
| Ethnicity | Arab | 35 (94.6%) | 17 (41.5%) | 0.001 |
| Asian | 2 (5.4%) | 24 (58.5%) | ||
| Number of diabetic patients | 10 (27%) | 13 (31%) | NS | |
| Number of patients on lipid lowering drugs | 33 (89%) | 23 (56%) | 0.001 |
Table 2.
Blood pressure values at enrollment (mean ± SD).
| Variables | Groups | PUFA group n = 37 | Comparison group n = 41 | p |
|---|---|---|---|---|
| Blood pressure of all participants | Systolic BP | 131.89 ± 18.64 | 138.76 ± 14.50 | NS |
| Diastolic BP | 85.27 ± 11.05 | 87.41 ± 10.61 | NS | |
| Blood pressure of diabetic patients | Systolic BP | 140.00 ± 25.386 | 141.54 ± 15.730 | NS |
| Diastolic BP | 87.50 ± 15.855 | 88.46 ± 12.810 | NS | |
| Blood pressure of patients on lipid lowering drugs | Systolic BP | 133.33 ± 19.15 | 137.09 ± 13.52 | NS |
| Diastolic BP | 86.21 ± 11.25 | 85.04 ± 9.89 | NS |
Table 3 shows the changes in blood pressure during the study. There was a significant reduction in both the systolic and diastolic blood pressures after 3 months of PUFA therapy. While the systolic BP showed a mean reduction of 9.7 mm Hg (95% CI of 2.3–15.7), the diastolic pressure reduced by a mean of 4.7 mm Hg (95% CI of 1.6–8.8) after PUFA therapy, when compared to the patients on antihypertensive medications alone.
Table 3.
Comparison of BP values at enrollment and at 3 months of follow up (mean ± SD).
| Variables | Enrollment |
Follow up |
||||
|---|---|---|---|---|---|---|
| PUFA group (n = 37) | Comparison group (n = 41) | p | PUFA group (n = 37) | Comparison group (n = 41) | p | |
| Systolic BP (mm Hg) | 131.89 ± 18.68 | 138.76 ± 14.50 | NS | 122.89 ± 12.18 | 132.59 ± 13.44 | <0.001 |
| Diastolic BP (mm Hg) | 85.27 ± 11.05 | 87.41 ± 10.61 | NS | 80.05 ± 6.33 | 84.78 ± 8.28 | <0.01 |
As the groups were not comparable with regards to gender, we used paired t-tests to analyze the response of different genders to PUFA therapy, with the intervention group. Following PUFA therapy the systolic blood pressure in males showed a significant reduction of 9.63 (95% CI of 0.2–17.1). Reduction was noted in the diastolic BP of males and both systolic and diastolic blood pressure values of the female participants as well, but this was not of statistical significance (Table 4).
Table 4.
Gender and changes in blood pressures following PUFA therapy.
| Gender | Variables | Enrollment | Follow up | p |
|---|---|---|---|---|
| Males (n = 22) | Systolic BP | 132.27 ± 17.34 | 123.64 ± 13.99 | <0.05 |
| Diastolic BP | 83.65 ± 10.02 | 80.45 ± 6.53 | NS | |
| Females (n = 15) | Systolic BP | 131.33 ± 21.00 | 121.80 ± 9.26 | NS |
| Diastolic BP | 80.33 ± 11.10 | 79.47 ± 6.20 | NS |
Hypertensive patients with diabetes showed reduction in their blood pressure values on PUFA therapy (CI for Systolic BP – 3.4–32.0, CI for diastolic BP – 3.0–15.6). However the reduction was not statistically significant. The systolic BP of non-diabetic patients showed a mean reduction of 7 mm Hg (95% CI of 0.22–14.30) while the diastolic BP reduced by 5 mm Hg (95% CI of 0.8–8.8) (Table 5).
Table 5.
Diabetic status and changes in BP with PUFA therapy.
| Disease groups | Variables | Enrollment | Follow up | p |
|---|---|---|---|---|
| Diabetic participants (n = 10) | Systolic BP | 140.00 ± 25.39 | 125.70 ± 6.87 | NS |
| Diastolic BP | 87.50 ± 15.85 | 81.20 ± 6.05 | NS | |
| Non diabetic participants (n = 27) | Systolic BP | 128.89 ± 15.02 | 121.85 ± 13.60 | <0.05 |
| Diastolic BP | 84.44 ± 8.91 | 79.63 ± 6.50 | <0.05 |
Significant blood pressures reduction of 13 mm Hg systolic (95% CI of 1.5–24.5) and 6.75 mm Hg diastolic (95% CI 1.6–11.9) was recorded, following PUFA therapy in participants with hypertension of 5 years or more duration. Though consumption of PUFA resulted in improvement of blood pressure values in patients with hypertension of less than 5 years duration, this was not statistically significant (Table 6).
Table 6.
Duration of hypertension and changes in BP with PUFA therapy.
| Duration of hypertension | Variables | Enrollment | Follow up | p |
|---|---|---|---|---|
| <5 years (n = 17) | Systolic BP | 125.88 ± 10.04 | 121.59 ± 7.93 | NS |
| Diastolic BP | 82.94 ± 7.71 | 79.53 ± 5.81 | NS | |
| ≥5 years (n = 20) | Systolic BP | 137.00 ± 22.73 | 124.00 ± 15.01 | <0.05 |
| Diastolic BP | 87.25 ± 13.13 | 80.50 ± 6.86 | <0.05 |
Consumption of PUFA along with statins resulted in a significant decrease in both systolic and diastolic BP of patients when compared to patients on statin therapy alone. Subgroup analysis could not be done to determine the difference in the effect of PUFA on the BP of patients who are on antihypertensives and patients who are on statin therapy along with antihypertensives due to insufficient sample size (Table 7).
Table 7.
BP changes in patients on statins in PUFA and comparison group (mean ± SD).
| Variable | Enrollment |
Follow up |
||||
|---|---|---|---|---|---|---|
| PUFA group (n = 33) | Comparison group (n = 23) | p | PUFA group (n = 33) | Comparison group (n = 23) | p | |
| Systolic BP (mm Hg) | 133.33 ± 19.15 | 137.09 ± 13.52 | NS | 123.24 ± 12.63 | 131.13 ± 14.25 | <0.05 |
| Diastolic BP (mm Hg) | 86.21 ± 11.25 | 85.04 ± 9.89 | NS | 79.76 ± 6.47 | 83.91 ± 7.22 | <0.05 |
4. Discussion
Dietary intake of ϖ3 PUFA is known to reduce the incidence of mortality and morbidity from cardiovascular diseases. The reduction in the risk for arrhythmias, inhibition of growth of atherosclerotic plaques, anti-inflammatory effects and decrease in triglyceride levels caused by ϖ3 PUFAs–DHA and EPA account for their advocated use in patients with cardiovascular diseases.11
The reduction caused by administration of PUFA in the systolic and diastolic blood pressure of hypertensive patients on treatment, as observed in our study is in accordance with the Meta-analysis findings of Morris et al, that showed a significant reduction in blood pressure of 3.4/2.0 mm Hg with the consumption of omega-3 fatty acids.12 While a systematic review of randomized controlled trials and cross over trials by Campbell et al (2013) reported significant reductions in systolic and diastolic BP; 2.56 mm Hg (95% CI 0.58–4.53) and 1.47 mm Hg (95% CI 0.41–2.53) of hypertensive patients, they did not find any significant reduction in BP values of normotensive patients.13 Studies have also stated that the reduction in systolic pressure is noted earlier in the course of treatment with PUFA than the improvement in diastolic blood pressure values.11 A cross sectional study conducted by Ueshima et al (2007) concluded that when compared to hypertensive individuals, consumption of foods containing PUFA had a stronger inverse association with blood pressure values of normotensives and in individuals who are not undergoing dietary and/or medical interventions.10
The beneficial effects of PUFA on vascular function are mediated by a wide range of biochemical and physiological alterations. EPA and DHA are acted upon by cyclo-oxygenase and lipo-oxygenase give rise to 3 series prostaglandins and 5 series leukotrienes. TxA3 is biologically inactive while PGI3 is equipotent to PGI2 in causing vasodilatation and inhibiting platelet aggregation hence the overall balance is shifted from vasoconstriction to vasodilatation.14
Studies in both humans and animals have revealed that long chain n-3 PUFAs inhibit the synthesis of TxA2 – a potent vasoconstrictor.15 This effect may be produced by altering the enzymes for its biosynthesis.16 Antagonism of TxA2 and PGH2 receptors is also another mechanism by which the blood vessel is kept dilated by n-3 PUFA.17 Another endothelium derived vasoconstrictor inhibited by EPA is Endothelin-1.
n-3 PUFAs increase endothelium-dependent relaxation by enhancing the release of NO.18 The antioxidant action of n-3 PUFA is also known to reduce endothelial free radical damage and thus restore the balance between VD and VC.19 Activation of vascular K+ channels and inhibition of Ca2+ channels leads to hyperpolarisation and relaxation of vascular smooth muscle was noted with treatment with PUFA.20
The metabolism of n-3 PUFAs by Δ5 and Δ6 desaturates is known to be modified by genetic and epigenetic factors. Genetic pleomorphism of genes for these enzymes result in altered content of n-3 PUFA in membrane phospholipids and hence their response in different individuals.21 This could be postulated to be the reason for the less improvement in BP values of females when compared to males in our study. In contrast to our findings there has been data suggesting no significant interaction between BP and gender.10,11 Results of meta-regression analysis conducted by Geleijnse (2002) suggest more significant reduction in women than in men; however, no direct comparison between BP reductions in the two genders was made. In view of these conflicting results more studies have to be conducted to analyze the interactions between female hormones and effect of PUFA.22
A rich source of dietary n-3 PUFA is known to reduce the increased systolic blood pressure associated with long-term diabetes in rats.23 Blood pressure also decreases in type 1 diabetic patients receiving supplements of n-3 PUFA.24 This is consistent with the findings of our study where a reduction was noted in the blood pressure values of hypertensive patients with diabetes. Obesity, malignancies and insulin resistance are among the acquired causes of modifications in the rate limiting enzymes of n-3 PUFA metabolism.7,25 This could probably be the reason why the improvement seen in non-diabetic hypertensives on PUFA therapy was significant when compared to patients with diabetes co-existing with hypertension in our study.
Endothelial dysfunction is present in various forms of cardiovascular disease. Long standing hypertension compounds endothelial dysfunction, decreasing responsiveness to medications. In hypertension, reduction of BP per se does not seem to restore endothelial function. Restoration of endothelial function is seen following treatment of only few underlying diseases.26 PUFAs by their mechanism of action reduce the endothelial damage and hence maintain responsiveness to antihypertensive therapy.
Treatment with 3 hydroxy3methylglutaryl-coenzyme A reductase inhibitors (statins) has a relatively small but statistically significant effect on blood pressure. A meta-analysis of the effect of statins on blood pressure in patients on concomitant antihypertensive treatment revealed significant reductions in both systolic and diastolic BP of patients taking statins, in comparison with placebo group.27 In our study, it was found that stains when consumed along with PUFA supplements caused significant reduction in blood pressures as compared to the group taking satins alone. This is in accordance with the findings of Cicero AF (2010) who found that PUFA supplementation for 1-year duration in hypertriglyceridemic patients on statin therapy with high normal blood pressure resulted in a significant reduction in their systolic BP, diastolic BP and pulse pressure. This hypotensive action was found to be independent of its hypotriglyceridemic effect.28
5. Limitations
Randomization was not performed, due to the fact that there were financial; constraints in making the PUFA supplement available to the patient. It was in such a scenario more feasible to observe patients who were in a position to pay for the same by themselves. We were not able to control the effect of the confounding factor of diet, due to lack of a validated questionnaire which is standardized for recording of dietary data of the population based in UAE.
6. Conclusion
From our observational study we found that supplementation with n-3 PUFA (1 g/d) for 3 months causes significant reduction in BP of patients with hypertension on treatment. The effect of PUFA was more pronounced in patients with long standing hypertension. Co-existence of diabetes was observed to cause a decrease in response of patients to n-3 PUFA supplements. Hence we like to conclude that in accordance with data already available Omega-3 PUFA dietary supplements have a beneficial effect as add-on modalities to augment pharmacotherapy in hypertension. However the observation that BP of females of Arab ethnicity shows a less response to PUFA is a finding which needs to be investigated by genetic studies.
Conflicts of interest
All authors have none to declare.
References
- 1.WHO Global Health Data Repository. 2008. http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/index.html Retrived on Feb 26th, 2013 from: [Google Scholar]
- 2.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Tu K., Chen Z., Lipscombe L. Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. Can Med Assoc J. 2008;178:1429–1435. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathan J.Y., Abdulle A.M., Obineche E.N. 2007. Hypertension in the United Arab Emirates.http://www.arabianbusiness.com/hypertension-in-united-arab-emirates-148672.html Retrived on Feb 15 2013 from: [Google Scholar]
- 5.Kris-Etherton P.M., Harris W.S., Appel L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 6.UK Scientific Advisory Committee on Nutrition . The Stationery Office; London, United Kingdom: 2004. Advice on Fish Consumption: Benefits and Risks. [Google Scholar]
- 7.Das U.N. Essential fatty acids—a review. Curr Pharm Biotechnol. 2006;7:467–482. doi: 10.2174/138920106779116856. [DOI] [PubMed] [Google Scholar]
- 8.Appel L.J., Brands M.W., Daniels S.R., Karanja N., Elmer P.J., Sacks F.M. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 9.Covington Maggie B. Omega-3 fatty acids. Am Fam Physician. 2004;70:133–140. [PubMed] [Google Scholar]
- 10.Ueshima H., Stamler J., Elliott P. Food omega-3 fatty acid intake of individuals (total, linoleic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block Robert C., Pearson Thomas A. The cardio vascular implications of Omega- 3 fatty acids. Folia Cardiol. 2006;13:557–569. [Google Scholar]
- 12.Leonarda De Rosa Maria. Can purified Omega-3 polyunsaturated fatty acids supplementation act blood pressure levels in untreated normal-high blood pressure subjects with hypertriglyceridemia? Pharmacol Pharm. 2012;3:234–239. [Google Scholar]
- 13.Morris M.C., Sacks F., Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 14.Campbell F., Dickenson H., Critchley J., Ford G., Bradburn M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur J Prev Cardiol. 2013;20:107–120. doi: 10.1177/2047487312437056. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt E.B. Fish consumption, n23 fatty acids in cell membranes, and heart rate variability in survivors of myocardial infarction with left ventricular dysfunction. Am J Cardiol. 1997;74:1670–1673. doi: 10.1016/s0002-9149(97)00220-8. [DOI] [PubMed] [Google Scholar]
- 16.Weber P.C. Clinical studies on the effects of n23 fatty acids on cells and eicosanoids in the cardiovascular system. J Intern Med. 1989;225:61–68. doi: 10.1111/j.1365-2796.1989.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 17.Abeywardena M.Y., McLennan P.L., Charnock J.S. Differential effects of dietary fish oil on myocardial prostaglandin I and thromboxane A production. Am J Physiol. 1992;260:H379–H385. doi: 10.1152/ajpheart.1991.260.2.H379. [DOI] [PubMed] [Google Scholar]
- 18.Abeywardena M.Y., Head R.J. Differential Antagonism by DHA and EPA at the Thromboxane-A and Isoprostane Receptors in Rat Aorta (abstr). 4th Congress of ISSFAL, June 4–9, Japan. 2000. p. 68. [Google Scholar]
- 19.Abeywardena M.Y., Head R.J. Long chain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res. 2001;52:361–371. doi: 10.1016/s0008-6363(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 20.Mori T.A., Puddey I.B., Burke V. Effect of v-3 fatty acids on oxidative stress in humans: GCMS measurement of urinary F2- isoprostane excretion. Redox Rep. 2000;5:45–46. doi: 10.1179/rer.2000.5.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Hirafuji M., Ebihara T., Kawahara F., Hamaue N., Endo T., Minami M. Inhibition by docosahexaenoic acid of receptor-mediated Ca(2+) influx in rat vascular smooth muscle cells stimulated with 5-hydroxytryptamine. Eur J Pharmacol. 2001;427:195–201. doi: 10.1016/s0014-2999(01)01274-2. [DOI] [PubMed] [Google Scholar]
- 22.Lattka E., Illig T., Koletzko B., Heinrich J. Genetic variantsof the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr Opin Lipidol. 2010;21:64–69. doi: 10.1097/MOL.0b013e3283327ca8. [DOI] [PubMed] [Google Scholar]
- 23.Geleijnse J., Giltay E., Grobbee D., Donders A., Kok F. Blood pressure response to fish oil supplementation. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Garman Joseph H., Mulroney Susan, Manigrasso Michaele, Flynn Elizabeth, Maric Christine. AJP - Ren Physiol. 2009;296:F306–F316. doi: 10.1152/ajprenal.90326.2008. [DOI] [PubMed] [Google Scholar]
- 25.Jensen T., Stender S., Goldstein K., Hølmer G., Deckert T. Partial normalization by dietary cod-liver oil of increased microvascular albumin leakage in patients with insulin-dependent diabetes and albuminuria. N Engl J Med. 1989;321:1572–1577. doi: 10.1056/NEJM198912073212304. [DOI] [PubMed] [Google Scholar]
- 26.Reese C., Fradet V., Witte J.S. ω-3 Fatty acids,genetic variants in COX-2 and prostate cancer. J Nutrigenetics Nutrigenomics. 2009;2:149–158,. doi: 10.1159/000235565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endemann D.H., Schiffrin L.E. Endothelial dysfunction. JASN. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 28.Strazzullo P., Kerry S.M., Barbato A., Versiero M., D'Elia L., Cappuccio F.P. Do statins reduce blood pressure?: a meta-analysis of randomized, controlled trials. Am J Hypertens. 2007;20:937–941. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]


