Abstract
Dothiorella and Spencermartinsia are two botryosphaeriaceous genera with dark 2-celled conidia and found in parasitic, saprophytic or endophytic association with various woody host plants. Based on ITS and EF1-α sequence data and morphology, eight new species are described from Iran, New Zealand, Portugal and Spain. Of these, five species are placed in Dothiorella, namely D. iranica, D. parva, D. prunicola, D. sempervirentis and D. striata, and three species belong to Spencermartinsia named as S. citricola, S. mangiferae and S. plurivora. An identification key to the species of each genus is provided.
Keywords: Botryosphaeriaceae, Dothiorella, ITS, phylogeny, Spencermartinsia, systematics, taxonomy
INTRODUCTION
Dothiorella was established by Saccardo in 1880 (Crous & Palm 1999) and according to Sutton (1980) Dothiorella pyrenophora Sacc. is the type species. Over time, the generic concept of Dothiorella has been debated and interpreted in various different ways. The taxonomic history of Dothiorella has been explained in details by Sutton (1977), Crous & Palm (1999), and Phillips et al. (2008, 2013). Crous & Palm (1999) studied the holotype of D. pyrenophora and concluded that Dothiorella is a synonym of Diplodia. This concept was followed by Denman et al. (2000), Zhou & Stanosz (2001) and Slippers et al. (2004), who recognised only two groups in Botryosphaeria corresponding to the asexual genera, Fusicoccum and Diplodia with hyaline and pigmented mature conidia, respectively. However, based on morphology and molecular data, Phillips et al. (2005) resurrected Dothiorella for species with conidia that become brown and 1-septate while they are still attached to the conidiogenous cells, and reserved Diplodia for species with conidia that are hyaline and become dark and septate only some time after discharge from the pycnidia. They also provided emended descriptions for Diplodia and Dothiorella. Since, there is no culture or DNA sequence for the type specimen and no authentic isolate or culture is available for the type species of Dothiorella, it is necessary to designate an epitype and ex-epitype culture for this important species.
Phillips et al. (2008) in a polyphasic approach using morphology and multi-gene sequence data (SSU, LSU, ITS, EF1-α and β-tubulin) introduced Spencermartinsia for species having brown, 1-septate ascospores with an apiculus at either end and transferred Dothiorella viticola to Spencermartinsia. Species of Dothiorella also have brown, 1-septate ascospores, but can be distinguished from Spencermartinsia by the absence of apiculi. In subsequent multi-gene phylogenetic analyses (Liu et al. 2012, Phillips et al. 2013) it was confirmed that Dothiorella and Spencermartinsia are two distinct genera in the Botryosphaeriaceae, though obviously closely related (see Slippers et al. 2013).
Species of Dothiorella and Spencermartinsia, as members of Botryosphaeriaceae, are known saprophytes, pathogens and endophytes in association with various woody plants. Until recently, like other members of Botryosphaeriaceae, species in Dothiorella were described based on host association, which led to the introduction of many species names. A search of Index Fungorum (March 2013; www.indexfungorum.org) lists 363 names in Dothiorella, while MycoBank lists 384 species names (accessed March 2013). Since, host association is not considered to be an important factor in species definition of the Botryosphaeriaceae (Slippers et al. 2004, 2013) most of these names are likely synonyms or they need to be transferred to Spencermartinsia.
A number of isolates morphologically resembling Dothiorella or Spencermartinsia were collected on different woody hosts in Iran, New Zealand, Portugal and Spain. The aim of this study was to characterise these isolates based on molecular data combined with morphology.
MATERIALS AND METHODS
Fungal isolates
Isolations were made by transferring conidia produced in pycnidia on twigs with canker or dieback symptoms to potato dextrose agar (1/2 PDA, Difco laboratories) or water agar (WA) supplemented with chloramphenicol (100 mg/L). After incubating overnight at 25 °C, single germinating conidia were transferred to fresh PDA plates and single conidium cultures were prepared for all isolates (Table 1).
Table 1.
Isolates considered in this study.
| Species | Culture no.1 | Host | Location | Collector | GenBank numbers2,3 |
|
|---|---|---|---|---|---|---|
| ITS | EF1-α | |||||
| Dothiorella iranica | IRAN1587C/CBS124722 | Olea europaea | Iran, Golestan | A. Javadi | KC898231 | KC898214 |
| D. parva | IRAN1579C/CBS124720 | Corylus avellana | Iran, Ardabil | J. Abdollahzadeh/A. Javadi | KC898234 | KC898217 |
| IRAN1585C/CBS124721 | C. avellana | Iran, Ardabil | J. Abdollahzadeh/A. Javadi | KC898235 | KC898218 | |
| D. pretoriensis | CMW36480 | Acacia karroo | South Africa | F. Jami/M. Gryzenhout | JQ239405 | JQ239392 |
| CMW36481 | A. karroo | South Africa | F. Jami/M. Gryzenhout | JQ239406 | JQ239393 | |
| D. prunicola | CBS124723/IRAN1541 | Prunus dulcis | Portugal, Algarve | E. Diogo | EU673313 | EU673280 |
| D. sempervirentis | IRAN1581C/CBS124719 | Cupressus sempervirens | Iran, Golestan | M.A. Aghajani | KC898237 | KC898220 |
| IRAN1583C/CBS124718 | C. sempervirens | Iran, Golestan | M.A. Aghajani | KC898236 | KC898219 | |
| IRAN1580C | C. sempervirens | Iran, Golestan | M.A. Aghajani | n.s. | n.s. | |
| IRAN1582C | C. sempervirens | Iran, Golestan | M.A. Aghajani | n.s. | n.s. | |
| IRAN1586C | C. sempervirens | Iran, Golestan | M.A. Aghajani | n.s. | n.s. | |
| D. striata | ICMP16819/CBS124730/IRAN1503C | Citrus sinensis | New Zealand | S.R. Pennycook/P.R. Johnston | EU673320 | EU673287 |
| ICMP16824/CBS124731/IRAN1572C | C. sinensis | New Zealand | S.R. Pennycook/P.R. Johnston | EU673321 | EU673288 | |
| D. uruguayensis | UY672/CBS124908 | Hexalamis edulis | Uruguay | C. Perez | EU080923 | EU863180 |
| Dothiorella sp. | IRAN1570C/CBS124717 | Juglans regia | Iran, Kermanshah | J. Abdollahzadeh/A. Javadi | KC898233 | KC898216 |
| IRAN1573C/CBS124716 | J. regia | Iran, Jolfa | J. Abdollahzadeh/A. Javadi | KC898232 | KC898215 | |
| IRAN1576C | J. regia | Iran, Kermanshah | J. Abdollahzadeh/A. Javadi | n.s. | n.s. | |
| IRAN1577C | J. regia | Iran, Kermanshah | J. Abdollahzadeh/A. Javadi | n.s. | n.s. | |
| Spencermartinsia citricola | ICMP16827/CBS124728/IRAN1504C | C. sinensis | New Zealand | S.R. Pennycook/P.R. Johnston | EU673322 | EU673289 |
| ICMP16828/CBS124729/IRAN1505C | C. sinensis | New Zealand | S.R. Pennycook/P.R. Johnston | EU673323 | EU673290 | |
| S. mangiferae | IRAN1545C | Mangifera indica | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898223 | KC898206 |
| IRAN1546C/CBS124726 | M. indica | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898222 | KC898205 | |
| IRAN1584C/CBS124727 | M. indica | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898221 | KC898204 | |
| S. plurivora | IRAN1537C | Citrus sp. | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898226 | KC898209 |
| IRAN1538C | C. sempervirens | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898229 | KC898212 | |
| IRAN1552C | Casuarina equisetifolia | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898228 | KC898211 | |
| IRAN1553C | Malus domestica | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898227 | KC898210 | |
| IRAN1556C/CBS124725 | Prunus armeniaca | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898230 | KC898213 | |
| IRAN1557C/CBS124724 | Citrus sp. | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898225 | KC898208 | |
| CJA257 | Eucalyptus sp. | Iran, Hormozgan | J. Abdollahzadeh/A. Javadi | KC898224 | KC898207 | |
1 Ex-type cultures are in bold face.
2 Sequence numbers in italics were obtained in the present study. All others were retrieved from GenBank.
3 n.s.: Not sequenced.
Morphology and culture characteristics
Sporulation was induced by growing isolates on 2 % WA supplemented with pieces of double-autoclaved halved poplar twigs. The plates were incubated at 25 °C under mixed black (nUV) and fluorescent light for 2–6 wk. Conidiomata were dissected and mounted in 100 % lactic acid. Observations and digital images were made with a light microscope and digital camera (Leica or Olympus). From measurements of 50 conidia the mean, standard deviation and 95 % confidence intervals were calculated. Dimensions are given as the range of measurements with extremes in brackets followed by 95 % confidence limits and mean ± standard deviation. Dimensions of other structures are given as the range of at least 20 measurements. Colony morphology, colour (Rayner 1970) and growth rates between 5 and 35 °C in 5 °C intervals, were determined on 2 % malt extract agar (MEA, Difco laboratories) incubated in the dark.
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from 4–7 d old cultures grown in 2 % malt extract broth (MEB) incubated at room temperature using the method as described by Abdollahzadeh et al. (2009). Amplification and sequencing of part of the ribosomal DNA (ITS region), translation elongation factor 1-alpha (EF1-α) and β-tubulin genes were performed as described previously (Alves et al. 2006, Abdollahzadeh et al. 2009).
Phylogenetic analyses
Sequences were checked with BioEdit v. 7.0.9.0 (Hall 2006). The ITS and EF1-α sequences of two outgroups (Neofusicoccun luteum CBS 110299, CBS 110497) and additional isolates were retrieved from GenBank. Sequences were aligned with ClustalX v. 1.83 (Thompson et al. 1997), using the following parameters: pairwise alignment parameters (gap opening = 10, gap extension = 0.1) and multiple alignment parameters (gap opening = 10, gap extension = 0.2, transition weight = 0.5, delay divergent sequences = 25 %). Alignments were checked and manual adjustments were made where necessary. Phylogenetic information contained in indels (gaps) was incorporated into the phylogenetic analyses using simple indel coding as implemented by GapCoder (Young & Healy 2003). Phylogenetic analyses were performed with PAUP v. 4.0b10 (Swofford 2003) for neighbour-joining (NJ) and maximum-parsimony (MP) analyses as described by Abdollahzadeh et al. (2010). A partition homogeneity test (PHT) was used to determine the congruence between the ITS, EF1-α and β-tubulin datasets (Farris et al. 1995, Huelsenbeck et al. 1996).
Bayesian analyses employing a Markov Chain Monte Carlo (MCMC) method were performed. The general time-reversible model of evolution (Rodriguez et al. 1990), including estimation of invariable sites and assuming a discrete gamma distribution with six rate categories (GTR+I+Γ) was used. Four MCMC chains were run simultaneously, starting from random trees, for 106 generations. Trees were sampled every 100th generation for a total of 104 trees. The first 103 trees were discarded as the burn-in phase of each analysis. Posterior probabilities (Rannala & Yang 1996) were determined from a majority-rule consensus tree generated from the remaining 9 000 trees. The analysis was repeated three times starting from different random trees to ensure trees from the same tree space were being sampled during each analysis. New sequences were deposited in GenBank (Table 1) and the alignment in TreeBASE (S14150).
RESULTS
Phylogenetic analyses
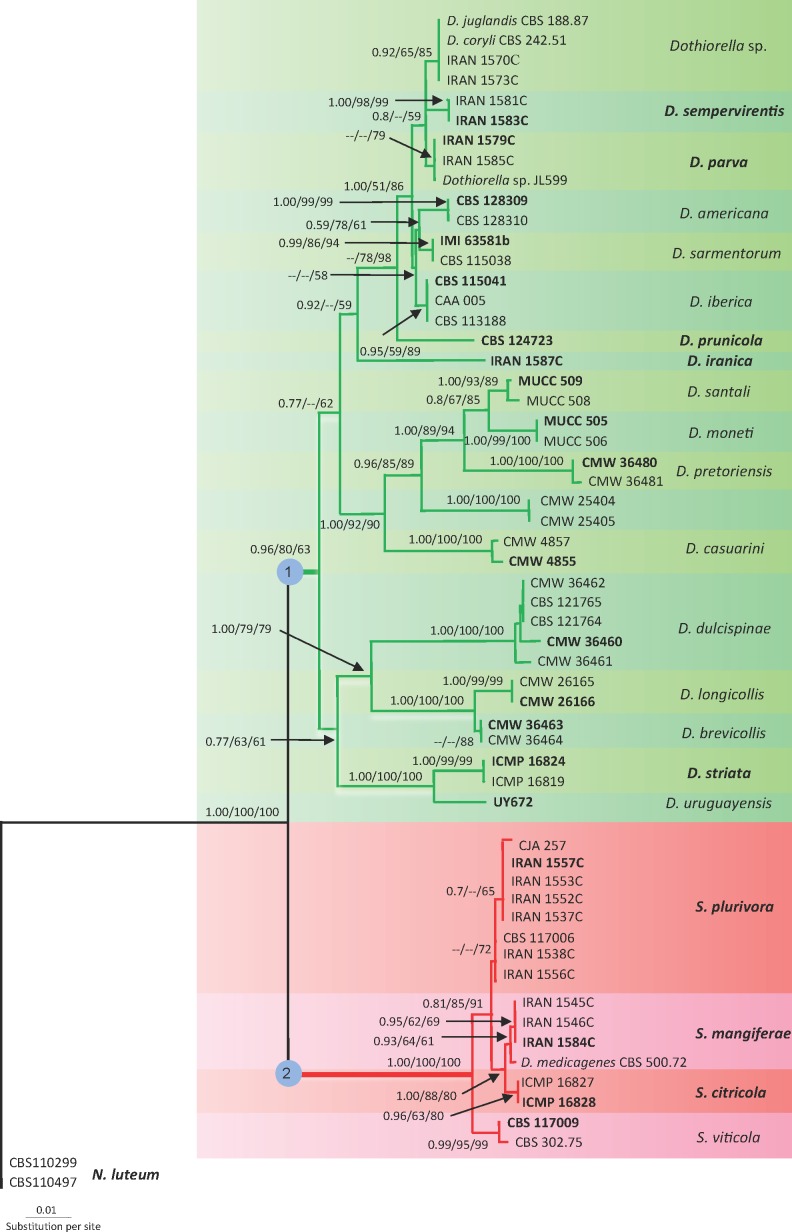
Phylogenetic analyses were done based on different combination of the three unlinked gene regions including ITS/EF1-α, ITS/β-tubulin and ITS/EF1-α/β-tubulin. The phylogenies resulted from ITS/EF1-α were stable and reproducible with highly supported internal nodes, while β-tubulin did not improve the phylogenies so that combination of ITS and β-tubulin datasets resulted in poor and distinct phylogenies in different analyses (data not shown). Furthermore, PHT test for combined ITS/EF1-α/β-tubulin datasets was significant (P < 0.01). Therefore, β-tubulin was excluded and phylogenetic analyses were based on ITS and EF1-α sequences. The partition homogeneity test (P = 0.11) indicated the phylogenies resulting from ITS and EF1-α were congruent so the ITS and EF1-α datasets were combined in a single analysis. The combined ITS and EF1-α sequences for 57 ingroup and 2 outgroup taxa contained 1 108 characters including alignment gaps, of which 334 characters were excluded, 528 were constant, 23 were variable and parsimony-uninformative and 223 were parsimony-informative. A heuristic search of the remaining 223 parsimony-informative characters resulted in a single most parsimonious tree of 426 steps (CI = 0.7, HI = 0.3, RI = 0.93). The Bayesian and NJ analyses produced phylogenetic trees with the same topology as the MP tree. The NJ tree is shown in Fig. 1 with BI/MP/NJ posterior probabilities and bootstrap support values at the nodes. MP and Bayesian trees are available in TreeBASE (S14150). In these phylogenetic analyses 22 subclades, representing 22 species of Botryosphaeriaceae with dark-walled 2-celled conidia, were recognized in two major clades corresponding to Dothiorella with 18 subclades, and Spencermartinsia with four subclades. Of these, nine subclades are recognised here as representatives of nine new species for the science.
Fig. 1.
Distance tree obtained using the K2P substitution model on the combined ITS and EF1-α sequence data. BI/MP/NJ posterior probabilities and bootstrap support values are given at the nodes. The tree is rooted to Neofusicoccum luteum (CBS 110299, CBS 110497). New species and ex-type strains are in bold face.
Taxonomy
All isolates in this study were induced to sporulate and produced pycnidia on poplar twigs on WA within 1–2 wk. No sexual structures were observed on the field specimens or in cultures. Based on ITS and EF1-α sequences, conidial and cultural characteristics and growth rate on MEA in the dark at 25 °C nine new species were identified. Of these, eight new species are described and illustrated here. Five species reside in the Dothiorella clade and the other three in the Spencermartinsia clade.
Key to Dothiorella species1
1. Conidiomata papillate . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. Conidiomata non-papillate . . . . . . . . . . . . . . . . . . . . . . . . . 8
2. Conidiomata with long necks (up to 1.5 mm) . . . . . . . . . . . . . . . . . . . . . . . . . D. longicollis
2. Conidiomata with short necks (< 0.5 mm) . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Conidia striate . . . . . . . . . . . . . . . . . . . . . . . . . D. striata
3. Conidia smooth . . . . . . . . . . . . . . . . . . . . . . . . . 4
4. Conidial length 16–22 μm . . . . . . . . . . . . . . . . . . . . . . . . . D. dulcispinae
4. Conidial length > 21 μm . . . . . . . . . . . . . . . . . . . . . . . . . 5
5. Colony growth rate on MEA in the dark at 25 °C > 30 mm/d (37 mm/d) . . . . . . . . . . . . . . . . . . . . . . . . . D. prunicola
5. Colony growth rate on MEA in the dark at 25 °C < 30 mm/d . . . . . . . . . . . . . . . . . . . . . . . . . 6
6. Colony growth rate on MEA in the dark at 25 °C > 20 mm/d . . . . . . . . . . . . . . . . . . . . . . . . . D. pretoriensis
6. Colony growth rate on MEA in the dark at 25 °C < 20 mm/d . . . . . . . . . . . . . . . . . . . . . . . . . 7
7. Colony growth rate on MEA in the dark at 25 °C < 15 mm (14 mm/d) . . . . . . . . . . . . . . . . . . . . . . . . . D. iranica
7. Colony growth rate on MEA in the dark at 25 °C > 15 mm (17.9 mm/d) . . . . . . . . . . . . . . . . . . . . . . . . . D. brevicollis
8. Conidial length < 16 μm (av. length 15 μm) . . . . . . . . . . . . . . . . . . . . . . . . . D. americana
8. Conidial length 16 μm or more (av. length > 18 μm) . . . . . . . . . . . . . . . . . . . . . . . . . 9
9. Average conidial width > 10 μm . . . . . . . . . . . . . . . . . . . . . . . . . 10
9. Average conidial width < 10 μm . . . . . . . . . . . . . . . . . . . . . . . . . 13
10. Average conidial length < 21 μm . . . . . . . . . . . . . . . . . . . . . . . . . 11
10. Average conidial length > 21 μm . . . . . . . . . . . . . . . . . . . . . . . . . 12
11. Average conidial width > 11 μm, conidial L/W ratio 1.7, colonies reaching 25–30 mm on MEA after 4 d in the dark at 25 °C . . . . . . . . . . . . . . . . . . . . . . . . . D. parva
11. Average conidial width < 11 μm, conidial L/W ratio 2, colonies reaching 50–70 mm on MEA after 4 d in the dark at 25 °C . . . . . . . . . . . . . . . . . . . . . . . . . D. sempervirentis
12. Conidia 23–31 × 9–11 μm (av. 27.1 × 10.8 μm) . . . . . . . . . . . . . . . . . . . . . . . . . D. casuarini
12. Conidia 23–23.4 × 10.8–11 μm (av. 23.2 × 10.9 μm) . . . . . . . . . . . . . . . . . . . . . . . . . D. iberica
13. Average conidial length < 20 μm . . . . . . . . . . . . . . . . . . . . . . . . . 14
13. Average conidial length > 20 μm . . . . . . . . . . . . . . . . . . . . . . . . . 15
14. Conidial L/W ratio 2 . . . . . . . . . . . . . . . . . . . . . . . . . D. santali
14. Conidial L/W ratio 2.4 . . . . . . . . . . . . . . . . . . . . . . . . . D. moneti
15. Conidia 21.4–21.9 × 9.7–9.9 μm (L/W ratio 2.2) . . . . . . . . . . . . . . . . . . . . . . . . . D. sarmentorum2
15. Conidia 22–22.5 × 9–9.5 μm (L/W ratio 2.4) . . . . . . . . . . . . . . . . . . . . . . . . . D. uruguayensis2
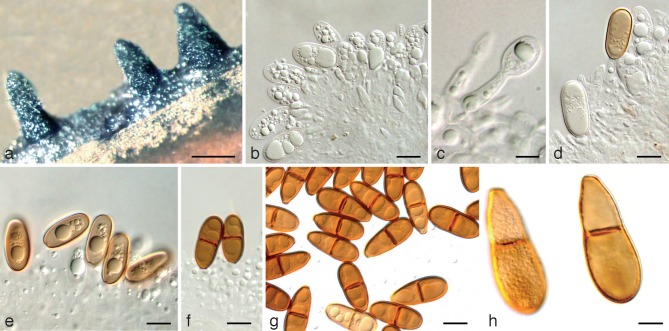
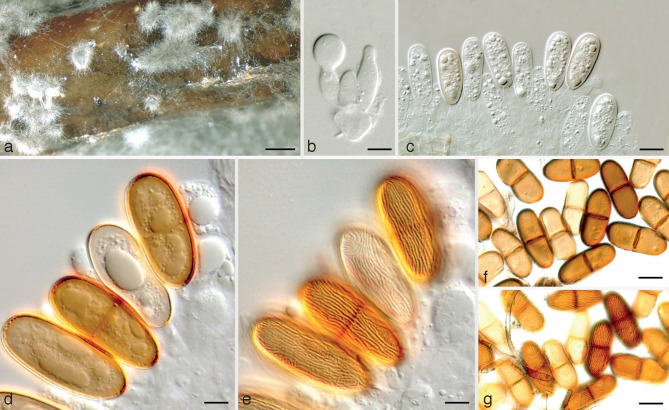
Dothiorella iranica Abdollahz. & A.J.L. Phillips, sp. nov. — MycoBank MB803988; Fig. 2
Fig. 2.
Dothiorella iranica holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline immature conidia developing on conidiogenous cells; d, e. brown aseptate conidia on conidiogenous cells; f. brown 1-septate conidia attached to the conidiogenous cells; g. mature conidia; h. mature conidia in two different focal planes. — Scale bars: a = 250 μm; b, d–g = 10 μm; c, h = 5 μm.
Etymology. Named for the country of origin, Iran.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 370 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (6.5–)9–12(–18.4) × 2–5 μm. Conidia subcylindrical to ellipsoid or ovoid, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends rounded, often with a truncate base, rarely becoming tapered at the base, (21.9–)23–26(–27.5) × (8.8–)9–11(–11.2) μm, 95 % confidence limits = 25–25.7 × 9.9–10.2 μm (av. ± S.D. = 25.3 ± 1.4 × 10.1 ± 0.6 μm, l/w ratio = 2.5 ± 0.2).
Culture characteristics — Colonies cottony with aerial mycelium, aerial mycelium becoming olivaceous-buff to grey-olivaceous at the surface and isabelline to dull green at the reverse after 2 wk in the dark at 25 °C. Colonies reaching 56 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Olea europaea.
Distribution — Northern Iran.
Specimens examined. IRAN. Golestan Province, Gorgan (Agriculture Research Center), on twigs of Olea europea, June 2007, A. Javadi, holotype IRAN 16253F, culture ex-type IRAN 1587C = CBS 124722.
Notes — Phylogenetically this species resides in a distinct subclade in Dothiorella and morphologically conidia of D. iranica are longer (25.3 ± 1.4 × 10.1 ± 0.6 μm, l/w ratio = 2.5 ± 0.2) than those of all other Dothiorella spp., except D. casuarini (27.1 × 10.8).
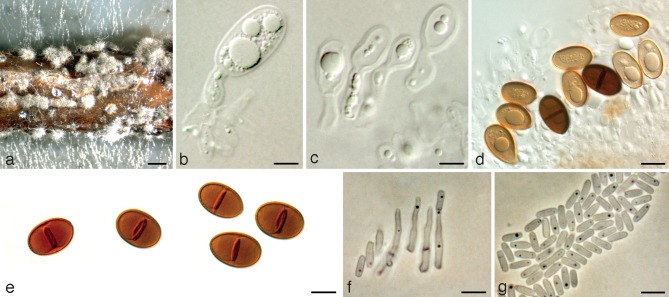
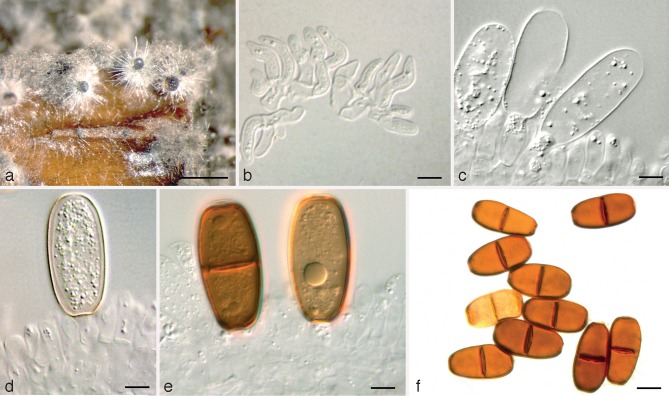
Dothiorella parva Abdollahz., Zare & A.J.L. Phillips, sp. nov. — MycoBank MB803989; Fig. 3
Fig. 3.
Dothiorella parva holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline immature conidia developing on conidiogenous cells; d. brown aseptate and 1-septate conidia attached to the conidiogenous cells; e. mature conidia; f. microconidiogenous cells; g. microconidia. — Scale bars: a = 1 000 μm; b, c, f, g = 5 μm; d, e = 10 μm.
Etymology. Named for its short conidia.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 350 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, non-papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (7–)8–11(–13.8) × 3–5 μm. Conidia ellipsoid to ovoid, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends rounded, often with a truncate base, (17.2–)18–21(–23.8) × (8.9–)10–13(–15.1) μm, 95 % confidence limits = 19.4–19.8 × 11.4–12 μm (av. ± S.D. = 19.6 ± 1.2 × 11.7 ± 1.6 μm, l/w ratio = 1.7 ± 0.2).
Culture characteristics — Colonies cottony with aerial mycelium, aerial mycelium becoming pale olivaceous-grey to iron-grey at the surface and olivaceous-buff to dull green at the reverse after 2 wk in the dark at 25 °C. Colonies reaching 25–30 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Corylus avellana.
Distribution — North-west of Iran.
Specimens examined. IRAN, Ardabil Province, Ardabil (Fandoghlo Forest Park), on twigs of Corylus avellana, July 2007, J. Abdollahzadeh and A. Javadi, holotype IRAN 14264F, culture ex-type IRAN 1579C = CBS 124720. Additional isolates are given in Table 1.
Notes — Phylogenetically, D. parva is closely related to D. sempervirentis and Dothiorella sp., but can be distinguished from these two species on account of its shorter and wider conidia (19.6 ± 1.2 × 11.7 ± 1.6 μm, l/w ratio = 1.7 ± 0.2) and slower growth rate on MEA in the dark at 25 °C. This species differed in nucleotide sequences from D. sempervirentis (three substitutions in ITS, five substitutions and 2 insertions/deletions in EF1-α) and Dothiorella sp. (one substitution in ITS, five substitutions and one insertion/deletion in EF1-α).
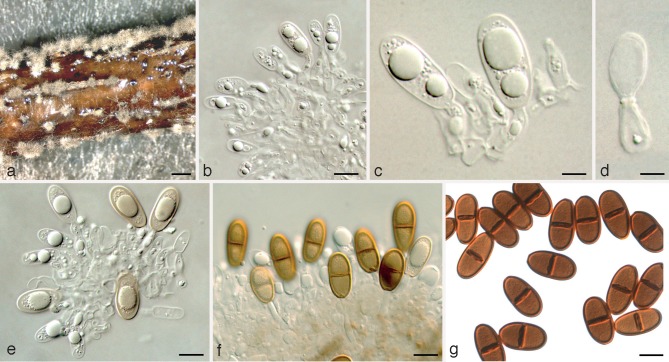
Dothiorella prunicola A.J.L. Phillips & Abdollahz., sp. nov. — MycoBank MB803991; Fig. 4
Fig. 4.
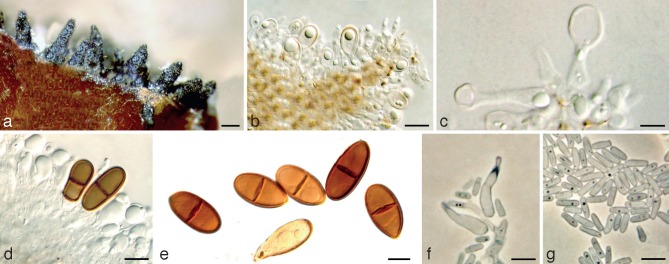
Dothiorella prunicola holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline immature conidia developing on conidiogenous cells; d. brown 1-septate conidia attached to the conidiogenous cells; e. mature conidia; f. microconidiogenous cells; g. microconidia. — Scale bars: a = 250 μm; b, c, f, g = 5 μm; d, e = 10 μm.
Etymology. Named for the host it was first isolated from, namely Prunus.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 370 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (5.9–)9–14(–20) × 3–5 μm. Conidia subcylindrical to ellipsoid or ovoid, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends rounded, often with a truncate base, (19–)22–27(–30.5) × (10.5–)11–14(–16.8) μm, 95 % confidence limits = 23.9–25.1 × 12.5–13.1 μm (av. ± S.D. = 24.5 ± 2.3 × 12.8 ± 1.2 μm, l/w ratio = 1.9 ± 0.2). Spermatiogenous cells discrete or integrated, hyaline, smooth, cylindrical, holoblastic or proliferating via phialides with periclinal thickenings, 5.8–17.3 × 1–3 μm. Spermatia hyaline, smooth, aseptate, rod-shaped with rounded ends, 3.5–5.3 × 1–2 μm.
Culture characteristics — Colonies rosette with lobed margins and aerial mycelium, white to cream at the surface and reverse after 2 wk in the dark at 25 °C. Colonies reaching 37 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Prunus dulcis.
Distribution — Portugal.
Specimens examined. PORTUGAL, Algarve Province, on twigs of Prunus dulcis, June 2007, E. Diogo, holotype IRAN 16252F, culture ex-type IRAN 1541C = CBS 124723.
Notes — This species resides in a completely distinct subclade within Dothiorella. Morphologically, D. prunicola resembles D. brevicollis, D. iranica and D. pretoriensis but can be distinguished on average conidial dimensions (24.5 ± 2.3 × 12.8 ± 1.2 μm, l/w ratio = 1.9 ± 0.2) and growth rate on MEA in the dark at 25 °C. Moreover, D. prunicola can be distinguished from all Dothiorella spp. on account of its white and creamy colony colour, a feature that is never seen in the family Botryosphaeriaceae.
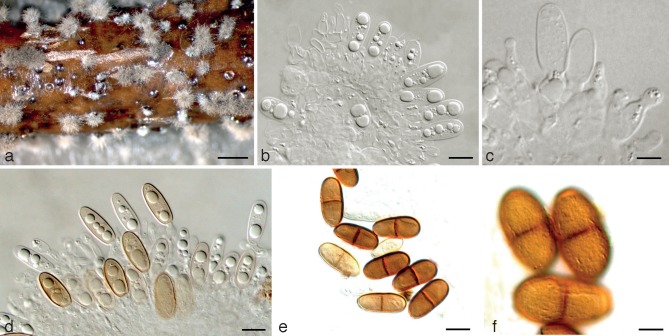
Dothiorella sempervirentis Abdollahz., Zare & A.J.L. Phillips, sp. nov. — MycoBank MB803987; Fig. 5
Fig. 5.
Dothiorella sempervirentis holotype. a. Conidiomata on poplar twigs in culture; b–d. hyaline immature conidia developing on conidiogenous cells; e, f. brown aseptate and 1-septate conidia attached to the conidiogenous cells; g. mature conidia. — Scale bars: a = 1 000 μm; b, e–g = 10 μm; c, d = 5 μm.
Etymology. Named for the host species it was first isolated from.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 510 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, non-papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings or rarely proliferating percurrently to form one or two annellations, hyaline, thin-walled, smooth, (8.2–)9–11(–17.3) × 3–5 μm. Conidia subcylindrical to ellipsoid or ovoid, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends rounded, often with a truncate base, (16.1–)18–20(–22.8) × (8.2–)9–11(–11.7) μm, 95 % confidence limits = 19.8–20.3 × 10–10.4 μm (av. ± S.D. = 20.1 ± 1.3 × 10.2 ± 0.9 μm, l/w ratio = 2 ± 0.2).
Culture characteristics — Colonies appressed with a sparse aerial mycelium at the margin, grey-olivaceous to olivaceous-black at the surface and greenish olivaceous to dull green in reverse after 2 wk in the dark at 25 °C. Colonies reaching 50–70 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs and cones of Cupressus sempervirens.
Distribution — Northern Iran.
Specimens examined. IRAN, Golestan Province, Gorgan (City Park), on twigs and cones of Cupressus sempervirens, Aug. 2006, M.A. Aghajani, holotype IRAN 14265F, culture ex-type IRAN 1583C = CBS 124718. Additional isolates are given in Table 1.
Notes — Phylogenetically, D. sempervirentis is closely related to D. parva and Dothiorella sp., but can be distinguished on average conidial dimensions (20.1 ± 1.3 × 10.2 ± 0.9 μm, l/w ratio = 2 ± 0.2) and growth rate on MEA in the dark at 25 °C. Compared with Dothiorella sp. nine differences were detected in ITS and EF1-α sequences (four substitutions in ITS, four substitutions and one insertion/deletion in EF1-α).
Dothiorella striata A.J.L. Phillips & Abdollahz., sp. nov. — MycoBank MB803990; Fig. 6
Fig. 6.
Dothiorella striata holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline immature conidia developing on conidiogenous cells; d. brown aseptate and 1-septate conidia attached to the conidiogenous cells; e. hyaline and brown striate conidia attached to the conidiogenous cells; f. mature conidia; g. mature conidia with striation. — Scale bars: a = 1 000 μm; b, d, e = 5 μm; c, f, g = 10 μm.
Etymology. Named for the distinctive striations on the conidial wall.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 420 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (5.9–)9–14(–20) × 3–5 μm. Conidia subcylindrical to ellipsoid or ovoid, brown, 1-septate, striate, thumb-like striations visible on hyaline conidia even while attached to conidiogenous cells, occasionally slightly constricted at septum, moderately thick-walled, internally finely verruculose, ends rounded, often with a truncate base, (21–)23–26(–29.4) × (8.9–)9–12(–15.1) μm, 95 % confidence limits = 24.9–25.4 × 10.5–11 μm (av. ± S.D. = 25.1 ± 1.4 × 10.7 ± 1.2 μm, l/w ratio = 2.4 ± 0.3).
Culture characteristics — Colonies with abundant aerial mycelia reaching the lid of Petri plates, aerial mycelium becoming smoke-grey to olivaceous-black at the surface and greenish olivaceous to dull green at the reverse after 2 wk in the dark at 25 °C. Colonies reaching 45–55 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Citrus sinensis.
Distribution — New Zealand.
Specimens examined. NEW ZEALAND, Northland, Kerikeri, Collins Orchard, Inlet Road, on twigs of C. sinensis, Sept. 2006, S.R. Pennycook, P.R. Johnston and B.C. Paulus, holotype PDD 92029, culture ex-type ICMP 16824 = CBS 124731. Additional isolates are given in Table 1.
Notes — Phylogenetically, D. striata is closely related to D. uruguayensis, but it can be distinguished from all Dothiorella spp. on account of the striate conidia, which was never seen in any other Dothiorella species. Furthermore, D. striata differs from all other species on account of colonies with abundant aerial mycelia reaching the lid of Petri plates.
Dothiorella sp.
Notes — Phylogenetically, Dothiorella sp. is closely related to D. parva and D. sempervirentis, but can be distinguished on account of the number of differences in ITS and EF1-α sequences, longer and narrower conidia and faster growth rate on MEA in the dark at 25 °C. This species is being further studied in relation to other species collected from Corylus and therefore is not formally described here.
Key to Spencermartinsia species1
1. Average conidial length < 20 μm . . . . . . . . . . . . . . . . . . . . . . . . . S. mangiferae
1. Average conidial length > 20 μm . . . . . . . . . . . . . . . . . . . . . . . . . 2
2. Conidia truncate at both ends (av. 25.8 × 12.2 μm) . . . . . . . . . . . . . . . . . . . . . . . . . S. citricola
2. Conidia round at apex, often with a truncate base . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Average conidial width > 10 μm (av. 22.5 × 11 μm) . . . . . . . . . . . . . . . . . . . . . . . . . S. plurivora
3. Average conidial width < 10 μm (av. 20.4 × 9.3 μm) . . . . . . . . . . . . . . . . . . . . . . . . . S. viticola
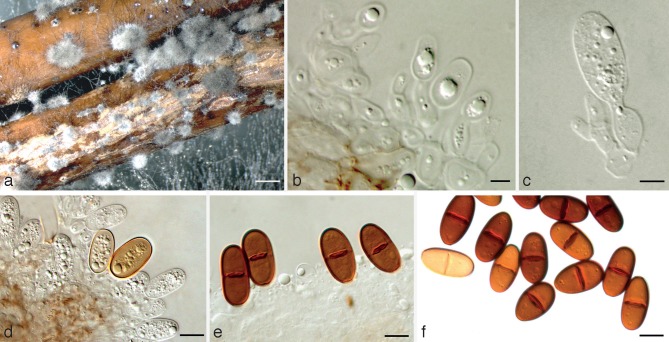
Spencermartinsia citricola A.J.L. Phillips & Abdollahz., sp. nov. — MycoBank MB803992; Fig. 7
Fig. 7.
Spencermartinsia citricola holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline immature conidia developing on conidiogenous cells; d, e. brown aseptate and 1-septate conidia attached to the conidiogenous cells; f. mature conidia. — Scale bars: a = 1 000 μm; b–e = 5 μm; f = 10 μm.
Etymology. Named for the host it was first isolated from, namely Citrus.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 460 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, non-papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (7.6–)9–11(–12) × 2–4 μm. Conidia oblong to subcylindrical, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends truncate, (23.7–)24–27(–28) × (9.5–)10–12(–14.1) μm, 95 % confidence limits = 25.5–26 × 11.9–12.5 μm (av. ± S.D. = 25.8 ± 1.1 × 12.2 ± 1.3 μm, l/w ratio = 2.1 ± 0.2).
Culture characteristics — Colonies cottony with dense aerial mycelium and crenate margins, aerial mycelium becoming smoke-grey to olivaceous-black at the surface and greenish olivaceous to dull green at the reverse after 2 wk in the dark at 25 °C. Colonies reaching 15–20 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Citrus sinensis.
Distribution — New Zealand.
Specimens examined. NEW ZEALAND, Northland, Kerikeri, Collins Orchard, Inlet Road, on twigs of C. sinensis, Sept. 2006, S.R. Pennycook, P.R. Johnston and B.C. Paulus, holotype PDD92023, culture ex-type ICMP16828 = CBS124729. Additional isolates are given in Table 1.
Notes — Phylogenetically, S. citricola is closely related to S. mangiferae and S. plurivora, but morphologically can be distinguished an account of conidial dimensions (25.8 ± 1.1 × 12.2 ± 1.3 μm), shape (truncated at either end) and slower growth rate on MEA in the dark at 25 °C. This species differed in nucleotide sequences from S. mangiferae (six substitutions and 2 insertions/deletions in EF1-α) and S. plurivora (two substitutions in ITS, six substitutions and three insertions/deletions in EF1-α).
Spencermartinsia mangiferae Abdollahz., Javadi & A.J.L. Phillips, sp. nov. — MycoBank MB803993; Fig. 8
Fig. 8.
Spencermartinsia mangiferae holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline immature conidia developing on conidiogenous cells; d. brown aseptate conidia on conidiogenous cells; e, f. mature conidia in two different focal planes. — Scale bars: a = 1 000 μm; b, d, e = 10 μm; c, f = 5 μm.
Etymology. Named for the host it was first isolated from, namely Mangifera.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 400 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, non-papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (5.2–)6–9(–11.8) × 3–5 μm. Conidia subcylindrical to ellipsoid or ovoid, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends rounded, often with a truncate base, (14.4–)17–22(–22.5) × (6.3–)8–10(–11) μm, 95 % confidence limits = 18.8–19.2 × 8.9–9.1 μm (av. ± S.D. = 19 ± 1.6 × 9 ± 0.9 μm, l/w ratio = 2.1 ± 0.2).
Culture characteristics — Colonies cottony with dense aerial mycelium, aerial mycelium becoming smoke-grey to olivaceous-grey at the surface and grey-olivaceous to olivaceous-black at the reverse after 2 wk in the dark at 25 °C. Colonies reaching 50–85 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Mangifera indica.
Distribution — Southern Iran.
Specimens examined. IRAN, Hormozgan Province, Bandar Abbas (Hajiabad-Siaho), on twigs of M. indica, Mar. 2007, J. Abdollahzadeh and A. Javadi, holotype IRAN 14266F, culture ex-type IRAN 1584C = CBS 124727.
Notes — Phylogenetically, S. mangiferae is closely related to S. citricola and S. plurivora, but in terms of morphology it is differed from all other species by having small conidia (19 ± 1.6 × 9 ± 0.9 μm). This species also differed in nucleotide sequences from S. citricola (six substitutions and two insertions/deletions in EF1-α) and S. plurivora (two substitutions in ITS, six substitutions and one insertion/deletion in EF1-α).
Spencermartinsia plurivora Abdollahz., Javadi & A.J.L. Phillips, sp. nov. — MycoBank MB803994; Fig. 9
Fig. 9.
Spencermartinsia plurivora holotype. a. Conidiomata on poplar twigs in culture; b, c. hyaline and immature conidia developing on conidiogenous cells; d, e. brown aseptate and 1-septate conidia attached to the conidiogenous cells; f. mature conidia. — Scale bars: a = 1 000 μm; b, c = 5 μm; d–f = 10 μm.
Etymology. Named for its broad host range.
Conidiomata pycnidial, produced on poplar twigs on WA within 1–2 wk, solitary or aggregated, individual conidiomata globose, up to 420 μm diam, superficial or semi-immersed, covered with hyphal hairs, uniloculate, thick-walled, non-papillate with a central ostiole. Conidiophores absent. Conidiogenous cells cylindrical to lageniform, discrete or integrated, holoblastic, indeterminate, proliferating at the same level giving rise to periclinal thickenings, hyaline, thin-walled, smooth, (5.1–)7–10(–11.9) × 3–5 μm. Conidia subcylindrical to ellipsoid or ovoid, brown, 1-septate, occasionally slightly constricted at septum, moderately thick-walled, externally smooth, internally finely verruculose, ends rounded, often with a truncate base, (18–)20–25(–27) × (8.9–)10–13(–14.4) μm, 95 % confidence limits = 22.3–22.7 × 10.8–11.2 μm (av. ± S.D. = 22.5 ± 1.7 × 11 ± 1.1 μm, l/w ratio = 2.1 ± 0.2).
Culture characteristics — Colonies cottony with dense aerial mycelium, aerial mycelium becoming smoke-grey to olivaceous-grey at the surface and grey-olivaceous to olivaceous-black at the reverse after 2 wk in the dark at 25 °C. Colonies reaching 62–84 mm on MEA after 4 d in the dark at 25 °C. Cardinal temperatures for growth: min. ≤ 5 °C, max. ≥ 35 °C, opt. 25 °C.
Substrate — Twigs of Casuarina sp., Citrus sp., Cupressus sempervirens, Eucalyptus sp., Juglans regia, Malus domestica, Prunus armeniaca and Vitis vinifera.
Distribution — Southern Iran, Spain.
Specimens examined. IRAN, Khuzestan Province, Dezful (Safiabad Citrus Research Centre), on twigs of Citrus sp., Nov. 2006, J. Abdollahzadeh and A. Javadi, holotype IRAN 14267F, culture ex-type IRAN 1557C = CBS 124724.
Notes — Phylogenetically, S. plurivora is closely related to S. citricola and S. mangiferae, but morphologically it resembles S. viticola. This species can be distinguished from other species on account of its conidial dimensions (22.5 ± 1.7 × 11 ± 1.1 μm) and colony growth rate on MEA at 25 °C. Moreover, this species differed in nucleotide sequences from S. citricola (two substitutions in ITS, six substitutions and three insertions/deletions in EF1-α) and S. mangiferae (two substitutions in ITS, six substitutions and one insertion/deletion in EF1-α).
DISCUSSION
In this study the phylogenetic analyses based on ITS and EF1-α sequences revealed two major clades corresponding to Dothiorella and Spencermartinsia. Within Dothiorella 18 subclades were resolved. Species names are available for 11 of these subclades, and five new species are introduced here from isolates collected from different woody hosts in Iran, New Zealand, Portugal and Spain. We refrain from giving species names to the subclade bearing Diplodia juglandis, Diplodia coryli and the two isolates IRAN 1570C and IRAN 1573C, since this will be dealt with in a separate paper currently being prepared on the species of Dothiorella on Corylus. The subclade bearing two isolates CMW 25404 and CMW 25405 was recognised by Jami et al. (2012) as Spencermartinsia sp., but they have not described it thus far. Four subclades were resolved in Spencermartinsia representing four species; S. viticola and three species which are newly described in this paper.
Spencermartinsia was introduced by Phillips et al. (2008) on account of its phylogenetic distinction from Dothiorella. The presence of apiculi on the ascospores of Spencermartinsia viticola was the only morphological character that separates it from Dothiorella, the ascospores of which do not have apiculi. However, a sexual state is known only for S. viticola, and there are no distinctive asexual characters that differentiate these two genera.
All Dothiorella species can be distinguished based on ITS and EF1-α sequence data supported with conidial dimensions (Table 2). In this survey, we also considered growth rate, a physiological character, on MEA in the dark at 25 °C to differentiate some closely related species, and apparently this is a useful character in some cases. Dothiorella striata was isolated from twigs of Citrus in New Zealand. In a phylogenetic study based on SSU, LSU, ITS, β-tubulin and EF1-α sequences the two isolates representing D. striata formed a distinct clade as a sister group to Spencermartinsia with quite low support in MP analysis (Phillips et al. 2008). The presence of striate conidia as a strong and unique morphological character led Phillips et al. (2008) to suspect that this clade could represent a separate genus. However, they declined to introduce a new genus and did not describe this species because the isolates failed to sporulate. In the present study isolates of D. striata clearly lie within the Dothiorella clade and cultures sporulating on poplar twigs confirmed the presence of conidial striation in this species. Therefore, conidial striation is interpreted as a distinctive morphological character at the species level in the Dothiorella clade.
Table 2.
Conidial dimensions of Dothiorella and Spencermartinsia species investigated in this study and previous studies.
| Species | Conidial dimensions (μm) | Average (μm) | L/W ratio | Reference |
|---|---|---|---|---|
| D. americana | 14.2–15.8 × 5.7–6.6 | 15 × 6.1 | 2.4 | Urbez Torres et al. 2012 |
| D. brevicollis | 21.5–26 × 9–12 | – | – | Jami et al. 2012 |
| D. casuarini | 23–31 × 9–12 | 27.1 × 10.8 | – | de Wet et al. 2009 |
| D. dulcispinae | 16–22 × 7–10 | – | – | Jami et al. 2012 |
| D. iberica | 23–23.4 × 10.8–11 | 23.2 × 10.9 | 2.2 | Phillips et al. 2005 |
| D. iranica | 23–26 × 9–11 | 25.3 × 10.1 | 2.5 | This study |
| D. longicollis | 19–22 × 8–9.5 | 20.4 × 8.7 | 2.3 | Pavlic et al. 2008 |
| D. moneti | 17–22 × 7–10 | 19.8 × 8.4 | 2.4 | Taylor et al. 2009 |
| D. parva | 18–21 × 10–13 | 19.6 × 11.7 | 1.7 | This study |
| D. pretoriensis | 20–28 × 7–14 | – | – | Jami et al. 2012 |
| D. prunicola | 22–27 × 11–14 | 24.5 × 12.8 | 1.9 | This study |
| D. santali | 16–20 × 7–11 | 18.2 × 9 | 2 | Taylor et al. 2009 |
| D. sarmentorum | 21.4–21.9 × 9.7–9.9 | 21.6 × 9.8 | 2.2 | Phillips et al. 2005 |
| D. sempervirentis | 18–20 × 9–11 | 20.1 × 10.2 | 2 | This study |
| D. striata | 23–26 × 9–12 | 25.1 × 10.7 | 2.4 | This study |
| D. uruguayensis | 22–22.5 × 9–9.5 | – | – | Pérez et al. 2010 |
| Dothiorella sp. | 21–27 × 8–10 | 24 × 9.9 | 2.2 | This study |
| S. citricola | 24–27 × 10–12 | 25.8 × 12.2 | 2.1 | This study |
| S. mangiferae | 17–22 × 8–10 | 19 × 9 | 2.1 | This study |
| S. plurivora | 20–25 × 10–13 | 22.5 × 11 | 2.1 | This study |
| S. viticola | 20.2–20.6 × 9.2–9.4 | 20.4 × 9.3 | 2.2 | Luque et al. 2005 |
Thus far, 18 species of Dothiorella, including those identified in this study, have been characterised from different hosts based on combined morphology and DNA sequence data (Luque et al. 2005, Phillips et al. 2005, Pavlic et al. 2008, Taylor et al. 2009, de Wet et al. 2009, Urbez-Torres et al. 2011, Jami et al. 2012). Of these, D. sarmentorum is cosmopolitan and has been isolated from 34 different host species across six continents, and D. iberica has been found on seven different tree species in Algeria, Italy, Spain and USA (http://nt.ars-grin.gov/fungaldatabases). However, other species have a very narrow host range and limited geographic distribution. To obtain a more realistic conclusion on host ranges, we require more sampling from various hosts in relatively unexplored regions.
Spencermartinsia was introduced as a monotypic genus typified with S. viticola, a species reported from four different woody hosts (mainly Vitis vinifera) in China, South Africa, Spain, USA and Uruguay (http://nt.ars-grin.gov/fungaldatabases). Based on an ITS and EF1-α phylogeny, Phillips et al. (2013) transferred the two recently described species, S. uruguayensis and S. pretoriensis to Dothiorella, and introduced two new combinations: D. uruguayensis and D. pretoriensis. In the present phylogenetic study we introduce a further three species. Therefore, only four species remain in Spencermartinsia. These species can be differentiated based on conidial dimensions (Table 2) and shape. As discussed in the case of Dothiorella, growth rate on MEA in the dark at 25 °C is obviously a helpful character to separate some closely related species in Spencermartinsia, as S. citricola is distinct from two closely related species, S. plurivora and S. mangiferae, with a much slower growth rate. According to Phillips et al. (2008) the isolate (CBS 117006) collected from V. vinifera in Spain was phylogenetically separate from S. viticola and produced a red-brown pigment. In the present study this isolate grouped with Iranian isolates from different woody hosts in a distinct clade corresponding to a new species we have named S. plurivora. However, in this study none of the Iranian isolates produced any pigment, which is consistent with the observations of Abdollahzadeh et al. (2010, 2013) about the limited taxonomic value of cultural characteristics in differentiation of Botryosphaeriaceae species. Furthermore, S. plurivora, the most common species, was characterised on eight different woody hosts in Iran (14 isolates) and Spain (1 isolate), while S. mangiferae was found on mango in Iran and S. citricola on citrus in New Zealand.
Although pathogenicity of the species described in this study has not been determined, according to pathogenicity tests previously conducted by different researchers (van Niekerk et al. 2004, Luque et al. 2005, Damm et al. 2007, Taylor et al. 2009, Inderbetzin et al. 2010, Urbez-Torres et al. 2012), the species of Dothiorella and Spencermartinsia appear to be minor pathogens or can be considered as saprophytic or endophytic fungi in association with different woody plants. Pathogenicity, host specificity and geographic distribution of the characterised species remain unknown issues that should be considered in future studies.
Acknowledgments
Part of this work was financed by the European Regional Development Fund and Fundação para a Ciência e a Tecnologia (FCT) Portugal under project PPCDT/AGR/56140/2004, and through grant PEst-OE/BIA/UI0457/2011. A.J.L. Phillips was supported by grant number SFRH/BCC/15810/2005 from FCT, and J. Abdollahzadeh received a grant from Studienstiftung Mykologie, Köln, Germany and Kurdistan provincial office. Shaun Pennycook and Peter Johnston, Landcare Research, Auckland, New Zealand provided isolates, type specimens and culture collection numbers for D. striata and S. citricola. M.A. Aghajani, Agricultural and Natural Resources Research Center of Golestan Province, Iran provided D. sempervirentis samples.
Footnotes
1 This key is based on conidial morphology and culture characteristics.
2 These two species are phylogenetically distant.
1 This key is based on conidial morphology.
REFERENCES
- Abdollahzadeh J, Javadi A, Mohammadi Goltapeh E, Zare R, Phillips AJL. 2010. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahzadeh J, Mohammadi Goltapeh E, Javadi A, Shams-bakhsh M, Zare R, Phillips AJL. 2009. Barriopsis iraniana and Phaeobotryon cupressi: two new species of the Botryosphaeriaceae from trees in Iran. Persoonia 23: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahzadeh J, Zare R, Phillips AJL. 2013. Phylogeny and taxonomy of Botryosphaeria and Neofusicoccum species in Iran, with description of Botryosphaeria scharifii sp. nov. Mycologia 105: 220–230 [DOI] [PubMed] [Google Scholar]
- Alves A, Correia A, Phillips AJL. 2006. Multi-gene genealogies and morphological data support Diplodia cupressi sp. nov., previously recognized as D. pinea f. sp. cupressi, as a distinct species. Fungal Diversity 23: 1–15 [Google Scholar]
- Crous PW, Palm ME. 1999. Reassessment of the anamorph genera Botryodiplodia, Dothiorella and Fusicoccum. Sydowia 51: 161–175 [Google Scholar]
- Damm U, Crous PW, Fourie PH. 2007. Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia 99: 664–680 [DOI] [PubMed] [Google Scholar]
- Denman S, Crous PW, Taylor JE, Kang JC, Pascoe I, Wingfield MJ. 2000. An overview of the taxonomic history of Botryosphaeria and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Studies in Mycology 45: 129–140 [Google Scholar]
- Farris JS, Kallersjo M, Kluge AG, Bult C. 1995. Testing significance of incongruence. Cladistics 10: 315–319 [Google Scholar]
- Hall T. 2006. Bioedit 7.5.0.3. Department of Microbiology, North Carolina State University; http://www.mbio.ncsu.edu/BioEdit/Bioedit.html [Google Scholar]
- Huelsenbeck JP, Bull JJ, Cunningham CV. 1996. Combining data in phylogenetic analysis. Trends in Ecology & Evolution 11: 152–158 [DOI] [PubMed] [Google Scholar]
- Inderbitzin P, Bostock RM, Trouillas FP, Michailides TJ. 2010. A six locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia 102: 1350–1368 [DOI] [PubMed] [Google Scholar]
- Jami F, Slippers B, Wingfield MJ, Gryzenhout M. 2012. Five new species of the Botryosphaeriaceae from Acacia karroo in South Africa. Cryptogamie Mycologie 33: 245–266 [Google Scholar]
- Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, et al. 2012. Towards a natural classification of Botryosphaeriales. Fungal Diversity 57: 149–210 [Google Scholar]
- Luque J, Martos S, Phillips AJL. 2005. Botryosphaeria viticola sp. nov. on grapevines: a new species with a Dothiorella anamorph. Mycologia 97: 1111–1121 [DOI] [PubMed] [Google Scholar]
- Niekerk JM van, Crous PW, Groenewald JZ, Fourie PH, Halleen F. 2004. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96: 781–798 [PubMed] [Google Scholar]
- Pavlic D, Wingfield MJ, Barber P, Slippers B, Hardy GEStJ, Burgess TI. 2008. Seven new species of the Botrysophaeriaceae from baobab and other native trees in Western Australia. Mycologia 100: 851–866 [DOI] [PubMed] [Google Scholar]
- Pérez CA, Wingfield MJ, Slippers B, Altier NA, Blanchette RA. 2010. Endophytic and canker-associated Botryosphaeriaceae occurring on non-native Eucalyptus and native Myrtaceae trees in Uruguay. Fungal Diversity 41: 53–69 [Google Scholar]
- Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, et al. 2013. The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76: 51–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJL, Alves A, Correia A, Luque J. 2005. Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97: 513–529 [DOI] [PubMed] [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. 2008. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannala B, Yang Z. 1996. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311 [DOI] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Kew, Surrey, UK: CMI and British Mycological Society [Google Scholar]
- Rodriguez F, Oliver JF, Marin A, Medina JR. 1990. The general stochastic model of nucleotide substitutions. Journal of Theoretical Biology 142: 485–501 [DOI] [PubMed] [Google Scholar]
- Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Wingfield MJ, et al. 2013. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Studies in Mycology 76: 31–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ. 2004. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96: 83–101 [PubMed] [Google Scholar]
- Sutton BC. 1977. Coelomycetes VI. Nomenclature of generic names proposed for Coelomycetes. Mycological Papers 141: 1–253 [Google Scholar]
- Sutton BC. 1980. The Coelomycetes, Fungi imperfecti with acervuli, pycnidia and stromata. Commonwealth Mycological Institute, Kew, UK [Google Scholar]
- Swofford DL. 2003. PAUP* 4.0b10: Phylogenetic Analysis Using Parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts [Google Scholar]
- Taylor K, Barber PA, Hardy GEStJ, Burgess TI. 2009. Botryosphaeriaceae from tuart (Eucalyptus gomphocephala) woodland, including descriptions of four new species. Mycological Research 113: 337–353 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbez-Torres JR, Peduto F, Striegler RK, Urrea-Romero KE, Rupe JC, et al. 2012. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Diversity 52: 169–189 [Google Scholar]
- Wet J de, Slippers B, Preisig O, Wingfield BD, Tsopelas P, Wingfield MJ. 2009. Molecular and morphological characterization of Dothiorella casuarini sp. nov. and other Botryosphaeriaceae with diplodia-like conidia. Mycologia 101: 503–511 [DOI] [PubMed] [Google Scholar]
- Young ND, Healy J. 2003. GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Stanosz GR. 2001. Relationships among Botryosphaeria species and associated anamorphic fungi inferred from the analyses of ITS and 5.8S rDNA sequences. Mycologia 93: 516–527 [Google Scholar]