Abstract
During the last decade several new orders were established in the class Chytridiomycetes on the basis of zoospore ultrastructure and molecular phylogeny. Here we present the ultrastructure and molecular phylogeny of strain x-51 CALU – a parasite of the alga Tribonema gayanum, originally described as Rhizophydium sp. based on light microscopy. Detailed investigation revealed that the zoospore ultrastructure of this strain has unique characters not found in any order of Chytridiomycetes: posterior ribosomal core unbounded by the endoplasmic reticulum and detached from the nucleus or microbody-lipid complex, and kinetosome composed of microtubular doublets. An isolated phylogenetic position of x-51 is further confirmed by the analysis of 18S and 28S rRNA sequences, and motivates the description of a new genus and species Gromochytrium mamkaevae. The sister position of G. mamkaevae branch relative to Mesochytrium and a cluster of environmental sequences, as well as the ultrastructural differences between Gromochytrium and Mesochytrium zoospores prompted us to establish two new orders: Gromochytriales and Mesochytriales.
Keywords: chytridiomycetes, Gromochytriales, Gromochytrium mamkaevae, Mesochytriales, Rhizophydium, strain x-51 CALU
INTRODUCTION
Molecular phylogeny has dramatically changed chytrid taxonomy. Investigation of gene sequences of nearly any species or strain initiates a revision of neighbour taxa and often permits authors to establish new taxa of higher rank, e.g. family, order and class, divisions normally supported by zoospore ultrastructure. In the past few years we have seen several big changes in chytrid taxonomy: Letcher et al. (2006) described the Rhizophydium clade (James et al. 2000, 2006) as the order Rhizophydiales; Mozley-Standridge et al. (2009) established the order Cladochytriales from the Cladochytrium clade (James et al. 2006) and Simmons et al. (2009) described the clade formerly represented in phylogenetic trees (James et al. 2006) by Chytriomyces angularis as the order Lobulomycetales. “This removal of clades from the polyphyletic Chytridiales better reflected the diversity of the Chytridiomycota and began the corrective process of classifying the Chytridiomycetes (chytrids) into phylogenetic groups according to the best tools available.” – wrote Longcore and Simmons in the introduction to the new order Polychytriales (Longcore & Simmons 2012: 276). This conclusion highlights the fact that we need molecular data for each traditionally described species of Chytridiomycetes to construct a meaningful and comprehensive classification of Chytridiomycetes.
Rhizophydium is one of the largest genera of Chytridiomycetes known from the middle of the 19th century (Rabenhorst 1868). It accounts for more than 225 species, which were described from freshwater, primarily as parasites of algae, and from soil as saprotrophs (Longcore 1996, Letcher et al. 2004). The data on this genus were significantly expanded in recent investigations (Letcher et al. 2006, 2008) and reviewed in a comprehensive taxonomic summary and revision of the genus (Letcher & Powell 2012).
Nevertheless, the list of species investigated with modern methods is still far from being complete, and new data on the ultrastructure and molecular phylogeny of other strains are always important for understanding the huge morphological and genetic diversity of this genus. Moreover, the transmission electron microscopy (TEM) sometimes reveals peculiarities that can be used as new taxonomic characters, or may show the unimportance of some commonly accepted ultrastructural characters.
Here we present the ultrastructure and molecular phylogeny of an algal parasite, strain x-51 CALU, which was described in a preliminary study as ‘Rhizophydium sp.’. We show that zoospore ultrastructure of this strain differs from that of other described species, and includes characters not described in any orders of Chytridiomycetes. These morphological data confirm an isolated phylogenetic position of x-51 obtained from the analysis of 18S and 28S rRNA sequences, and serve as the basis for the description of a new species and genus. Sister position of the x-51 branch relative to a cluster of environmental sequences, which includes Mesochytrium penetrans, and the ultrastructural differences of x-51 and Mesochytrium zoospores prompt us to establish two new orders: Gromochytriales and Mesochytriales.
MATERIALS AND METHODS
Strain CALU x-51 was isolated from a water sample collected from a ditch by the highway near town Kirovsk, Leningrad Region (Russia) in the autumn of 1999 by B.V. Gromov, and maintained on the host culture of filamentous, freshwater yellow-green alga Tribonema gayanum Pascher CALU 20 cultivated on No. 1 liquid organic medium (Gromov & Titova 1991). A dual clonal culture was incubated at 25 °C under continuous illumination of 25 μmol photon m-2·s-1 supplied by 40 W cool white fluorescent tubes.
For light microscopy, the parasite was examined with a Zeiss phase-contrast microscope.
For electron microscopy, the dual culture material was prefixed with 0.5 % OsO4 for 10 min followed by 2.5 % glutaraldehyde in 0.05 M cacodylate buffer at 4 °C for 2 h. The samples were then incubated with buffered 1 % osmium tetroxide for 1 h at 4 °C. After centrifugation the pellet was dehydrated with a graded ethanol series, and embedded in Spurr’s resin. Thin sections were stained with uranyl acetate and lead citrate, and examined with a Jeol 1011 electron microscope at 80 kV.
After inoculation of host strain with x-51, the cultures were incubated until the maximum infection of host cells was reached. Zoospores were then harvested by centrifugation and used directly for DNA extraction. The DNA was extracted with Diatom DNA Prep (IsoGen Lab, Moscow). The rRNA gene sequences were amplified using Encyclo PCR kit (Evrogen, Moscow) and a set of primers (Medlin et al. 1988, van der Auwera et al. 1994) and sequenced directly with Applied Biosystems 3730 DNA Analyzer. The assembled contig sequence was deposited in GenBank under accession number KF586842.
Molecular phylogenetic analysis
Ribosomal DNA sequences of x-51 were aligned with 113 OTUs from zoosporic fungi and closely related uncultured clones collected from the GenBank database. Sequences were selected based on the following scheme. First, all chytrid LSU genes that had sufficiently large length (> 2 000 bp) were added to the list of OTUs, and SSU genes were selected for all listed species. Second, all fragments of chytrid SSU and LSU rRNA genes were selected from cultured strains and environmental samples that occupied isolated positions on the distance tree. Third, all sequences of uncultured clones available in GenBank as of January 2013 were selected that grouped closely with x-51 CALU and Mesochytrium penetrans x-10 CALU. For environmental sample sequences that formed particularly long branches on the distance tree we performed an additional verification step that involved breaking the sequence into two or more non-overlapping fragments that were then used as independent OTUs for preliminary phylogenetic analysis (data not shown). This method identified seven sequences (accession numbers: EU162637, EF196798, EF196785, EF196773, EF196750, FJ592495, HQ191339) from three independent environmental samples as potentially chimaeric. The parts of sequences EU162637 and FJ592495 that presumably have fungal source were retained; the remainder and the other four sequences were excluded from the phylogenetic analysis. To minimise missing data a small number of sequences was assembled by fusing or constructing a consensus of sequences from different isolates of the same species or by fusing partial sequences that have a 98–100 % overlap identity. The full list of consensus and chimaeric sequences constructed for the purpose of phylogenetic analysis is presented in Table 1 and Fig. 2. The sequences of early-branching fungal taxa – Rozella allomycis and Amoeboaphelidium protococcarum were chosen as outgroup (James et al. 2006, Karpov et al. 2013). Alignments were generated with MUSCLE (Edgar 2004) and refined manually using BioEdit (Hall 1999). After discarding ambiguously aligned nucleotide positions and concatenating the alignments of 18S, 5.8S and 28S rRNA genes, the alignment consisted of 4 850 positions. Tree search for the concatenated alignment was performed using the Bayesian method implemented by MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). The tree reconstruction used GTR+G12+I model and partition by genes (18S, 5.8S, and 28S) with all parameters unlinked, except the topology and branch lengths. Four independent runs of eight Markov Chain Monte Carlo (MCMC) were performed to evaluate the convergence. Chains were run for 10 million generations sampling trees every 1 000 generations after discarding the first 8 million as burn-in. Sampled trees were used to generate a majority rule consensus tree with Bayesian posterior probabilities. Bootstrap support values for the consensus tree reconstructed by MrBayes were generated using RAxML v. 7.2.6 (Stamatakis 2006) on the basis of 1 000 replicates under the GTR+G+I model.
Table 1.
List of rRNA genes used in phylogenetic analysis.
| Taxon | Isolate number | GenBank accession no. |
Cumulative length (%) | ||
|---|---|---|---|---|---|
| 18S | 5.8S | 28S | |||
| Outgroup: aphelids and rozellids | |||||
| Amoeboaphelidium protococcarum | CALU X-5 | JX507298 | JX507298 | JX507298 | 99 |
| Rozella allomycis | UCB 47-054 (AFTOL-ID 297) | AY635838 | AY997087 | DQ273803 | 99 |
| Rozella sp. | JEL347 (AFTOL-ID 16) | AY601707 | AY997086 | DQ273766 | 98 |
| Blastocladiomycota | |||||
| Blastocladiella emersonii | M54937 | AY997032 | X90411 | 98 | |
| Allomyces arbuscula | AFTOL-ID 300 | AY552524 | AY997028 | AY552525 | 98 |
| Physoderma maydis | AFTOL-ID 19 | AY601708 | AY997072 | DQ273768 | 96 |
| Neocallimastigomycota | |||||
| Neocallimastix sp. | GE13 (AFTOL-ID 638) | DQ322625 | AY997064 | DQ273822 | 97 |
| Orpinomyces sp. | OUS1 | AJ864616, AJ864475 | AJ864475 | AJ864475 | 98 |
| Cyllamyces aberensis | EO14 (AFTOL-ID 846) | DQ536481 | AY997042 | DQ273829 | 100 |
| D3 | uncultured | EU910609 | 36 | ||
| Monoblepharidomycetes | |||||
| Monoblepharella mexicana | BK 78-1 (AFTOL-ID 33) | AF164337 | AY997061 | DQ273777 | 98 |
| Gonapodya prolifera | JEL478 | JGI v. 1.0 | JGI v. 1.0 | JGI v. 1.0 | 100 |
| Oedogoniomyces sp. | CR84 (AFTOL-ID 298) | AY635839 | AY997066 | DQ273804 | 99 |
| Hyaloraphidium curvatum | SAG 235-1 (AFTOL-ID 26) | Y17504 | AY997055 | DQ273771 | 91 |
| PFE7AU2004 | uncultured | DQ244008 | 36 | ||
| L73_ML_156 | uncultured | FJ354068 | 22 | ||
| Elev_18S_563 | uncultured | EF024210 | 36 | ||
| Gromochytriales, ord. nov. | |||||
| Gromochytrium mamkaevae | CALU X-51 | KF586842 | KF586842 | KF586842 | 99 |
| kor_110904_17 | uncultured | FJ157331 | 33 | ||
| IIN1-34 | uncultured | EU516964 | EU516964 | 15 | |
| Mesochytriales, ord. nov. | |||||
| Mesochytrium penetrans | CALU X-10 | FJ804149 | FJ804153 | 37 | |
| WS 10-E02 | uncultured | AJ867629 | 34 | ||
| WS 10-E14 | uncultured | AJ867630 | 36 | ||
| WS 10-E15 | uncultured | AJ867631 | 36 | ||
| Spring_08 | uncultured | JX069031 | 11 | ||
| Spring_37 | uncultured | JX069054 | 11 | ||
| Spring_57 | uncultured | JX069067 | 11 | ||
| Spring_71 | uncultured | JX069077 | 11 | ||
| T2P1AeB05 | uncultured | GQ995415 | 36 | ||
| T2P1AeF04 | uncultured | GQ995412 | 36 | ||
| T3P1AeC03 | uncultured | GQ995413 | 36 | ||
| T5P2AeC07 | uncultured | GQ995414 | 36 | ||
| SAPA5_E7 | uncultured | FJ483310 | 15 | ||
| P60E-9 | uncultured | DQ104060 | 13 | ||
| P60E-29 | uncultured | DQ104068 | 14 | ||
| Clones from a lake in China | uncultured | JX426910, JX426918, JX426923, JX426937, JX426998, JX427002, JX427011 | 7 | ||
| Clones from Lake Bourget (BI74, B1, B43, B44, B46-138, B49, B52, B56, BI78, BI88, BI100, BI104, BI107, BI121, BI123, BI15, BI72, BI76, B86-161, BI5) | uncultured | EF196711, EF196713, EF196728, EF196729, EF196731, EF196734, EF196735, EF196738, EF196745, EF196749, EF196751, EF196753, EF196755, EF196762, EF196763, EF196765, EF196775, EF196776, EF196786, EF196799 | 20 | ||
| PFF5SP2005 | uncultured | EU162641 | 36 | ||
| PFD6SP2005 | uncultured | EU162637 3’-end | 30 | ||
| PFA12SP2005 | uncultured | EU162643 | 36 | ||
| Pa2007C10 | uncultured | JQ689425 | 35 | ||
| F08_SE1B | uncultured | FJ592495 3’-end | 17 | ||
| ThJAR2B-48 | uncultured | JF972676 | 33 | ||
| 528-O25 | uncultured | EF586095 | 17 | ||
| GA069 | uncultured | HM486988 | 28 | ||
| GF29312 | uncultured | JX417945 | 16 | ||
| PFG9SP2005 | uncultured | EU162638 | 36 | ||
| PA2009C3 | uncultured | HQ191369 | HQ191369 | 40 | |
| PA2009B6 | uncultured | HQ191400 | HQ191400 | 40 | |
| PA2009D8 | uncultured | HQ191406 | HQ191406 | 40 | |
| PA2009E7 | uncultured | HQ191286 | HQ191286 | 40 | |
| Va2007BB6 | uncultured | JQ689445 | 35 | ||
| FV23_1H5 | uncultured | DQ310332 | 29 | ||
| Order Lobulomycetales | |||||
| Lobulomyces angularis | JEL45 (AFTOL-ID 630) | AF164253 | AY997036 | DQ273815 | 100 |
| Lobulomyces angularis | PL70 | EF443138 | EU352774 | EF443143 | 53 |
| Gen. sp. | AF011 | EF432819 | EF432819 | EF432819 | 57 |
| Maunachytrium keaense | AF021 | EF432822 | EF432822 | EF432822 | 54 |
| CCW64 | uncultured | AY180029 | 35 | ||
| RSC-CHU-20 | uncultured | AJ506002 | 32 | ||
| D2P03D7 | uncultured | EF100268 | 29 | ||
| AY2009B4 | uncultured | HQ219419 | HQ219419 | 40 | |
| IIS1-20 | uncultured | EU517013 | EU517013 | 14 | |
| Family Synchytriaceae | |||||
| Synchytrium decipiens | AFTOL-ID 634 | DQ536475 | AY997094 | DQ273819 | 91 |
| Synchytrium macrosporum | DUH0009363 (AFTOL-ID 635) | DQ322623 | AY997095 | DQ273820 | 99 |
| Synchytrium endobioticum | P-58 and Sluknov | AJ784274, AY854021 | 36 | ||
| Synchytrium endobioticum | AS-1 | JF795580 | JF795579 | 16 | |
| OTU97-188 | uncultured | JQ310927 | 11 | ||
| OTU97-621 | uncultured | JQ311409 | 11 | ||
| Order Polychytriales | |||||
| Polychytrium aggregatum | JEL109 (AFTOL-ID 24) | AY601711 | AY997074 | AY546686 | 100 |
| Lacustromyces hiemalis | JEL31 | AH009039 | HQ901700 | 49 | |
| Arkaya lepida | JEL93 (AFTOL-ID 629) | AF164278 | AY997056 | DQ273814 | 100 |
| Neokarlingia chitinophila | JEL510 | HQ901766 | HQ901703 | 52 | |
| Karlingiomyces asterocystis | JEL572 | HQ901769 | HQ901708 | 52 | |
| Order Cladochytriales | |||||
| Cladochytrium replicatum | JEL180 (AFTOL-ID 27) | AY546683 | AY997037 | AY546688 | 98 |
| Endochytrium sp. | JEL325 | AY349046 | AY349081 | 33 | |
| Allochytridium luteum | JEL324 (AFTOL-ID 631) | AY635844 | AY997044 | DQ273816 | 97 |
| Nephrochytrium sp. | JEL125 | AH009049 | EU828511 | 41 | |
| Diplochytridium lagenarium | JEL72 | AH009044 | AY349109 | AY349083 | 50 |
| Nowakowskiella sp. | JEL127 (AFTOL-ID 146) | AY635835 | AY997065 | DQ273798 | 98 |
| Order Chytridiales | |||||
| Podochytrium dentatum | JEL30 (AFTOL-ID 1539) | AH009055 | DQ53650 | DQ273838 | 95 |
| Chytriomyces sp. | JEL378 (AFTOL-ID 1532) | DQ536483 | DQ273832 | 73 | |
| Rhizoclosmatium sp. | JEL347-h (AFTOL-ID 20) | AY601709 | AY997076 | DQ273769 | 98 |
| Chytriomyces spinosus | JEL59 (AFTOL-ID 1540) | AH009063 | DQ273839 | 86 | |
| Chytridiales sp. | JEL187 (AFTOL-ID 39) | AY635825 | AY997035 | DQ273783 | 98 |
| Chytriomyces sp. | WB235A (AFTOL-ID 1536) | DQ536486 | DQ536498 | DQ536493 | 98 |
| Chytriomyces hyalinus | AFTOL-ID 1537 | DQ536487 | DQ536499 | DQ273836 | 98 |
| Chytriomyces sp. | JEL341 (AFTOL-ID 1531) | DQ536482 | DQ273831 | 92 | |
| Rhizidium endosporangiatum | JEL221 (AFTOL-ID 1534) | DQ536484 | DQ536496 | DQ273834 | 100 |
| «Rhizophydium» sp. | JEL354 (AFTOL-ID 41) | AY635827 | AY997083 | DQ273785 | 100 |
| Phlyctochytrium planicorne | JEL47 (AFTOL-ID 628) | DQ536473 | AY997070 | DQ273813 | 99 |
| Order Spizellomycetales | |||||
| Spizellomyces punctatus | ATCC 48900 (AFTOL-ID 182) | AY546684 | AY997092 | AY546692 | 92 |
| Powellomyces sp. | JEL95 (AFTOL-ID 32) | AF164245 | AY997075 | DQ273776 | 98 |
| Triparticalcar arcticum | AFTOL-ID 696 | DQ536480 | AY997096 | DQ273826 | 100 |
| Gaertneriomyces semiglobiferus | BK91-10 | AF164247 | |||
| Gaertneriomyces semiglobiferus | AFTOL-ID 34 | AY997051 | DQ273778 | 99 | |
| Order Rhizophlyctidales | |||||
| Rhizophlyctis rosea | JEL 318 (AFTOL-ID 43) | AY635829 | AY997078 | DQ273787. | 99 |
| Catenomyces sp. | JEL342 (AFTOL-ID 47) | AY635830 | AY997033 | DQ273789 | 99 |
| Blyttiomyces helicus | AFTOL-ID 2006 | DQ536491 | 34 | ||
| P34.43 | uncultured | AY642701 | 36 | ||
| Order Rhizophydiales | |||||
| ‘Rhizophlyctis’ harderi | JEL171 (AFTOL-ID 31) | AF164272 | AY997077 | DQ273775 | 98 |
| Rhizophydium sp. | JEL316 (AFTOL-ID 1535) | DQ536485 | DQ536497 | DQ273835 | 99 |
| Rhizophydium sp. | JEL317 (AFTOL-ID 35) | AY635821 | AY997081 | DQ273779 | 98 |
| Rhizophydium brooksianum | JEL136 (AFTOL-ID 22) | AY601710 | AY997079 | DQ273770 | 99 |
| Boothiomyces macrosporum | PL AUS 21 (AFTOL-ID 689) | DQ322622 | AY997084 | DQ273823 | 99 |
| Kappamyces laurelensis | AFTOL-ID 690 | DQ536478 | DQ536494 | DQ273824 | 99 |
| Rhizophydium sphaerotheca | AFTOL-ID 37 | AY635823 | AY997082 | DQ273781 | 97 |
| Rhizophydium sp. | JEL151 (AFTOL-ID 30) | AF164270 | AY997080 | DQ273774 | 96 |
| Entophlyctis sp. | JEL174 (AFTOL-ID 38) | AY635824 | AY997049 | DQ273782 | 93 |
| Entophlyctis sp. DU-DC1 | DU-DC1 | AF164255 | 20 | ||
| Entophlyctis helioformis | JEL326 (AFTOL-ID 40) | AY635826 | AY997048 | DQ273784 | 97 |
| Homoloaphlyctis polyrhiza | JEL142 | AFSM01005055 | AFSM01005055 | AFSM01005055 | 99 |
| Batrachochytrium dendrobatidis | AAHL-97-845 | AF051932 | |||
| Batrachochytrium dendrobatidis | JEL197 (AFTOL-ID 21) | AY997031 | AY546693 | 96 | |
| incertae sedis | |||||
| 18s1-47 | uncultured | EU733554 | 21 | ||
| 18s3 24 | uncultured | EU733608 | 21 | ||
| LLSG10_1 PML-2011t | uncultured | JN049552 | 13 | ||
Fig. 2.
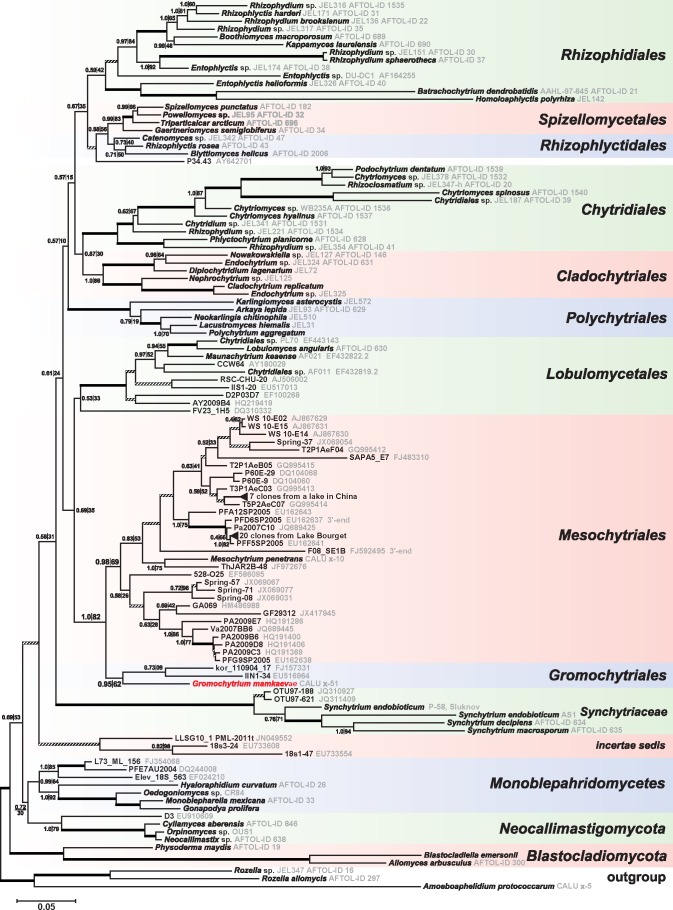
Bayesian phylogenetic tree based on concatenated rDNA sequences (18S, 5.8S, 28S). Node support values are given by Bayesian posterior probability (left of the vertical line) and Maximum Likelihood bootstrap support (right of the vertical line). Support values are omitted for nodes that score above 95 % in both analyses (edges drawn with thick lines) and nodes that score less than 50 % in both analyses (edges drawn with striated lines). The strain x-51 - Gromochytrium mamkaevae is highlighted with red. Two groups of nearly identical clones in the Mesochytriales clade are collapsed into single branches (represented by triangles).
RESULTS
Light microscopy
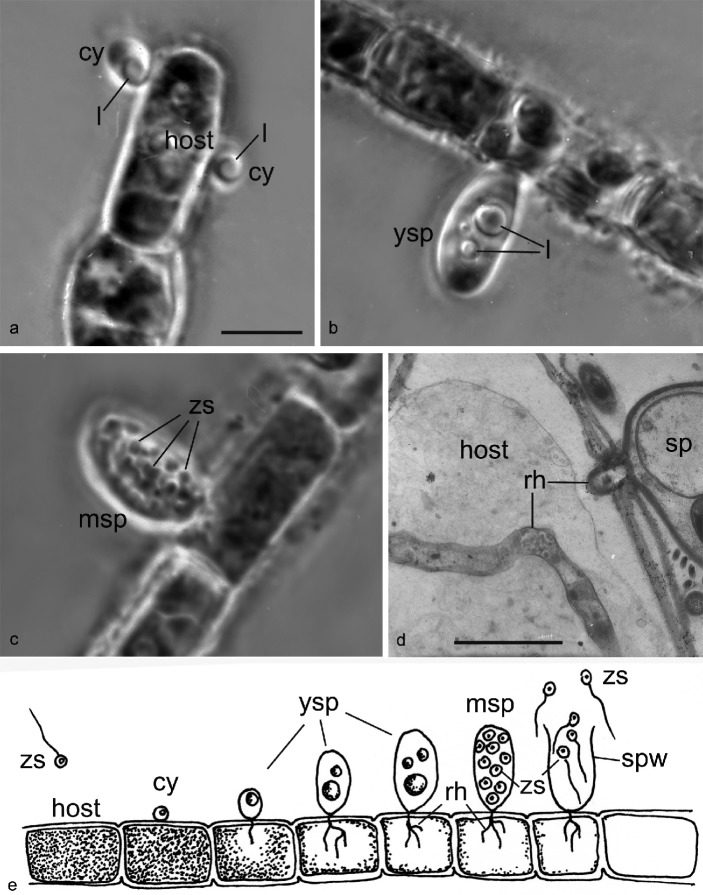
The parasite has a typical chytrid endogenous life cycle with tiny (~ 2 μm diam) zoospores that attach to the host cell surface, retract the flagellum and encyst. After the germ tube enters the host the zoospore cyst enlarges; a prominent lipid globule is clearly visible at this early stage (Fig. 1a). The young sporangium has homogenous contents with few lipid globules of different size (Fig. 1b), and the mature sporangium contains zoospores, which are released through an apical pore. The inoperculate sporangium is long ovoid (~ 18 × 10 μm diam) without a differentiated apical papilla (Fig. 1c). The apical pore varies in its dimensions: from narrow to as broad as the diameter of the sporangium or even broader (Fig. 1e). The delicate rhizoidal system is poorly visible, but can be estimated as weakly branched with short rhizoids emerging from a slender main axis (Fig. 1d, e). According to this description the fungus could be identified as Rhizophydium mammillatum (A. Braun) A. Fish. (1892) or, less likely, R. melosirae (1952) (Sparrow 1960, Letcher & Powell 2012), and therefore it was identified as R. mammillatum (Mamkaeva et al. 2006).
Fig. 1.
Stages of the life cycle of Gromochytrium mamkaevae (x-51 CALU) on the host Tribonema gayanum. — a–c: LM images of living parasite on filament of host Tribonema, phase contrast. – a. Two cysts with a lipid globule; b. young sporangium with 3 lipid globules; c. mature sporangium contains zoospores. – d. Rhizoid in the host cell in TEM. – e. Drawing of the life cycle. — Abbreviations: cy = cyst; l = lipid globule; msp = mature sporangium; rh = rhizoid; sp = sporangium; spw = sporangium wall; ysp = young sporangium; zs = zoospores. — Scale bars: a–c = 10 μm; d = 2 μm.
Molecular phylogeny
The rDNA sequences of strain x-51 occupy an isolated position in the tree (Fig. 2); its closest relatives are three uncultured clones: one from Lake Koronia in Greece (clone kor_110904_17), another from snow-covered soil in alpine Austria (clone IIN1-34), and one more from a hyposaline soda lake in Kenya, East Africa (Genitsaris et al. 2009, Kuhnert et al. 2012, Luo et al. 2013). Together these sequences form a new phylogenetic group. Among the described organisms, the closest relative of this group is Mesochytrium penetrans, which was classified in the Chytridiomycetes incertae sedis (Karpov et al. 2010). Mesochytrium penetrans is the only described species of a diverse group of uncultured fungi from soil, freshwater and hydrobiont gut samples collected from temperate zone of Eurasia and North America (Table 2). This group was recognised earlier as an order-level ‘Novel clade I’ within the Chytridiomycetes (Lefèvre et al. 2008, Jobard et al. 2012). Another name for ‘clade I’ is ‘snow chytrids’ (‘Snow Clade 1’ or SC1) according to Naff et al. (2013). The rDNA data places the clade uniting x-51 and the ‘clade I’ (Lefèvre et al. 2008) sister to Lobulomycetales (Simmons et al. 2009), albeit with relatively low support (Fig. 2). The distances inside the clusters of OTUs that contain x-51 and M. penetrans on the rDNA tree are comparable to the distances inside the established orders of Chytridiomycota, and distances between the OTUs in these clusters and the members of Lobulomycetales are no less than the distances between different orders of Chytridiomycota (Fig. 2).
Table 2.
List of environmental clones of the Mesochytriales and Gromochytriales.
| Name | GenBank accession no. | Habitat / Geographic location | Characterisation/Season | Reference |
|---|---|---|---|---|
| Gromochytrium mamkaevae | KF586842 | Ditch near town Kirovsk, Leningrad Region | parasite of yellow-green alga Tribonemagayanum | This paper |
| CALU x-51 | ||||
| Mesochytrium penetrans | FJ804149; FJ804153 | Small lake in Karelia (Northern Europe) | parasite of green alga Chlorococcum minutum | Karpov et al. (2010) |
| CALU x-10 | ||||
| 528-O25 | EF586095 | Opanuku Stream biofilm, Auckland, New Zealand | Dopheide et al. (2008) | |
| PFD6SP2005, PFG9SP2005, PFF5SP2005, PFA12SP2005 | EU162637, EU162638, EU162641, EU162643 | Oligo-mesotrophic mountain Lake Pavin, France | May – June | Lefèvre et al. (2008) |
| BI74, B1, B43, B44, B46-138, B49, B52, B56, BI78, BI88, BI100, BI104, BI107, BI121, BI123, BI15, BI72, BI76, B86-161, BI5 | EF196711, EF196713, EF196728, EF196729, EF196731, EF196734, EF196735, EF196738, EF196745, EF196749, EF196751, EF196753, EF196755, EF196762, EF196763, EF196765, EF196775, EF196776, EF196786, EF196799 | Large mesotrophic alpine Lake Bourget, France | May – August | Lepère et al. (2008) |
| F08_SE1B | FJ592495 | Cold-fumarole soil, Socompa Volcano, Andes (elev. 5824 m) | April | Costello et al. (2009) |
| P60E-9, P60E-29 | DQ104060, DQ104068 | Glacial ice from Tibetan plateau | 150-yr-old ice | Zhang et al. (2009) |
| T2P1AeB05, T2P1AeF04, T3P1AeC03, T5P2AeC07 | GQ995415, GQ995412, GQ995413, GQ995414 | High-elevation soil not far from ice and snow | July – October | Freeman et al. (2009) |
| PA2009C3, PA2009B6, PA2009D8, PA2009E7 | HQ191369, HQ191400, HQ191406, HQ191286 | Oligo-mesotrophic mountain Lake Pavin, France | July | Monchy et al. (2011) |
| SAPA5_E7 | FJ483310 | Salt marsh, USA: RI | Summer | Mohamed & Martiny (2011) |
| ThJAR2B-48 | JF972676 | Air sample, Greece | October | Genitsaris (2011) |
| GA069 | HM486988 | Feces from a detritus-feeding crustacean Gammarus tigrinus; Canada | September – October | Sridhar et al. (2011) |
| Spring_08, Spring_37, Spring_57, Spring_71 | JX069031, JX069054, JX069067, JX069077 | River site, Southern Alberta, Canada | Spring | Thomas et al. (2012) |
| Pa2007C10 | JQ689425 | Oligo-mesotrophic mountain Lake Pavin, France | April | Jobard et al. (2012) |
| Va2007BB6 | JQ689445 | Large brown-coloured humic and mesotrophic Lake Vassivière, France | May | Jobard et al. (2012) |
| WS 10-E02, WS 10-E14, WS 10-E15 | AJ867629, AJ867630, AJ867631 | Melted white snow water, alpine Lake Joeri XIII, Switzerland | – | Unpubl. data |
| GF29312 | JX417945 | Greenhouse soil, China | – | Unpubl. data |
| Seven clones from a freshwater lake in China | JX426910, JX426918, JX426923, JX426937, JX426998, JX427002, JX427011 | Freshwater lake, China | – | Unpubl. data |
| kor_110904_17 | FJ157331 | Lake Koronia water column, Greece | Nov. | Genitsaris et al. (2009) |
| IIN1-34 | EU516964 | Alpine snow-covered soil, Alpes, Austria | – | Unpubl. data |
| Nineteen clones: E109_XXX, E107_XXX | KC561936–KC561954 | High mountain soil Nepal | October | Naff et al. (2013) |
| Five clones: R11a_XX | KC561955–KC561959 | Rocky Mountain talus snow, Colorado, USA | July – August | Naff et al. (2013) |
| Sixteen clones: º T31a_XX, T31b_XX | KC561960–KC561975 | Rocky Mountain talus snow, Colorado, USA | July – August | Naff et al. (2013) |
| NKS146 | JX296576 | Hyposaline soda lake Nakuru, Kenya, East Africa | November | Luo et al. (2013) |
Zoospore ultrastructure
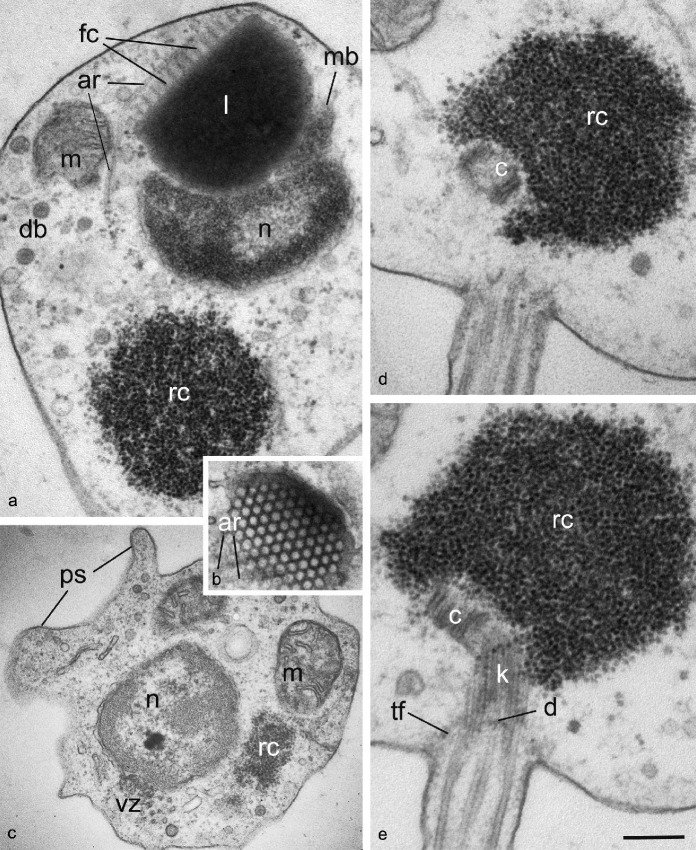
The spherical zoospore has a posterior flagellum and sometimes produces short anterior filopodia (Fig. 3c). A core of aggregated ribosomes is located in the posterior part of the cell. The ribosomal aggregation is relatively small and does not have surrounding endoplasmic reticulum (Fig. 3, 4). The ribosomes fill the space between the flagellar base and the nucleus and have no connection with nucleus, mitochondria or other membrane bounded organelles.
Fig. 3.
General ultrastructure of Gromochytrium mamkaevae (x-51 CALU) zoospore. — a. General disposition of nucleus and other organelles at LS; b. tangential section of fenestrated cisterna crossed by anterior microtubular root; c. pseudopodia at cell anterior; d, e. two consecutive sections of the kinetid. — Abbreviations: ar = anterior microtubular root; c = centriole; d = kinetosome diaphragm; db = dense bodies; fc = fenestrated cisterna; k = kinetosome; l = lipid globule; m = mitochondrion; mb = microbody; n = nucleus; ps = pseudopodia; rc = ribosomal core; tf = transitional fibers (props); vz = vesicular zone. — Scale bar on E: a = 300 nm; b, c = 400 nm; d, e = 200 nm.
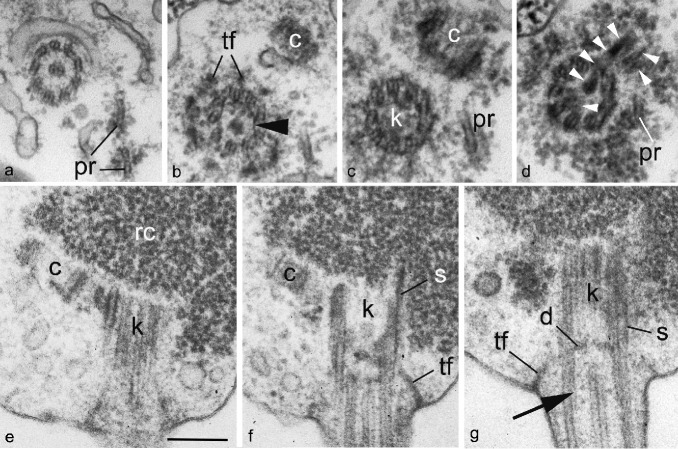
Fig. 4.
Kinetid structure of Gromochytrium mamkaevae (x-51 CALU) zoospore. a–d. Selected serial TS of the kinetid from distal to proximal. View from flagellar base to top. Arrowhead on b shows a spiral fiber. Arrowheads on d mark the bridge between kinetosome and centriole; e–g. selected serial LS of the kinetid. Arrow on g shows a spiral fiber. — Abbreviations: c = centriole; d = kinetosome diaphragm; k = kinetosome; pr = posterior microtubular root; rc = ribosomal core; s = spur; tf = transitional fibers (props). — Scale bar on E: a–d = 300 nm, e–g = 200 nm.
Several mitochondria with flat cristae reside at the cell periphery. A nearly central nucleus associates with anteriorly adpressed narrow microbody and a single large lipid globule anteriorly attached to the microbody (Fig. 3a). The anterior flat side of the lipid globule is bounded by a prominent fenestrated cisterna (rumposome) oriented to the cell exterior. Thus, the microbody-lipid globule complex (MLC) contains a single microbody enveloping a large anterior lipid globule with fenestrated cisterna.
Endoplasmic reticulum cisternae are rare and are normally found at the cell periphery. A vesicle rich zone occupies an area from one side of the ribosomal core extending from the nucleus to the centriole (Fig. 3a, c). Several small vesicles with electron-opaque contents (dense bodies) are present in the cytoplasm of the anterior part of the cell.
Kinetid structure
The structure of the flagellar apparatus was investigated with serial sections of six released zoospores. The kinetosome and centriole are embedded in the ribosomal core (Fig. 3d, e, 4). The kinetosome is c. 400 nm long and composed of microtubular doublets (not triplets) with developed transitional fibers (props) (Fig. 4b–d). The flagellar transition zone is simple without transversal plate, but with a slightly inward curved diaphragm at the distal end of kinetosome (Fig. 4g). Two thin lines parallel to the peripheral microtubular doublets are present above the diaphragm, and seem to correspond to the spiral fiber, or cylinder (Fig. 4g). The centriole is about 100 nm long and lies at an angle of c. 30° to the kinetosome (Fig. 3e, 4b, c, e, f). The kinetosome is connected to the centriole by a broad fibrillar bridge composed of at least three thick connectors (Fig. 4d). The longest middle connector passes through the bottom of kinetosome to the side of centriole. The structure of interconnecting bridge seems to be an unstable character. The bridge looks rather broad and prominent, connecting the sides of kinetosome and centriole at the longitudinal sections (Fig 4e, f), but it is not visible at the corresponding transverse sections (Fig. 4b–d). Approximately 1/3 of all serial sections had the broad bridge connecting the sides of kinetosome and centriole and in 2/3 of the series the bridge connects the bottom of kinetosome to the side of centriole. The diagram (Fig. 5a) shows the more common state.
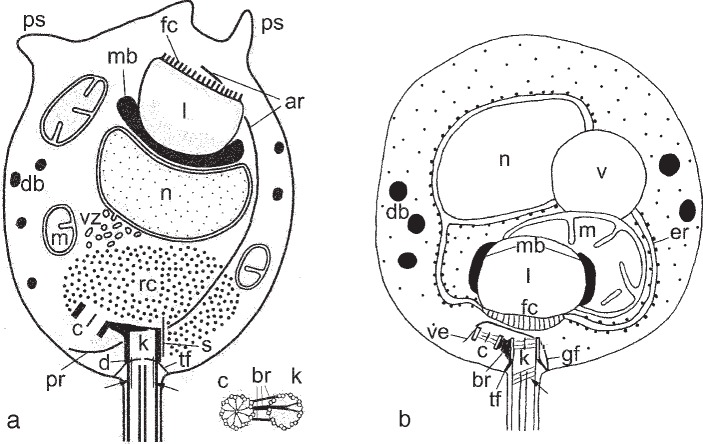
Fig. 5.
General scheme of zoospore structure. — a. Gromochytrium mamkaevae (x-51 CALU); b. Mesochytrium penetrans (x-10 CALU). Arrows show the spiral fiber in flagellar transition zone (b: after Karpov et al. (2010) with modified abbreviations).— Abbreviations: ar = anterior microtubular root; br = bridge between kinetosome and centriole; c = centriole; d = kinetosome diaphragm; db = dense bodies; er = endoplasmic reticulum; fc = fenestrated cisterna; gf = girdle fiber; k = kinetosome; l = lipid globule; m = mitochondrion; mb = microbody; n = nucleus; pr = posterior microtubular root; ps = pseudopodia; rc = ribosomal core; s = spur; tf = transitional fibers (props); v = vacuole; ve = veil; vz = vesicular zone.
The kinetosome produces at least two microtubular roots. The anterior root consists of two microtubules and passes laterally in the direction of the lipid globule crossing the surface of fenestrated cisterna (Fig. 3a, 5). The posterior root is much shorter, composed of one or two microtubules and is directed right about the anterior root (Fig. 4a–d). Their origin is not clear: anterior root emerges in the vicinity of kinetosome, and posterior root appears somewhere in between the kinetosome and the centriole.
One more kinetosomal derivate, a spur, lies close to the outer surface of the kinetosome on the side opposite the centriole (Fig. 4f, g). The spur is thin and short, projecting about 70–100 nm from the kinetosome into the ribosomal core (Fig. 4f).
A general scheme of zoospore ultrastructure is illustrated in Fig. 5a.
DISCUSSION
According to the morphology of strain x-51 at different life cycle stages it belongs to the genus Rhizophydium sensu Sparrow (1960). It has a simple thallus composed of inoperculate monocentric epibiotic elongated sporangium. It bears a single slightly branching rhizoidal axis. Judging by the shape of the sporangium and its dimensions this strain could be Rh. mammillatum, however, contrary to Rh. mammillatum, the sporangium of x-51 has no papilla. Our study has shown that zoospore ultrastructure of x-51 differs cardinally from that of Rhizophydium and other members of Rhizophydiales (Letcher et al. 2006, 2008). The order Rhizophydiales has 18 zoospore types that are rather different from each other, but none have a posterior ribosomal core without delimiting ER and mitochondria separated from MLC as in x-51. The MLC structure in the zoospore of x-51 has similarities with that of the recently established Gorgonomyces, which unlike other rhizophydiales has a close association of nucleus with microbody and lipid globule (Letcher et al. 2008), but in all other respects the zoospore of Gorgonomyces is different.
Molecular phylogeny places the strain x-51 far from Rhizophydiales, as a sister to ‘clade I’ – a cluster containing many environmental sequences of the Chytridiomycetes (Lefèvre et al. 2008, Jobard et al. 2012) besides a formally described species Mesochytrium penetrans, which was earlier shown to have a rather isolated position among the Chytridiomycetes (Karpov et al. 2010). The features that distinguish Mesochytrium are the partial penetration of the host cell by the sporangium and a zoospore with a unique ultrastructural organization.
Thus, we have to compare the zoospore structure of strain x-51 with that of M. penetrans. Two strains of M. penetrans (x-10 and x-46 CALU) were studied by electron microscopy, and 18S and 28S rRNA genes were sequenced for x-10 (Gromov et al. 2000, Karpov et al. 2010). Their general organization differs from that of x-51; unlike x-51 the M. penetrans has no ribosomal aggregation, its mitochondrion with MLC is enclosed by ER, a fenestrated cisterna faces the posterior of the cell, and a vacuole is present (Fig. 5b). At the same time, some morphological characters are similar in x-51 and x-10; both have small dense vesicles in the cytoplasm, which are common for the Chytridiomycetes; the kinetosomes lie at the same angle to each other and the flagellar transition zones contain a spiral element or a cylinder (Fig. 5). The kinetid structure also has some differences; x-51 has two microtubular roots which are absent in M. penetrans, a bridge in x-51 connects the bottom of kinetosome to the lateral surface of the centriole, not the lateral surfaces of kinetosome and centriole as in M. penetrans and the kinetosome of x-51 is composed of microtubular doublets. The spur structure and shape are also different; in x-51 the spur is inconspicuous and straight and in M. penetrans it is long and curved enclosing both the kinetosome and the centriole (Fig. 5).
We conclude, that the overall organization and kinetid structure of the zoospores of M. penetrans and x-51 differ considerably. According to the modern paradigm stemming from D. Barr’s studies (e.g. Barr 1978, Barr & Hadland-Hartmann 1978, Powell 1978, Longcore 1995, 1996, Letcher et al. 2006, 2008, Simmons 2009), their zoospores certainly have enough peculiarities to separate them at the taxonomic level of order. Moreover, their zoospores can be regarded as having a unique organization among the chytridiomycetes. We have already shown this for M. penetrans (Karpov et al. 2010). For the strain x-51 the unique characters are: the posterior core of ribosomes is not bounded by ER membranes, mitochondria are not associated with MLC, and a bridge connects the bottom of kinetosome to centriole.
The nearest branch to the x-51/Meshochytrium cluster is the order Lobulomycetales (Fig. 2), a group that was recently established on the basis of SSU and partial LSU gene phylogeny and ultrastructural analysis of zoospores (Simmons et al. 2009). In the previous study, the 18S and 28S sequences of M. penetrans (strain x-10 CALU) also placed this strain as a sister lineage to Lobulomycetales but with a rather low support (Karpov et al. 2010). ‘Snow chytrids’ were also suggested as a deep divergent branch sister to Lobulomycetales (Naff et al. 2013). In the present study the increased taxon sampling through the addition of environmental sequences results in better support for the sister group position of the x-51/Meshochytrium cluster relative to Lobulomycetales (Fig. 2).
Zoospores of Lobulomycetales (Lobulomyces angularis, Clydaea vesicula and Maunachytrium keaense) differ from those of x-51 and Meshochytrium in a number of ways: kinetids of lobulomycetes have parallel centrioles, an electron-opaque plug is present in the flagellar transition zone, and no spur or flagellar roots are found; the ribosomal core in Lobulomycetes is bounded by the ER, and the vacuole and 1–2 lipid globules lie posteriorly (Simmons et al. 2009). The presence of a rumposome (fenestrated cisterna) was noted in the text, but not shown in the pictures of the above cited article, therefore its precise position is unknown for Lobulomycetales.
Thus, our morphological data strongly support an isolated position of x-51/Meshochytrium cluster on the phylogenetic tree.
Taxonomy
An isolated position of Mesochytrium was shown by 18S+28S rRNA gene phylogeny and zoospore morphology of two strains: x-46 CALU (Gromov et al. 2000) and x-10 (Karpov et al. 2010), and recapitulated by molecular phylogenetic analysis in the present paper. The sequence of M. penetrans clusters with a large number of environmental sequences forming a clear monophyletic branch with good statistical support (Fig. 2). Molecular phylogenetic analysis of this genus does not reveal family or ordinal level affinity of M. penetrans, consequently in the previous paper we referred to it as incertae sedis (Karpov et al. 2010). Here we have a better resolved tree with a number of environmental sequences and a new neighbour of this branch that includes isolate x-51. Because of the molecular phylogeny of M. penetrans and CALU x-51, together with each having a unique organisation of zoospores, we establish new orders and families for both, plus a new genus and species for CALU x-51.
Gromochytriales Karpov & Aleoshin, ord. nov. — MycoBank MB805305
Zoospore with posterior ribosomal aggregation not bounded by endoplasmic reticulum. Microbody-lipid complex adpressed to the nucleus and containing a single microbody enveloping a large anterior lipid globule with anteriorly oriented fenestrated cisterna. Several mitochondria are separated from MLC. Small dense bodies present in peripheral cytoplasm. Kinetosome and centriole embedded in posterior side of the ribosomal core. Flagellar transition zone contains a spiral fiber, or a cylinder. Centriole at an angle of c. 30° to kinetosome; bottom of kinetosome connected by a broad fibrillar bridge to centriole. Anterior and posterior microtubular roots and a short straight spur associated with kinetosome.
Gromochytriaceae Karpov & Aleoshin, fam. nov. — MycoBank MB805306
Type genus. Gromochytrium Karpov & Aleoshin.
Description as for Gromochytriales: simple thallus with inoperculate, monocentric, epibiotic sporangium having endogenous development and single slightly branching rhizoidal axis.
Gromochytrium Karpov & Aleoshin, gen. nov. — MycoBank MB805307
Type species. Gromochytrium mamkaevaeKarpov & Aleoshin.
Simple thallus with inoperculate, monocentric, epibiotic sporangium having endogenous development and single slightly branching rhizoidal axis. Zoospore with posterior ribosomal aggregation unbounded by endoplasmic reticulum. Microbody-lipid-complex adpressed to the nucleus and contains a single microbody enveloping a large anterior lipid globule with anteriorly oriented fenestrated cisterna. Several mitochondria are separated from MLC. Small dense bodies present in peripheral cytoplasm. Kinetosome and centriole embedded in posterior side of the ribosomal core. Flagellar transition zone contains a spiral fiber, or a cylinder. Centriole at an angle of c. 30° to kinetosome; bottom of kinetosome connected by a broad fibrillar bridge to centriole. Anterior and posterior microtubular roots and a short straight spur associated with kinetosome composed of microtubular doublets.
Gromochytrium mamkaevaeKarpov & Aleoshin, sp. nov. — MycoBank MB805308, GenBank KF586842; Fig. 1, 2, 3, 4, 5a
Etymology. Genus named in honour of Boris V. Gromov, a prominent Russian microbiologist, and species named in honour of his spouse, colleague and co-author, Kira A. Mamkaeva.
Mature inoperculate epibiotic sporangium long ovoid (18 × 10 μm) without papillae. Zoospores released through apical pore. Delicate, weakly branched rhizoidal system with short rhizoids emerging from a slender main axis. Zoospores 2 μm diam with single lipid globule.
Specimen examined. RUSSIA, Leningrad Region, ditch near town Kirovsk, parasite of Tribonema gayanum. Holotype x-51 presented by fixed specimen embedded in resin block for electron microscopy. Deposited in CALU (Biological Faculty of St. Petersburg State University, St. Petersburg 199034, Russia).
Mesochytriales Karpov & Aleoshin, ord. nov. — MycoBank MB805303
Zoospores with unique ultrastructural organisation; centriole at an angle of c. 30° to kinetosome; ribosomes dispersed through the cytoplasm; mitochondrion and MLC surrounded by rough endoplasmic reticulum.
Mesochytriaceae Karpov & Aleoshin, fam. nov. — MycoBank MB805304
Description as for Mesochytriales. Sporangium inoperculate, monocentric, epibiotic, endogenous, semi absorbed by host cell.
Mesochytrium B.V. Gromov, Mamkaeva & Pljusch. Nova Hedwigia 71: 159. 2000, emend. Karpov
Type species. Mesochytrium penetrans B.V. Gromov, Mamkaeva & Pljusch.
Zoosporangium sessile, partially penetrating host cell. Delicate branched rhizoids emerge near the sporangial base. Zoospores spherical to oval with single lipid globule and dispersed ribosomes. Microbody-lipid-complex composed of a single mitochondrion and a single lipid globule partially covered with microbody and posterior fenestrated cisterna; centriole with veil at an angle of c. 30° to kinetosome, the two being connected by a broad, dense fibrillar bridge. Flagellar transition zone contains a spiral fiber. Resting spore endobiotic, spherical with smooth thick wall.
Mesochytrium penetrans B.V. Gromov, Mamkaeva & Pljusch. Nova Hedwigia 71: 159. 2000, emend. Karpov
Sporangium pyriform 10–14 × 6–7.5 μm with thin smooth wall and apical papilla. Zoospores spherical 2–2.5 μm diam with a 5–14 μm long flagellum. Parasite of green alga Chlorococcum minutum.
Specimen examined. Small lake Pryazha in Karelia, parasite of Chlorococcum minutum. Holotype CALU x-46.
Diversity and abundance of Mesochytriales and Gromochytriales in nature
The fact that Mesochytrium penetrans and Gromochytrium mamkaevae have thus far not been found during environmental DNA studies indicates that these species are not prevalent in the sampled ecosystems, at least not during the time of sampling. This fact emphasizes the incompleteness of our current knowledge of chytrid diversity and the importance of collecting new samples for exhaustive description of fungal diversity. At the same time, some of the undescribed species from the Mesochytriales clade that are represented by almost identical clones were repeatedly recovered in several environmental samples. Such clusters are formed by clones shown on Fig. 2 as small black triangles: one is presented by PFG9SP2005, PA2009C3, PA2009B6, PA2009D8 (Lefèvre et al. 2008, Monchy et al. 2011), another by PFF5SP2005, PFD6SP2005 (3’-end), Pa2007C10 and 20 clones are from Lake Bourget (Lefèvre et al. 2008, Lepère et al. 2008, Jobard et al. 2012), collected during the course of several years from lakes in France. Moreover, the clones of Mesochytriales from Lake Bourget form a substantial fraction of all fungal clones in the sample, which implies that their zoospores were ubiquitous during the time of sampling. It is likely that the abundance of Mesochytriales may vary by season. Ribosomal DNA clones of Mesochytriales accounted for about 50 % of the number of fungal rDNA clones from Lake Pavin (France) in spring and summer seasons (Lefèvre et al. 2008, Jobard et al. 2012), but they were not detected there in autumn (Lefèvre et al. 2007). Similarly to M. penetrans and G. mamkaevae, these clones probably can be attributed to parasites of algae. The diversity and abundance of rDNA clones from undescribed members in these environmental samples suggest that members of the Mesochytriales may play an important role as regulators of phytoplankton populations (Lefèvre et al. 2008, Lepère et al. 2008, Genitsaris et al. 2009, Monchy et al. 2011).
Acknowledgments
This work was supported by grants from the Russian Foundation for Basic Research (projects No 12-04-00154, 12-04-01486, 12-04-31870 and 13-04-10177) and by the program ‘Problems of life origin and biosphere development’, launched by the Presidium of the Russian Academy of Sciences. We are grateful to the staff of the Chebyshev and Lomonosov Supercomputer Center of the Moscow State University (http://parallel.ru/cluster) and the Bioportal of the University of Oslo (www.bioportal.uio.no), whose resources we used for computation.
REFERENCES
- Auwera G van der, Chapelle S, Wachter R de. 1994. Structure of the large ribosomal subunit RNA of Phytophthora megasperma, and phylogeny of the oomycetes. FEBS Letters 338: 133–136 [DOI] [PubMed] [Google Scholar]
- Barr DJS. 1978. Taxonomy and phylogeny of Chytrids. BioSystems 10: 153–162 [DOI] [PubMed] [Google Scholar]
- Barr DJS, Hadland-Hartmann VE. 1978. Zoospore ultrastructure in the genus Rhizophydium (Chytridiales). Canadian Journal of Botany 56: 2380–2404 [Google Scholar]
- Costello EK, Halloy SR, Reed SC, Sowell P, Schmidt SK. 2009. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation land‐scape on Socompa Volcano, Puna de Atacama, Andes. Applied and Environmental Microbiology 75: 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide A, Lear G, Stott R, Lewis G. 2008. Molecular characterization of ciliate diversity in stream biofilms. Applied and Environmental Microbiology 74: 1740–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KR, Martin AP, Karki D, Lynch RC, Mitter MS, et al. 2009. Evidence that chytrids dominate fungal communities in high-elevation soils. Proceedings of the Natural Academy of Sciences USA 106: 18315–18320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genitsaris S. 2011. Airborne microorganisms in urban areas: biodiversity, succession and dispersion. PhD thesis, Aristotle University of Thessaloniki, Greece (in Greek). http://invenio.lib.auth.gr/record/128157/files/GRI-2011-7695.pdf?version=1 [Google Scholar]
- Genitsaris S, Kormas KA, Moustaka-Gouni M. 2009. Microscopic eukaryotes living in a dying lake (Lake Koronia, Greece). FEMS Microbiology Ecology 69: 75–83 [DOI] [PubMed] [Google Scholar]
- Gromov BV, Mamkaeva KA, Pljusch AV. 2000. Mesochytrium penetrans gen. et sp. nov. (Chytridiales) – a parasite of the green algae Chlorococcum minutum (Chlorococcales), with an unusual behaviour of the sporangia. Nova Hedwigia 71: 151–160 [Google Scholar]
- Gromov BV, Titova NN. 1991. CALU-collection of algal cultures in the laboratory of microbiology of Biological Institute of Sankt-Petersburg University. In: Catalogue of microalgal cultures in the collections of the USSR: 76–125. RAS, Moskow [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Research S41: 95–98 [Google Scholar]
- James TY, Letcher PM, Longcore JE, Mozley-Standridge SE, Porter D, Powell MJ, Griffith GW, Vilgalys R. 2006. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 98: 860–871 [DOI] [PubMed] [Google Scholar]
- James TY, Porter D, Leander CA, Vilgalys R, Longcore JE. 2000. Molecular phylogenetics of the Chytridiomycota supports the utility of ultrastructural data in chytrid systematics. Canadian Journal of Botany 78: 336–350 [Google Scholar]
- Jobard M, Rasconi S, Solinhac L, Cauchie HM, Sime-Ngando T. 2012. Molecular and morphological diversity of fungi and the associated functions in three European nearby lakes. Environmental Microbiology 14: 2480–2494 [DOI] [PubMed] [Google Scholar]
- Karpov SA, Letcher PM, Mamkaeva MA, Mamkaeva KA. 2010. Phylogenetic position of the genus Mesochytrium (Chytridiomycota) based on zoospore ultrastructure and 18S and 28S rRNA gene sequences. Nova Hedwigia 90: 81–94 [Google Scholar]
- Karpov SA, Mikhailov KV, Mirzaeva GS, Mirabdullaev IM, Mamkaeva KA, Titova NN, Aleoshin VV. 2013. Obligately phagotrophic aphelids turned out to branch with the earliest-diverging Fungi. Protist 164: 195–205 [DOI] [PubMed] [Google Scholar]
- Kuhnert R, Oberkofler I, Peintner U. 2012. Fungal growth and biomass development is boosted by plants in snow-covered soil. Microbial Ecology 64: 79–90 [DOI] [PubMed] [Google Scholar]
- Lefèvre E, Bardot C, Noël C, Carrias JF, Viscogliosi E, Amblard C, Sime-Ngando T. 2007. Unveiling fungal zooflagellates as members of fresh‐water picoeukaryotes: evidence from a molecular diversity study in a deep meromictic lake. Environmental Microbiology 9: 61–71 [DOI] [PubMed] [Google Scholar]
- Lefèvre E, Roussel B, Amblard C, Sime-Ngando T. 2008. The molecular diversity of freshwater picoeukaryotes reveals high occurrence of putative parasitoids in the plankton. PLoS One 3: e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepère C, Domaizon I, Debroas D. 2008. Unexpected importance of potential parasites in the composition of the freshwater small-eukaryote community. Applied and Environmental Microbiology 74: 2940–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher PM, Powell MJ. 2012. A taxonomic summary and revision of Rhizophydium (Rhizophydiales, Chytridiomycota). Alabama University Printing, No. 1. Imprint Tuscaloosa, USA [Google Scholar]
- Letcher PM, Powell MJ, Barr DJS, Churchill PF, Wakefield WS, Picard KT. 2008. Rhizophlyctidales – a new order in Chytridiomycota. Mycological Research 112: 1031–1048 [DOI] [PubMed] [Google Scholar]
- Letcher PM, Powell MJ, Chambers JG, Holznagel WE. 2004. Phylogenetic relationships among Rhizophydium isolates from North America and Australia. Mycologia 96: 1339–1351 [PubMed] [Google Scholar]
- Letcher PM, Powell MJ, Churchill PF, Chambers JG. 2006. Ultrastructural and molecular phylogenetic delineation of a new order, the Rhizophydiales. Mycological Research 110: 898–915 [DOI] [PubMed] [Google Scholar]
- Longcore JE. 1995. Morphology and zoospore ultrastructure of Entophlyctis luteolus sp. nov. (Chytridiales): implications for chytrid taxonomy. Mycologia 87: 25–33 [Google Scholar]
- Longcore JE. 1996. Chytridiomycete taxonomy since 1960. Mycotaxon 60: 149–174 [Google Scholar]
- Longcore JE, Simmons DR. 2012. The Polychytriales ord. nov. contains chitinophilic members of the rhizophlyctoid alliance. Mycologia 104: 276–294 [DOI] [PubMed] [Google Scholar]
- Luo W, Kotut K, Krienitz L. 2013. Hidden diversity of eukaryotic plankton in the soda lake Nakuru, Kenya, during a phase of low salinity revealed by a SSU rRNA gene clone library. Hydrobiologia 702: 95–103 [Google Scholar]
- Mamkaeva MA, Pljusch AV, Mamkaeva KA. 2006. Some peculiarities of Rhizophydium mammillatum strains isolated from fresh-water reservoirs of North-West region of Russia. Mycologia i Phytopathologia 40: 402–410 In Russian. [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71: 491–499 [DOI] [PubMed] [Google Scholar]
- Mohamed DJ, Martiny JB. 2011. Patterns of fungal diversity and composition along a salinity gradient. ISME Journal 5: 379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchy S, Sanciu G, Jobard M, Rasconi S, Gerphagnon M, et al. 2011. Exploring and quantifying fungal diversity in freshwater lake ecosystems using rDNA cloning/sequencing and SSU tag pyrosequencing. Environmental Microbiology 13: 1433–1453 [DOI] [PubMed] [Google Scholar]
- Mozley-Standridge SE, Letcher PM, Longcore JE, Porter D, Simmons DR. 2009. Cladochytriales – a new order in Chytridiomycota. Mycological Research 113: 498–507 [DOI] [PubMed] [Google Scholar]
- Naff CS, Darcy JL, Schmidt SK. 2013. Phylogeny and biogeography of an uncultured clade of snow chytrids. Environmental Microbiology 15: 2672–2680 [DOI] [PubMed] [Google Scholar]
- Powell MJ. 1978. Phylogenetic implications of the microbody-lipid globule complex in zoosporic fungi. Biosystems 10: 167–180 [DOI] [PubMed] [Google Scholar]
- Rabenhorst L. 1864–1868. Flora Europaea Algarum Aquae dulcis et Submarinae. Vol. 3 Kummer, Leipzig, Germany [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Simmons DR. 2009. Cladochytriales – a new order in the Chytridiomycota. Mycological Research 113: 498–507 [DOI] [PubMed] [Google Scholar]
- Simmons DR, James TY, Meyer AF, Longcore JE. 2009. Lobulomycetales, a new order in the Chytridiomycota. Mycological Research 113: 450–460 [DOI] [PubMed] [Google Scholar]
- Sparrow FK. 1960. Aquatic Phycomycetes, 2nd edn.University of Michigan Press, Ann Arbor, USA [Google Scholar]
- Sridhar KR, Beaton M, Bärlocher F. 2011. Fungal propagules and DNA in feces of two detritus-feeding amphipods. Microbial Ecology 61: 31–40 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Thomas MC, Selinger LB, Inglis GD. 2012. Seasonal diversity of planktonic protists in Southwestern Alberta rivers over a 1-year period as revealed by terminal restriction fragment length polymorphism and 18S rRNA gene library analyses. Applied and Environmental Microbiology 78: 5653–5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ma X, Wang N, Yao T. 2009. New subgroup of Bacteroidetes and diverse microorganisms in Tibetan plateau glacial ice provide a biological record of environmental conditions. FEMS Microbiology Ecology 67: 21–29 [DOI] [PubMed] [Google Scholar]