Abstract
This study reveals the concordance, or lack thereof, between morphological and phylogenetic species concepts within Entoloma subg. Leptonia in boreal-temperate Eurasia, combining a critical morphological examination with a multigene phylogeny based on nrITS, nrLSU and mtSSU sequences. A total of 16 taxa was investigated. Emended concepts of subg. Leptonia and sect. Leptonia as well as the new sect. Dichroi are presented. Two species (Entoloma percoelestinum and E. sublaevisporum) and one variety (E. tjallingiorum var. laricinum) are described as new to science. On the basis of the morphological and phylogenetical evidence E. alnetorum is reduced to a variety of E. tjallingiorum, and E. venustum is considered a variety of E. callichroum. Accordingly, the new combinations E. tjallingiorum var. alnetorum and E. callichroum var. venustum are proposed. Entoloma lepidissimum var. pauciangulatum is now treated as a synonym of E. chytrophilum. Neotypes for E. dichroum, E. euchroum and E. lampropus are designated.
Keywords: Entolomataceae, morphology, multiple gene phylogeny, neotypes, new species
INTRODUCTION
The genus Entoloma s.l. is very species-rich and morphologically diverse. It contains more than 1 500 species and occurs worldwide from arctic to tropical habitats (e.g., Largent 1977, 1994, Romagnesi & Gilles 1979, Horak 1980, 2008, Noordeloos 1988, 1992, 2004, 2008, Gates & Noordeloos 2007, Noordeloos & Hausknecht 2007, Vila & Caballero, 2007, 2009, Noordeloos & Gates 2009, 2012, Morozova et al. 2012). Recent molecularly based phylogenetic studies have revealed that the genus is monophyletic and sister to the Clitopilus/Rhodocybe clade (Moncalvo et al. 2002, Matheny et al. 2006, Co-David et al. 2009, Baroni & Matheny 2011). Besides agaricoid basidiocarp types, the genus also comprises gasteroid, pleurotoid and cyphelloid forms (Co-David et al. 2009, Baroni & Matheny 2011). Life-style is equally varied, from saprotrophs to parasites, or mycorrhizal symbionts. The classification of Entolomatoid agarics traditionally followes two lines. The first group of authors interprets it as a single species-rich entity with an elaborate infrageneric classification (e.g., Romagnesi 1974, 1978, Noordeloos 1992, 2004), while the second segregates Entoloma s.l. in up to 13 genera (e.g., Largent & Baroni 1988, Orton 1991a, b, Largent 1994, Baroni & Matheny 2011). Delimitation of (sub)generic entities, has long been based solely on morphological characters (e.g. in Romagnesi & Gilles 1979, Noordeloos 1981, 2004). As a result of the phylogenetic studies mentioned above, it also became apparent that many of the (sub)generic divisions appear to be paraphyletic. Recently Baroni et al. (2011) described the new genus Entocybe within the Entolomatoid clade to accommodate species with basidiospore morphology intermediate between Entoloma and Rhodocybe, supported also by the molecular data. Ongoing studies, however, suggest that also this entity is polyphyletic (Morgado et al. 2013). Moreover, Baroni et al. (2011) have demonstrated the paraphyly of the Entolomataceae. Continued phylogenetic studies, based on both morphological characters and molecular markers (He et al. 2013, Morgado et al. 2013, Vila et al. 2013) reveal more insight into the interrelation between morphological and phylogenetic species concepts, as well as into the evolution of the Entolomataceae, that will result in future in a more natural classification.
Also subg. Leptonia in the sense of Noordeloos (2004) is polyphyletic. Sect. Leptonia of the subgenus belongs to the Nolanea-Claudopus clade, and Cyanula and Griseorubida to the Inocephalus-Cyanula clade (Co-David et al. 2009). Based on these data Cyanula recently has been raised to the subgenus level (Noordeloos & Gates 2012).
This paper is an attempt to clarify the phylogeny and species concept of a morphologically distinct group within the genus Entoloma, viz. subg. Leptonia sect. Leptonia in the classification of Noordeloos (2004). Despite the fact that most species are rare, some of them were described as far back as in the 19th century. The protologues of these species were short and incomplete, and the type specimens were not preserved. The morphological variability of Leptonia species appears to be very high and depending also on the age of basidiomata and weather conditions. Due to these factors, a large number of misunderstandings and incorrect identifications are found in the literature. This paper aims to describe morphological variability of each phylogenetic species resulting in emended descriptions and an identification tool, as well as to reconstruct an infrageneric classification of subg. Leptonia.
MATERIALS AND METHODS
Taxa sampling
To clarify the taxonomic status of 16 taxa of Entoloma subg. Leptonia as well as their position within the genus, 98 specimens of this subgenus or previously considered as belonging to this subgenus were selected for morphological study and molecular sampling (Table 1). ITS1-5.8S-ITS2 sequences were obtained for all of them. Type material, if possible, was included in the analysis. LSU sequences were obtained for 1–3 collections from each taxon. Species for which DNA extraction from type specimens appeared to be impossible or unsuccessful and where no additional reliable collections were available (E. austriacum, E. cedretorum, E. insidiosum, E. juniperinum, E. klofacianum, E. lidbergii, E. syringicolor and E. wynnei) are not considered in the present work. The outgroup choice and taxa sampling to determine the position of studied species in the system were primarily based on the recent global study on the phylogeny of the Entolomataceae (Co-David et al. 2009). Therefore, the representatives of the main subgenera of the crown Entoloma clade – Nolanea and Claudopus (Nolanea-Claudopus clade), Inocephalus and Cyanula (Inocephalus-Cyanula clade), as well as Entoloma, Pouzarella, Alboleptonia, and Trichopilus were included in the phylogenetic analyses (Table 2). Two species of subg. Entoloma (Prunuloides clade, which occupies basal position towards the groups treated above (Co-David et al. 2009)), were selected as outgroup in all analyses. A total of 114 specimens was included in the work. Most of the sequences were obtained from the present study. Additional 8 nrITS1-5.8S-ITS2, 19 nrLSU and 19 mtSSU sequences were retrieved from the Genbank: with the acronym GQ – Co-David et al. 2009; GU – Baroni et al. 2011; JQ – He et al. 2013; JX – Vila et al. 2013. The geographic origin of the collections includes Europe, the Caucasus and extratropical Asia from the Urals to the Russian Far East.
Table 1.
Specimens and GenBank accession numbers of DNA sequences used in the molecular analyses. Entoloma subg. Leptonia.
| Species | Location | Collector, voucher number | GenBank accession no. |
||
|---|---|---|---|---|---|
| nrITS | mtSSU | nrLSU | |||
| E. allochroum | Austria | K.F. Reinwald, A. Hausknecht (L 9860) | KC898370 | – | – |
| Netherlands | L. Bos (L, 7-08-1993, as E. tjallingiorum) | KC898368 | – | – | |
| Netherlands | R. Chrispijn (L 14-08-2000) | KC898371 | – | – | |
| Netherlands | Kits van Waveren (L, 29-07-1973, holotype) | KC898372 | – | – | |
| Russia: Caucasus | A. Kiyashko (LE262984) | KC898375 | – | – | |
| Russia: Caucasus | K. Potapov (LE254324) | KC898374 | – | – | |
| Spain | J. Vila, F. Caballero & A. Mayoral (JVG 1060902-1) | KC898376 | KC898488 | KC898522 | |
| Spain | F. Caballero (EFC 1272008-137) | KC898455 | – | – | |
| Spain | F. Caballero (EFC 2482008-148) | KC898456 | – | – | |
| Switzerland | G. Wölfel (L: E0809) | KC898369 | – | – | |
| Germany | G. Wölfel (L: E4884, as E. dichroum) | KC898373 | – | – | |
| E. callichroum var. callichroum | Switzerland | E. Horak (ZT 71/58, holotype) | KC898350 | – | – |
| E. callichroum var. venustum | Belarus | P. Kolmakov (LE226909, as E. lepidissimum) | KC898356 | – | – |
| Germany | G. Wölfel, F. Hampe (L, Wö E17/10, holotype E. venustum) | KC898355 | KC898490 | KC898523 | |
| Russia: Zhiguli | E. Malysheva (LE227532, as E. lepidissimum) | KC898357 | – | – | |
| Russia: Altaj | V. Malysheva (LE254312) | KC898351 | KC898489 | KC898521 | |
| Russia: Novosibirsk | T. Bulyonkova (LE254313) | KC898352 | – | – | |
| Russia: Novosibirsk | T. Bulyonkova (LE254314) | KC898353 | – | – | |
| Russia: Primorsky Territory | M. Nazarova (VLA M-20528, as Rhodophyllus lampropus) | KC898354 | – | – | |
| E. chytrophilum | Germany | M. Enderle, (M, as E. lepidissimum var. pauciangulatum, holotype) | KC898435 | – | – |
| Poland | M. Wantoch-Rekowska, 8 Aug. 2010 | KC898425 | – | – | |
| Poland | J. Soboń, 27 Aug. 2010 | KC898426 | – | – | |
| Poland | M. Wantoch-Rekowski, 3 Sept. 2010 | KC898427 | – | – | |
| Russia: Altaj | E. Malysheva (LE262994) | KC898429 | KC898480 | KC898520 | |
| Russia: Caucasus | E. Malysheva (LE262993) | KC898424 | – | – | |
| Russia: Caucasus | K. Potapov (LE254337) | KC898428 | – | – | |
| Russia: Novosibirsk | T. Bulyonkova (LE254326) | KC898430 | – | – | |
| Russia: Moscow Region | Yu. Rebriev (LE 254325) | KC898431 | – | – | |
| Russia: Vologda Region | O. Kirillova (LE 235259) | KC898432 | – | – | |
| Russia: Novgorod Region | S. Arslanov (LE 254336) | KC898433 | – | – | |
| Spain: Canary Islands | R.M. Dähncke (L 855, holotype) | KC898434 | KC898479 | KC898519 | |
| E. coelestinum | Russia: Sverdlovsk Region | L. Marina (LE258103) | KC898362 | KC898494 | KC898524 |
| E. dichroum | Russia: Zhiguli | E. Malysheva (LE227472, neotype) | KC898440 | – | – |
| Russia: Zhiguli | E. Malysheva (LE234260) | KC898442 | KC898487 | KC898528 | |
| Spain | J. Vila & F. Caballero (JVG 1070821-4) | KC898441 | KC898486 | KC898527 | |
| Spain | S. Català (SGC 16-10-11) | KC898454 | – | – | |
| E. euchroum | Germany | L. Krieglsteiner (KR-M-0032474, neotype) | KC898421 | – | – |
| Germany | L. Krieglsteiner (KR-M-0005673) | KC898423 | KC898485 | – | |
| Germany | M. Scholler (KR-M-0033332) | KC898420 | – | – | |
| Germany | L. Krieglsteiner (KR-M-0032221) | KC898422 | – | – | |
| Netherlands | C. Bas (L 6502, as E. tjallingiorum) | KC898415 | – | – | |
| Russia: Caucasus | E. Popov (LE262995) | KC898417 | KC898483 | KC898516 | |
| Russia: Leningrad Region | E. Popov (LE254334) | KC898416 | – | – | |
| Russia: Ryazan Region | E. Malysheva (LE254332) | KC898419 | KC898484 | KC898517 | |
| Russia: Tomsk | N. Agafonova (LE254329) | KC898418 | – | – | |
| Spain | J. Vila (JVG 1020827-2) | KC898461 | – | – | |
| Spain: Canary Islands | D. Chávez et al. (JVG 1091115A) | KC898462 | – | – | |
| E. eugenei | Russia: Primorsky Territory | E. Popov (LE253771 holotype) | KC898438 | – | KC898529 |
| Russia: Primorsky Territory | T. Svetasheva (LE254347) | KC898439 | – | KC898530 | |
| E. lampropus | Austria | F. Sucti (WU 13198, as E. dichroum) | KC898379 | – | – |
| Austria | A. Hausknecht (WU 24148, as E. dichroum) | KC898391 | – | – | |
| Austria | A. Hausknecht (WU 10092, as E. dichroum) | KC898393 | – | – | |
| Germany | G. Wölfel (L 1509) | KC898382 | – | – | |
| Russia: Bryansk Region | A. Fedosova (LE254339) | KC898392 | – | – | |
| Russia: Caucasus | O. Morozova (LE 254316) | KC898390 | – | – | |
| Russia: Kamchatka Region | O. Morozova (LE254349) | KC898389 | – | KC898507 | |
| Russia: Murmansk Region | L. Mikhailovsky (LE9121, as Leptonia placida) | KC898378 | – | – | |
| Russia: Novosibirsk | T. Bulyonkova (LE 262992) | KC898388 | – | – | |
| Russia: Orenburg Region | O. Desyatova, (LE253584, as E. tjallingiorum) | KC898387 | – | – | |
| Russia: Tatarstan Republic | K. Potapov (LE262991) | KC898384 | KC898470 | KC898505 | |
| Russia: Tomsk | N. Agafonova (LE262985) | KC898383 | – | – | |
| Russia: Sverdlovsk Region | L. Marina (LE258111) | KC898381 | – | – | |
| Russia: Udmurtia Republic | V. Kapitonov (LE254315) | KC898386 | – | – | |
| Russia: Udmurtia Republic | V. Kapitonov (LE254338) | KC898385 | – | – | |
| Russia: Vologda Region | O. Kirillova (LE235263) | KC898380 | – | – | |
| Sweden | T. Læssøe (UPS:BOT:F-176490, as E. placidum, designated here as neotype) | KC898377 | KC898471 | KC898506 | |
| Spain | S. Català (RM0855) | KC898458 | – | – | |
| E. lepidissimum | Czech Republic | M. Svrček (PRM 755801, holotype, as Leptonia lepidissima) | KC898364 | – | KC898532 |
| Russia: Novgorod Region | E. Popov (LE234755) | KC898365 | KC898491 | – | |
| Russia: Novgorod Region | E. Popov (LE254871) | KC898363 | KC898493 | KC898531 | |
| Russia: Novgorod Region | E. Popov (LE234751) | KC898367 | KC898492 | KC898534 | |
| Russia: Primorsky Territory | E. Malysheva (LE254311) | KC898366 | – | KC898533 | |
| E. percoelestinum | Russia: Novosibirsk | T. Bulyonkova (LE254327) | KC898359 | KC898496 | KC898526 |
| Russia: Novosibirsk | N. Filippova (LE254341) | KC898361 | – | – | |
| Russia: Penza Region | A. Ivanov (LE18913, as E. lepidissimum) | KC898358 | KC898495 | KC898525 | |
| Spain | J. Vila, X. Llimona (LE254390, holotype) | KF745927 | – | KF745928 | |
| Spain | J. Vila, F. Caballero (JVG 1061111-7, as E. coelestinum) | KC898360 | – | – | |
| E. placidum | Russia: Caucasus | O. Morozova (LE 254335) | KC898397 | – | – |
| Spain | J. Carreras, J. Vila, F. Caballero, A. Duran & A. Mayoral (JVG 1060830-6) | KC898395 | KC898482 | KC898515 | |
| Spain | J. Vila, F. Caballero & A. Mayoral (JVG 1060902-2) | KC898396 | – | – | |
| Spain | F. Caballero (EFC 2682008-151) | KC898457 | – | – | |
| Sweden | S. Lundell (5276) & G. Haglund (UPS:BOT:F-121714, epitype, as Leptonia placida) | KC898394 | KC898481 | KC898514 | |
| E. sublaevisporum | Austria | A. Hausknecht (MEN 9858) | KC898437 | – | – |
| Spain | J. Vila & F. Caballero (LIP JVG 1070823T, holotype) | KC898436 | KC898478 | KC898518 | |
| E. tjallingiorum var. tjallingiorum | |||||
| Russia: Moscow Region | E. Lukashina (LE254320) | KC898409 | – | – | |
| Russia: Leningrad Region | R. Singer (LE9123) | KC898410 | – | – | |
| Russia: Ulyanovsk Region | E. Ilyukhin (LE254319) | KC898408 | – | – | |
| Russia: Novgorod Region | O. Morozova (LE254318) | KC898411 | KC898472 | KC898510 | |
| Russia: Tatarstan Republic | K. Potapov (LE254317) | KC898407 | KC898475 | KC898511 | |
| Spain | F. Caballero (SFC 081019-01) | KC898459 | – | – | |
| Spain | F. Caballero (SFC 081005-01) | KC898460 | – | – | |
| Sweden | S. Ryman (6124) (UPS:BOT:F-016378, holotype) | KC898412 | KC898474 | KC898509 | |
| E. cf. tjallingiorum var. tjallingiorum | Russia: Zhiguli | O. Morozova (LE227507) | KC898404 | – | – |
| Russia: Zhiguli | E. Malysheva (LE227584, as E. placidum) | KC898405 | – | – | |
| Russia: Zhiguli | E. Malysheva (LE234285) | KC898406 | – | – | |
| E. tjallingiorum var. alnetorum | Russia: Leningrad Region | E. Popov (LE254321) | KC898398 | – | – |
| Russia: Tumen Region | E. Zvyagina (LE254322) | KC898402 | – | – | |
| Russia: Zhiguli | E. Malysheva (LE227527) | KC898401 | – | – | |
| Russia: Zhiguli | E. Malysheva (LE234287) | KC898403 | – | – | |
| Switzerland | O. Röllin (29-05-1994) | KC898399 | – | – | |
| Switzerland | O. Röllin (G 00111402, holotype) | KC898400 | KC898473 | KC898508 | |
| E. tjallingiorum var. laricinum | Russia: Kamchatka Region | E. Popov, O. Morozova (LE254343, holotype) | KC898413 | KC898477 | KC898513 |
| Russia: Kamchatka Region | E. Popov, O. Morozova (LE254344). | KC898414 | KC898476 | KC898512 | |
Table 2.
Specimens and GenBank accession numbers of DNA sequences used in the molecular analyses. Entoloma subg. Claudopus, Cyanula, Entoloma, Inocephalus, Nolanea, Pouzarella. The symbol * is placed before the names of the species for which sequences was taken from the Genbank.
| Species | Location | Collector, voucher No | GenBank accession no. |
||
|---|---|---|---|---|---|
| nrITS | mtSSU | nrLSU | |||
| *Entoloma abortivum | Canada | H. den Bakker (92) | – | GQ289290 | GQ289150 |
| *E. abortivum | China: Jilin | X.L. He et al. (HMJAU 1955) | JQ281483 | – | – |
| *E. aprile | Japan | C. Takehashi et al. (TNS:F-24626) | AB520845 | – | – |
| *E. araneosum | Belgium | M.E. Noordeloos (200314) | – | GQ289293 | GQ289153 |
| E. cetratum | Russia: Leningrad Region | O. Morozova (LE235480) | KC898450 | – | – |
| E. chalybeum | Russia: Leningrad Region | E. Morozova (LE254353) | KC898445 | KC898465 | KC898500 |
| E. clypeatum | Russia: Stavropol Region | I. Ukhanova (LE254350) | KC898349 | KC898497 | KC898535 |
| *E. cocles | Finland | J. Vauras (9770F) | – | GQ289299 | GQ289159 |
| *E. conferendum | Belgium | M.E. Noordeloos (200313) | – | GQ289330 | GQ289160 |
| *E. crassicystidiatum | China: Guangdong | X.L. He et al. (GDGM 27357) | – | JQ993056 | JQ291569 |
| *E. furfuraceum | China: Jilin | X.L. He et al. (GDGM 28818) | – | JQ993062 | JQ993094 |
| E. griseocyaneum | Russia: Caucasus | O. Morozova (LE254351) | KC898444 | KC898463 | KC898498 |
| *E. hebes | Netherlands | C. Hartman (1992-10-28) | – | GQ289310 | GQ289170 |
| E. indutoides | Russia: Leningrad Region | O. Morozova (LE254354) | KC898451 | KC898468 | KC898503 |
| *E. infula | Spain | J. Vila et al. (JVG 1080907-13) | JX454837 | – | – |
| E. inocephalum | Vietnam | O. Morozova (LE262922) | KC898449 | – | – |
| E. insidiosum | Norway | M.E. Noordeloos (L 376) | KC898443 | – | – |
| *E. minutum | Spain | J. Vila et al. (LIP PAM 00072307) | JX454829 | – | – |
| E. mougeotii | Russia: Caucasus | K. Potapov (LE254352) | KC898446 | KC898464 | KC898499 |
| *E. murrayi | V. Hofstetter (VHAs02.02) | – | GU384590 | GU384620 | |
| *E. nitidum | Italy | E. Campo (287) | JF907989 | – | – |
| *E. nitidum | Slovakia | M.E. Noordeloos (200426) | – | GQ289315 | GQ289175 |
| *E. parasiticum | Belgium | M.E. Noordeloos (200330) | – | GQ289317 | GQ289177 |
| *E. porphyrescens | Tasmania: Australia | M.E. Noordeloos (2004113) | – | GQ289322 | GQ289182 |
| *E. prunuloides | Slovakia | M.E. Noordeloos (200340) | – | GQ289324 | GQ289184 |
| E. quadratum | Russia: Primorsky Territory | E. Malysheva (LE254355) | KC898452 | KC898469 | KC898504 |
| *E. sericatum | Slovakia | M.E. Noordeloos (200328) | – | GQ289329 | GQ289189 |
| E. sericellum | Russia: Caucasus | O. Morozova (LE254362) | KC898453 | – | – |
| *E. sericellum | Belgium | M.E. Noordeloos (200315) | – | GQ289330 | GQ289190 |
| *E. sericeum | Slovakia | M.E. Noordeloos (200329) | – | GQ289331 | GQ289191 |
| E. serrulatum | Russia: Caucasus | O. Morozova (LE254361) | KC898447 | KC898466 | KC898501 |
| *E. sinuatum | Netherlands | J. Wisman (2003-09-19) | – | GQ289333 | GQ289193 |
| *E. sordidulum | Belgium | Co-David (2003) | – | GQ289334 | GQ289194 |
| E. tectonicola | India | P. Manimohan (741, holotype) | – | GQ289336 | GQ289196 |
| *E. turbidum | Italy | E. Campo (16176) | JF908005 | – | – |
| *E. turbidum | Slovakia | M.E. Noordeloos (200351) | – | GQ289341 | GQ289201 |
| *E. undatum | Belgium | M.E. Noordeloos (200327) | – | GQ289342 | GQ289202 |
| *E. undatum | Italy | E. Bizio, E. Campo (16854) | JF908007 | – | – |
| *E. valdeumbonatum | Germany | M. Meusers (E4565, holotype) | – | GQ289343 | GQ289203 |
| *E. violaceovillosum | India: Kerala | P. Manomohan (645, holotype) | – | GQ289345 | GQ289205 |
| E. violaceozonatum | Estonia | V. Liiv (L 275, holotype) | KC898448 | KC898467 | KC898502 |
Morphological analyses
The study was based both on recently collected material and collections kept in European and Asian herbaria (KR, L, LE, M, PRM, UPS, VLA, WU, ZT). The specimens were collected, documented and preserved using standard methods. Macroscopic descriptions are based on the study of the fresh material as well as on analysis of the photos. The dried material was examined using standard microscopic techniques. Spores, basidia and cystidia were observed in squash preparations of small parts of the lamellae in 5 % KOH or 1 % Congo Red in concentrated NH4OH. The pileipellis was examined in a preparation of the radial section of the pileus in 5 % KOH. Microscopic measurements and drawings were made with AxioImager A1 microscopes. Basidiospore dimensions are based on observing 20 spores, cystidia and basidia dimensions on observing at least 10 structures per collection. Basidia were measured without sterigmata, and the spores without hilum. Spore length to width ratios are reported as Q. The collected material is deposited in the Naturalis Biodiversity Center, section Botany (L), in the Mycological Herbarium of the Komarov Botanical Institute (LE) and in the collection of J. Vila (JVG) and S. Català (SGC). The holotype of E. sublaevisporum is deposited in LIP (Lille, France).
DNA extraction, amplification and sequencing
DNA was extracted from herbarium material using a CTAB extraction buffer technique with the following steps of consecutive addition of chloroform-isoamyl alcohol mixture, then isopropyl alcohol-3M sodium acetate solution for precipitation, 70 % ethanol for washing and finally water for dissolution. The alternative method of extraction DNA was using Axy Prep Multisourse Genomic DNA Miniprep Kit (Axygen Biosciences).
The ribosomal ITS1-5.8S-ITS2 region was amplified by PCR with the fungal specific primers ITS1F and ITS4B (Gardes & Bruns 1993; http://www.biology.duke.edu/fungi/mycolab/primers.htm). Sequences of nrLSU-rDNA were generated using primers LR0R and LR5 (Vilgalys & Hester 1990), and sequences of mtSSU – using primers MS1 and MS2 (http://nature.berkeley.edu/brunslab/tour/primers.html). PCR products were visualized using agarose gel electrophoresis and Gel Red staining, and subsequently purified with the kit AxyPrep PCR Cleanup Kit (Axygen Biosciences). Sequencing was performed with ABI model 3130 Genetic Analyzer (Applied Biosystems) using BigDyeTM Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) with the same primers. The raw data were processed using Sequencing Analysis 5.3.1 (Applied Biosystems).
Alignments and phylogenetic analysis
The sequences were aligned with MAFFT web tool (http://align.bmr.kyushu-u.ac.jp/mafft/online/server/) with Q-INS-I strategy and default settings for other options. The final alignment was corrected manually using MEGA version 5 (Tamura et al. 2011).
Phylogenetic reconstructions were performed with maximum likelihood (ML), maximum parsimony (MP) and Bayesian (BA) analyses. Representatives of the basal Entoloma clade (Co-David et al. 2009), E. turbidum and E. nitidum, were selected as outgroup for all analyses.
MP analysis was performed using PAUP*4.0.b10 (Swofford 2002). One hundred heuristic searches were conducted by stepwise addition with random sequence addition and tree bisection-reconnection (TBR) branch-swapping algorithm. One tree was held at each step during stepwise addition. All characters were treated as unordered and of equal weight. Parsimony bootstrap values were calculated from 1 000 replicates. Only clades with a support ≥ 50 % were retained. Gaps were treated as missing data.
The ML analysis was run in the RAxML servers (http://phylobench.vital-it.ch/raxml-bb/index.php; which implements the search protocol of Stamatakis et al. (2008)), under a GTR+G model with one hundred rapid bootstrap replicates.
Bayesian analysis was performed using MrBayes 3.1 (Ronquist & Huelsenbeck 2003) for two independent runs, each with 2 000 000 generations with sampling every 100 generations, with GTR+G model and four chains. Posterior probability (PP) value ≥ 0.95 are considered significant.
Species were delineated on the base of phylogenetic species concept referring to the examples from fungi in Taylor et al. (2000). Monophyletic clades are recognized as phylogenetic species when they are concordantly supported by the majority of the received phylogenetic trees. Additionally, ITS sequence differences were taken into account. Genetic distances between ITS sequences were estimated using PAUP*4.0.b10 (Swofford 2002). We consider a p-distance greater than 3 % to be a criterion that we will use to recognize new species, following Petersen et al. (2008) and Hughes et al. (2009). This approach was based on the data for within-species variation and heterozygosity from the Great Smoky Mountains National Park in the United States. According to these data, aproximatelly 2–3 % sequence divergence usually represents different species for Basidiomycotina. Morphological criteria were also taken into account. In the case where the morphological differences between separate monophyletic clades are not evident or p-distance is less than 3 % we prefer to consider these as varieties within a phylogenetic species.
RESULTS AND DISCUSSION
Phylogenetic analysis
Analysis of the nrITS1-5.8S-ITS2 dataset
The full alignment contained 122 ITS sequences with 1 047 characters. We first excluded from the alignment two large ambiguously aligned regions in ITS1. The first region (282 bp) corresponded to a presumptive insertion characteristic for the Entoloma dichroum group. In addition, E. eugenei from this group had another insertion of 51 bp that was also excluded from the full analysis.
The analysed dataset included 720 characters (gaps included), of which 327 were parsimony-informative. In the MP analysis, the 100 most parsimonious trees (MPTs) were saved (length = 1738, CI = 0.4177, HI = 0.5823, RI = 0.8402, RC = 0.3510). The ML, MP and BA analyses revealed nearly identical topologies.
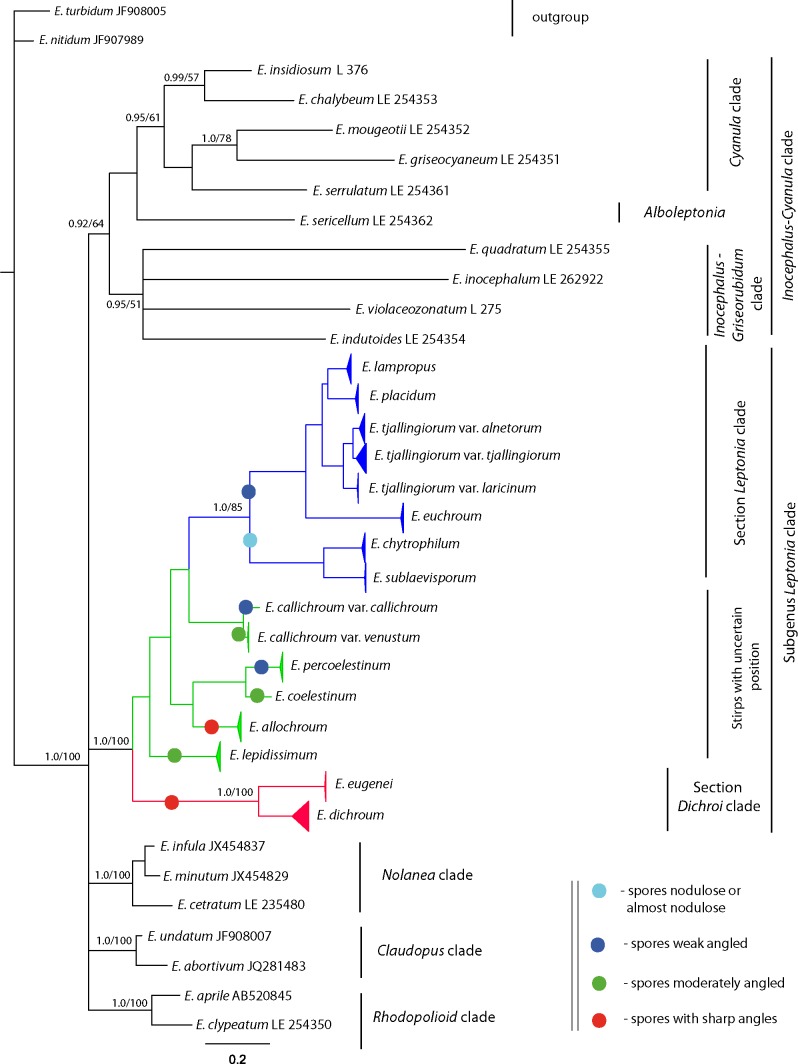
First of all, the analyses show a well-supported Leptonia clade (1.0/100 – BA/MP – hereinafter), separated from both the Nolanea-Claudopus and the Inocephalus-Cyanula clades (Fig. 1). As was shown by Co-David et al. (2009) and confirmed by the present work, subg. Leptonia in the traditional sense (Noordeloos 1992, 2004) is not monophyletic and must be considered without sections Cyanula and Griseorubida. Section Cyanula recently has been raised to the subgenus level (Noordeloos & Gates 2012). Representatives of sect. Griseorubida (E. indutoides) are nested within the Inocephalus subclade, and the exact taxonomic position of the section must be clarified. Therefore, the available data allowed treating subg. Leptonia in the strict sense, corresponding to sect. Leptonia in the sense of Noordeloos (1992, 2004) and Largent (1994).
Fig. 1.
Phylogenetic tree derived from Bayesian analysis, based on nrITS1-5.8S-ITS2 data. 1. Branches of subg. Leptonia subtree collapsed. Posterior probability (PP) values from the Bayesian analysis followed by bootstrap values from the Maximum Parsimony (BS, %) analysis are added to the left of a node (PP/BS). PP values ≥ 0.95 and BS values > 50 % are shown.
The holotype of E. violaceozonatum is grouped together with E. inocephalum (type species of subg. Inocephalus) with significant support of BA value (0.95/51) in the Inocephalus-Griseorubidum clade; it must therefore be excluded from subg. Leptonia. It is a similar situation with E. insidiosum. A specimen identified as E. insidiosum is nested within the Cyanula clade (0.99/57), hence it cannot belong to subg. Leptonia. Unfortunately, we were not successful in DNA isolation from the holotype of this species.
Two highly supported subclades are recognized in the Leptonia clade (Fig. 1, 2) corresponding to two different taxonomic sections – sect. Leptonia (1.0/85) and sect. Dichroi (1.0/100), newly recognized here. Entoloma callichroum, E. coelestinum, E. lepidissimum and E. allochroum form independent subclades.
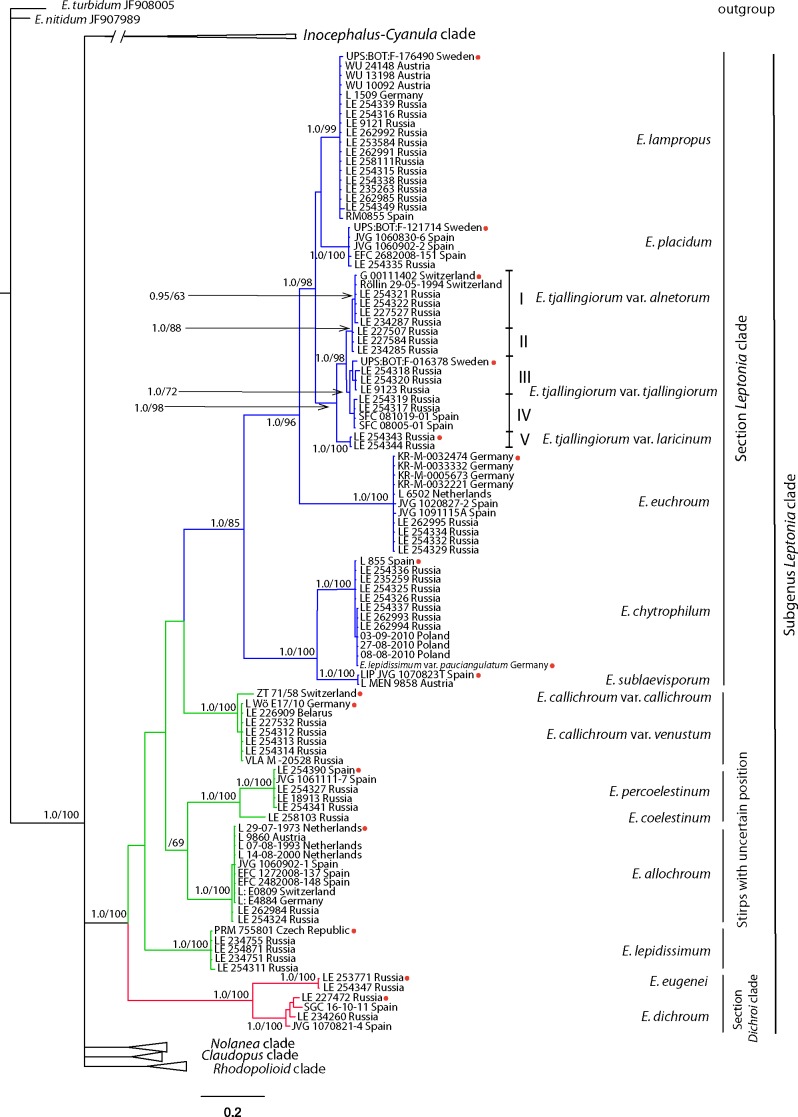
Fig. 2.
Phylogenetic tree derived from Bayesian analysis, based on nrITS1-5.8S-ITS2 data. 2. Main subtree – subg. Leptonia clade. Posterior probability (PP) values from the Bayesian analysis followed by bootstrap values from the Maximum Parsimony (BS, %) analysis are added to the left of a node (PP/BS). Type specimens are marked with red points.
In sect. Leptonia two subclades are recognized (Fig. 2). First of them – stirps Leptonia – is composed of E. euchroum (type species of subg. Leptonia), E. lampropus, E. placidum and E. tjallingiorum. Morphologically the weak angled and thin walled basidiospores are characteristic for species listed above. Entoloma lampropus, E. placidum and E. tjallingiorum possess also similar colours – grey-brown pileus and bluish stipe. Despite the rather high morphological variability expressed by the numerous cases of misidentifications of these taxa in several herbaria, E. euchroum, E. lampropus and E. placidum show genetic similarity since the internal topology for each species is unresolved as the sequences are almost 100 % identical (Fig. 2). Neotypes for E. euchroum and E. lampropus are designated in the present work. Specimens collected as close as possible to the type locality were chosen: E. euchroum KR-M-0032474 from Germany and E. lampropus UPS:BOT:F-176490 from Sweden, as well as an epitype for E. placidum – UPS:BOT:F-121714, collected close to the neotype locality (as DNA extraction from the neotype was impossible).
The ITS sequences for taxa in the E. tjallingiorum clade are highly variable. Even with this extreme variability, it is possible to identify five well-supported subclades that are arranged in three groups (Fig. 2). The first group (subclade I and II) includes the holotype of the E. alnetorum and it is well supported (1.0/88). Subclade I unites specimens morphologically corresponding to E. alnetorum – with a pale coloured pileus, fruiting in Alnus spp. forests early in the season (May-June, rarely July (in northern taiga), except LE227527, collected in August. The holotype of E. alnetorum is nested here. However, subclade II includes three specimens from one locality morphologically similar to E. tjallingiorum. The second group (subclade III and IV) unites specimens morphologically corresponding to E. tjallingiorum (with grey-brown pileus, growing in August on soil or on wood of various decidious trees), including the holotype of E. tjallingiorum and it is also well supported (1.0/72). As the p-distance between the holotypes of E. alnetorum and E. tjallingiorum is only 1.3 %, we consider E. alnetorum a variety of E. tjallingiorum. The divergence between the sequences as explained by the geographical distribution can be also recognized within the E. tjallingiorum clade. Subclade III (1.0/87) unites specimens with more northern distribution. It involves holotype of E. tjallingiorum from Sweden, as well as specimens from the Leningrad, Novgorod and Moscow regions of Russia. In subclade IV (0.99/72) specimens are found with southern origin – Tatarstan Republic and Ulyanovsk Region of Russia, and Spain. The third group, involving subclade V (1.0/98) only, takes a rather distant position within the E. tjallingiorum clade. It can be explained by the geographic reason also – the isolation between European and Far Eastern populations (p-distance between sequences – 3.3 %). Morphologically this distinction is supported by the presence of a sterile lamella edge made up of abundant, septate terminal elements of the hymenophoral trama. Also the habitat is different, as it is growing on coniferous wood. In spite of the p-distance between subclade V sequences and holotype of E. tjallingiorum is more than 3 %, we decided to recognize a new variety E. tjallingiorum var. laricinum only, as the morphological differences are only small.
The Entoloma chytrophilum–E. sublaevisporum subclade also nested within the section Leptonia-clade, despite the rather isolated position. These species are characterized by the nodulose or almost nodulose basidiospores with extremely weak and numerous angles. They definitely belong therefore to sect. Leptonia of subg. Leptonia. The holotype of E. lepidissimum var. pauciangulatum is nested within the E. chytrophilum-clade, so these names must be considered synonymous. This evidence is confirmed by the morphological features – almost nodulose spores and blue colour of the whole basidiomata. It is noteworthy that E. sublaevisporum macromorphologically is similar to species with differently coloured pileus and stipe, such as E. placidum, E. lampropus, E. callichroum, and at first it was considered closely related to E. callichroum. The phylogenetically informative feature in this case is the shape of basidiospores. Therefore, E. sublaevisporum is recognized here as a new species due to genetical evidence and spore morphology (viz. nodulose form) combined with the difference in colour between the pileus and stipe (see discussion in the taxonomic part).
Although sect. Dichroi has few species, it, however, forms a clearly distinct group. Morphologically it includes species with very pronounced, sharply angled spores such as E. dichroum and E. eugenei. Entoloma allochroum, of uncertain phylogenetic position, is the only other species with similar spores. Section Dichroi is also distinguished genetically by the presence of large insertions in the ITS1 region (282 bp). ITS region in E. dichroum is rather variable, but the p-distance between the specimens studied does not exceed 2 %. Morphological intraspecific variability in E. dichroum is also pronounced (as the variation in colour of the pileus) (Fig. 17a–c). Entoloma dichroum was described by Persoon in 1801. As the holotype does not exist and the type locality is unknown we selected a neotype from the available material. We studied 8 collections previously identified as E. dichroum, and only 4 of them correspond to the current concept of this species. We selected therefore LE227472 (Zhiguli, Russia) as it fits best the protologue.
Fig. 17.
a–c: Entoloma dichroum. a. LE227472 (photo taken at the neotype locality); b. herbarium SGC; c. JVG 1070821-4. — d. E. eugenei LE 253771, holotype. — e, f. E. allochroum LE254324. — Scale bars = 1 cm. — Photos by: a, d. O. Morozova; b. S. Català; c. J. Vila; e, f. K. Potapov.
The E. callichroum-clade consists of two branches. One: the holotype of E. callichroum only and the other including the holotype of E. venustum and 7 specimens which are conspeciphic with it. As the difference between the holotypes is low (p-distance 1.8 % bp difference) and morphological characters vary within the range of genetically identical specimens of E. venustum (basidiospore size, presence and amount of the cheilocystidia, the intensity of the bluish tinge in lamellae – see discussion in the taxonomic part) so that some specimens are indistinguishable from the E. callichroum, we propose to treat E. venustum as a variety of E. callichroum.
Two clades corresponding to the morphological concept of E. coelestinum were recovered in all analyses. Specimens previously identified as E. coelestinum form a well-supported clade, which, however, consists of two sister clades that can be distinguished morphologically. One collection characterized by more pronounced angled, not nodulose spores and a polished stipe fits well with the protologue of the type, and the current concept of E. coelestinum (Noordeloos 2004). The larger clade (5.3 % divergence with previous) is represented by specimens characterized by vaguely angled, almost nodulose spores and a longitudinally fibrillose-striate stipe. A new species, E. percoelestinum, is proposed for this taxon below. The E. allochroum clade involves almost identical collections, including the holotype, and only the Caucasian one slightly stands out among them. The E. lepidissimum clade is also highly supported, homogeneous, and includes the holotype specimen.
The position of E. callichroum, E. coelestinum, E. lepidissimum and E. allochroum within subg. Leptonia clade is uncertain. In the BA, ML and MP analyses they nest in the section Leptonia clade with low support. Morphologically these species occupy an intermediate position between sect. Leptonia and sect. Dichroi. The first three species have basidiospores with moderately developed angles, but E. allochroum has sharply angled basidiospores. It can be concluded that the degree of development of angles in basidiospores for members of the Entolomataceae generally is a phylogenetically informative feature, but with some restrictions. Similarly shaped basidiospores have developed independently in the sister clades. A wider sampling of species assigned to subg. Leptonia from other geographic regions will be necessary to resolve the phylogeny within subg. Leptonia.
Analysis of the nrLSU and mtSSU datasets
To accommodate subg. Leptonia in an understanding of the Entolomataceae tree produced by Co-David et al. (2009), analyses of the LSU, mtSSU and combined LSU-mtSSU datasets were performed on almost all taxa of Leptonia examined in this study. The PCR with MS1 and MS2 primers was unsuccessful for E. callichroum var. callichroum and for E. eugenei. The analyses were performed without these species.
Analysis of the nrLSU dataset
The dataset contained 57 LSU sequences with 775 characters (gaps included), of which 162 were parsimony-informative. The 100 equally most parsimonious trees (MPTs) were saved (length = 854, CI = 0.3255, HI = 0.6745, RI = 0.4043, RC = 0.1316). There were some topological differences and contradictions among the MPTs, the strict consensus tree from the MP analysis the best tree from the ML analysis, and the 50 % majority rule consensus tree from the BA. There was a high number of clades in each of these trees with low support.
In total, LSU trees are less informative than nrITS and mtSSU ones. Topological relations among the species of Leptonia and other subgenera of Entoloma were not confirmed by sufficient support because of low difference between LSU sequences. So, we do not present any LSU trees in this work as ambiguous.
Analysis of the mtSSU dataset
The dataset contained 54 mtSSU sequences that represent the main subgenera of the Entoloma s.l. taxa comprising the main branch of the Entolomataceae as well as representatives of almost all species of Leptonia considered in the present work. The analysed dataset included 658 characters (gaps included), of which 175 were parsimony-informative. The saved 100 most parsimonious trees had the following characteristics: length = 612, CI = 0.4776, HI = 0.5224, RI = 0.7245, RC = 0.3788. The ML, MP and BA analyses revealed nearly identical topologies.
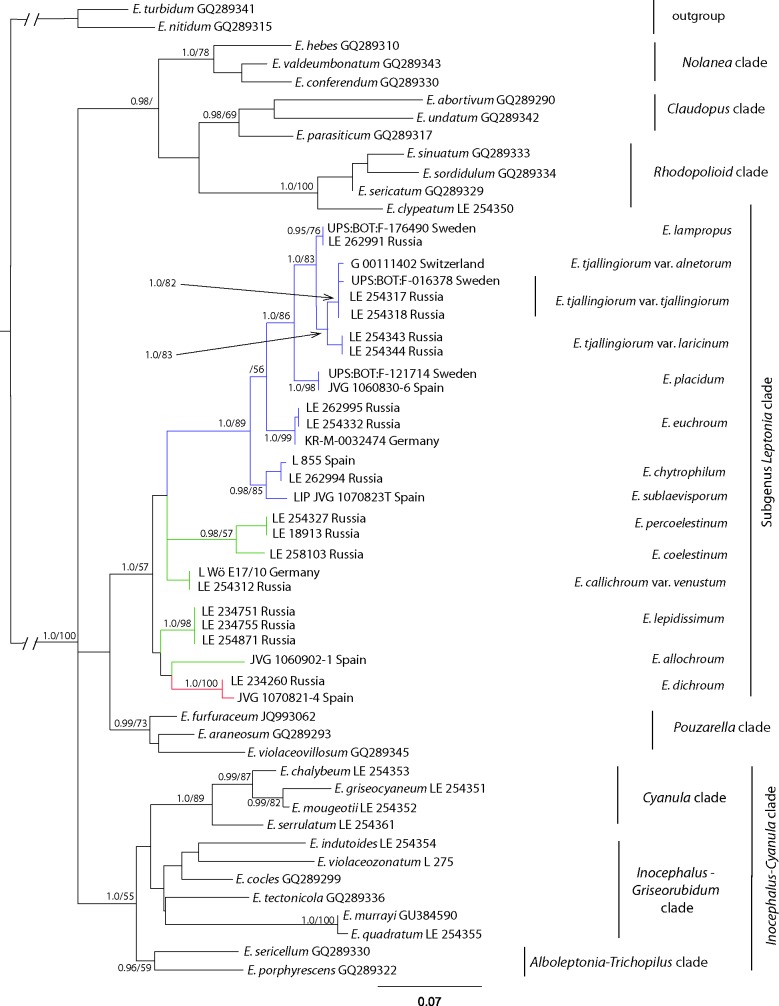
The main clades of the crown part of the Entolomataceae tree indicated by Co-David et al. (2009) were mostly confirmed (Fig. 3). The Rhodopolioid (1.0/100), Nolanea (1.0/78), Claudopus (0.98/69), Pouzarella (0.99/73), Inocephalus-Cyanula (1.0/55) and the Leptonia clades (1.0/57) received the highest support, especially in BA. The holotype of E. violaceozonatum was nested in the Inocephalus-Cyanula clade, therefore this species must be excluded from subg. Leptonia. Inside the Inocephalus-Cyanula clade three subclades can be revealed: the highly supported Cyanula and Alboleptonia-Trichopilus clades and the lowly supported Inocephalus-Griseorubidum clade. So, the position of the E. violaceozonatum within this clade is rather uncertain.
Fig. 3.
Phylogenetic tree derived from Maximum Likelihood, based on mtSSU data. Posterior probability (PP) values followed by bootstrap values from the Maximum Parsimony (BS, %) analysis are added to the left of a node (PP/BS).
The analyses performed confirm the results of Co-David et al. (2009), that subg. Leptonia in the sense of Noordeloos (2004) is polyphyletic and sections Leptonia, Cyanula and Griseorubidum do not form a monophyletic clade (Fig. 3). Cyanula, containing most known species of former subg. Leptonia, recently has been raised to the subgenus level (Noordeloos & Gates 2012). It is characterized by the presence of brilliant granules, absence of clamp-connections in all tissues and a fibrillose to polished stipe. We suggest that the Griseorubidum must be also excluded from subg. Leptonia based on phylogenetic affinity and morphological resemblance to Inocephalus species, like the presence of brilliant granules and well differentiated cheilocystidia. Section Leptonia (1.0/89) clearly stands out within the clade represented by subg. Leptonia, and it is characterized morphologically by the presence of clamp-connections, absence of brilliant granules and more or less fibrillose to squamulose stipe.
The analyses of the mtSSU dataset confirm the separation of the new species E. percoelestinum and E. sublaevisporum and the variety E. tjallingiorum var. laricinum.
Analysis of the combined mtSSU-LSU dataset
The dataset contained 52 sequences as in the previous analyses. The analysed dataset included 1 433 characters (gaps included), of which 334 were parsimony-informative. In the MP analysis, 93 most parsimonious trees were recovered (length = 1313, CI = 0.4501, HI = 0.6113, RI = 0.6204, RC = 0.2793). The ML, MP and BA analyses revealed nearly identical topologies, although the MP bootstrap values were rather low.
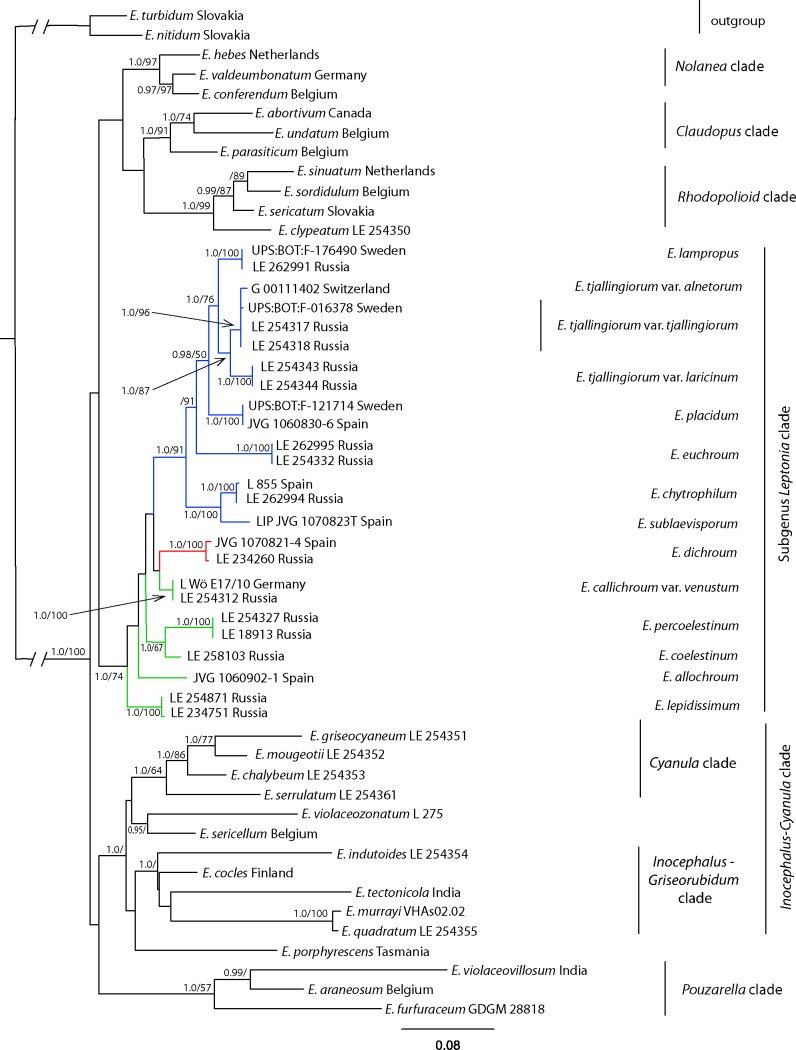
The topology revealed by the analysis of the combined data is similar to the results of the analysis of the mtSSU data (Fig. 4). The main subgenera of Entoloma form their clades with high support: Rhodopolioid clade (1.0/99), Nolanea (1.0/97), Claudopus (1.0/91), Pouzarella (1.0/57) and Inocephalus-Cyanula clade (1.0/), supported only by the BA. Subg. Leptonia clade received slightly higher support in the MP analysis (1.0/74). Sect. Leptonia (1.0/91) is also well revealed. Etoloma allochroum, E. callichroum, E. coelestinum-percoelestinum clade, E. dichroum, E. lepidissimum occupy isolated uncertain positions within subg. Leptonia. The Cyanula clade (1.0/64) and the Inocephalus-Griseorubidum clade (1.0/) are well indicated within the Inocephalus-Cyanula clade. Etoloma violaceozonatum also nests within this clade, but groups together with E. sericellum. Its taxonomic position needs to be carefully studied in the future.
Fig. 4.
Phylogenetic tree derived from Maximum Likelihood, based on combined mtSSU-nrLSU data. Posterior probability (PP) values followed by bootstrap values from the Maximum Parsimony (BS, %) analysis are added to the left of a node (PP/BS).
The analyses of the combined mtSSU-LSU dataset also confirm the separation of new species E. percoelestinum and E. sublaevisporum and the variety E. tjallingiorum var. laricinum.
TAXONOMIC PART
Entoloma subgenus Leptonia (Fr.: Fr.) Noordel. emend. O.V. Morozova, Noordel. & Vila
Entoloma subg. Leptonia sect. Leptonia (Fr.: Fr.) Noordel., Persoonia 11: 146. 1981. — Agaricus trib. Leptonia Fr., Syst. Mycol. (Lundae) 1: 10, 201. 1821. — Lectotype (Clements & Shear 1931: 349): Agaricus euchrous Pers.: Fr.
Rhodophyllus sect. Leptoniarii Romagn., Bull. Soc. Mycol. France 53: 332. 1937 (nom. nud.; no Latin diagnosis). — Lectotype (Noordeloos 1981: 146): Agaricus euchrous Pers.: Fr.
Leptonia sect. Lampropodae Konrad & Maubl., Les Agaricales: 259. 1948 (nom. nud., no Latin diagnosis). — Lectotype (Noordeloos 1982a: 453): Agaricus lampropus Pers.: Fr.
Rhodophyllus sect. Lampropodes (Kühner & Romagn., Fl. Anal.: 208. 1953) ex Romagn., Bull. Mens. Soc. Linn. Lyon 43: 328. 1974. — Holotype: R. lampropus (Pers.: Fr.) Quél.
Habit mycenoid, collybioid or tricholomatoid; pileus conico-convex or plano-convex, rarely with papilla or umbo, sometimes depressed; lamellae almost free to emarginate, sometimes adnate, rarely with decurrent tooth; stipe fibrillose-striate or flocculose-scaly, rarely almost smooth; lamellae edge sterile, fertile or heterogeneous; cheilocystidia present or absent, basidiospores with both weak or sharp angles, or almost nodulose, pileipellis more or less a trichoderm of inflated elements with intracellular, and often additionally encrusting, pigment; brilliant granules absent; clamp-connections present and often frequent; lignicolous or terrestrial, mostly in forests.
Entoloma subg. Leptonia (Fr.: Fr.) Noordel. is emended here by excluding sections Cyanula (Romagn.) Noordel. (that now is considered subg. Cyanula (Romagn.) Noordel. (Noordeloos & Gates 2012)), Griseorubida (Romagn.) Noordel. and Rhamphocystotae (Largent) Noordel. The subgenus now is characterized by species previously considered in sect. Leptonia only, and characterized by the presence of clamp-connections, absence of brilliant granules, and more or less fibrillose to squamulose stipe.
Synopsis of the phylogenetic species in subgenus Leptonia from boreal-temperate Eurasia
Section Leptonia (Fr.: Fr.) Noordel. emend. O.V. Morozova, Noordel. & Vila
1. Entoloma chytrophilum Wölfel, Noordel. & Dähncke
2. Entoloma euchroum (Pers.: Fr.) Donk
3. Entoloma lampropus (Fr.: Fr.) Hesler
4. Entoloma placidum (Fr.) Noordel.
5. Entoloma sublaevisporum Vila, Noordel. & O.V. Morozova
6a. Entoloma tjallingiorum Noordel. var. tjallingiorum
6b. Entoloma tjallingiorum var. alnetorum (Monthoux & Röllin) O.V. Morozova, Noordel. & Vila
6c. Entoloma tjallingiorum var. laricinum O.V. Morozova, Noordel., Vila & E.S. Popov
Section Dichroi O.V. Morozova, Noordel. & Vila
7. Entoloma dichroum (Pers.: Fr.) P. Kumm.
8. Entoloma eugenei Noordel. & O.V. Morozova
Species of uncertain position
Stirpe Allochroum
9. Entoloma allochroum Noordel.
Stirpe Callichroum
10a. Entoloma callichroum E. Horak & Noordel. var. callichroum
10b. Entoloma callichroum var. venustum (Wölfel & F. Hampe) O.V. Morozova, Noordel. & Vila
Stirpe Coelestinum
11. Entoloma coelestinum (Fr.) Hesler
12. Entoloma percoelestinum O.V. Morozova, Noordel., Vila & Bulyonkova
Stirpe Lepidissimum
13. Entoloma lepidissimum (Svrček) Noordel.
Certainly, the list of potential phylogenetic species within subg. Leptonia is much larger. Several morphospecies are still in need of molecular analysis in order to determine their phylogenetic position. The following additional morphospecies are known from boreal and temperate Eurasia: E. austriacum Courtec., E. cedretorum (Romagn. & Riousset) Noordel., E. insidiosum Noordel., E. juniperinum Barkman & Noordel., E. klofacianum Noordel., Wölfel & Hauskn., E. lidbergii Noordel. and E. wynneae (Berk. & Broome) Sacc. (Noordeloos 2004); E. syringicolor E. Ludw. & Noordel., E. dichroum var. leptosporum E. Ludw. & Noordel. (Ludwig 2007); E. dichroum var. corsicum (Romagn.) Courtec. (Courtecuisse 2008); E. legionense Blanco-Dios (Blanco-Dios ‘2012’ 2013). In the present study, the DNA extraction of these morphospecies failed, either due to the age and condition of the (type) specimens, or material was not available for destructive sampling.
For a sound phylogeny of subg. Leptonia, extra-European species (some of which are listed below) must also be taken into account, viz. from North America: E. velatum Hesler (Hesler 1967); E. kauffmanii Malloch (Malloch 2010); E. cyaneum [Peck→] Sacc., Leptonia approximata Largent, L. carnea Largent, L. convexa Largent, L. cyaneonita Largent, L. insueta Largent, L. occidentalis Murrill, L. subeuchroa Kauffman, L. subgracilis Largent, L. violaceonigra Largent, L. subcoelestina Largent, L. violacea (Kauffman) Largent, L. zanthophylla Largent (Largent 1994); Leptoniella acericola Murrill; from India: E. indoviolaceum Manim. & Noordel. (Manimohan et al. 2006); from New Guinea: E. egregium E. Horak (Horak 1980); and from Australia: E. panniculus (Berk.) Sacc., E. tomentosolilacinum G.M. Gates & Noordel., E. violascens G.M. Gates & Noordel. and E. endotum Noordel. & G.M. Gates (Noordeloos & Gates 2012).
Key to the species of subgenus Leptonia in boreal and temperate Eurasia
For the convenience in identifying following morphospecies have been added to the key, on account of the evidence in Noordeloos (2004): E. austriacum, E. cedretorum, E. insidiosum, E. juniperinum, E. klofacianum, E. lidbergii and E. wynnei. Also E. violaceozonatum Noordel. & Liiv is included, despite its placement in the Inocephalus-Cyanula clade.
1. Pileus and stipe blue, violaceous or pinkish lilaceous; lamellae white or coloured when young . . . . . . . . . . . . . . . . . . . . . 2
1. Pileus yellow-brown, brown, grey-brown or ochraceous, sometimes with blue or violaceous tinge near the cap margin; stipe blue or violaceous; lamellae white when young . . . . . . . . . . . . . . . . . . . . . 16
2. Lamellae coloured when young . . . . . . . . . . . . . . . . . . . . . 3
2. Lamellae white when young . . . . . . . . . . . . . . . . . . . . . 7
3. Lamellae firstly brownish then reddish brown with fimbriate concolorous edge; pileus fibrillose-zonate, lamellae edge entirely sterile with dense clusters of very long (up to 300 μm) subcylindrical cheilocystidia . . . . . . . . . . . . . . . . . . . . . E. violaceozonatum
3. Lamellae with bluish or violaceous-blue tinge when young, lamellae edge sterile or heterogeneous, cheilocystidia of another type, smaller . . . . . . . . . . . . . . . . . . . . . 4
4. Lamellae violaceous-blue with brownish violaceous edge when young; fruitbodies entirely deep violaceous-blue; on wood, preferably of Alnus . . . . . . . . . . . . . . . . . . . . . 2. E. euchroum
4. Lamellae pale to moderately dark blue or violaceous-blue with concolorous or slightly paler edge when young . . . . . . . . . . . . . . . . . . . . . 5
5. Pileus and stipe with the same deep blue colour, lamellae bluish with concolorous or slightly paler edge; spores 7.5–11.5 × 6.0–8.0 μm . . . . . . . . . . . . . . . . . . . . . . 13. E. lepidissimum
5. Pileus pinkish lilaceous to violaceous, stipe dark blue . . . . . . . . . . . . . . . . . . . . . 6
6. Spores with blunt angles, cheilocystidia absent . . . . . . . . . . . . . . . . . . . . . 10a. E. callichroum var. callichroum
6. Spores with moderately pronounced angles, cheilocystidia broadly clavate, intermixed with basidia . . . . . . . . . . . . . . . . . . . . . 10b. E. callichroum var. venustum
7. Pileus and stipe blue or violaceous-blue . . . . . . . . . . . . . . . . . . . . . 8
7. Pileus and stipe violaceous, brown-violaceous or pinkish lilaceous . . . . . . . . . . . . . . . . . . . . . 14
8. Spores isodiametrical, up to 9 μm diam . . . . . . . . . . . . . . . . . . . . . E. klofacianum
8. Spores heterodiametrical . . . . . . . . . . . . . . . . . . . . . 9
9. Spores 6.5–8.5(–9.0) × 5.5–6.5 μm, pileus conical to campanulate, hardly expanding, stipe smooth to subfibrillose . . . . . . . . . . . . . . . . . . . . . 10
9. Spores larger, pileus hemisphaerical, convex to applanate, stipe fibrillose or minutely squamulose . . . . . . . . . . . . . . . . . . . . . 11
10. Spores moderately angled, stipe smooth to subfibrillose . . . . . . . . . . . . . . . . . . . . . 11. E. coelestinum
10. Spores weakly angled, stipe fibrillose or minutely squamulose . . . . . . . . . . . . . . . . . . . . . 12. E. percoelestinum
11. Spores distinctly nodulose, 8.5–11.5 × 6.0–7.0 μm, pileus convex to applanate . . . . . . . . . . . . . . . . . . . . . 1. E. chytrophilum
11. Spores with 5–7 pronounced angles . . . . . . . . . . . . . . . . . . . . . 12
12. Cheilocystidia absent . . . . . . . . . . . . . . . . . . . . . E. cedretorum
12. Cheilocystidia present . . . . . . . . . . . . . . . . . . . . . 13
13. Fruitbody collybioid . . . . . . . . . . . . . . . . . . . . . 7. E. dichroum
13. Fruitbody tricholomatoid . . . . . . . . . . . . . . . . . . . . . 8. E. eugenei
14. Spores with 7–9 weak angles; 9.0–12.2 × 6.0–8.2 μm; pileus a tender pinkish ochre with brown with lilac or violaceous tint at centre . . . . . . . . . . . . . . . . . . . . . E. austriacum
14. Spores with 5–7 pronounced angles . . . . . . . . . . . . . . . . . . . . . 15
15. Fruitbodies very small; pileus up to 10 mm, pink . . . . . . . . . . . . . . . . . . . . . E. lidbergii
15. Fruitbodies larger; pileus 20–40 mm, greyish brown with lilaceous tinge . . . . . . . . . . . . . . . . . . . . . . 9. E. allochroum
16. Spores 10.0–16.0(–18.0) × 7.0–10.0 μm; pileus warm brown contrasting with the blue stipe . . . . . . . . . . . . . . . . . . . . . E. wynnei
16. Spores smaller . . . . . . . . . . . . . . . . . . . . . 17
17. Spores with 5–7 pronounced angles . . . . . . . . . . . . . . . . . . . . . 18
17. Spores nodulose or with 6–9 weak angles . . . . . . . . . . . . . . . . . . . . . 19
18. Stipe blue, flocculose-squamulose . . . . . . . . . . . . . . . . . . . . . . 7. E. dichroum (pale form)
18. Stipe violaceous, longitudinally fibrillose . . . . . . . . . . . . . . . . . . . . . 9. E. allochroum
19. Spores distinctly nodulose . . . . . . . . . . . . . . . . . . . . . 5. E. sublaevisporum
19. Spores with 6–9 weak angles . . . . . . . . . . . . . . . . . . . . . 20
20. Stipe glabrous, polished . . . . . . . . . . . . . . . . . . . . . 21
20. Stipe longitudinally fibrillose or squamulose . . . . . . . . . . . . . . . . . . . . . 23
21. Spores isodiametrical to subisodiametrical . . . . . . . . . . . . . . . . . . . . . 22
21. Spores heterodiametrical . . . . . . . . . . . . . . . . . . . . . E. insidiosum
22. Spores 8.0–10.0 × 6.0–8.0 μm; pileus dark brown when young . . . . . . . . . . . . . . . . . . . . . E. juniperinum
22. Spores 7.5–9.0 × 6.5–8.5 μm; pileus blue when young . . . . . . . . . . . . . . . . . . . . . E. klofacianum
23. Stipe flocculose-squamulose . . . . . . . . . . . . . . . . . . . . . 24
23. Stipe fibrillose-striate . . . . . . . . . . . . . . . . . . . . . 26
24. Pileus pale ochraceous; on wood of Alnus, early in season . . . . . . . . . . . . . . . . . . . . . 6b. E. tjallingiorum var. alnetorum
24. Pileus brown or grey-brown . . . . . . . . . . . . . . . . . . . . . 25
25. Lamellae edge heterogeneous, cheilocystidia lageniform, intermixed with basidia; on soil or on wood of deciduous trees . . . . . . . . . . . . . . . . . . . . . 6a. E. tjallingiorum var. tjallingiorum
25. Lamellae edge sterile, cheilocystidia as strands of terminal elements of hyphae of trama hymenial . . . . . . . . . . . . . . . . . . . . . 6c. E. tjallingiorum var. laricinum
26. Pileipellis with intracellular pigment; cheilocystidia absent; smell farinaceous; on wood of deciduous trees, mainly of Fagus . . . . . . . . . . . . . . . . . . . . . 4. E. placidum
26. Pileipellis with both intracellular and encrusting pigment; cheilocystidia present or absent; smell none; on soil or on wood of coniferous trees . . . . . . . . . . . . . . . . . . . . . 3. E. lampropus
Descriptions of the species
Below we provide descriptions of the species included in the analysis (in the key indicated with the number that corresponds with the descriptive text of this paper). Most of them are rarely collected and exact and complete information was difficult to find. Therefore the descriptions below contain new data on ecology, substratum, colour variation, spore shape, and presence/absence of cheilocystidia. One section, two species and one variety are described as new to science.
Section Leptonia (Fr.: Fr.) Noordel. emend. O.V. Morozova, Noordel. & Vila
Lectotype (Clements & Shear 1931: 349): Agaricus euchrous Pers.: Fr.
Habit collybioid to almost tricholomatoid; pileus conico-convex or plano-convex, rarely with papilla or umbo, sometimes depressed; lamellae almost free to emarginate, sometimes adnate, rarely with decurrent tooth; stipe fibrillose-striate or flocculose-scaly; lamella edge sterile, fertile or heterogeneous; cheilocystidia present or absent, spores with poor angles to almost nodulose, pileipellis more or less a trichoderm of inflated elements with intracellular, and often additionally encrusting, pigment; pileitrama regular; brilliant granules absent; clamp-connections present and often frequent; lignicolous or terrestrial, mostly in forests.
Section Leptonia is emended here to unite only those species with weakly angled, almost nodulose spores, which form a well-supported monophyletic clade, as opposed to the new section Dichroi which includes species with pronouncedly acutely angled spores. Several species with uncertain phylogenetic position (Entoloma allochroum, E. callichroum, E. coelestinum, E. lepidissimum, E. percoelestinum) also are not included in sect. Leptonia.
1. Entoloma chytrophilum Wölfel, Noordel. & Dähncke, Öst. Z. Pilzk. 10: 190. 2001. — Fig. 5a, b, 6
Fig. 5.
a, b: Entoloma chytrophilum. a. LE262994; b. LE254326. — c–f: E. euchroum. c, d. LE254329 (photo’s taken at the same locality but at different times); e. JVG 1091115A; f. LE262995. — Scale bars = 1 cm. — Photos by: a, f. O. Morozova; b. T. Bulyonkova; c. N. Agafonova; d. S. Gashkov; e. M.Á. Ribes.
Fig. 6.
Entoloma chytrophilum. a. Basidium; b. spores (LE262994). — Scale bars = 10 μm.
Syn.: Entoloma lepidissimum var. pauciangulatum Gminder & Enderle, Beitr. Kenntn. Pilze Mitteleur. 10: 60. 1996.
Pileus 2–15 mm broad, plano-convex to concave with depressed centre, not hygrophanous, not translucently striate, with straight then deflexed undulating margin, radially fibrillose, in centre squamulose, dark blue, slightly discolouring to bluish violaceus. Lamellae moderately distant, adnate-emarginate with small decurrent tooth, ventricose, white, becoming pinkish, with entire concolorous edge. Stipe 20–40 × 1–2 mm, cylindrical, fibrillose-striate, slightly squamulose in the upper part, concolourous with pileus or grey-blue, with white basal tomentum. Smell strong, fungoid, reminiscent of Cantharellus cibarius or indistinct, taste not reported. Spores 8.4–11.5 × 5.8–7.0 μm, Q = 1.3–1.8, nodulose, heterodiametrical. Basidia 37.8–48.2 × 10.2–12.3 μm, 1–4-spored, clavate, clamped. Lamellae edge fertile. Cheilocystidia absent. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 10–20 μm wide with blue intracellular pigment. Clamp-connections present.
Habitat — Type specimen on chips of Pinus bark in pot with Cymbidium in garden. In natural conditions on rotten wood (mostly of coniferous trees) in coniferous and mixed forests.
Known distribution — Western, Central and Eastern Europe, Canary Islands (holotype), Caucasus, Western Siberia.
Specimens examined. GERMANY, Baden-Württemberg, Langenau-Lindenau, 500 m NN, MTB 7426/4, among conifer needles, 9 Aug. 1985, M. Enderle, (M, as Entoloma lepidissimum var. pauciangulatum, holotype). – POLAND, Trójmiejski Landscape Park, Gdansk, Oliwa districy, on Picea abies cones in mixed forest (Fagus sylvatica, Pinus sylvestris, Picea abies, Betula pendula), 8 Aug. 2010, M. Wantoch-Rekowska; ibid., on Fagus cupules and woody debris in mixed forest (Fagus sylvatica, Pinus sylvestris, Picea abies, Betula pendula), 3 Sept. 2010, M. Wantoch-Rekowski; Sudety Mts, Kaczawskie foothills, Mount Szeroka, on Pinus branches in Pinus sylvestris-Larix sp. forest, 27 Aug. 2010, J. Soboń. – RUSSIA, Vologda Region, ‘Russky Sever’ National Park, Nilovetskoye forestry, on soil in Picea abies-Pinus sylvestris forest, 20 Aug. 2004, O. Kirillova (LE235259, as E. lepidissimum); Novgorod Region, Malaya Vishera District, vicinities of Syujska village, among mosses in Picea abies-Populus tremula forest, 7 Aug. 2012, S. Arslanov (LE254336); Moscow Region, Zvenigorod Biological Station of Moscow State University, on rotten stump, 24 July 2012, Yu. Rebriev (LE254325); Karachaevo-Cherkesia Republik, Teberda Biosphere Reserve, valley of Baduk River, on rotten stump in Picea orientalis-Abies nordmanniana forest, 8 Aug. 2009, E. Malysheva (LE262993); ibid., vicinities of Teberda, on rotten stump, K. Potapov, 22 Aug. 2012 (LE254337); Altaj Republic, Altajsky Nature Reserve, Atkichu, on rotten stumps in flood plain forest, 18 Aug. 2008, E. Malysheva (LE262994); Novosibirsk, Akademgorodok, on rotten birch stump near the ICG SB RAS mosel animal breeding facility, 15 June 2008, T. Bulyonkova (LE254348); ibid., on rotten pine stump in mixed forest (Pinus sylvestris, Acer negundo, Betula pendula) near ICG SB RAS module, 12 Aug. 2011, T. Bulyonkova (LE254326); ibid., on rotten mossy stump in young birch grove bordering mixed forest, with old rotting stumps, 14 July 2010, T. Bulyonkova (LE254328). – SPAIN, Canary Islands, La Palma, in a pot with an orchid, 31 Aug. 1994, R.M. Dähncke (855, L, holotype).
Notes — This species has originally been described from the island of La Palma (Islas Canarias, Spain), where it was found on chips of Pinus bark in pot with Cymbidium in a garden. After that it was collected several times in different types of natural habitats in Europe, Caucasus, Western Siberia. These records fit the original description well (Wölfel & Noordeloos 2001, Noordeloos 2004), and molecular data confirm the con-specifity of the new records with the holotype. Entoloma chytrophilum can be recognized by the bright blue colour of the fruitbodies, plano-convex shape of the pileus and lignicolous habit on coniferous wood. From the other blue coloured species (E. coelestinum, E. dichroum, E. lepidissimum) it differs first of all by the rather large, thin-walled and nodulose spores. Entoloma lepidissimum var. pauciangulatum must be considered a synonym of E. chytrophilum due to morphological (almost nodulose spores and blue colour of both pileus and stipe (Gminder & Enderle 1996)) and phylogenetic evidence. Despite a later publication, the epithet ‘chytrophilum’ has priority over ‘pauciangulatum’ at the rank of species, because the name ‘pauciangulatum’ has priority only in the rank of variety in which it was published (Art. 11.2).
2. Entoloma euchroum (Pers.: Fr.) Donk, Bull. Bot. Gard. Buitenzorg, ser. III, 18: 157. 1949. — Fig. 5c–f, 7
Fig. 7.
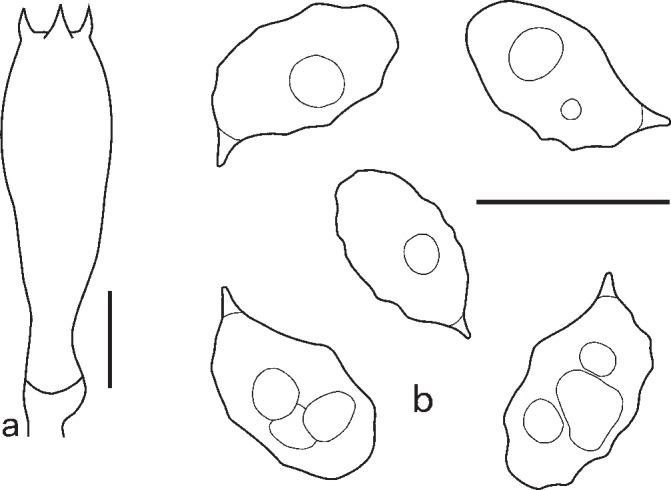
Entoloma euchroum. a. Spores; b. basidium; c. cheilocystidia (KR 0032474, neotype). — Scale bars = 10 μm.
Syn.: Agaricus euchrous Pers., Syn. Meth. Fung. (Göttingen) 2: 343. 1801; Agaricus euchrous Pers.: Fr., Syst. Mycol. 1: 203. 1821; Leptonia euchroa (Pers.: Fr.) P. Kumm., Führer Pilzk. (Zwickau): 24, 96. 1871; Rhodophyllus euchrous (Pers.: Fr.) Quél., Enchir. Fung.: 60. 1886; Hyporrhodius euchrous (Pers.: Fr.) J. Schröt. in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.1 (33–40): 615. 1889.
Neotype (designated here). GERMANY, Baden-Württemberg, Schwäbisch-Fränkischer Wald, Ks. Ostalbkreis, Durlangen, W Täferrot, Rottal, on Alnus sp., 25 Aug. 2005, L. Krieglsteiner (KR-M-0032474).
Pileus 5–40 mm broad, broadly conical, hemisphaerical or campanulate, then convex, often with central depression, not hygrophanous, not translucently striate, radially fibrillose to squamulose all over, violaceous blue, discolouring dark or pale brown with violaceous or purplish tint. Lamellae adnate-emarginate, to adnexed often with decurrent tooth, violaceous blue, dark brownish violet, or sometimes discolouring to pale blue, with irregular to serrulate brownish, violaceous or bluish edge. Stipe 20–70 × 1–6 mm, cylindrical, slightly broadened to base or distinctly bulbose, solid, longitudinally fibrillose, squamulose in the upper part, violaceous blue, then brownish blue with violaceous or purplish tint, base whitely tomentose. Context concolorous with surface or paler. Smell sweet, aromatic like violet flowers, or indistinct, taste unpleasant. Spores 9.0–11.5 × 6.0–8.0 μm, Q = 1.2–1.7, heterodiametrical with 6–8 rather blunt angles in side view. Basidia 45.8–48.3 × 10.1–12.8 μm, 4-spored, clavate, clamped. Lamellae edge heterogeneous. Cheilocystidia cylindrical, narrowly clavate or lageniform, sometimes septate, often thick walled in the upper part, hyaline or with violaceous brown content. Pileipellis a trichoderm in the centre, plagiotrichoderm towards the pileus margin, made up of cylindrical hyphae with inflated terminal elements, 8–15 μm wide with blue-violaceous or violaceous brown intracellular pigment. Caulocystidia present as septate clamped hairs up to 300 μm, colourless or with blue-violaceous intracellular pigment. Hyphae of the trama of both the pileus and the stipe with incrusted pigment. Clamp-connections abundant.
Habitat — On dead and living deciduous trees (mostly Alnus but also Quercus, Sorbus, Corylus, Acer, Prunus), exceptionally on coniferous tree (JVG 1091115A; Noordeloos 1982a).
Known distribution — Western and Eastern Europe, Canary Islands, Caucasus, Western Siberia, Russian Far East.
Additional specimens examined. GERMANY, Brandenburg, Ks. Uckermark, Gartz, NSG Fauler Ort, on Alnus sp., 22 Sept. 1990, M. Scholler (KR-M-0033332); Bayern, Rhön, Gefäll, Seebachtal, Buntsandstein, 450 m, MTB/Q 5625/4, Carici remotae-Fraxinetum, on Alnus sp., 11 Oct. 2003, L. Krieglsteiner (KR-M-0005673); Baden-Württemberg, Lorch, N Wachthaus, Haselbachtal, Welzheimer Wald, on Quercus robur, 6 Sept. 2007, L. Krieglsteiner (KR-M-0032221). – NETHERLANDS, Bloemendaal, Koningshof, 2 Nov. 1974, C. Bas (L 6502, as E. tjallingiorum). – RUSSIA, Leningrad Region, Kingisepp District, Kotelsky Sanctuary, Sept. 2005, E. Popov (LE254334); Novgorod Region, vicinities of the Opechensky Pasad, Gornaya Msta, on the Alnus glutinosa stump, Yu. Rebriev (LE253879); Moscow Region, Taldom District, Bolshoye Strashevo, 27 Sept. 2010, C. Lukashin (LE254333); Ryazan Region, Oksky Nature Reserve, on Alnus glutinosa, 2006, E. Malysheva (LE254332); Stavropol Territory, on Acer platanoides (?) stump, 25 Aug. 2009, I. Ukhanova (LE254331); Karachaevo-Cherkesia Republic, Teberdinsky Nature Reserve, valley of Teberda River, on the base of Alnus glutinosa, 10 Aug. 2009, E. Popov (LE262995); Tomsk, in the planting of birch and pine, on Prunus padus stump, 2 Oct. 2007, N. Agafonova (LE254329). – SPAIN, Saga, Cerdanya, Girona, alt. 1050 m, on Alnus glutinosa decaying wood, 27 Aug. 2002, J. Vila (JVG 1020827-2); Canary Islands, La Malgarida, La Palma, alt. 1080 m, on decaying wood of Pinus canariensis, 15 Nov. 2009, D. Chávez, V. Escobio, J.F. López, J.I. Velaz, J.L. Lantigua and M.Á. Ribes (JVG 1091115A).
Notes — Entoloma euchroum is a very distinctive species distributed all over Europe and reported also from Siberia. Usually it is easy to recognize due to its entirely blue-violaceous basidiomes, lignicolous habitat and sweet, flowerlike smell. Sometimes it can be rather variable in colour, depending on the growing conditions, but violaceus blue tinges are always present in all parts of the basidioma, especially in the lamellae. It can be distinguished from the other taxa that can possess more or less violaceous-blue lamellae (E. callichroum var. venustum, E. lepidissimum) by the presence of cheilocystidia with a thick-walled upper part, often filled with violaceous brown content cheilocystidia. Cheilocystidia are absent in E. callichroum var. venustum, rare, and intermixed with basidia in E. lepidissimum. Also the squamulose stem and lignicolous habitat help to distinguish E. euchroum.
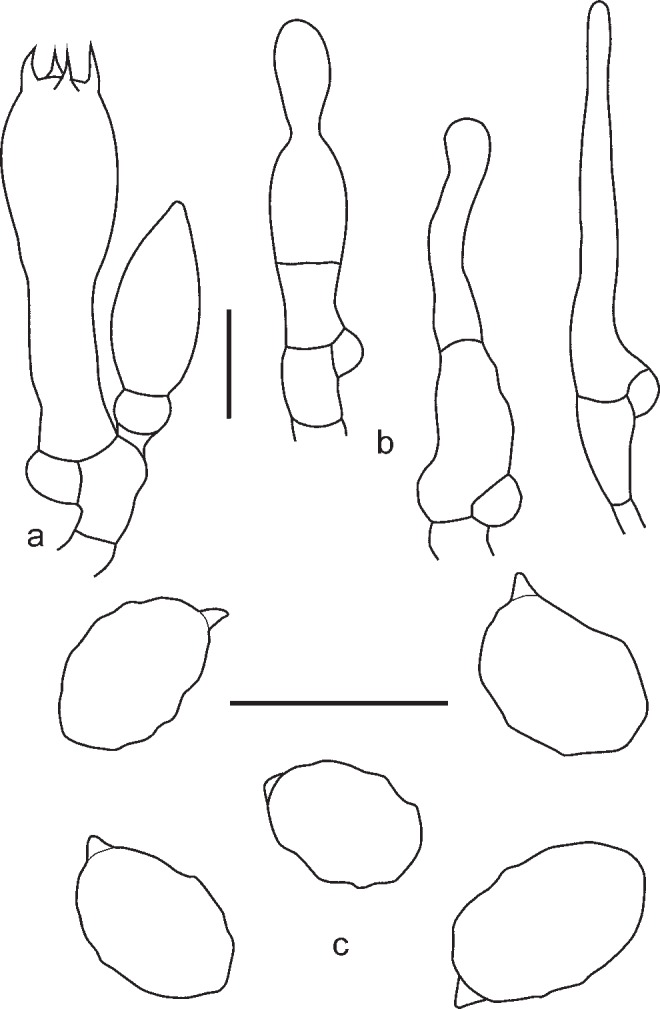
3. Entoloma lampropus (Fr.: Fr.) Hesler, Beih. Nova Hedwigia 23: 154. 1967. — Fig. 8, 9a, b
Fig. 8.
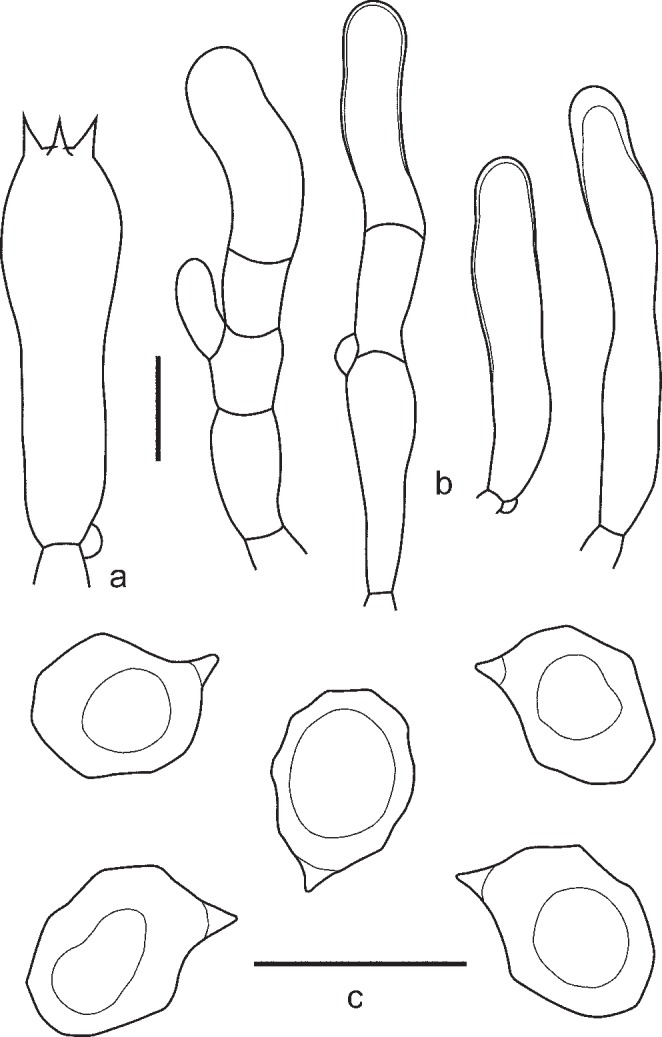
Entoloma lampropus. a. Basidium; b. cheilocystidia; c. spores (UPS:BOT:F-176490, neotype). — Scale bars = 10 μm.
Fig. 9.
a, b: Entoloma lampropus. a. RM0855; b. LE254315. — c. E. placidum. JVG 1060830-6. — d. E. sublaevisporum LIP JVG 1070823T, holotype. — Scale bars = 1 cm. — Photos by: a. S. Català; b. V. Kapitonov; c, d. J. Vila.
Syn.: Agaricus lampropus Fr., Observ. Mycol. 1: 19. 1815; Agaricus lampropus Fr.: Fr., Syst. Mycol. 1: 203. 1821; Leptonia lampropus (Fr.: Fr.) Quél., Mém. Soc. Émul. Montbéliard, sér. 2, 5: 121. 1872; Rhodophyllus lampropus (Fr.: Fr.) Quél., Enchir. Fung.: 60. 1886.
Excl.: Rhodophyllus lampropus sensu J.E. Lange, Fl. Agar. Dan. 2, pl. 76C. 1937 [=? E. corvinum (Kühner) Noordel.]; Leptonia lampropus sensu Bres., Iconogr. Mycol. XII, pl. 570-1. 1929; P.D. Orton, Trans. Brit. Mycol. Soc. 43, Suppl.: 105. 1960; Entoloma lampropus sensu Hesler, Beih. Nova Hedwigia 23: 154. 1967 [= E. sodale Kühner & Romagn. ex Noordel.].
Neotype (designated here): SWEDEN, Medelpad, Liden, Sundsjöåsen, T. Læssøe (as E. placidum), 31 Aug. 1999 (UPS:BOT:F-176490).
Pileus 10–40 mm broad, conical or hemispherical expanding to plano-convex with incurved margin and usually slightly depressed centre, not hygrophanous, not translucently striate, entirely radially fibrillose to squamulose at centre, with small dark grey-brown squamules on paler background, most dense at centre, varying from rather pale beige-grey to moderately dark grey-brown, often darker, almost black in centre, sometimes with bluish or lilac tint especially near pileus margin but not very often. Lamellae moderately distant, narrowly adnate, emarginate or adnate with small decurrent tooth, sometimes arcuate, whitish to cream becoming greyish or brownish pink, with irregular concolorous edge. Stipe 25–65 × 1–3.5 mm, cylindrical or slightly broadened towards base, often twisted, steel blue, brownish or greyish blue, distinctly longitudinally fibrillose-striate with dark blue fibrils, usually glabrous but sometimes flocculose with white or bluish floccules in upper part, base with white tomentum. Flesh whitish, blue beneath the stipe surface. Smell not distinctive, taste mild or slightly bitter. Spores 8.8–11.5 × 6.0–7.5 μm, Q = 1.3–1.7, heterodiametrical, with 6–9 rather blunt angles in side view. Basidia 28.6–35.4 × 9.3–10.5 μm, 4-spored, narrowly clavate, clamped. Lamellae edge fertile or heterogeneous. True cheilocystidia absent, but terminal elements of tramal hyphae often well developed, 29.0–60.0 × 6.0–13.0 μm, cylindrical, often septate, colourless, clamped. Pileipellis trichoderm at centre, a plagiotrichoderm towards margin, composed of cylindrical to slightly inflated hyphae 15–25 μm wide, with both pale intracellular and incrusting pigment and abundant clamp-connections.
Habitat — On dead wood of coniferous trees or on soil in forests and open places, including grasslands.
Known distribution — Northern, Western and Eastern Europe, Caucasus, Western Siberia, Russian Far East.
Additional specimens examined. AUSTRIA, Frankenberg, Ried, Hinterzeining, 22 Sept 1994, F. Sucti (WU 13198, as E. dichroum); Rastenfeld, NW Dobra, on wood, 9 Sept. 2009, A. Hausknecht (WU 24148, as E. dichroum); Burgenland, Oberpullendorf, Tschnurdorf, Seltzabachtal, Pflanzen with Salix and Alnus, 12 Oct. 1991, W. Klofoc & A. Hausknecht (WU 10092, as E. dichroum) (WU 10092, as E. dichroum). – GERMANY, Nettetal, 3 Oct. 2009, G. Wölfel (1509, L). – RUSSIA, Murmansk Region, Khibiny, Botanical Garden, on soil in Picea-Betula forest, 4 Sept. 1974, L. Mikhailovsky (LE9121, as Leptonia placida); Vologda Region, Kirillov District, Kovarzino, N59°44’28" E038°23’33", on soil in Picea abies forest, 26 Aug. 2005, O. Kirillova (LE235263); Bryansk Region, Syzemka District, ‘Bryansky Les’ Nature Reserve, Chykhrai Village, on the base of Fraxinus exelcior trunk in broad-leaved forest, 24 Oct. 2012, A. Fedosova (LE254339); Tatarstan Republic, Zelenodolsk Region, on decaying wood in Pinus sylvestris-Tilia cordata forest, 27 Sept. 2010, K. Potapov (LE262991); Udmurtia Republic, vicinities of Izhevsk, on rotten Picea stump, 19 July 2009, V. Kapitonov (LE254315); ibid., on rotten Picea stump in mixed forest, 19 July 2009, V. Kapitonov (LE254338); Karachaevo-Cherkesia Republic, Teberda Biosphere Reserve, Arkhyz, on soil in Abies nordmanniana-Fagus orientalis forest, 19 Aug. 2009, O. Morozova (LE254316); Sverdlovsk Region, Visimsky Natura Reserve, Lipovy Sutuk Mt, N57°24’18" E059°43’34", on burnt soil, L. Marina, 3 Sept. 1999 (LE258111); Orenburg Region, Tyulgan District, Tashla Village, on soil in broad-leaved forest, 13 July 2007, O. Desyatova, (LE253584, as E. tjallingiorum); Novosibirsk, Akademgorodok, mixed forest (Pinus sylvestris, Acer negundo, Betula pendula) near ICG SB RAS module, 11 Aug. 2011, T. Bulyonkova (LE262992); Tomsk Region, near airport, on Larix stump, 11 Aug. 2006, N. Agafonova (LE262985); Kamchatka Region, vicinities of Esso, on decaying wood, 8 Aug. 2005, O. Morozova (LE254349). – SPAIN, València, La Puigmola, alt. 350 m, on Corylus avellana, among mosses, 16 Oct. 2011, S. Català (RM0855).
Notes — Entoloma lampropus is a rather confusing species. It was described by Fries as a species with “pileo subcarnoso convexo cinereo-griseo fibrilloso, lamellis albidis denticulo-adnatis, stipite nitido coeruleo fistuloso” (Fries 1815). In the sanctioning work it was characterized by “pileo demum umbilicate fibrilloso griseo, lamellis adnatis albido-griseis, stipite fistuloso coeruleo” (Fries 1821). The brevity of the description led to various interpretations of the species. In the concept of Lange (1937, pl. 76C), Bresadola (1929, pl. 570-1), Hesler (1967) it represents a species with a smooth stipe without clamp connections, and is considered a member of subg. Cyanula. The present work follows the interpretation of Kühner & Romagnesi (1953), which was based on a key phrase in the Fries’s description “primo obtutu simillimus priori (Agaricus placidus)”, stressing the resemblance to E. placidum. This interpretation was adopted by Noordeloos (1982a, 1992). The misapplied interpretation of Agaricus lampropus mentioned above, was described as a new species, Rhodophyllus sodalis Kühner & Romagn., and later on accepted as E. sodale Kühner & Romagn. ex Noordel. Noordeloos (1982a) published a modern description of E. lampropus, but nevertheless, due to the limited number of records the species continued to be insufficiently known and lot of misidentifications were still encountered in several herbaria during our study. The present study expands the concept of E. lampropus with more morphological, ecological and molecular data, and its phylogenetic position is now known, and fixed with a neotype.
Entoloma lampropus belongs to the group of species characterized by the many-angled nodulose spores and the presence of ‘cheilocystidia’ in form of hyphal elements, often septate, arising from the hymenophoral trama. The predominantly grey brown pileus sometimes has a slight lilac tinge near the margin, and the stipe is blue and longitudinally fibrillose, without squamules (contrary to E. tjallingiorum). It grows both terrestrial and on rotten wood (mainly coniferous).
4. Entoloma placidum (Fr.: Fr.) Noordel., Persoonia 11, 2: 150. 1981. — Fig. 9c, 10
Fig. 10.
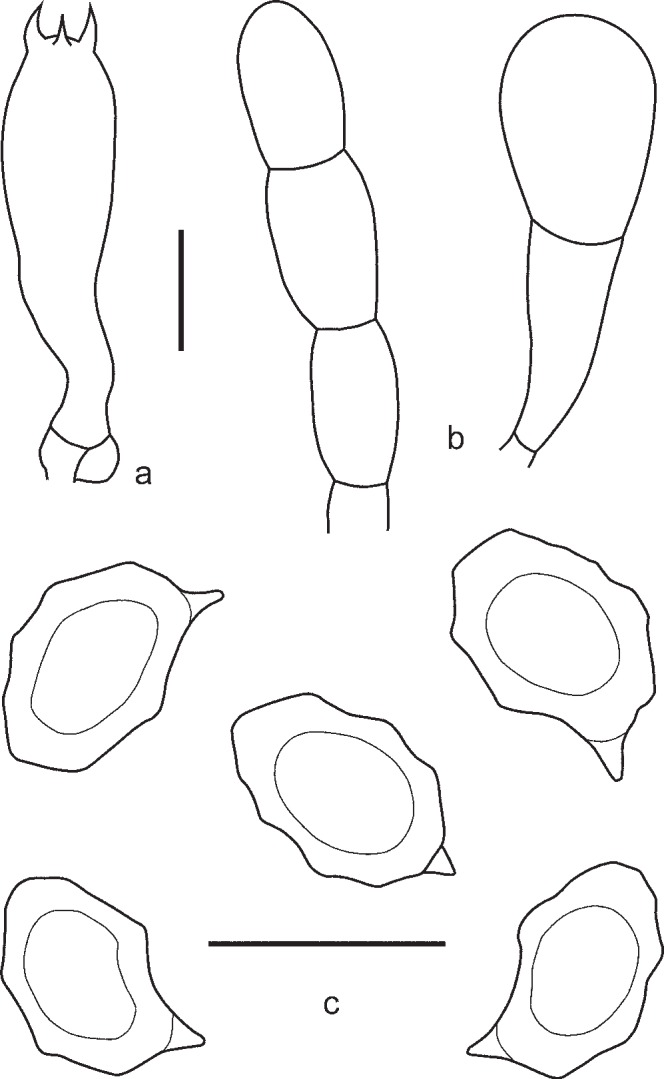
Entoloma placidum. a. Basidium; b. spores (UPS:BOT:F-121714, epitype). — Scale bars = 10 μm.
Syn.: Agaricus placidus Fr., Observ. Mycol. 2: 94. 1818; Agaricus placidus Fr.: Fr., Syst. Mycol. 1: 202. 1821; Leptonia placida (Fr.: Fr.) P. Kumm., Führer Pilzk.: 96. 1871; Rhodophyllus lampropus (Fr.: Fr.) Quél., Enchir. Fung.: 60. 1886; Entoloma placidum (Fr.) Zerova, in Zerov et al., Viznachnik Ukraĭnĭ 5 Basidiomycetes: 104. 1979 [nom. inval., Art. 33.2].
Epitype (designated here): SWEDEN, Småland, Femsjö, Hägnen, NW part, on beech stump, 10 Sept. 1948, S. Lundell (5276) and G. Haglund (UPS: BOT:F-121714, as Leptonia placida).
Pileus 10–30 mm broad, conical to convex with straight margin and slightly depressed or umbonate centre, not hygrophanous, not translucently striate, entirely radially fibrillose to minutely squamulose, especially in the centre, with small dark grey-brown squamules on greyish background. Lamellae moderately distant, adnate or emarginate, with small decurrent tooth, whitish to cream becoming pink, with concolorous edge. Stipe 25–65 × 1–3 mm, cylindrical or slightly broadened towards base, distinctly longitudinally fibrillose-striate with whitish or pale blue fibrils on deep blue background, pruinose in upper part, base with white tomentum. Context whitish, blue beneath the stipe surface. Smell and taste farinaceous. Spores 8.0–11.0(–11.5) × 6.0–7.0(–7.5) μm, Q = 1.2–1.6, heterodiametrical, with 6–8 blunt angles in side view. Basidia 27.0–33.0 × 8.8–11.5 μm, 4-spored, narrowly clavate, clamped. Lamellae edge fertile or heterogeneous. Scattered cystidia-like elements sometimes present in the edge of the lamellae as vacuolised basidioles or septate terminal cells of hyphae of the trama. Pileipellis a trichoderm in centre, plagiotrichoderm towards margin, composed of cylindrical to slightly inflated hyphae 10–25 μm wide, with intracellular pigment and abundant clamp-connections.
Habitat — On dead wood of deciduous trees, mostly Fagus, but also Corylus, Betula, etc.
Known distribution — Western and Eastern Europe, Caucasus.
Specimens examined. RUSSIA, Karachaevo-Cherkesia Republic, Teberda Biosphere Reserve, vicinities of Teberda, on beech trunk in Abies nordmanniana-Fagus orientalis forest, 7 Aug. 2009, O. Morozova (LE254335). – SPAIN, Lleida, Bòsc d’Aubàs, Bossòst, alt. 1090 m, on plant remnants, especially on small twigs of Corylus avellana and Betula pendula, 30 Aug. 2006, J. Carreras, J. Vila, F. Caballero, A. Duran & A. Mayoral (JVG 1060830-6); ibid., 2 Sept. 2006, J. Vila, F. Caballero & A. Mayoral (JVG 1060902-2); ibid., 26 Aug. 2008, F. Caballero (EFC 2682008-151). – SWEDEN, Småland (Inre), Femsjö, Hägnen, NW part, on beech stump, 30 Aug. 1949, S. Lundell (6020) and J. Stordal (UPS:BOT:F-121715, neotype).
Notes — Entoloma placidum is very similar to E. lampropus due to the grey-brown pileus, blue longitudinally fibrillose stipe without squamules, and more or less nodulose spores. True cheilocystidia are absent in both species, but in some specimens cystidia-like elements can be observed as vacuolised basidioles (Noordeloos 1982a, b) or septate terminal endings of the hyphae of the trama (Vila & Caballero 2007). Entoloma placidum can be recognized by the farinaceous smell, distinctly nodulose spores, and habitat on the wood of deciduous trees, especially Fagus. For a long time it was known as a species growing exclusively on beech wood. Recently some records were made on other deciduous trees (Corylus avellana, Betula pendula) (Vila & Caballero 2007). Molecular data confirm that they all belong to E. placidum. Entoloma lampropus grows on conifers or on soil. Entoloma tjallingiorum is also very similar but differs by the distinctly squamulose stipe and well differentiated cheilocystidia.
5. Entoloma sublaevisporum Vila, Noordel. & O.V. Morozova, sp. nov. — Mycobank MB803971; Fig. 9d, 11, 12
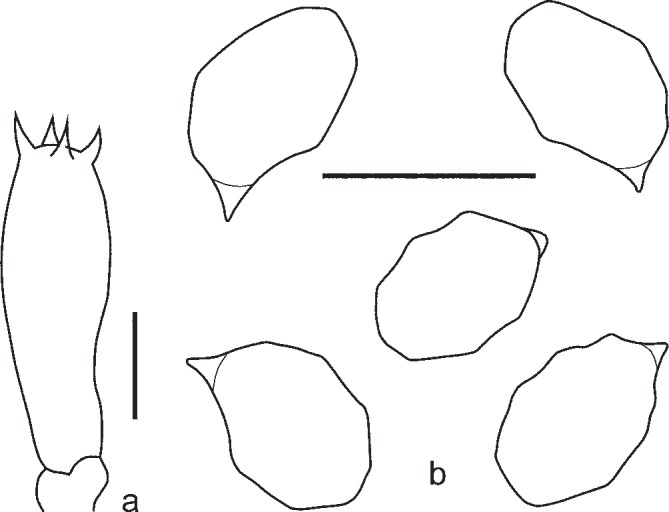
Fig. 11.
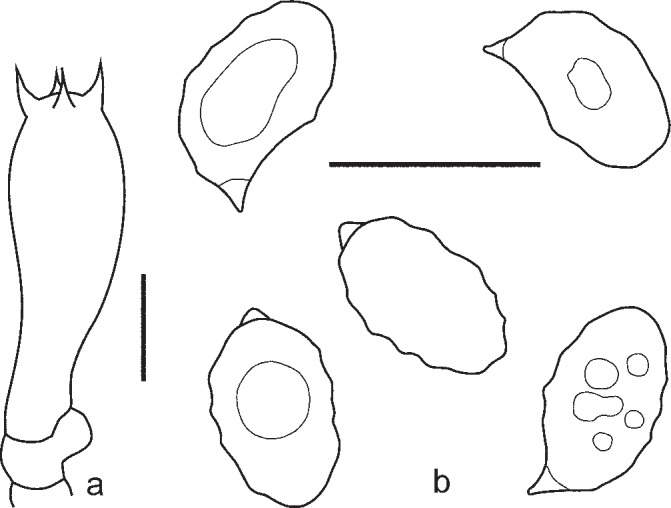
Entoloma sublaevisporum. a. Basidium; b. spores (LIP JVG 1070823T, holotype). — Scale bars = 10 μm.
Fig. 12.
Entoloma sublaevisporum. a. Basidium; b. cheilocystidia; c. spores (MEN 9858). — Scale bars = 10 μm.
Etymology. From latin ‘laevus’ (smooth), referring to the very weakly angled, subnodulose to almost smooth spores.
Holotype. SPAIN, Ripollès, Girona, Vall de Carlat, Setcases, alt. 1550 m, in acid soil, under Pinus uncinata, Sarothamnus sp. and Urtica sp., 23 Aug. 2007, J. Vila & F. Caballero (LIP JVG 1070823T).
Diagnosis. The species is characterized by the grey-brown pileus, bluish finely longitudinally striate stipe combined with the many-angled, nodulose spores.
Pileus up to 20 mm broad, flattened or slightly convex, with a shallow central depression; grey to pale grey-brown, with darker centre, without violaceous tinges or only a hint in central depression; not hygrophanous, not translucently striate, with fine to heavy fibrils, especially in the apex, where it is subsquamulose; margin straight to revolute, protruding above the lamellae. Lamellae moderately distant (L = 15–20) with abundant lamellulae (1 : 3 to 1 : 5), adnate to emarginate, thin, slightly ventricose or not; whitish, turning pale pinkish when spores mature; edge of the same colour, entire or somewhat irregular. Stipe central, up to 40 × 2 mm, cylindrical, straight or slightly curved; dark blue to bluish grey; surface smooth or with weak fibrils, finely longitudinally striate; pruinose at apex and with white basal tomentum. Flesh very thin, greyish on the pileus and grey-bluish grey on the stipe; smell fungoid. Spores 7.7–9.3 × 4.8–5.9 μm, average 8.5 × 5.3 μm, Q = 1.4–1.8, Qm = 1.6, heterodiametrical, with very weak angles, subnodulose to almost smooth. Basidia 27.6–32.8 × 9.7–11.7 μm, 4-spored, rarely 2-spored, narrowly clavate, clamped. Lamellae edge fertile or heterogeneous. Cheilocystidia absent in holotype, sometimes present, intermixed with basidia, 23.2–45.0 × 5.0–7.8, subcylindrical, flexuose to lageniform, sometimes with long tapering neck, frequently septate. Hymenial trama cyanophilous. Pileipellis a trichoderm structure composed of cylindrical hyphae, 20–28 μm wide, with fusiform terminal elements; pigment mixed, brownish vacuolar pigment abundant, also encrusting epiparietal present, especially on thin hyphae. Clamp-connections very abundant in hymenium structures, scarce on the hyphae of the pileipellis.
Habitat — On soil, in pine (Pinus uncinata) forest.
Known distribution — Western Europe (Austria, Spain (holotype)).
Additional material examined. AUSTRIA, Kärnten, Eisenkappel, Vellacher Kotscha, 7 Sept. 1998, A. Hausknecht (MEN 9858).
Notes — According to the phylogenetic analysis the new species is close to E. chytrophilum. Morphologically this similarity is confirmed by the spore morphology. Entoloma sublaevisporum differs from E. chytrophilum particularly by the greyish brown colour of the pileus and smaller spores. The many-angled, nodulose spores distinguish it from the other species with grey-brown pileus (E. lampropus, E. placidum, E. tjallingiorum). Cheilocystidia were found only in the specimen from Austria. The p-distance between E. chytrophilum and E. sublaevisporum is 7.4 %.
6. Entoloma tjallingiorum Noordel., Persoonia 11: 465. 1982
Syn.: Leptonia tjallingiorum (Noordel.) P.D. Orton, Mycologist 5, 3: 135. 1991.
Misapplied names: Agaricus dichrous sensu Fr., Summa Veg. Scand. 2: 287. 1849; Agaricus dichrous sensu Fr., Ic. Sel. Hymenomyc. 1, pl. 92. 1867; Entoloma dichroum sensu Bres., Iconogr. Mycol. 12, pl. 554. 1929; Entoloma dichroum sensu Konrad & Maubl., Icon. Sel. Fung. 2, pl. 190, f. 2. 1932; Rhodophyllus dichrous sensu J.E. Lange, Fl. Agar. Dan. 2, pl. 72A. 1937; Rhodophyllus dichrous sensu Romagn., Bull. Soc. Mycol. France 92: 292. 1976; Agaricus placidus sensu Fr., Ic. Sel. Hymenomyc. 1, pl. 97, f. 1. 1867.
a. var. tjallingiorum — Fig. 13a–c, 14
Fig. 13.
a–c: Entoloma tjallingiorum var. tjallingiorum. a. LE254317; b. JVG 1081116-5; c. LE227507. — d, e. E. tjallingiorum var. alnetorum LE254321. — f, g: E. tjallingiorum var. laricinum. f. LE254343, holotype; g. LE254344. — Scale bars = 1 cm. — Photos by: a. K. Potapov; b. J. Vila; c. A. Kovalenko; d, e, g. O. Morozova.
Fig. 14.
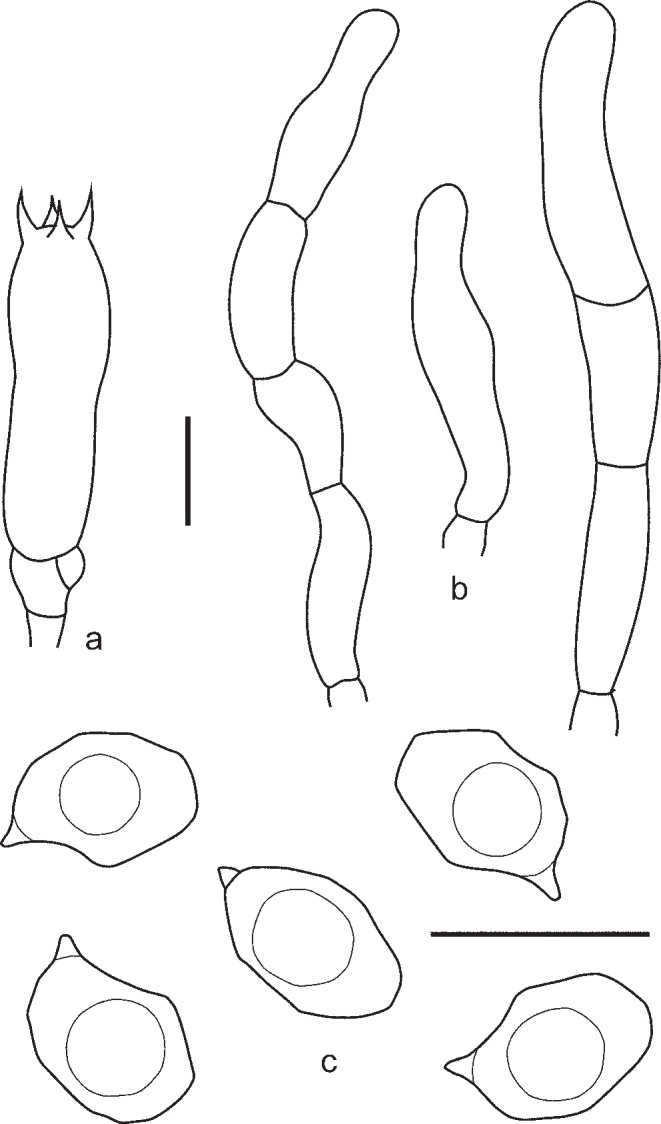
Entoloma tjallingiorum var. tjallingiorum. a. Basidium; b. cheilocystidia; c. spores (LE254318). — Scale bars = 10 μm.
Pileus 20–80 mm broad, hemispherical expanding to conico-convex or applanate, with low broad umbo, sometimes depressed, with involute then straight margin, not hygrophanous, not translucently striate, entirely radially fibrillose to squamulose in centre, light to moderately dark grey-brown, often with violaceous blue tinges near pileus margin. Lamellae adnate with small decurrent tooth or arcuate, whitish to cream in youth becoming pink, with irregular concolorous edge. Stipe 25–100 × 5–10 mm, cylindrical or broadened towards base, fibrillose, entirely squamulose with dark blue squamules, more intensively coloured in upper part, greyish blue or violaceous-blue below, base with white tomentum. Flesh whitish, blue beneath the stipe surface. Smell indistinct, taste mild or bitterish. Spores 8.0–11.0(–12.0) × 5.8–7.2(–7.6) μm, Q = 1.3–1.5, heterodiametrical, with 6–9 rather blunt angles in side view. Basidia 32.6–40.4 × 8.6–10.3 μm, 4-spored, narrowly clavate, clamped. Lamellae edge heterogeneous or, rarely, sterile. Cheilocystidia 29.7–71.1 × 5.9–8.7 μm, cylindrical, narrowly lageniform or irregularly shaped, sometimes septate, sometimes represented by strands of terminal elements of hyphae of the hymenial trama, colourless. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 7–20 μm wide, with both brown intracellular and incrusting pigment and abundant clamp-connections. Caulocystidia present as long, up to 250 μm, septate, clamped hairs, with cylindrical or lageniform terminal elements, 60–120 × 8–12 μm, colourless or with incrusting and blue intracellular pigment.
Habitat — On soil or on dead wood of mostly broad-leaved trees (Quercus spp.), but also on Betula and Alnus in deciduous and mixed forests.
Known distribution — Western and Eastern Europe, Western Siberia.
Specimens examined. RUSSIA, Leningrad Region, Sovkhozy, Sept. 1936, around trunks in Alnus forest, R. Singer (LE9123, as Leptonia placida); Novgorod Region, ‘Valdajsky’ National Park, Valdaj District, Poddub’e, 22 Sept. 2011, on soil in broad-leaved-coniferous (Picea abies) forest, O. Morozova (LE254318); Moscow Region, Taldom District, Bolshoye Strashevo, 27 Sept. 2010, E. Lukashina (LE254320); Tatarstan Republic, Kazan’, Sovetsky District, ‘Skotskiye Gory’ Park, on soil in broad-leaved forest (Tilia cordata, Quercus robur, Betula pendula), 29 Sept. 2011, K. Potapov (LE254317); Ulyanovsk Region, on soil in broad-leaved forest, 14 Aug. 2009, E. Ilyukhin (LE254319). – SPAIN, Barcelona, Can Romegosa, Sant Fost de Campsentelles, alt. 140 m, on trunk of Quercus pubescens, 5 Oct. 2008, F. Caballero (SFC 081005-01); ibid., F. Caballero (SFC 081019-01); ibid., 16 Nov. 2008, J. Vila & F. Caballero (JVG 1081116-5). – SWEDEN, Uppland, Bondkyrka, Predikstolen, on soil and rotten wood of Quercus, 4 Oct. 1980, S. Ryman (6124) (UPS:BOT:F-016378, holotype).
Additional specimens examined (grouped together with the E. alnetorum holotype). RUSSIA, Samara Region, Zhigulevsky Nature Reserve, vicinities of Bakhilova Polyana, Maloye Kamennoye Pole, Tilia cordata-Acer platanoides forest, 16 Aug. 2004, O. Morozova (LE227507); ibid., 17 Sept. 2004, E. Malysheva (LE234285); ibid., vicinities of Bakhilova Polyana, Tilia cordata-Acer platanoides forest, 1 Sept. 2004, E. Malysheva (LE227584, as Entoloma placidum).
Notes — Entoloma tjallingiorum belongs to the group of species characterized by the many-angled almost nodulose spores and septate terminal elements of the hymenophoral trama that protrude through the hymenium (‘cheilocystidia’). Among the species with greyish brown pileus and blue stipe it stands out by the stouter basidiocarps and distinctly squamulose stipe.
In an earlier paper (Noordeloos 1982a, 1992) some collections with a bluish tinge in the lamellae and pigmented cheilocystidia were also assigned to E. tjallingiorum. Molecular data show that these specimens are discolored forms of E. euchroum. The current concept of E. tjallingiorum therefore excludes forms with blue tinges in the lamellae.
As was shown by the phylogenetic analysis, the evolutionary process within this species continues at the present time: several lineages in the early stage of divergence were revealed. As a result, based on molecular and morphological differences, as well as geographical distribution, two more varieties of E. tjallingiorum can be distinguished. But the limits between these varieties are sometimes vague and intermediate forms have been found. So, the specimens from Zhiguli (LE227507, LE227584, LE234285) despite the genetic affinity with var. alnetorum are morphologically closer to the type variety. The specimen from Novgorod (LE254318) possesses in some lamellae rather large sites of sterile edge which are characteristic for var. laricinum.
b. var. alnetorum (Monthoux & Röllin) O.V. Morozova, Noordel. & Vila, comb. nov. — Mycobank MB803972; Fig. 13d, e, 15
Fig. 15.
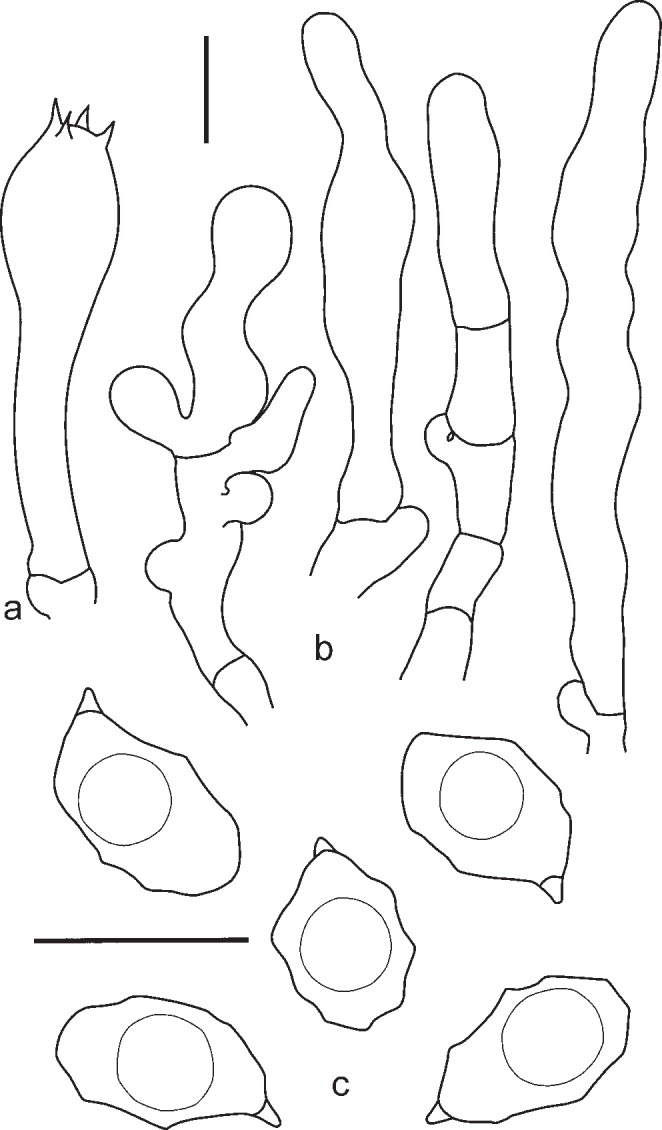
Entoloma tjallingiorum var. alnetorum. a. Basidium; b. cheilocystidia; c. spores (LE254321). — Scale bars = 10 μm.
Basionym. Entoloma alnetorum Monthoux & Röllin, Mycol. Helv. 3, 1: 43. 1988.
Pileus 20–40 mm broad, hemispherical expanding to plano-convex with incurved fimbriate margin, not hygrophanous, not translucently striate, entirely tomentose when young, becoming radially fibrillose to squamulose at centre, cream to pale ochraceous at first, then sordid ochre. Lamellae adnate with small decurrent tooth or arcuate, whitish to cream in youth becoming pink, with irregular concolorous edge. Stipe 25–80 × 5–10 mm, cylindrical, broadened towards base, fibrillose-striate, entirely squamulose with dark squamules, violaceous in upper part and whitish below, base with white tomentum. Flesh whitish, blue beneath the stipe surface. Smell fruitish, taste mild or slightly spermatic. Spores 8.1–11.3 × 5.5–7.2 μm, Q = 1.4–1.8(–1.9), heterodiametrical, with 6–9 rather blunt angles in side view. Basidia 37.0–39.6 × 11.7–13.0 μm, 2- or 4-spored, narrowly clavate, clamped. Lamellae edge sterile or heterogeneous. Cheilocystidia 39.8–69.1 × 6.6–15 μm, cylindrical, narrowly lageniform or irregularly shaped, sometimes septate, colourless. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 15–25 μm wide, with both pale intracellular and incrusting pigment and abundant clamp-connections. Caulo-cystidia present as long, up to 250 μm, septate clamped hairs, with lageniform, cylindrical or subcapitate terminal elements 30–90 × 6–12 μm, colourless or with blue or violaceous intracellular pigment.
Habitat — On dead wood of Alnus incana in deciduous forests in May–June, rarely also in July or August.
Known distribution — Western and Eastern Europe, Western Siberia.
Specimens examined. RUSSIA, Leningrad Region, Kirovsk District, Vasilkovo, left bank of Lava River, on dead trunk of Alnus incana in Alnus incana-Ulmus glabra forest, 11 June 2009, E. Popov (LE254321); Samara Region, Zhigulevsky Nature Reserve, vicinities of Bakhilova Polyana, on soil in Alnus glutinosa flood plain forest, 15 June 2004, E. Malysheva (LE234287); ibid., on soil in Acer platanoides-Tilia cordata forest, 18 Aug. 2004, E. Malysheva (LE227527); Tumen Region, Surgut district, Yugansky Nature Reserve, Kamenny, on soil in Pinus sibirica-Abies sibirica forest, 9 July 2006, E. Zvyagina (LE254322). – SWITZERLAND, Nant-Bride, Sixt, on old trunks and branches of Alnus incana in submontane Alnus forest, alt. ± 850 m, 28 June 1986, O. Röllin (G00111402, holotype); ibid., 29 May 1994, O. Röllin (L).
Notes — Entoloma alnetorum has been described as a species very similar to E. tjallingiorum due to the thin-walled, almost nodulose spores, however it is distinguished by the pale ochraceus pileus and the vernal appearance in Alnus forests (Monthoux & Röllin 1988). Phylogenetic analysis shows that all specimens possessing these features are grouped together. At the same time the difference between them and typical E. tjallingiorum is very small (p-distance between holotypes is 1.3 %). Some specimens (LE227507, LE227584, LE234285) with the typical habit of E. tjallingiorum occur in the E. alnetorum-clade with the difference only in 0.2 %. For this reason we decided to consider E. alnetorum as a variety of E. tjallingiorum. It is noteworthy that in E. dichroum also specimens with a pale pileus can be encountered (JVG 1070821-4). They can be separated from the species of the tjallingiorum-group by the characteristic spores with sharp angles.
c. var. laricinum O.V. Morozova, Noordel., Vila & E.S. Popov, var. nov. — Mycobank MB803973; Fig. 13f, g, 16
Fig. 16.
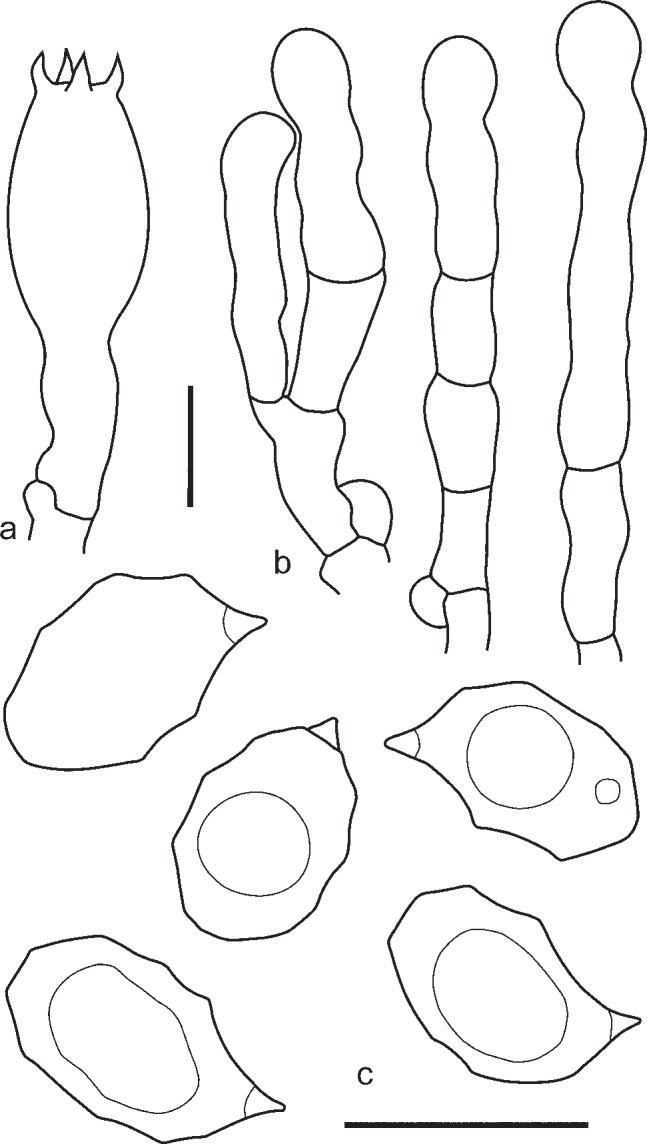
Entoloma tjallingiorum var. laricinum. a. Basidium; b. cheilocystidia; c. spores (LE254343, holotype). — Scale bars = 10 μm.
Etymology. The name refers to the substrate on which it has been found (Larix cajanderi).
Holotype. RUSSIA, Kamchatka Region, vicinities of Esso, on dead wood of Larix cajanderi, 8 Aug. 2005, E. Popov & O. Morozova (LE254343).
Diagnosis. Entoloma tjallingiorum var. laricinum differs from the type variety by the sterile lamellae edge with long septate ‘cheilocystidia’ (terminal elements of the hymenophoral trama) as well as by growing on Larix wood.
Pileus 20–60 mm broad, hemispherical expanding to conico-convex or applanate, with low broad umbo, with involute then straight margin, not hygrophanous, not translucently striate, entirely radially fibrillose with light grey-brown fibrils and squamules on whitish background, with violaceous blue tinge near pileus margin. Lamellae adnate with small decurrent tooth or arcuate, whitish to cream in youth becoming pink, with irregular concolorous edge. Stipe 25–80 × 5–15 mm, cylindrical or broadened towards base up to 20 mm, fibrillose, entirely squamulose with dark violaceous-blue or violaceous-grey squamules on paler, almost white background, base with white tomentum. Flesh whitish, blue beneath the stipe surface. Smell indistinct, taste mild. Spores 8.5–11.3 × 5.5–7.4 μm, Q = 1.4–1.7, heterodiametrical, with 6–9 rather blunt angles in side view. Basidia 35.4–42.2 × 10.3–13.1 μm, 4-spored, clavate, clamped. Lamellae edge sterile. Cheilocystidia 29.7–71.1 × 5.9–8.7 μm, cylindrical, flexuous, septate, represented by strands of terminal elements of hyphae of the hymenial trama, colourless. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 7–20 μm wide, with both brown intracellular and incrusting pigments and abundant clamp-connections. Caulocystidia present as long, up to 350 μm, septate clamped hairs, with lageniform or cylindrical terminal elements 50–120 × 6–12 μm, colourless or with incrusting and blue intracellular pigment.
Habitat — On rotten wood of Larix in mixed L. cajanderi-Betula ermanii forest.
Known distribution — Russian Far East.
Additional specimen examined. RUSSIA, Kamchatka Region, vicinities of Esso, on burnt wood of Larix cajanderi, 8 Aug. 2005, E. Popov & O. Morozova (LE254344).
Notes — Although E. tjallingiorum var. laricinum can be distinguished from the typical variety by the sterile lamella edge with abundant, septate terminal elements of the hymenophoral trama, this character is not reliable enough to make a clear-cut morphological distinction. Sometimes we find this character also in the typical variety. The main distinctive features are the habitat (growing on Larix cajanderi) and the geographic origin, the rather isolated Kamchatka Peninsula. This may explain the significant genetic divergence (p-distance 3.3 %).
Section Dichroi O.V. Morozova, Noordel. & Vila, sect. nov. — Mycobank MB803974
Type species. Entoloma dichroum (Pers.: Fr.) P. Kumm.
Habit collybioid or tricholomatoid; pileus conico-convex or plano-convex; lamellae almost free to emarginate; stipe flocculose to squamulose; lamellae edge sterile or heterogeneous; cheilocystidia present, spores with sharp angles, pileipellis more or less trichoderm of inflated elements with intracellular pigment; brilliant granules absent; clamp-connections present and often frequent; terrestrial, in forests.
7. Entoloma dichroum (Pers.: Fr.) P. Kumm., Führer Pilzk.: 97. 1871. — Fig. 17a–c, 18
Fig. 18.
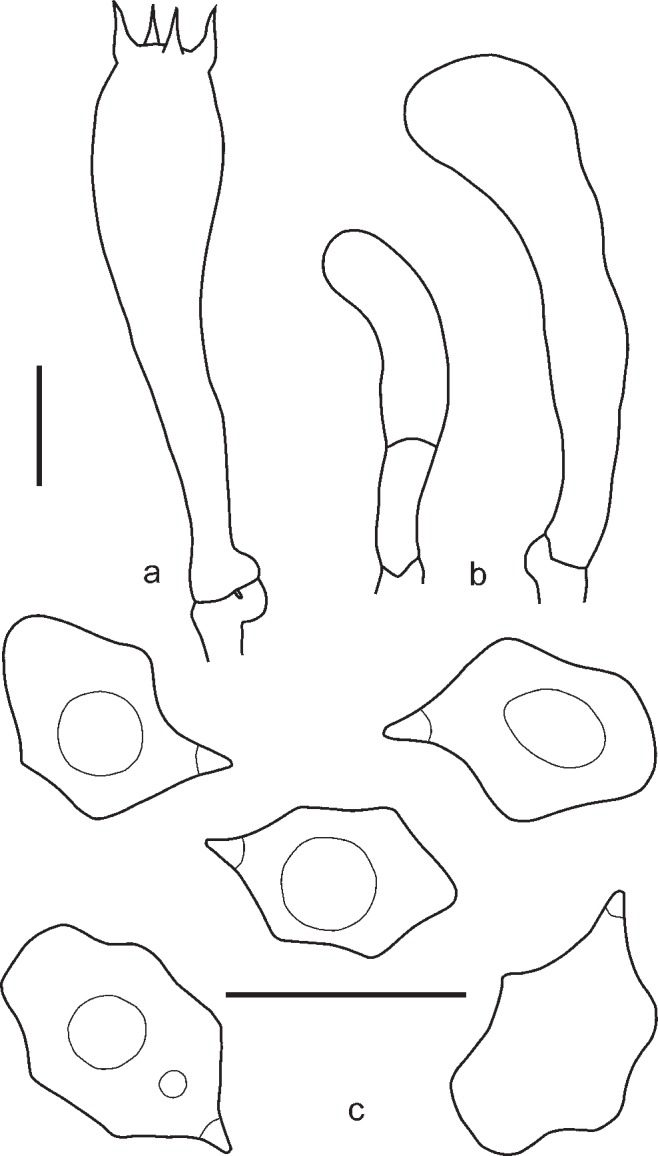
Entoloma dichroum. a. Basidium; b. cheilocystidia; c. spores (LE227472, neotype). — Scale bars = 10 μm.
Syn.: Agaricus dichrous Pers., Syn. Meth. Fung. (Göttingen) 2: 343. 1801; Rhodophyllus dichrous (Pers.: Fr.) Quél., Enchir. Fung. (Paris): 58. 1886; Leptonia dichroa (Pers.) P.D. Orton, Mycologist 5, 3: 132. 1991.
Excl.: Agaricus dichrous sensu Fries 1849, 1867; Entoloma dichroum sensu Bresadola 1929; sensu Konrad & Maublanc 1932; Rhodophyllus dichrous sensu Lange 1937; sensu Kühner & Romagnesi 1953 (= E. tjallingiorum).
Neotype (designated here). RUSSIA, Samara Region, Zhigulevsky Nature Reserve, vicinities of Bakhilova Polyana, Malaya Bakhilova Hill, broad-leaved forest, 26 Aug. 2003, E. Malysheva (LE227472).
Pileus 5–35 mm broad, conical or hemispherical, hardly expanding with age, with involute then straight margin, not hygrophanous, not translucently striate, dark violaceous-blue, purplish brown, or very pale, greyish with lilac tinge, entirely granular-fibrillose, becoming squamulose with violaceous-blue squamules on pale background. Lamellae adnate-emarginate with decurrent tooth, white then pinkish, with entire concolorous edge. Stipe 30–80 × 2–5 mm, clavate or cylindrical with slightly swollen base, deep blue, different from colour of pileus, longitudinally fibrillose, with dark blue squamules on the paler background, base with white tomentum. Flesh white, dark blue under the surface. Smell indistinct, taste unpleasant. Spores 9.2–11.5 × 6.4–7.7 μm, Q = 1.3–1.7, heterodiametrical, with 5–7 pronounced angles in side view. Basidia 44.2–51.4 × 8.5–10.5 μm, clavate, clamped. Lamellae edge heterogeneous. Cheilocystidia 20–45 × 5–11 μm, cylindrical, narrowly lageniform or irregularly shaped, sometimes septate, colourless, scattered among basidia. Pileipellis a trichoderm of cylindrical hyphae with terminal elements 35–120 × 15–35 μm with blue intracellular pigment and abundant clamp-connections. Caulocystidia present as long, up to 300 μm, septate clamped hairs, with tapering terminal elements, 50–110 × 8–12 μm, with dark blue intracellular pigment.
Habitat — On soil in deciduous forests.
Known distribution — Western and Eastern Europe.
Additional specimens examined. RUSSIA, Samara Region, Zhigulevsky Nature Reserve, vicinities of Bakhilova Polyana, Maloye Kamennoye Pole, Tilia cordata forest, 3 July 2005, E. Malysheva (LE234260). – SPAIN, Girona, Torrent Burgil, Pardines, alt. 1425 m, in acid soil, under Buxus sempervirens, 21 Aug. 2007, J. Vila & F. Caballero (JVG 1070821-4); València, La Puigmola, alt. 350 m, in basic soil, under Pinus halepensis, among mosses, 16 Oct. 2011, S. Català (herbarium SGC).
Notes — Entoloma dichroum together with E. eugenei forms a separate clade genetically characterized by the large insertion in the ITS1-region. Morphologically, E. dichroum can be recognized by the bright blue squamulose stipe and spores with 5–7 sharp angles. The pileus colour, however, varies considerably among the studied collections, from bright blue to violaceous-blue, violaceous-brown and pale brown. The ITS-sequences slightly vary, however this variability (p-distance 1.4–2 % base-pair difference) might well be acceptable within a species. More material would possibly allow for the distinction of varieties. Entoloma allochroum, another species with sharply-angled spores possesses a lilaceous or violaceous, less squamulose, more longitudinally fibrillose stipe. Entoloma dichroum differs from the closely related E. eugenei mainly by the slender collybioid habit, the heterogeneous lamellae edge, and slightly smaller and less pronouncedly angled spores.
8. Entoloma eugenei Noordel. & O.V. Morozova, Mycotaxon 112: 234. 2010. — Fig. 17d, 19
Fig. 19.
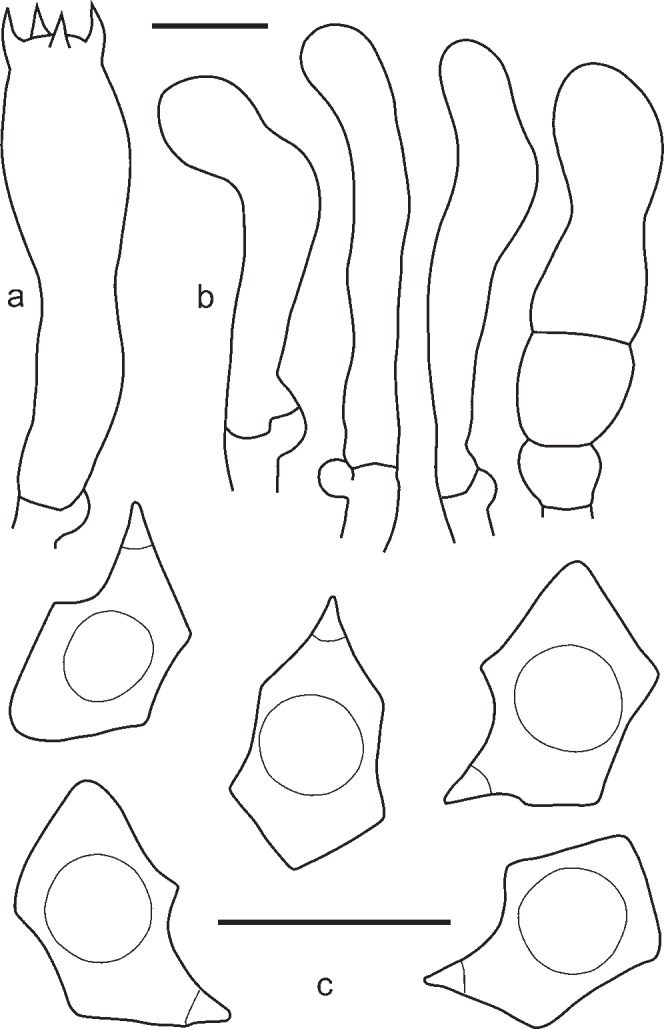
Entoloma eugenei. a. Basidium; b. cheilocystidia; c. spores (LE253771, holotype). — Scale bars = 10 μm.
Pileus 13–60 mm broad, hemispherical expanding to plano-convex with incurved margin, fleshy, not hygrophanous, not translucently striate, entirely velvety when young, becoming glabrous at the margin, uniformly deep blue (Indian blue) at first, then with violet tinge at margin. Lamellae adnate-emarginate with decurrent tooth, pure white then pinkish, with irregular concolorous edge. Stipe 30–80 × 4–8 mm, clavate or cylindrical with swollen base (to 15 mm), concolourous with the pileus or slightly paler, entirely squamulose with concolorous squamules, base white tomentouse. Flesh white, dark blue beneath the surface. Smell slightly spicy, taste mild. Spores 10.0–12.5 × 6.0–8.0 μm, Q = 1.3–1.7, heterodiametrical, with 5–7 angles in side view. Basidia 34–44 × 9–12 μm, clavate, clamped. Lamellae edge sterile. Cheilocystidia 28.0–39.0 × 6.5–15.5 μm, cylindrical, narrowly lageniform or irregularly shaped, colourless. Pileipellis a trichoderm of cylindrical hyphae with terminal elements 90–200 × 12–20 μm with blue intracellular pigment and abundant clamp-connections. Caulocystidia present as long, up to 250 μm, septate clamped hairs, with tapering or cylindrical terminal elements 50–100 × 8–12 μm, with dark blue intracellular pigment.
Habitat — On soil in the flood plain forest.
Known distribution — Russian Far East, Japan (GenBank AB509605, as Entoloma aff. kujuense).
Specimens examined. RUSSIA, Primorsky Territory, Kedrovaya Pad Nature Reserve, the right bank of the Kedrovaya River, N43°05’51" E131°33’34", 24 Aug. 2005, E. Popov (LE253771, holotype); ibid., 8 Sept. 1994, E. Bulach (VLA M-3556); ibid., Leopardovy Sanctuary, watershed of the rivers Gryaznaya and Ananjevka, on the base of dead trunk, 1 Sept. 2011, A. Kovalenko (LE254340); ibid., 1 Sept. 2011, T. Svetasheva (LE254347).
Notes — Entoloma eugenei is morphologically very close to E. dichroum. The main morphological difference is in its tri-cholomatoid habit, the sterile lamellae edge, and slightly larger and more pronouncedly angled spores. Genetically it differs from E. dichroum among other things in one rather large (about 40 base-pair) insertion in the ITS1-region. The significant divergence between these two species (p-distance 9.8 % base-pair difference) could be explained by geographical reasons – the natural isolated habitat of E. eugenei in the Southern Far East and Japan with unique climatic conditions (Noordeloos & Morozova 2010), while E. dichroum is known from Europe.
INCERTAE SEDIS
9. Entoloma allochroum Noordel., Persoonia 11, 4: 463. 1982. — Fig. 17e, f, 20
Fig. 20.
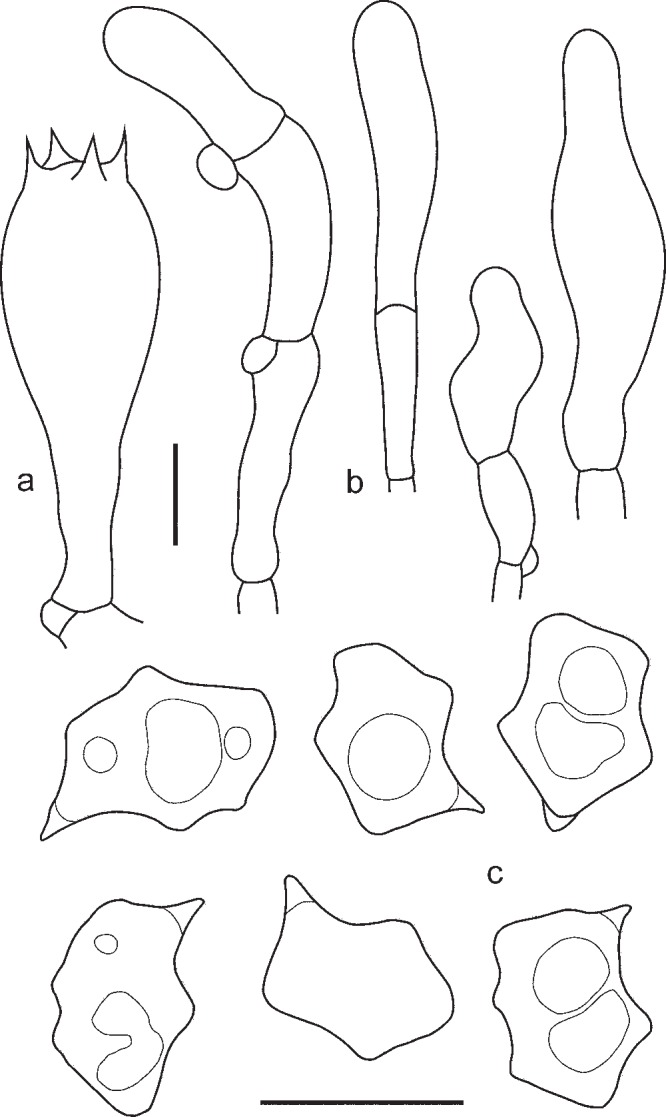
Entoloma allochroum. a. Basidium; b. cheilocystidia; c. spores (LE262984). — Scale bars = 10 μm.
Pileus 20–40 mm diam, conical, then convex with umbo, expanded to plano-convex, not or hardly hygrophanous, not translucently striate or at margin only, with straight margin, first with greyish brownish lilaceus tinged velvety covering later breaking up into brownish lilac squamules on pale background. Lamellae moderately crowded, adnate-emarginate, adnexed to almost free, ventricose, whitish, becoming pinkish, with entire to irregular concolorous edge. Stipe 40–70 × 3–6 mm, cylindrical, broadened towards the base, pale violaceous, entirely covered with darker fibrillose squamules, with white tomentum at base. Context greyish, darker under the surface, yellowish at the stem base. Smell agreeable or indistinct, taste indistinct. Spores 8.3–12.5 × 6.2–9.5 μm, Q = 1.3–1.6, heterodiametrical, with 5–8 rather pronounced angles. Basidia 32.6–44.2 × 11.9–15.7 μm, 4-spored, clavate, clamped. Lamellae edge heterogeneous. Cheilocystidia 32.4–57.4 × 5.5–15.7 μm, cylindrical to flexuose, septate, rare or absent. Pileipellis a trichoderm of cylindrical to inflated hyphae with terminal elements 15–40 μm wide with brownish violaceous intracellular pigment. Some hyphae of pileitrama incrusted. Clamp-connections present.
Habitat — On soil and plant remains in broad-leaved forests and parks.
Known distribution — Western Europe, Caucasus.
Specimens examined. AUSTRIA, Vellacher Kotscha, Eisenkappel, Karinthia, 7 Sept. 1998, K.F. Reinwald & A. Hausknecht (L9860). – GERMANY, Mühltal bei Willisau, im feuchten Eschen-haselnusswald zwischen Moosen und Schachtelhalmen auf feuchter, lehmiger Erde in der Nähe des Bachufers, 21 Sept. 1984, G. Wölfel (L: E4884, as E. dichroum (Noordeloos 2004: 1276, upper fig.). – NETHERLANDS, Aerdenhout, 29 July 1973, Kits van Waveren (holotype L); Apeldoorn, Vellertsdijk, 7 Aug. 1993, L. Bos (L, as E. tjallingiorum); Paterswolde, Vennebroek, 14 Aug. 2000, R. Chrispijn (L). – RUSSIA, Caucasus Biosphere Reserve, valley of the Pslukh River, on soil in broad-leaved forest with Fagus orientalis, Alnus glutinosa, Abies nordmanniana, 29 Aug. 2006, A. Kiyashko (LE262984); Karachaevo-Cherkesia Republik, Teberda Biosphere Reserve, Dombaj, on rotten stump in Abies nordmanniana-Fagus orientalis forest, 14 Aug. 2012, T. Svetasheva (LE254342); ibid., Teberda, on rotten stump in Fagus orientalis forest, 19 Aug. 2012, K. Potapov (LE254324). – SPAIN, Val d’Aran, Lleida, alt. 1090 m, on plant debris in forest of Alnus glutinosa, Populus sp., Fraxinus excelsior, 2 Sept. 2006, J. Vila, F. Caballero, A. Mayoral (JVG 1060902-1); ibid., 24 Aug. 2008, F. Caballero (EFC 2482008-148); Espinavell, Girona, alt. 1350 m, in acid soil, under Corylus avellana, 12 July 2008, F. Caballero (EFC 1272008-137). – SWITZERLAND, Schonau, Tunau, 20 Sept. 2009, G. Wölfel (L, E0809).
Notes — Entoloma allochroum is an easily recognizable species due to the presence of the lilaceous or violaceous colours both in the pileus and, especially, in the stipe, white lamellae, as well as rather thick-walled and pronouncedly angled spores. Due to the sharply-angled spores E. allochroum is similar to E. dichroum and E. eugenei, however the molecular evidence does not allow the placing of this species in sect. Dichroi (Fig. 2).
10. Entoloma callichroum E. Horak & Noordel., in Noordeloos, Cryptog. Mycol. 4, 1: 33. 1983
a. var. callichroum — Fig. 21
Fig. 21.
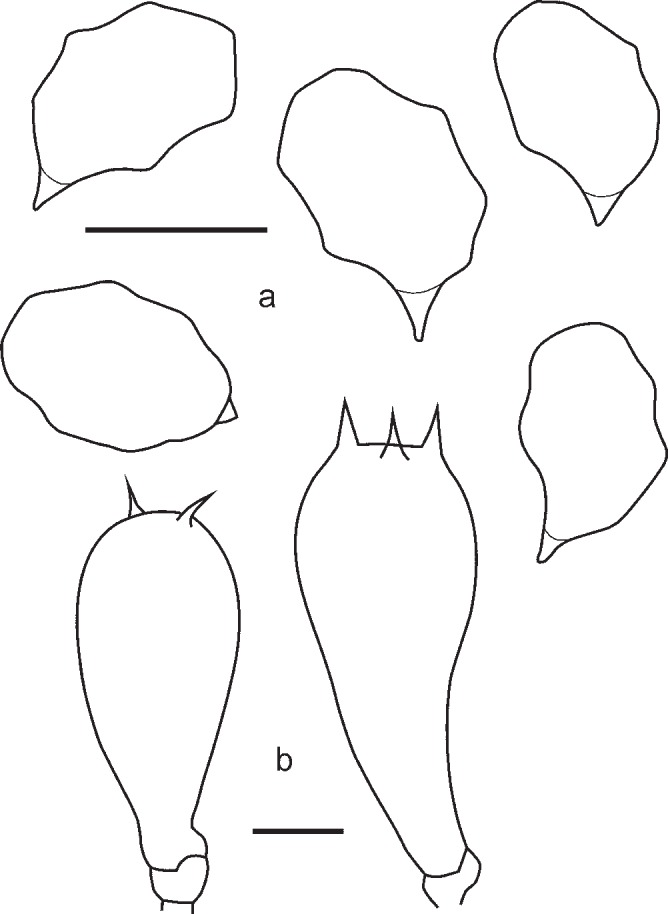
Entoloma callichroum var. callichroum. a. Spores; b. basidia (ZT 71/58, holotype). — Scale bars = 10 μm.
Pileus to 22 mm broad, convex with small papilla, not hygrophanous, not translucently striate, radially fibrillose, lilaceous-pink. Lamellae distant, emarginate, ventricose, whitish with lilac tint towards entire concolorous edge. Stipe 40 × 2 mm, cylindrical, steel-blue, base with white tomentum, fistulose. Smell and taste not reported. Spores 9.5–13.2 × 7.2–9.4 μm, Q = 1.4–1.6, heterodiametrical, with 6–9 blunt angles in side view, almost nodulose. Basidia 39.0–48.9 × 14.4–20.1 μm, 4-spored, broadly clavate, clamped. Lamellae edge fertile. Cheilocystidia absent. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 10–18 μm wide, with intracellular pigment. Clamp-connections present.
Habitat — On soil in Alnus incana forest.
Known distribution — Western Europe.
Specimen examined. SWITZERLAND, Graubunden, Forna, 3 Aug. 1971, E. Horak (ZT 71/58, holotype).
Notes — This rare species is characterized by pinkish violaceous tinge in the pileus in combination with steel blue stipe. The type variety is distinguished by the broad, almost nodulose spores, and the absence of cheilocystidia.
b. var. venustum (Wölfel & F. Hampe) O.V. Morozova, Noordel. & Vila, comb. nov. — Mycobank MB804535; Fig. 22, 23a, b
Fig. 22.
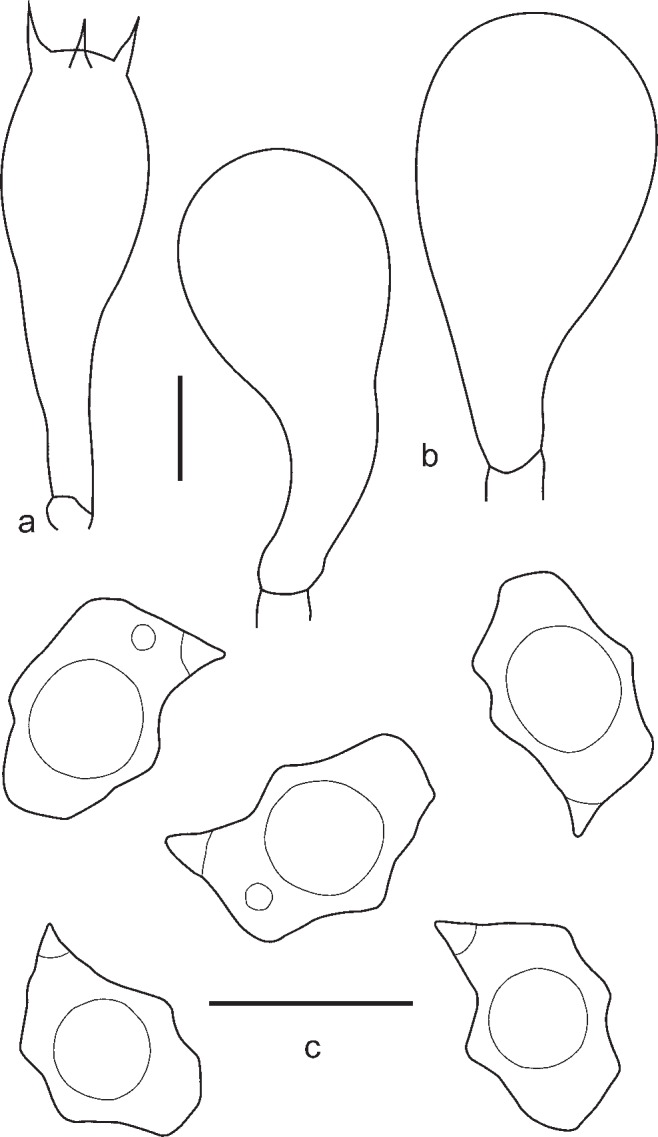
Entoloma callichroum var. venustum. a. Basidium; b. cheilocystidia; c. spores (Wö E17/10, holotype). — Scale bars = 10 μm.
Fig. 23.
a, b: Entoloma callichroum var. venustum. a. Wö E17/10, holotype; b. LE254312. — c. E. coelestinum LE258103. — d, e: E. percoelestinum. d. LE254390 (JVG 1130925-24), holotype; e. LE254341. — f, g: E. lepidissimum f. LE254871; g. LE234751. — Scale bars = 1 cm. — Photos by: a. F. Hampe (from Wölfel & Hampe 2011); b, f, g. O. Morozova; c. L. Marina; d. J. Vila; e. T. Bulyonkova
Basionym. Entoloma venustum Wölfel & F. Hampe, Z. Mykol. 77/2: 185. 2011.
Pileus 0.5–30 mm broad, convex to plano-convex, not hygrophanous, not translucently striate, radially silky fibrillose to squamulose in centre, pinkish lilaceous to violaceous, becoming brownish pink with age. Lamellae moderately distant, adnate-emarginate with small decurrent tooth, ventricose, brightly pinkish lilaceous, pinkish violaceus or whitish with blue tinge towards entire concolorous edge, becoming pink. Stipe 20–50 × 0.3–2.5 mm, cylindrical, longitudinally fibrillose-striate, dark blue, steel blue or violaceous-blue, base with white tomentum, fistulose. Smell of fruits or flowers (viola), taste unknown. Spores 11.5–13.0(–16.0) × 5.7–8.6 μm, Q = 1.3–1.8(–2.5), heterodiametrical, with 6–8 moderately pronounced angles in side view. Basidia 35.0–45.0 × 12.0–18.0 μm, 4-spored, broadly clavate, clamped. Lamellae edge heterogeneous. Cheilocystidia 30–60 × 15–28 μm, broadly clavate or sphaeropedunculate, intermixed with the basidia, sometimes hardly separated from the basidioles. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 10–20 μm wide, with intracellular pigment. Clamp-connections present.
Habitat — On soil in grasslands and in wet deciduous forest.
Known distribution — Western Europe, Western Siberia, Russian Far East.
Specimens examined. BELARUS, Vitebsk Region, Verkhnedvinsk District, vicinities of Rositsa Village, on soil in Alnus incana forest, 19 Aug. 2003, P. Kolmakov (LE226909, as E. lepidissimum). – GERMANY, Hannover-Nord, Kuglfanger, 13 Nov. 2010, G. Wölfel & F. Hampe (Wö E17/10, L, as E. venustum, holotype). – RUSSIA, Samara Region, Zhigulevsky Nature Reserve, Bakhilova Polyana, on soil in Betula pendula forest, 23 Aug. 2003, E. Malysheva (LE227532, as E. lepidissimum); Altaj Republic, Altajsky Nature Reserve, Komga, on soil in flood plain forest, 18 Aug. 2008, V. Malysheva (LE254312); Novosibirsk, Akademgorodok, on rotten birch stump, 15 June 2008, T. Bulyonkova (LE254313); ibid., planted forest between the Sobolev Institute of Mathematics and the Computing Center, 16 Aug. 2011, T. Bulyonkova (LE254314); Primorsky Territory, Ussuriysky Nature Reserve, 17 Sept. 1963, M. Nazarova (VLA M-20528, as Rhodophyllus lampropus).
Notes — The phylogenetic analysis shows that E. venustum is very close to E. callichroum (p-distance 1.8 % base-pair difference) and, therefore, could be considered its variety. Both species are morphologically distinct by the pinkish violaceous pileus; more or less lilaceous-blue tinges in the lamellae, the steel blue or violaceous-blue stipe, and the size of the spores. The description of E. venustum as a new species was based on the bright colour of the basidiomata and on the presence of well developed cheilocystidia, which, however, do not form a sterile gill edge and are often hardly distinguishable from basidioles (Wölfel & Hampe 2011). These characters can significantly vary within the range of genetically (nrITS) identical specimens. A more reliable feature for delimitation of these two taxa is spore form. Spores of E. venustum are narrower and possess more pronounced angles. Also the presence of a number of extremely long (up to 16 μm) germinating (?) spores has been reported from the holotype and other specimens (Table 3).
Table 3.
Comparison between Entoloma callichroum var. callichroum and E. callichroum var. venustum.
| Specimen | Spores size (μm) | Q | Spores form | Cheilocystidia | Lamellae colour |
|---|---|---|---|---|---|
| E. callichroum var. callichroum (holotype) | 10.0–13.2 × 7.0–9.4 | 1.4–1.6 | 5–9 angled, almost nodulose | absent, some cystidia like clavate cells present | whitish with lilac tinge towards edge |
| E. callichroum var. venustum (holotype) | 8.4–12.7(16.0) × 6.0–8.6 | 1.3–1.6(2.5) | with 6–8 moderately pronounced angles | broadly clavate or sphaero-pedunculate, intermixed with basidia, 45–60 × 15–28 μm | brightly pinkish lilaceous, pinkish violaceus |
| E. callichroum var. venustum (LE254313) | 9.3–12.7 × 6.4–8.2 | 1.4–1.8 | with 6–8 moderately pronounced angles | rare, broadly clavate or sphaero-pedunculate, hardly distinguishable from the basidioles, 30.9–42.8 × 12.0–19.0 μm | whitish with lilac tinge towards edge |
| E. callichroum var. venustum (LE254312) | 9.5–13.0(14.0) × 5.7–7.2 | 1.4–1.8(2.0) | with 6–8 moderately pronounced angles | rare, broadly clavate or sphaero-pedunculate hardly distinguishable from the basidioles, 29.8–42.7 × 12.9–21.0 μm | whitish with bluish tinge towards edge |
11. Entoloma coelestinum (Fr.) Hesler, Beih. Nova Hedwigia 23: 111. 1967. — Fig. 23c, 24
Fig. 24.
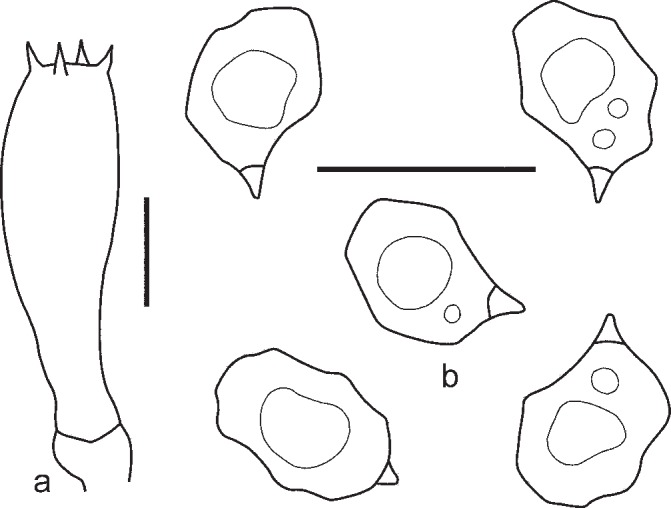
Entoloma coelestinum. a. Basidium; b. spores (LE258103). — Scale bars = 10 μm.
Syn.: Agaricus coelestinus Fr., Epicr. Syst. Mycol. (Upsaliae): 158. 1838 ‘1836–1838’; Nolanea coelestina (Fr.) Gillet, Mém. Soc. Émul. Montbéliard, sér. 2 5: 536. 1875; Rhodophyllus coelestinus (Fr.) Quél., Enchir. Fung. (Paris): 65. 1886; Leptonia coelestina (Fr.) P.D. Orton, Trans. Brit. Mycol. Soc. 43, 2: 177. 1960.
Pileus 7–10 mm broad, conical to hemispherical with umbo, not hygrophanous, not translucently striate, with straight margin, radially silky fibrillose, squamulose at centre, uniformly dark blue. Lamellae moderately distant, adnate-emarginate, ventricose, white, becoming pinkish, with entire concolorous edge. Stipe 20–50 × 1–2 mm, cylindrical, smooth or almost smooth, glabrous, concolourous with pileus, white tomentose at base. Smell and taste indistinct. Spores 6.9–8.3(–8.9) × 5.2–6.2 μm, Q = 1.3–1.5, heterodiametrical, 5–7-angled in side-view. Basidia 29.5–37.0 × 8.1–9.6 μm, 4-spored, clavate, clamped. Lamellae edge fertile. Cheilocystidia absent. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 10–20 μm wide with blue intracellular pigment. Clamp-connections present.
Habitat — On soil in broad-leaved forest.
Known distribution — Ural.
Specimens examined. RUSSIA, Sverdlovsk Region, Visimsky Nature Reserve, on soil in Acer platanoides-Fraxinus excelsior forest, 21 Aug. 2004, L. Marina (LE258103).
Notes — Entoloma coelestinum is distinguished by the tiny, very dark blue to black basidiocarps with conical hardly expanded pileus combined with the small spores. In the course of the phylogenetic analysis specimens previously identified as E. coelestinum ended up in a well-supported clade, which, however, consists itself of two sister clades that can be distinguished morphologically. The larger clade is characterized by almost nodulose spores and a longitudinally fibrillose-striate stipe. It includes blue-coloured basidiomes and entirely black ones (Fig. 22e). The other clade consist of one collection characterized by more pronouncedly angled, not nodulose spores and a polished stipe. This collection fits well with the protologue, and the current concept of E. coelestinum (Noordeloos 2004). Considering these morphological differences, and the significant p-distance between these clades (5.3 % base-pair difference) it was decided to describe the first clade as the new species, E. percoelestinum below. Unfortunately we were unable to design a neotype for E. coelestinum since the limited material studied is not from the original geographic area. More material from Europe, especially from Sweden is needed to do so.
12. Entoloma percoelestinum O.V. Morozova, Noordel., Vila & Bulyonkova, sp. nov. — Mycobank MB803975; Fig. 23d, e, 25
Fig. 25.
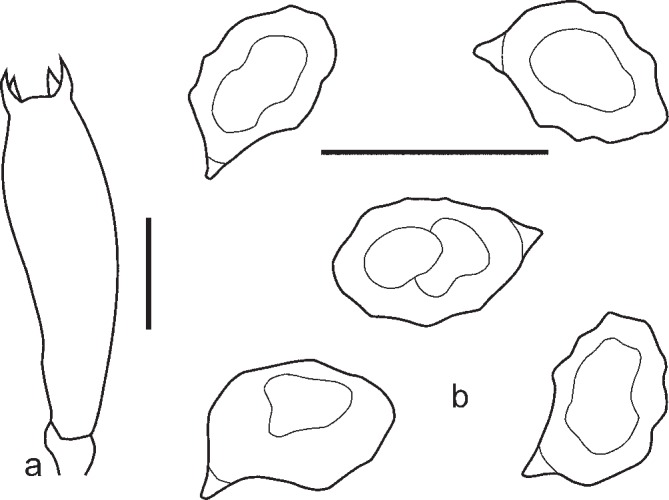
Entoloma percoelestinum. a. Basidium; b. spores (LE254390, holotype). — Scale bars = 10 μm.
Etymology. Named after its similarity to E. coelestinum.
Diagnosis. The new species is close to E. coelestinum from which it differs by almost nodulose spores and a longitudinally fibrillose-striate stipe.
Holotype. SPAIN, Osona, Barcelona, La Devesa, Rupit, alt. 1050 m, among grasses and mosses, near Quercus pubescens and Fagus sylvatica, on basic soil, 25 Sept. 2013, J. Vila & X. Llimona (LE254390), isotype in JVG 1130925-24.
Pileus 5–12 mm broad, conical or hemispherical with umbo, not hygrophanous, not translucently striate, with straight margin, radially fibrillose, squamulose at centre, uniformly dark blue, blackish blue or black. Lamellae moderately distant, adnate-emarginate, ventricose, white, becoming pinkish, with entire concolorous edge. Stipe 20–40 × 1–2 mm, cylindrical, longitudinally fibrillose-striate or almost smooth, concolourous with pileus, whitely tomentose at base. Context thin, concolorous with the surface. Smell indistinct or fungoid, taste not reported. Spores 6.5–8.5(–9.0) × 5.0–6.5 μm, Q = 1.3–1.5(–1.7), heterodiametrical, with 7–9 blunt angles in side-view, almost nodulose. Basidia 27.9–37.0(–45.4) × 8.1–9.6(–13.7) μm, 4-spored, narrowly clavate to subcylindrical, clamped. Lamellae edge fertile. Cheilocystidia absent. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 10–20 μm wide with blue intracellular pigment. Clamp-connections present.
Habitat — On soil in broad-leaved, mixed and pine (Pinus sylvestris) forests.
Known distribution — Western and Eastern Europe, Western Siberia.
Additional specimens examined. RUSSIA, Penza Region, vicinities of Poperechnoye, on soil in Fraxinus excelsior forest, 7 Aug. 1990, A. Ivanov (LE18913, as E. lepidissimum); Novosibirsk, Akademgorodok, on soil in planted Pinus sylvestris forest SW of Lavrentieva 6/1, 12 Oct. 2011, T. Bulyonkova (LE254327); ibid., on soil in mixed forest, 20 Oct. 2010, N. Filippova (LE254341). – SPAIN, Barcelona, Mas Joan, Espinelves, alt. 730 m, on plant debris’s (Rhododendron sp., Sequoiadendron giganteum, Picea sp. and Abies alba), 11 Nov. 2006, J. Vila & F. Caballero (JVG1061111-7, as E. coelestinum (Vila & Caballero 2007)).
Notes — In the boreal-temperate Eurasia several species with small blue or blackish blue basidiomata are recognized. Entoloma percoelestinum differs from E. coelestinum by almost nodulose spores and a longitudinally fibrillose-striate stipe, from E. chytrophilum by the smaller spores and conical, hardly expanding pileus, and from E. lepidissimum by the smaller spores and lack of the blue tinge in young lamellae. Entoloma klofacianum is characterized by the isodiametrical spores. North American Leptonia subcoelestina is also close but it differs by the larger spores and by the pileipellis which lacks clamps and is composed of submoniliform cells.
13. Entoloma lepidissimum (Svrček) Noordel., Persoonia 11: 460. 1982. — Fig. 23f, g, 26
Fig. 26.
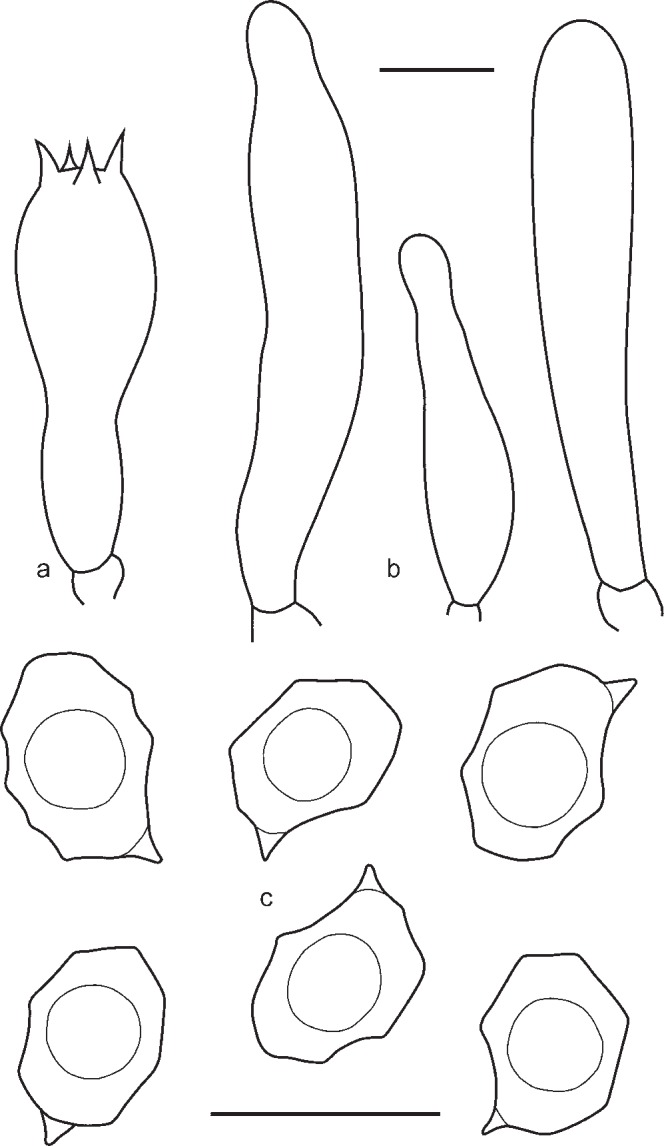
Entoloma lepidissimum. a. Basidium; b. cheilocystidia; c. spores (LE254871). — Scale bars = 10 μm.
Syn.: Leptonia lepidissima Svrček, Czech Mycol. 18: 205. 1964; Rhodophyllus lepidissimus (Svrček) M.M. Moser, Rohrlinge-Blatterpilze, 4 Aufl., 2, b/2: 203. 1978.
Pileus 5–25 mm broad, conical, broadly conical, hemispherical to convex with small umbo, not hygrophanous, not translucently striate, with straight margin, radially fibrillose to slightly squamulose at centre, deep blue to blackish blue, sometimes discolouring to greyish violet. Lamellae moderately distant, adnate-emarginate or almost free, ventricose, bluish, greyish blue or bluish violaceous, becoming greyish pink, with entire concolorous or paler edge. Stipe 20–60 × 1–3 mm, cylindrical, longitudinally fibrillose-striate or almost smooth, concolourous with pileus, white tomentose at base. Context concolorous with the surface. Smell indistinct, taste not reported. Spores 7.5–11.0 × 6.0–8.0 μm, Q = 1.3–1.6(–1.7), heterodiametrical, with 6–8 angles in side-view. Basidia 27.9–37.0(–45.4) × 8.1–9.6(–13.7) μm, 4-spored, narrowly clavate to subcylindrical, clamped. Lamellae edge fertile or heterogeneous. Cheilocystidia cylindrical, lageniform or narrowly clavate, intermixed with basidia, sometimes rare or absent. Pileipellis a plagiotrichoderm of cylindrical to slightly inflated hyphae 10–20 μm wide, with swollen terminal elements and blue intracellular pigment. Clamp-connections present.
Habitat — On soil in coniferous and deciduous forests.
Known distribution — Western, Central and Eastern Europe, Russian Far East.
Specimens examined. CZECH REPUBLIC, Bohemia merid., Vrabské near Cimelice, on fallen twigs of Alnus glutinosa in swamp Alnus forest, 20 Oct. 1963, M. Svrček (PRM755801, holotype, as Leptonia lepidissima). – RUSSIA, Novgorod Region, Valdajsky National Park, cost of Uzhyn Lake, on soil in Picea abies forest, 20 Aug. 2003, E. Popov (LE234755); ibid., vicinities of Sokolovo, Krasnaya Gorka, on soil in Quercus robur forest, 22 Aug. 2003, E. Popov (LE234751); ibid., valley of Poneretka River, on soil in Pinus sylvestris forest, 23 Sept. 2011, E. Popov (LE254871); Primorsky Territory, Sikhote-Alin Nature Reserve, Kabanuj, 25 Aug. 2011, E. Malysheva (LE254311).
Notes — Entoloma lepidissimum is recognized by the dark blue basidiomata with bluish lamellae. Microscopically the scattered cheilocystidia also can be distinctive. Despite the fact that the blue tinge of the lamellae was not mentioned in the protologue, all studied specimens are characterized by bluish lamellae. Molecular data support their identity with the holotype. The similar species E. coelestinum is distinguished by the white lamellae, smaller spores and more conical pileus. Entoloma chytrophilum possesses white lamellae, nodulose spores and a more applanate pileus.
Acknowledgments
We are very grateful to the following curators of herbaria for the loan of the specimens: Drs Ph. Clerc (G), M. Scholler (KR), D. Triebel (M), J. Holec (PRM), S. Ryman (UPS), E. Bulakh (VLA), W. Till (WU) and R. Berndt (ZT). This work would not be possible without the participation of our friends and colleagues, who contributed with material and photos for the study: E. Malysheva, E. Popov, T. Bulyonkova, M.Á. Ribes, S. Català, F. Caballero, G. Wölfel, F. Hampe, A. Hausknecht, Y. Rebriev, K. Potapov, N. Agafonova, S. Gashkov, B. Gierzyk, V. Kapitonov, A. Kovalenko, V. Malysheva, A. Kiyashko, T. Svetasheva, L. Marina, O. Desyatova, O. Shiryaeva (Kirillova), A. Fedosova, E. Ilyukhin, E. Lukashina, S. Lukashin, E. Zvyagina, I. Ukhanova, and S. Arslanov. We express our sincere thanks to all of them. We are also grateful to the anonymous reviewers of the manuscript for their valuable and constructive comments. This work was supported in part by the Russian Foundation for Basic Research (project N 12-04-33018 mol-a-ved and N 13-04-00838 a).
REFERENCES
- Baroni TJ, Hofstetter V, Largent DL, Vilgalys R. 2011. Entocybe is proposed as a new genus in the Entolomataceae (Agaricomycetes, Basidiomycota) based on morphological and molecular evidence. North American Fungi 6: 1–19 [Google Scholar]
- Baroni TJ, Matheny PB. 2011. A re-evaluation of gasteroid and cyphelloid species of Entolomataceae from eastern North America. Harvard Papers in Botany 16, 2: 293–310 [Google Scholar]
- Blanco-Dios JB. ‘2012’ 2013. Notas sobre el género Entoloma en el Noroeste de la Península Ibérica (IV): Entoloma legionense, una nueva especie del subgénero Leptonia. Revista Catalana de Micologia 34: 13–18 [Google Scholar]
- Bresadola J. 1929. Iconographia Mycologica. Vol. 12 Mediolani [Google Scholar]
- Clements FE, Shear CL. 1931. The genera of fungi. Wilson & Co., New York [Google Scholar]
- Co-David D, Langeveld D, Noordeloos ME. 2009. Molecular phylogeny and spore evolution of Entolomataceae. Persoonia 23: 147–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtecuisse R. 2008. Novitates 5. Nouvelles combinaisons et nouveaux noms nécessaires suite à la mise au point du référentiel des noms de champignons présents sur le territoire national métropolitain (1 - Basidiomycètes). Documents Mycologiques 34 (135-136): 49 [Google Scholar]
- Fries EM. 1815. Observationes Mycologicae 1. Havniae [Google Scholar]
- Fries EM. 1821. Systema mycologicum. 1. Lundae [Google Scholar]
- Fries EM. 1849. Summa vegetabilium Scandinaviae. Sectio Posterior. Holmiae & Lipsiae [Google Scholar]
- Fries EM. 1867. Icones selectae Hymenomycetum nondum delineatorum. I. Holmiae [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118 [DOI] [PubMed] [Google Scholar]
- Gates GM, Noordeloos ME. 2007. Preliminary studies in the genus Entoloma in Tasmania – I. Persoonia 19: 157–226 [Google Scholar]
- Gminder A, Enderle M. 1996. [On the variability of Entoloma lepidissimum (Svrček) Noordeloos]. Beiträge zur Kenntnis der Pilze Mitteleuropas 10: 59–64 [Google Scholar]
- He XL, Li TH, Xi PG, Jiang ZD, Shen YH. 2013. Phylogeny of Entoloma s.l. subgenus Pouzarella, with descriptions of five new species from China. Fungal Diversity 58: 227–243 [Google Scholar]
- Hesler LR. 1967. Entoloma in southeastern North America. Beihefte Nova Hedwigia 23: 1–245 Cramer, Germany [Google Scholar]
- Horak E. 1980. Entoloma (Agaricales) in Indomalaya and Australasia. Beihefte Nova Hedwigia 65: 341–346 Cramer, Germany [Google Scholar]
- Horak E. 2008. The fungi of New Zealand. Vol. 5: Agaricales of New Zealand 1. Pluteaceae – Entolomataceae. Fungal Diversity Research Series, vol. 19: 1–305 Fungal Diversity Press, Hong Kong [Google Scholar]
- Hughes KW, Petersen RH, Lickey EB. 2009. Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species’ delimitation across basidiomycete fungi. New Phytologist 182: 795–798 [DOI] [PubMed] [Google Scholar]
- Konrad P, Maublanc A. 1932. Icones Selectae Fungorum. Lechevallier, Paris [Google Scholar]
- Kühner R, Romagnesi H. 1953. Flore analytique des champignons superieus (Agarics, Bolets, Chanterelles). Paris [Google Scholar]
- Lange JE. 1937. Flora agaricina Danica. Vol. 2 Copenhagen [Google Scholar]
- Largent DL. 1977. The genus Leptonia on the Pacific Coast of the United States including a study of North American types. Bibliotheca Mycologica 55 Cramer, Germany [Google Scholar]
- Largent DL. 1994. Entolomatoid fungi of the Pacific Northwest and Alaska. Mad River Press, USA [Google Scholar]
- Largent DL, Baroni TJ. 1988. How to identify mushrooms to genus VI: Modern genera. Mad River Press, Eureka, CA [Google Scholar]
- Ludwig E. 2007. Pilzkompendium. Band 2. Die größeren Gattungen der Agaricales mit farbigem Sporenpulver (ausgenommen Cortinariaceae). Beschreibungen + Abbildungen. Fungicon-Verlag; Berlin [Google Scholar]
- Malloch D. 2010. Fleshy fungi (Basidiomycota) of the Atlantic Maritime Ecozone / Assessment of species diversity in the Atlantic Maritime Ecozone: 107–151 [Google Scholar]
- Manimohan P, Noordeloos ME, Dhanya AM. 2006. Studies on the genus Entoloma (Basidiomycetes, Agaricales) in Kerala State, India. Persoonia 19: 45–94 [Google Scholar]
- Matheny PB, Curtis JM, Hofstetter V, et al. 2006. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 984–997 [DOI] [PubMed] [Google Scholar]
- Moncalvo J-M, Vilgalys R, Redhead SA, et al. 2002. One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400 [DOI] [PubMed] [Google Scholar]
- Monthoux O, Röllin O. 1988. Une nouvelle espèce d’Agaricales: Entoloma alnetorum Month. & Röll. Mycologia Helvetica 3, 1: 43–51 [Google Scholar]
- Morgado LN, Noordeloos ME, Lamoureux Y, Geml J. 2013. Multi-gene phylogenetic analyses reveal species limits, phylogeographic patterns, and evolutionary histories of key morphological traits in Entoloma (Agaricales, Basidiomycota). Persoonia 31: 159–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova OV, Popov ES, Kovalenko AE. 2012. Studies on mycobiota of Vietnam. I. Genus Entoloma: new records and new species. Mikologiya i fitopatologiya, 46, 3: 182–200 [Google Scholar]
- Noordeloos ME. 1981. Introduction to the taxonomy of the genus Entoloma sensu lato (Agaricales). Persoonia 11: 121–151 [Google Scholar]
- Noordeloos ME. 1982a. Entoloma subgenus Leptonia in northwestern Europe – I. Introduction and a revision of its section Leptonia. Persoonia 11: 451–471 [Google Scholar]
- Noordeloos ME. 1982b. Notes on Entoloma. New and rare species of Entoloma from Scandinavia. New names and combinations. Nordic Journal of Botany 2: 155–162 [Google Scholar]
- Noordeloos ME. 1988. Entoloma in North America. The species described by L.R. Hesler, A.H. Smith & S.J. Mazzer: type-species and comments. Cryptogamic Studies, Vol. 2 Gustav Fisher Verlag, Stuttgart, Germany [Google Scholar]
- Noordeloos ME. 1992. Entoloma s.l. Fungi Europaei, vol. 5 Giovanna Biella, Saronno, Italy [Google Scholar]
- Noordeloos ME. 2004. Entoloma s.l. Fungi Europaei, vol. 5a Edizione Candusso, Italy [Google Scholar]
- Noordeloos ME. 2008. Entoloma in North America 2: the species described by C.H. Peck – type studies and comments. Österreichische Zeitschrift für Pilzkunde 17: 87–152 [Google Scholar]
- Noordeloos ME, Gates GM. 2009. Preliminary studies in the genus Entoloma in Tasmania II. Cryptogamie, Mycologie 30: 107–140 [Google Scholar]
- Noordeloos ME, Gates GM. 2012. The Entolomataceae of Tasmania. Fungal Diversity Research Series. Vol. 22 Springer Dordrecht, Heidelberg, New York, London [Google Scholar]
- Noordeloos ME, Hausknecht A. 2007. The genus Entoloma (Basidiomycetes, Agaricales) of the Mascarenes and Seychelles. Fungal Diversity 27: 111–144 [Google Scholar]
- Noordeloos ME, Morozova OV. 2010. New and noteworthy Entoloma species from the Primorsky Territory, Russian Far East. Mycotaxon 112: 231–255 [Google Scholar]
- Orton PD. 1991a. A revised list of the British species of Entoloma sensu lato. The Mycologist 5: 123–138 [Google Scholar]
- Orton PD. 1991b. A revised list of the British species of Entoloma sensu lato. The Mycologist 5: 172–176 [Google Scholar]
- Persoon CH. 1801. Synopsis methodica fungorum. Vol. 2 Gottingae, Apud H; Dieterich [Google Scholar]
- Petersen RH, Hughes KW, Lickey EB, Kovalenko AE, Morozova OV, Psurtseva NV. 2008. A new genus, Cruentomycena, with Mycena viscidocruenta as type species. Mycotaxon 105: 119–136 [Google Scholar]
- Romagnesi H. 1974. Essai d’une classification des Rhodophylles. Bulletin Mensuel de la Societe Linneenne de Lyon 43: 325–332 [Google Scholar]
- Romagnesi H. 1978. Les fondements de la taxinomie des Rhodophylles et leur classification. Beihefte Nova Hedwigia 59: 1–80. Cramer, Germany [Google Scholar]
- Romagnesi H, Gilles G. 1979. Les Rhodophylles des fôrets côtières du Gabon et de la Côte d’ Ivoire. Beihefte Nova Hedwigia 59 [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758–771 [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002. PAUP*: Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, Sinauer Associates [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31, 1: 21–32 [DOI] [PubMed] [Google Scholar]
- Vila J, Caballero F. 2007. Entoloma nuevos o interesantes de la Península Ibérica. Fungi non Delineati. Pars XXXVIII. Edizione Candusso, Italy [Google Scholar]
- Vila J, Caballero F. 2009. Entoloma nuevos o interesantes de la Península Ibérica (2). Fungi non Delineati. Pars XLV. Edizione Candusso, Italy [Google Scholar]
- Vila J, Carbó J, Caballero F, Català S, Llimona X, Noordeloos ME. 2013. A first approach to the study of the genus Entoloma subgenus Nolanea sensu lato using molecular and morphological data. Fungi non Delineati. LXVI (Studies on Entoloma): 3–62, 93–135 (iconography). Edizione Candusso, Italy [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococus. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel G, Hampe F. 2011. Entoloma-Forschung in Mitteleuropa I – Zwei neue Entoloma-Arten aus Deutschland (Entoloma studies in Central Europe I – Two new Entoloma species described from Germany.) Zeitschrift für Mykologie, Band 77/2: 181–190 [Google Scholar]
- Wölfel G, Noordeloos ME. 2001. Neue oder bemerkenswerte Entoloma – Arten der Kanarischen Inseln. Österreichische Zeitschrift für Pilzkunde 10: 185–200 [Google Scholar]