Abstract
Based on analyses of concatenated internal transcribed spacer regions of the nrDNA operon (ITS), large subunit rDNA (LSU), γ-actin and β-tubulin gene sequences the taxonomy of coniothyrium-like fungi belonging in the family Montagnulaceae, order Pleosporales, was re-assessed. Two new genera are proposed, Alloconiothyrium, to accommodate A. aptrootii sp. nov., and Dendrothyrium for D. longisporum sp. nov. and D. variisporum sp. nov. One new species is described in Paraconiothyrium, viz. Parac. archidendri sp. nov., while two species so far classified in Paraconiothyrium are transferred to Paraphaeosphaeria, viz. Paraph. minitans comb. nov. and Paraph. sporulosa comb. nov. In Paraphaeosphaeria five new species are described based on asexual morphs, viz. Paraph. arecacearum sp. nov., Paraph. neglecta sp. nov., Paraph. sardoa sp. nov., Paraph. verruculosa sp. nov., and Paraph. viridescens sp. nov. Macro- and micromorphological characteristics are fully described.
Keywords: γ-actin, β-tubulin, ITS, LSU, Microsphaeropsis, Paraconiothyrium, taxonomy
INTRODUCTION
Coniothyrium-like fungi are coelomycetous asexual morphs of Pleosporales and other Dothideomycetes (Ascomycota), characterised by pycnidial or stromatic conidiomata producing mostly relatively small, subhyaline to pigmented, 1- or 2-celled conidia. Most species have been classified in the genera Coniothyrium or Microsphaeropsis. They are often of considerable importance to society, being destructive as plant pathogens or beneficial as effective biological control agents (Carisse et al. 2001, Carisse & Bernier 2002a, b, El-Bassam et al. 2002) or bioremediators (da Silva et al. 2003a, b). They are also being reported from clinical cases with invasive cutaneous infections in immunocompromised or transplant patients (Balajee et al. 2007, Gordon et al. 2012, de Gruyter et al. 2012). The taxonomy of most coniothyrium-like fungi is problematic, due to the simplicity, plasticity and variability of morphological features exhibited by these coelomycetes. Attempts to delimit the genera based on features such as conidiomatal structure, conidiogenesis and conidial morphology have not been successful (Sutton 1980). Species of Coniothyrium and Microsphaeropsis described from plant material were largely distinguished by host-plant taxonomy (Wollenweber & Hochapfel 1937, Bestagno Biga et al. 1958, Sutton 1974), and for the majority no type or other reference cultures are available to date. Soils are also rich in coniothyrium-like fungi (Domsch et al. 2007), but the small number of species formally described from soil today does not cover the extant diversity, and the variability seen in such isolates hampers reliable identification. DNA sequences are still scarcely available and mostly of doubtful identity (Verkley et al. 2004).
Recent molecular phylogenetic studies focussing on sexual and asexual genera of Pleosporales have demonstrated that Coniothyrium and Microsphaeropsis, and also the ubiquitous and speciose coelomycete genus Phoma, are polyphyletic, with species occurring in several clades of the order Pleosporales, which are now being used as a firm basis for redefining families (Verkley et al. 2004, 2013, Schoch et al. 2009, Zhang et al. 2009, 2012, Aveskamp et al. 2010, de Gruyter et al. 2010, 2012, Quaedvlieg et al. 2013). The position of the type species of Microsphaeropsis, M. olivacea, was confirmed within the family Didymellaceae and that of Coniothyrium, C. palmarum, within the Leptosphaeriaceae. Several Coniothyrium species were grouped in the well-supported clade of Montagnulaceae, together with Paraphaeosphaeria (including Paraph. michotii, the type species of this genus) and the genera Kalmusia, Bimuria, Didymocrea, Letendraea and Montagnula (Zhang et al. 2009). In early recognition of the genetic distance from Coniothyrium s.str., Verkley et al. (2004) introduced the new genus Paraconiothyrium for a number of these asexual morphs grouping with Paraphaeosphaeria, and described four new Paraconiothyrium species, viz. Parac. estuarinum (the type species of this genus), Parac. brasiliense, Parac. cyclothyrioides and Parac. fungicola. Based on molecular phylogenetic evidence, the frequently reported soil-borne fungus Coniothyrium sporulosum and the important biocontrol agent C. minitans were recombined to Paraconiothyrium. Damm et al. (2008) described a further two new Paraconiothyrium species, Parac. africanum and Parac. variabile, and also transferred Microdiplodia hawaiiensis to Paraconiothyrium. Budziszewska et al. (2011) described Parac. babiogorense, an endophyte of the clubmoss Huperzia. Based on LSU sequence analyses, de Gruyter et al. (2012) transferred the coelomycetes Phoma falvescens, Plenodomus fuscomaculans, Asteromella tiliae and Phoma lini to Paraconiothyrium, while they also described a new species, Paraconiothyrium maculicutis. Paraconiothyrium currently holds 15 species, and only one of these, Parac. fuckelii, has a known sexual morph (Verkley et al. 2004, Damm et al. 2008, de Gruyter et al. 2012). Other novel genera are sporadically being proposed to accommodate coniothyrium-like fungi in other clades of Dothideomycetes as well. For example, the genus Xenoconiothyrium Crous & Marinc. was recently erected for coniothyrium-like fungi belonging to Teratosphaeriaceae (Crous et al. 2011).
In the course of many decades strains of coniothyrium-like fungi have been deposited in culture collections world-wide to serve as reference material for important research. These cultures represent a valuable resource of genetic diversity that has thus far been under-investigated. The culture collection of CBS (CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands) holds several hundreds of these strains. The main purpose of this study was to assess the genetic diversity of isolates preserved in CBS with special attention to strains grouping in the family of Montagnulaceae, and to delimit and formally describe novel species by comparing the obtained molecular phylogenetic and morphological data of cultures and their sporulating structures.
MATERIAL AND METHODS
Culture studies and morphological analyses
Cultures preserved in the CBS-KNAW, Utrecht, The Netherlands were used for the present study. Cultures were activated from lyophilised or cryopreserved material and inoculated on oatmeal (OA) and 3 % malt extract (MEA, Oxoid) agars, prepared according to Crous et al. (2009). For culture studies, 5-d-old cultures were transferred to fresh plates and incubated in the laboratory in diffuse daylight (20 °C), and in an incubator under n-UV light (12 h light, 12 h dark) at 18 °C to promote sporulation. Colony diameter measurements were taken from OA plates placed in the incubator with UV, after 10 d. Colours were described according to Rayner (1970). Sporulating structures obtained from cultures were used for the morphological description. Structures were mounted in water and examined with an Olympus BX 50 microscope mounted with bright field and differential interference contrast (DIC) objectives, and photographed using a mounted Nikon Digital Sight DS-5M camera. Photographs of culture plates were taken after 10 and 14 d on a photo stand with daylight tubes with a Pentax K110 D digital camera. Conidial masses from OA plates were mounted in water and 30 spores measured. Length/width (L/W) ratio was calculated for each spore and average L/W ratio calculated (N = 30). Descriptions and nomenclature of taxonomic novelties were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
DNA isolation, PCR and sequencing
Total genomic DNA was extracted from material preserved in liquid nitrogen or from living cultures, using the Genomed Jetquick general DNA clean-up kit or a high-throughput 96-well plate extraction (Ivanova et al. 2006) following the given protocols. The PCR reactions for amplification of the recently ratified universal fungal barcode ITS1-5.8S-ITS2 of the nuclear ribosomal DNA operon (Schoch et al. 2012), using ITS5/ITS1 and ITS4 were performed under standard or semi-nested conditions (White et al. 1990, Stielow et al. 2010). PCR conditions for amplifying the partial LSU rDNA using the standard primers LR0R and LR5 only differed in their annealing temperature (55 °C instead of 60 °C) and increased cycle extension time (90 s per cycle). Amplification of partial γ-actin (ACT), covering the more variable 5’-end containing two small introns, and partial β-tubulin (TUB), covering the variable 5’-end containing four small introns, followed the protocol of Aveskamp et al. (2009) and Carbone & Kohn (1999) using the primers ACT-512f, ACT783r, TUB4Rd and TUB4Fd, respectively. PCR products were directly purified using FastAP thermosensitive alkaline phosphatase and shrimp alkaline phosphatase (Fermentas, Thermo Scientific). The cycle-sequencing reaction was set up using ABI big dye terminator v. 3.1, using a quarter of the suggested volumes (modified manufacturers’ protocol), followed by bidirectional sequencing with a laboratory capillary electrophoresis system (Life Technologies 3730XL DNA analyser). Sequences were stored, manually corrected for sequencing artefacts and forward and reverse sequences assembled using the Biolomics database (www.bio-aware.com) (Vu et al. 2012). Sequences were deposited at NCBI GenBank under the accession numbers provided in Table 1. Alignments were deposited in TreeBASE.
Table 1.
Overview of species and isolates used in this study with their CBS accession numbers, former names and, where applicable, corrected taxon names according to findings in this study.
| Species | CBS accession nr. | Former identification | INSDC ITS | INSDC TUB | INSDC LSU | INSDC ACT | Substrate | Host |
|---|---|---|---|---|---|---|---|---|
| Alloconiothyrium aptrootii | CBS 980.95T | Coniothyrium sp. | JX496121 | JX496460 | JX496234 | JX496347 | Soil | ~ |
| CBS 981.95 | Coniothyrium sp. | JX496122 | JX496461 | JX496235 | JX496348 | Soil | ~ | |
| Ampelomyces quisqualis | CBS 128.79 | ~ | ~ | ~ | JX681063 | ~ | Lesion | Cucumber mildew |
| CBS 129.79 | ~ | ~ | ~ | JX681064 | ~ | Lesion | Cucumber mildew | |
| CBS 131.31 | ~ | ~ | ~ | JX681066 | ~ | Lesion | Erysiphe cichoracearum on Helianthus tuberosus | |
| CBS 131.79 | ~ | ~ | ~ | JX681065 | ~ | Lesion | Cucumber mildew | |
| CBS 133.32 | ~ | ~ | ~ | JX681067 | ~ | Lesion | Microsphaera alni on Lonicera sp. | |
| Aplosporella aquifolii | CBS 103.68 | ~ | ~ | ~ | JX681068 | ~ | Dead leaf | Ilex aquifolium |
| A. hesperidica | CBS 208.37 | ~ | ~ | ~ | JX681069 | ~ | Early stem-end rot | Citrus sinensis |
| A. mali | CBS 519.75 | ~ | ~ | ~ | JX681070 | ~ | Fruit | Malus sylvestris |
| A. prunicola | CBS 121167 | ~ | ~ | ~ | JX681071 | ~ | Bark | Prunus persica var. nucipersica |
| A. ruborum | CBS 117.82 | ~ | ~ | ~ | JX681072 | ~ | Dead stem | Rubus sp. |
| A. sterculiae | CBS 342.78 | ~ | ~ | ~ | JX681073 | ~ | ~ | Sterculia oblonga |
| Boeremia exigua var. exigua | CBS 431.74 | ~ | ~ | ~ | JX681074 | ~ | Tuber with gangrene | Solanum tuberosum |
| Coniothyrina agaves | CBS 470.69 | ~ | ~ | ~ | JX681075 | ~ | Spot on dead leaf | Agave americana |
| Coniothyrium cerealis | CBS 157.78 | ~ | ~ | ~ | JX681080 | ~ | Stem | Triticum aestivum |
| CBS 518.74 | ~ | ~ | ~ | JX681079 | ~ | ~ | Phleum pratense | |
| Con. juniperi | CBS 610.72 | ~ | ~ | ~ | JX681081 | ~ | ~ | Juniperus sp. |
| Con. nitidae | CBS 111302 | ~ | ~ | ~ | JX681082 | ~ | ~ | Protea nitida |
| CBS 111321 | ~ | ~ | ~ | JX681083 | ~ | ~ | Protea nitida | |
| Con. palmarum | CBS 400.71 | ~ | EU754153 | ~ | JX681084 | ~ | Dead petiole | Chamaerops humilis |
| CBS 758.73 | ~ | EU040225 | ~ | JX681085 | ~ | Leaf spots | Phoenix dactylifera | |
| EU754154 | ||||||||
| Con. palmicola | CBS 161.37 | ~ | ~ | ~ | JX681086 | ~ | Stem | Pandanus tectoriae |
| Coniothyrium sp. | CBS 122.76 | ~ | ~ | ~ | JX681077 | ~ | ~ | Cocos nucifera |
| CBS 302.72 | ~ | JX496065 | JX496404 | JX496178 | JX496291 | Leaf | Azalea sp. | |
| CBS 423.92 | ~ | ~ | ~ | JX681078 | ~ | Root | Hordeum vulgare | |
| Cucurbidothis pityophila | CBS 149.32 | ~ | ~ | ~ | JX681087 | ~ | Root, young tree | Picea sp. |
| Cucurbitaria berberidis | CBS 394.84 | ~ | ~ | ~ | JX681088 | ~ | Dead branches | Berberis julianae |
| Dendrothyrium longisporum | CBS 582.83T | Coniothyrium sp. | JX496097 | JX496436 | JX496210 | JX496323 | ~ | Arceuthobium pusillum |
| CBS 824.84 | Coniothyrium cerealis | JX496115 | JX496454 | JX496228 | JX496341 | Leaf spot | Triticum aestivum | |
| D. variisporum | CBS 121517T | Coniothyrium sp. | JX496030 | JX496369 | JX496143 | JX496256 | Declined grape vine | Vitis vinifera |
| CBS 197.82 | Coniothyrium sp. | JX496053 | JX496392 | JX496166 | JX496279 | ~ | Erica carnea | |
| Didymella exigua | CBS 183.55 | ~ | EU754155.1 | ~ | JX681089 | ~ | ~ | Rumex arifolius |
| Keissleriella cladophila | CBS 104.55 | ~ | ~ | ~ | JX681090 | ~ | ~ | Smilax parvifolia |
| Leptosphaeria doliolum subsp. errabunda | CBS 541.66 | ~ | ~ | ~ | JX681093 | ~ | Stem | Rudbeckia sp. |
| Leptosphaeria doliolum var. doliolum | CBS 297.51 | ~ | ~ | ~ | JX681094 | ~ | ~ | Papaver rhoeas |
| CBS 504.75 | ~ | ~ | ~ | JX681095 | ~ | Stem | Urtica dioica | |
| L. maculans | CBS 260.94 | ~ | ~ | ~ | JX681096 | ~ | ~ | Brassica oleracea |
| L. australis | CBS 100575 | ~ | ~ | ~ | JX681099 | ~ | Soil | ~ |
| CBS 939.69 | ~ | ~ | ~ | JX681098 | ~ | Soil | ~ | |
| Massaria platani | CBS 221.37 | ~ | DQ678065 | ~ | JX681100 | ~ | ~ | Platanus occidentalis |
| ‘Microsphaeropsis arundinis’ | CBS 100243 | ~ | JX496010 | JX496349 | JX496123 | JX496236 | Soil | ~ |
| M. olivacea | CBS 233.77 | ~ | GU237988 | ~ | JX681103 | ~ | Needle | Pinus laricio |
| CBS 303.68 | ~ | ~ | ~ | JX681101 | ~ | Leaf spots | Ligustrum vulgare | |
| CBS 432.71 | ~ | GU237987 | ~ | JX681102 | ~ | Dead twig and pod | Sarothamnus sp. | |
| Neophaeosphaeria filamentosa | CBS 102203 | ~ | ~ | ~ | JX681104 | ~ | ~ | Yucca rostrata |
| Paraconiothyrium africanum | CBS 121166T | ~ | JX496029 | JX496368 | JX496142 | JX496255 | ~ | Prunus persica |
| Paracon. archidendri | CBS 168.77T | Coniothyrium sp. | JX496049 | JX496388 | JX496162 | JX496275 | Leaf spot | Pithecelobium bigeminum |
| Paracon. brasiliense | CBS 100299T | ~ | AY642531 | JX496350 | JX496124 | JX496237 | Fruit | Coffea arabica |
| CBS 115.92 | Coniothyrium sp. | JX496022 | JX496361 | JX496135 | JX496248 | Phyllosphere | Olea europaea | |
| CBS 122319 | ~ | JX496032 | JX496371 | JX496145 | JX496258 | ~ | ~ | |
| CBS 122320 | ~ | JX496033 | JX496372 | JX496146 | JX496259 | Pruning cut | Actinidia chinensis var. Hort16A | |
| CBS 122321 | ~ | JX496034 | JX496373 | JX496147 | JX496260 | Browning wood | Platanus ′ acerifolia | |
| CBS 122851 | ~ | JX496036 | JX496375 | JX496149 | JX496262 | ~ | Juglans regia | |
| CBS 159.60 | Coniothyrium sp. | JX496044 | JX496383 | JX496157 | JX496270 | ~ | ~ | |
| CBS 254.88 | Coniothyrium sp. | JX496058 | JX496397 | JX496171 | JX496284 | ~ | Magnolia sp. | |
| CBS 395.87 | Coniothyrium sp. | JX496083 | JX496422 | JX496196 | JX496309 | Soil | ~ | |
| CBS 587.84 | Coniothyrium sp. | JX496099 | JX496438 | JX496212 | JX496325 | Bark | Vitis vinifera | |
| Paracon. cyclothyrioides | CBS 432.75 | Coniothyrium sp. | JX496088 | JX496427 | JX496201 | JX496314 | Soil | Hevea brasiliensis |
| CBS 972.95T | ~ | JX496119 | JX496458 | JX496232 | JX496345 | Soil | ~ | |
| Paracon. estuarinum | CBS 109850T | ~ | JX496016 | JX496355 | JX496129 | JX496242 | Sediment from estuarine habitat | ~ |
| Paracon. fuckelii | CBS 508.94 | Coniothyrium rosarum | JX496096 | JX496435 | JX496209 | JX496322 | Stem cancer | Rosa sp. |
| CBS 584.69 | Coniothyrium fuckelii | JX496098 | JX496437 | JX496211 | JX496324 | Root of gymnosperm | ~ | |
| CBS 653.85 | Coniothyrium sp. | JX496104 | JX496443 | JX496217 | JX496330 | Canker | Picea abies | |
| CBS 764.71B | Paraconiothyrium minitans | JX496112 | JX496451 | JX496225 | JX496338 | Academic hospital | Human | |
| CBS 797.95 | Coniothyrium fuckelii | JX496113 | JX496452 | JX496226 | JX496339 | Dead stem | Rubus sp. | |
| Paracon. fungicola | CBS 113269T | ~ | JX496020 | JX496359 | JX496133 | JX496246 | Resupinate polypore fungus | ~ |
| Paracon. hawaiiense | CBS 120025T | ~ | JX496027 | JX496366 | JX496140 | JX496253 | Stem | Sophora chrysophylla |
| Paraconiothyrium sp. | CBS 119485 | ~ | EF055359 | ~ | ~ | ~ | Wood | Actinidia chinensis var. Hort 16A |
| CBS 194.82 | Coniothyrium sp. | JX496052 | JX496391 | JX496165 | JX496278 | ~ | Lycopodium annotinum | |
| Paraconiothyrium sp. 1 | CBS 113682 | Microsphaeropsis pseudaspera | JX496021 | JX496360 | JX496134 | JX496247 | Air sample | ~ |
| CBS 251.87 | Coniothyrium sp. | JX496057 | JX496396 | JX496170 | JX496283 | Nail | Human | |
| Paracon. variabile | CBS 112.72 | Coniothyrium sp. | JX496019 | JX496358 | JX496132 | JX496245 | Dead stem ex herbarium specimen | Dianthus sp. |
| CBS 119486 | ~ | JX496023 | JX496362 | JX496136 | JX496249 | Wood | Actinidia chinensis var. Hort 16A | |
| CBS 119633 | Paraconiothyrium sp. | JX496024 | JX496363 | JX496137 | JX496250 | ~ | Laurus nobilis | |
| CBS 120014 | Paraconiothyrium variabile | JX496026 | JX496365 | JX496139 | JX496252 | Wood | Actinidia chinensis var. Hort 16A | |
| CBS 121163 | ~ | EU295639 | ~ | ~ | ~ | ~ | Prunus persica | |
| CBS 121164 | ~ | JX496028 | JX496367 | JX496141 | JX496254 | ~ | Prunus persica | |
| CBS 121754 | ~ | JX496031 | JX496370 | JX496144 | JX496257 | ~ | Prunus salicina | |
| CBS 122322 | Paraconiothyrium sp. | JX496035 | JX496374 | JX496148 | JX496261 | Pruning cut | Actinidia chinensis var Hot16A | |
| CBS 168.69 | Paraconiothyrium sporulosum | JX496048 | JX496387 | JX496161 | JX496274 | Soil | Acer pseudoplatanus | |
| CBS 269.74 | Coniothyrium sp. | JX496060 | JX496399 | JX496173 | JX496286 | Desert soil | ~ | |
| CBS 413.84 | Coniothyrium sp. | JX496086 | JX496425 | JX496199 | JX496312 | Stem and leaf | Lepidosperma longitudinale | |
| CBS 433.71 | Coniothyrium palmigenum | JX496089 | JX496428 | JX496202 | JX496315 | Dead leaf | Chamaerops humilis | |
| CBS 461.90 | Coniothyrium platani | JX496093 | JX496432 | JX496206 | JX496319 | Pruning wound | Platanus acerifolia | |
| CBS 504.84 | Coniothyrium sp. | JX496095 | JX496434 | JX496208 | JX496321 | Teleutosorus | Puccinia allii | |
| CBS 638.93 | Microsphaeropsis sp. | JX496102 | JX496441 | JX496215 | JX496328 | Disintegrated calcareous sandstone | ~ | |
| CBS 680.83 | Coniothyrium sp. | JX496105 | JX496444 | JX496218 | JX496331 | Toe nail | Human | |
| CBS 882.70 | Coniothyrium sp. | JX496118 | JX496457 | JX496231 | JX496344 | Dead stem | Spartium junceum | |
| Paraphaeosphaeria angularis | CBS 167.70T | Coniothyrium sp. | JX496047 | JX496386 | JX496160 | JX496273 | Leaf | Saccharum officinarum |
| Paraph. arecacearum | CBS 158.75T | Coniothyrium sp. | JX496043 | JX496382 | JX496156 | JX496269 | Soil | Elaeis guineensis |
| CBS 614.75 | Coniothyrium sp. | JX496100 | JX496439 | JX496213 | JX496326 | Cocos nucifera | Cocos nucifera | |
| Paraph. michotii | CBS 340.86 | Coniothyrium sp. | JX496079 | JX496418 | JX496192 | JX496305 | Leaf | Phragmites australis |
| CBS 652.86 | ~ | JX496103 | JX496442 | JX496216 | JX496329 | ~ | Typha latifolia | |
| Paraph. minitans | CBS 111750 | Paraconiothyrium minitans | JX496017 | JX496356 | JX496130 | JX496243 | Sclerotia | Sclerotinia sclerotorium, Lucerne |
| CBS 111752 | Paraconiothyrium minitans | JX496018 | JX496357 | JX496131 | JX496244 | ~ | Unknown | |
| CBS 151.96 | Paraconiothyrium minitans | JX496042 | JX496381 | JX496155 | JX496268 | Sclerotia | Sclerotinia sclerotiorum | |
| CBS 286.81 | Paraconiothyrium minitans | JX496063 | JX496402 | JX496176 | JX496289 | Stem | Solanum tuberosum | |
| CBS 859.71 | Paraconiothyrium minitans | JX496116 | JX496455 | JX496229 | JX496342 | Sclerotium, in soil | Sclerotinia trifoliorum | |
| CBS 860.71 | Paraconiothyrium minitans | JX496117 | JX496456 | JX496230 | JX496343 | Sclerotium, in soil | Solanum tuberosum | |
| Paraph. neglecta | CBS 119637 | Paraconiothyrium sporulosum | JX496025 | JX496364 | JX496138 | JX496251 | Inner ear | Human |
| CBS 124076 | Paraconiothyrium sp. | JX496037 | JX496376 | JX496150 | JX496263 | Wood | Actinidia chinensis var. Hort16A | |
| CBS 124077 | Paraconiothyrium sp. | JX496038 | JX496377 | JX496151 | JX496264 | Wood | Actinidia chinensis var. Hort16A | |
| CBS 124078 | Paraconiothyrium sp. | JX496039 | JX496378 | JX496152 | JX496265 | Wood | Actinidia chinensis var. Hort16A | |
| CBS 180.61 | Coniothyrium fuckelii | JX496051 | JX496390 | JX496164 | JX496277 | Acid mull soil, with very well decomposed leaves | ~ | |
| CBS 300.72 | Coniothyrium sp. | JX496064 | JX496403 | JX496177 | JX496290 | Leaf | Azalea sp. | |
| CBS 303.77 | Paraconiothyrium sporulosum | JX496067 | JX496406 | JX496180 | JX496293 | Taxus baccata | Taxus baccata | |
| CBS 305.77 | Paraconiothyrium sporulosum | JX496070 | JX496409 | JX496183 | JX496296 | ~ | Taxus baccata | |
| CBS 306.77 | Paraconiothyrium sporulosum | JX496071 | JX496410 | JX496184 | JX496297 | ~ | Juniperus chinensis | |
| CBS 307.77 | Paraconiothyrium sporulosum | JX496072 | JX496411 | JX496185 | JX496298 | ~ | Cupressocyparis leylandii | |
| CBS 335.78 | Coniothyrium sp. | JX496076 | JX496415 | JX496189 | JX496302 | Decayed wood | ~ | |
| CBS 337.78 | Paraconiothyrium sporulosum | JX496077 | JX496416 | JX496190 | JX496303 | Rotten wood | ~ | |
| CBS 359.75 | Paraconiothyrium sporulosum | JX496081 | JX496420 | JX496194 | JX496307 | Canker | Juniperus sp. | |
| CBS 431.77 | Paraconiothyrium sporulosum | JX496087 | JX496426 | JX496200 | JX496313 | ~ | Unknown | |
| CBS 434.71A | Paraconiothyrium minitans | JX496090 | JX496429 | JX496203 | JX496316 | ~ | Erica carnea | |
| CBS 434.71B | Paraconiothyrium minitans | JX496091 | JX496430 | JX496204 | JX496317 | ~ | Pyrola rotundifolia | |
| CBS 452.81 | Paraconiothyrium sporulosum | JX496092 | JX496431 | JX496205 | JX496318 | Dead branches | Pyrus malus | |
| CBS 627.94 | Paraconiothyrium sporulosum | JX496101 | JX496440 | JX496214 | JX496327 | Decaying leaf | Mahonia nervosa | |
| CBS 683.83 | Paraconiothyrium sporulosum | JX496107 | JX496446 | JX496220 | JX496333 | Seed | Quercus robur | |
| Paraph. pilleata | CBS 102207 | ~ | JX496013 | JX496352 | JX496126 | JX496239 | ~ | Juncus roemerianus |
| Paraph. sardoa | CBS 501.71T | Coniothyrium sp. | JX496094 | JX496433 | JX496207 | JX496320 | Dead leaf | Smilax aspera |
| Paraphaeosphaeria sp. | CBS 101464 | Microsphaeropsis rugosa | JX496012 | JX496351 | JX496125 | JX496238 | Soil | ~ |
| CBS 978.95 | Microsphaeropsis sp. | JX496120 | JX496459 | JX496233 | JX496346 | Soil | ~ | |
| Paraph. sporulosa | CBS 105.76 | Paraconiothyrium sporulosum | JX496014 | JX496353 | JX496127 | JX496240 | Root | Picea abies |
| CBS 109.72 | Coniothyrium sp. | JX496015 | JX496354 | JX496128 | JX496241 | Agricultural soil | ~ | |
| CBS 146.69 | Paraconiothyrium sporulosum | JX496040 | JX496379 | JX496153 | JX496266 | Agricultural soil | ~ | |
| CBS 150.32 | Coniothyrium rosarum | JX496041 | JX496380 | JX496154 | JX496267 | ~ | Rosa canina | |
| CBS 162.69 | Coniothyrium sp. | JX496045 | JX496384 | JX496158 | JX496271 | Soil | ~ | |
| CBS 163.69 | Coniothyrium sp. | JX496046 | JX496385 | JX496159 | JX496272 | Soil | ~ | |
| CBS 177.59 | Paraconiothyrium sporulosum | JX496050 | JX496389 | JX496163 | JX496276 | Artificially inoculated soil | ~ | |
| CBS 218.68T | Paraconiothyrium sporulosum | JX496054 | JX496393 | JX496167 | JX496280 | Wheat-field soil | ~ | |
| CBS 221.78 | Coniothyrium sp. | JX496055 | JX496394 | JX496168 | JX496281 | Soil | ~ | |
| CBS 245.76 | Coniothyrium sp. | JX496056 | JX496395 | JX496169 | JX496282 | ~ | ~ | |
| CBS 271.78 | Coniothyrium sp. | JX496061 | JX496400 | JX496174 | JX496287 | Rhizosphere of grass | ~ | |
| CBS 281.81 | Coniothyrium sp. | JX496062 | JX496401 | JX496175 | JX496288 | ~ | Clematis sp. | |
| CBS 302.77 | Coniothyrium sp. | JX496066 | JX496405 | JX496179 | JX496292 | ~ | Calluna vulgaris | |
| CBS 304.80 | Coniothyrium sp. | JX496068 | JX496407 | JX496181 | JX496294 | Root | Malus sylvestris | |
| CBS 305.68 | Microsphaeropsis olivacea | JX496069 | JX496408 | JX496181 | JX496295 | ~ | Opuntia sp. | |
| CBS 308.81 | Coniothyrium sp. | JX496073 | JX496412 | JX496186 | JX496299 | Soil, potato field | Solanum tuberosum | |
| CBS 317.81 | Paraconiothyrium sporulosum | JX496074 | JX496413 | JX496187 | JX496300 | River water | ~ | |
| CBS 329.76 | Coniothyrium sp. | JX496075 | JX496414 | JX496188 | JX496301 | ~ | Picea abies | |
| CBS 340.85 | Coniothyrium sp. | JX496078 | JX496417 | JX496191 | JX496304 | Cyst, buried in soil | Globodera rostochiensis | |
| CBS 391.86 | Coniothyrium sp. | JX496082 | JX496421 | JX496195 | JX496308 | ~ | Triticum aestivum | |
| CBS 401.71 | Coniothyrium sp. | JX496084 | JX496423 | JX496197 | JX496310 | ~ | Fragaria vesca | |
| CBS 688.70B | Paraconiothyrium sporulosum | JX496108 | JX496447 | JX496221 | JX496334 | Soil | ~ | |
| CBS 688.70C | Paraconiothyrium sporulosum | JX496109 | JX496448 | JX496222 | JX496335 | Soil | ~ | |
| CBS 690.70 | Coniothyrium fuckelii | JX496110 | JX496449 | JX496223 | JX496336 | ~ | Secale cereale | |
| CBS 764.71A | Paraconiothyrium minitans | JX496111 | JX496450 | JX496224 | JX496337 | Greenhouse soil | ~ | |
| CBS 824.68 | Coniothyrium cydoniae | JX496114 | JX496453 | JX496227 | JX496340 | Leaf spot | Cydonia oblonga | |
| Paraph. verruculosa | CBS 263.85 | Coniothyrium sp. | JX496059 | JX496398 | JX496172 | JX496285 | Needle | Picea abies |
| CBS 354.80 | Coniothyrium sp. | JX496080 | JX496419 | JX496193 | JX496306 | Páramo soil, after burning | ~ | |
| CBS 682.84 | Coniothyrium sp. | JX496106 | JX496445 | JX496219 | JX496332 | Wood | Pinus radiata | |
| Paraph. viridescens | CBS 854.73T | Coniothyrium sp. | JX496085 | JX496424 | JX681076 | JX496311 | Fresh water | ~ |
| Parastagonospora nodorum | CBS 272.59 | ~ | ~ | ~ | JX681114 | ~ | Grain | Triticum aestivum |
| CBS 273.59 | ~ | ~ | ~ | JX681115 | ~ | Leaf | Triticum aestivum | |
| CBS 287.52 | ~ | ~ | ~ | JX681113 | ~ | Decaying straw | Triticum aestivum | |
| Peyronellaea glomerata | CBS 528.66 | ~ | ~ | ~ | JX681105 | ~ | Wood cutting | Chrysanthemum |
| Phaeocytostroma plurivorum | CBS 113835 | ~ | ~ | ~ | JX681106 | ~ | ~ | Helianthus annuus |
| P. sacchari | CBS 275.34 | ~ | ~ | ~ | JX681107 | ~ | ~ | Unknown |
| Phaeosphaeria avenaria f.sp. triticae | CBS 289.52 | ~ | ~ | ~ | JX681108 | ~ | Leaf | Triticum aestivum |
| CBS 385.86 | ~ | ~ | ~ | JX681109 | ~ | ~ | Triticum aestivum | |
| P. brevispora | CBS 120248 | ~ | ~ | ~ | JX681110 | ~ | Culms | Sasa sp. |
| P. eustoma | CBS 307.71 | ~ | ~ | ~ | JX681111 | ~ | ~ | Juncus alpinus |
| CBS 724.92 | ~ | ~ | ~ | JX681112 | ~ | ~ | Ramalina sp. (lichen) | |
| P. occulta | CBS 582.86 | ~ | ~ | ~ | JX681116 | ~ | ~ | Carex hirta |
| P. parvula | CBS 260.49 | ~ | ~ | ~ | JX681117 | ~ | Dead leaf | Iris pseudacorus |
| CBS 605.86 | ~ | ~ | ~ | JX681118 | ~ | ~ | Iris pseudacorus | |
| Phaeosphaeriopsis obtusispora | CBS 246.64 | ~ | ~ | ~ | JX681119 | ~ | Dead leaf | Aloe arborescens |
| Plenodomus lingam (syn. Leptosphaeria maculans) | CBS 147.24 | ~ | ~ | JX681097 | ~ | ~ | Unknown | |
| Plenodomus biglobosus (syn. Leptosphaeria biglobosa) | CBS 475.81 | ~ | ~ | JX681091 | ~ | Leaf spots | Brassica oleracea | |
| CBS 476.81 | ~ | ~ | JX681092 | ~ | Black leaf spots | Brassica oleracea | ||
| Pleospora herbarum var. herbarum | CBS 191.86 | ~ | ~ | ~ | JX681120 | ~ | Leaf | Medicago sativa |
| Thyridaria rubronotata | CBS 385.39 | ~ | ~ | ~ | JX681121 | ~ | ~ | Acer sp. |
* GenBank accession numbers of ITS, TUB, LSU and ACT sequences (starting with ‘JX’ for newly generated sequences in this study), substrate, and host organism. ‘T’ following the CBS accession number indicates ex-type strains.
Sequence alignment and phylogenetic analysis
Sequences were aligned with MAFFT v. 6.850b, using the ‘–genafpair’ option but default settings otherwise (Katoh et al. 2005). All introns and exons were aligned separately. Regions containing many leading or trailing gaps were removed from the ITS and LSU alignments prior to tree building. Phylogenetic analysis under the maximum-likelihood (ML) criterion (Felsenstein 1981) was conducted with RAxML v. 7.2.8, using its novel rapid bootstrap option combined with the autoMRE bootstopping criterion (Pattengale et al. 2009) with subsequent search for the best tree under the GTRMIX approach (Stamatakis et al. 2008). The resulting best-known ML tree was rooted using the midpoint-rooting method (Farris 1972, Hess & de Moraes Russo 2007). Bootstrapping under the maximum-parsimony (MP) criterion (Fitch 1971) was done with PAUP v. 4.0b10 (Swofford 2002), treating gaps as missing data, collapsing branches of zero minimum length, and using, per bootstrap replicate, five rounds of random sequence addition followed by TBR branch swapping, saving only one tree per round. In MP bootstrapping, 1 000 replicates were conducted. Search for the best MP tree(s) was done in the same manner but using 1 000 rounds of random sequence addition, saving no more than ten trees per round, and the strict consensus of tree all most-parsimonious trees determined.
The relative performance of the four loci (ITS, LSU, ACT and TUB) in phylogenetic inference for the group was assessed as follows. ML bootstrap analyses of the four alignments were conducted separately (using the same settings as above), the support values from each gene mapped to the best ML tree from combined analysis using RAxML, and each average bootstrap support determined, both absolute and relative to the number of variable characters per alignment. Under MP, partitioned Bremer support (Baker & DeSalle 1997, Baker et al. 1998) was determined using the ‘bremer.tcl’ script (Göker et al. 2009b) in conjunction with PAUP (heuristic-search settings were as above but with 100 rounds for each Bremer search), visualised using a heatmap as implemented in the opm package for R (Vaas et al. 2012) and summed up over all nodes for each gene, both absolute and relative to the number of parsimony-informative characters per alignment (partitioned Bremer support trees are available upon request). The suitability of the four loci for molecular taxonomy of the group was investigated using OPTSIL (Göker et al. 2009a, Stielow et al. 2011) with the revised classification of the group (as detailed below) as reference partition, thus optimizing sequence dissimilarity thresholds for F values between 0.0 and 1.0 (with a step width of 0.05), and measuring the resulting best agreement between the clustering and the reference partition. The F value determines the shape of the clusters; we here considered the full range between single-linkage (0.0) and complete-linkage (1.0) clustering; see Göker et al. (2009a) for details. The underlying distance matrices were calculated with PAUP, using uncorrected (‘p’) distances.
RESULTS
Sequence alignment and phylogenetic analyses
The aligned LSU dataset used for determining the relationships between coniothyrium-like members of Pleosporales and their relatives comprised 172 organisms and 890 characters, including 290 variable and 248 parsimony-informative characters. The resulting ML tree is presented in Fig. 1 together with ML and MP bootstrap values. Strains representing the dark-spored coelomycete genera Asplosporella (Botryosphaeriales) and Phaeocytostroma (Diaporthales) form the outgroup and a small ingroup clade sister to all other ingroup clades, respectively. The pleosporalean taxa that constitute the major part of this tree group in clades that correspond to families that have previously been resolved in other molecular phylogenetic studies of Pleosporales (Schoch et al. 2009, Zhang et al. 2009, Aveskamp et al. 2010). One monophyletic group comprising 41 strains representing various families (bootstrap support 96/83 %) includes two strains of Coniothyrium palmarum, of the recently reinstated family Coniothyriaceae (de Gruyter et al. 2012), and Cucurbitaria berberidis (CBS 394.84) of the Cucurbitariaceae. Its subclade (89/63 %) representing the family Phaeosphaeriaceae comprises four subclades of its own, viz. a clade (98/79 %) of two strains identified as ‘Coniothyrium’ cerealis (CBS 518.74, 157.78), a second, well-supported (92/94 %) subclade of five strains of Ampelomyces quisqualis which reveals at least two distinct genotypes based on LSU. According to de Gruyter et al. (2009) Ampelomyces is heterogenous, with the type species A. quisqualis belonging in the Phaeosphaeriaceae, and A. quercinus in the Didymellaceae. Our data indicate that the three strains originating from cucumber mildew in Canada (CBS 128.79, 129.79, 131.79) are specifically distinct from USA strains CBS 131.31 and 133.32, from Erysiphe cichoracearum on Helianthus tuberosus and Microsphaera alni on Lonicera sp., respectively. A third, rather weakly supported (74/< 60 %) subclade with Phaeosphaeriopsis obtusispora (CBS 246.64), Phaeosphaeria occulta (CBS 582.86) and Parastagonospora nodorum (CBS 287.52, 272.59, 273.59), and a fourth, strongly supported subclade (100/98 %) with Phaeosph. avenaria (CBS 289.52, 385.86), Phaeosph. parvula (CBS 260.49, 605.86) and Phaeosph. eustoma (CBS 724.92, 307.71). The Didymellaceae clade (99/100 %) contains 10 strains, including Didymella exigua (CBS 183.51), the type species of the genus Didymella, Microsphaeropsis olivacea (CBS 233.77, 432.71) and two strains of ‘Coniothyrium’ nitidae (CBS 111302, 111321). Its unsupported sister group of miscellaneous fungi comprises Neophaeosphaeria filamentosa (CBS 102203), Coniothyrina agaves (CBS 470.69) (type species of the genus is C. agavicola), a well-supported subclade (100/100 %) with Leptosphaeria doliolum vars doliolum (CBS 297.51, 504.75) and errabunda (CBS 541.66), agreeing with Leptosphaeriaceae clade B of de Gruyter et al. (2012), and an incompletely resolved clade containing Plenodomus biglobosus (syn. Leptosphaeria biglobosa) (CBS 475.81, 476.81), Plenodomus lingam (syn. Leptosphaeria maculans, Phoma lingam) (CBS 147.24, 260.94) and Pleospora herbarum (CBS 191.86).
Fig. 1.
Midpoint-rooted maximum-likelihood phylogeny of Coniothyrium-like Pleosporales and their relatives inferred from 890 LSU characters. The numbers abovenext to the branches are ML (left) and MP (right) bootstrap support values. Several large branches (marked by “//”) have been scaled to 25 % of original length to better fit the tree on page. Highlighted sections indicate affiliations to families.
Keisleria cladophila (CBS 104.55) and Massaria platani (CBS 221.37) classified in the Lentitheciaceae, and Thyridaria rubronotata (CBS 385.39) of uncertain familial affinity (‘Clade J’ in Schoch et al. 2009) form the sister group of the Montagnulaceae clade (< 60/72 %), which consist of 120 strains, and the sister group of the latter two clades, respectively. LSU apparently does not provide sufficient variation within Montagnulaceae, most inner branches show poor or no support, and thus the genera cannot be sufficiently resolved here. But the following well-supported subclades can be noted: the new monotypic genus Alloconiothyrium (CBS 980.95T, 981.95) and the new genus Dendrothyrium with D. longisporum (CBS 582.83T, 824.84) and D. variisporum (CBS 121517T, 197.82). CBS 161.37 preserved as ‘Coniothyrium’ palmicola also groups here, and ITS shows 99 % similarity to the type strain of Dendrothyrium longisporum. No additional sequences could be obtained for CBS 161.37 and the identity of this strain therefore remains uncertain. CBS 120248 also groups here, confirming the position of Phaeosphaeria brevispora in Montagnulaceae (Schoch et al. 2009). Furthermore, Parac. estuarinum (CBS 109850T), Parac. cyclothyrioides (CBS 972.95T), CBS 122.76 and 432.75 ‘Coniothyrium sp.’, as well as CBS 100243 ‘Microsphaeropsis’ arundinis group together (94/91 %).
The performance of the four concatenated gene alignments (ITS, LSU, ACT and TUB) in combined and separate phylogenetic inference is shown in Table 2. The measures agreed that TUB provided overall the most support in combined and separate analysis, followed by ACT, ITS and LSU. Relative to the number of variable and parsimony-informative characters, however, ACT performed best, followed by LSU, TUB and ITS (in this respect, LSU performed even better than ACT when analysed separately). In the multi-locus phylogeny inferred from the combined dataset shown in Fig. 2, several well-supported clades can be identified, which are interpreted as appropriate for the delimitation of genera. The outgroup of the tree is formed by two highly supported clades representing the genera Alloconiothyrium (100/100 %) and Dendrothyrium (100/100 %). The Dendrothyrium clade comprises two species, with two isolates of D. longisporum (CBS 824.84, 582.83T) and the type strain of D. variisporum (CBS 121517T). A second strain, CBS 197.82, is also assigned to this species based on morphological similarities to the type strain, even though this renders the species paraphyletic in the presently postulated phylogeny, but without support. Another well-supported (100/98 %) clade forming the major part of the ingroup of the tree comprises 64 strains assigned to the genus Paraphaeosphaeria, with two isolates of Paraph. michotii, the type species of the genus, and the highly supported clades of the following species: Paraph. sporulosa (26 strains), Paraph. minitans (6), the new species Paraph. sardoa (1), Paraph. angularis (1), which clusters with Paraph. michotii and Paraph. pilleata, and furthermore Paraph. verruculosa (3), Paraph. neglecta (19), Paraph. arecacearum (2) and Paraph. viridescens (1). The intraspecific sequence variability regarding TUB is somewhat higher in Paraph. neglecta than in the other species of the genus with multiple strains in the tree, as indicated by partitioned Bremer support values for the interior branches of the Paraph. neglecta clade of 1–5 steps for TUB but ≤ 2 for the other genes (data not shown). CBS 101464 from Malawi deposited in CBS as Microsphaeropsis rugospora is found within the Paraphaeosphaeria clade (close to its base), and is preliminarily re-identified as Paraphaeosphaeria sp. The type of M. rugospora originated from cultivated soil in southern Japan (Someya et al. 1997).
Table 2.
Performance of the four loci in separate and combined phylogenetic analyses of Montagnulaceae and in molecular taxonomy.
| LSU | ITS | ACT | TUB | |
|---|---|---|---|---|
| # characters | 887 | 615 | 302 | 482 |
| # variable | 74 | 194 | 156 | 238 |
| # MP-informative | 44 | 159 | 136 | 218 |
| SPBrS | 62.12 | 174.90 | 221.14 | 266.83 |
| ...per character | 1.41 | 1.10 | 1.63 | 1.22 |
| ABS, combined | 14.34 | 24.65 | 31.90 | 39.80 |
| ...per character | 0.19 | 0.13 | 0.20 | 0.17 |
| ABS, separate | 26.38 | 34.69 | 45.12 | 52.61 |
| ...per character | 0.36 | 0.18 | 0.29 | 0.22 |
| highest MRI | 0.9682 | 0.9952 | 0.9795 | 0.9927 |
| ...for F value(s) | 0.8 | 0.35–0.5 | 0.0–1.0 | 0.0–1.0 |
| ...for threshold(s) | 0.29 % | 1.37 % | 3.005–3.64 % | 4.585–4.71 % |
| # clusters | 23 | 25 | 28 | 28 |
Note: SPbrS, sum of partitioned Bremer-support values over all nodes; ABS, average bootstrap support (under ML, either in combined or separate analysis); MRI, modified Rand index (indicating the agreement, at most 1.0, between sequence clustering and proposed classification). Normalization ‘per character’ was conducted per number of parsimony-informative characters for SPBrS and per number of variable characters for all other measures.
Fig. 2.
Midpoint-rooted maximum-likelihood phylogeny of Montagnulaceae inferred from four concatenated gene alignments (ITS, LSU, ACT and TUB) yielding a total of 2 286 characters. The numbers next to the branches are ML (left) and MP (right) bootstrap support values. The affiliations to species are highlighted. Species named in bold indicate taxa proposed in this study.
Paraconiothyrium estuarinum (CBS 109850T), the type species of Paraconiothyrium, groups together with Parac. cyclothyrioides (CBS 972.95T) and CBS 432.75, regarded conspecific with it, in a well-supported (100/100 %) clade also comprising CBS 100243, identified as Microsphaeropsis arundinis. A second Paraconiothyrium subclade comprises Parac. variabile (16 strains), the Parac. brasiliense complex (10), and a group containing CBS 113682 and 251.87, ‘Paraconiothyrium sp. 1’. A third Paraconiothyrium subclade comprises CBS 120025 and 121166, the type strains of Parac. hawaiiense and Parac. africanum, respectively. Two additional clades correspond to Parac. fuckelii (5 strains) and the novel species Parac. archidendri (CBS 168.77T), respectively.
The results of optimising sequence-clustering parameters for the concatenated alignment and each gene individually with OPTSIL are included in Table 2. Expectedly, LSU performed worst, failing to differentiate between a number of species (see also Fig. 1), but also dividing Paraconiothyrium fuckelii and Paraphaeosphaeria michotii into two clusters, respectively (details not shown, but compare Fig. 1). ACT and TUB divided Paraconiothyrium brasiliense into three or two clusters, respectively; in addition, ACT merged Parac. cyclothyrioides and Parac. estuarinum. ITS merged these two species and also Dendrothyrium longisporum and D. variisporum; as ITS divided no species, it thus yielded the highest overall agreement, minimally larger than the one obtained with TUB, as the conflicting species were only represented by few specimens. The best clustering obtained with the entire dataset was identical to the optimal one for ITS. The data also indicate, however, that once the Parac. brasiliense complex could convincingly be split into two species, TUB sequence clustering would yield 100 % agreement with the classification for a single choice of sequence dissimilarity threshold applied to all included taxa, independent of the clustering parameter F (Table 2). That F = 0.0 is included in the optimal values also indicates the presence of a TUB barcoding gap for the species under study.
Taxonomy
Alloconiothyrium Verkley, Göker & Stielow, gen. nov. — MycoBank MB800756
Type species. Alloconiothyrium aptrootii Verkley, Göker & Stielow.
Etymology. Named after its morphological resemblance to Coniothyrium in contrast to the phylogenetic distance between both genera.
Conidiomata pycnidial or eustromatic. Conidiogenous cells holoblastic, annellidic. Conidia olivaceous-brown and irregular in outline, surface roughened. Sexual morph unknown.
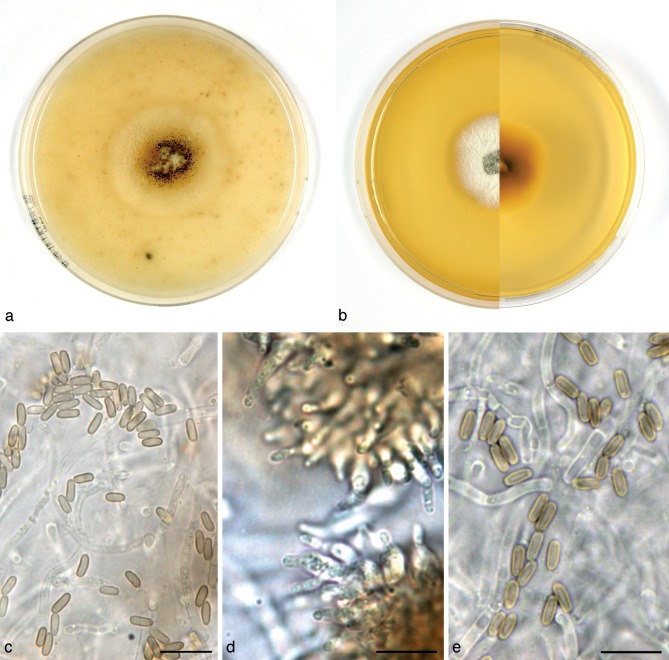
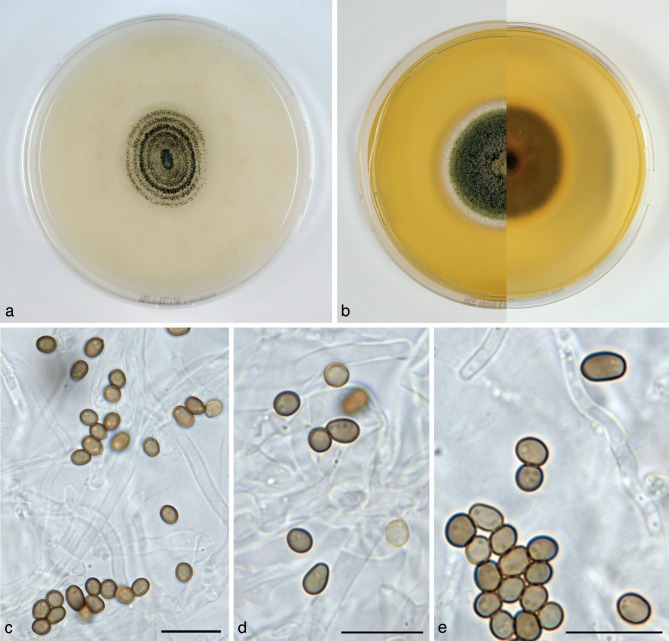
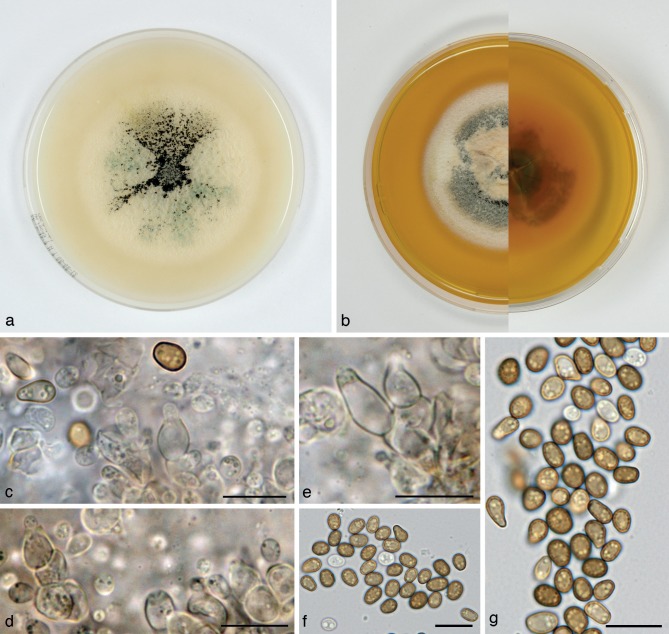
Alloconiothyrium aptrootii Verkley, Göker & Stielow, sp. nov. — MycoBank MB800757; Fig. 3
Fig. 3.
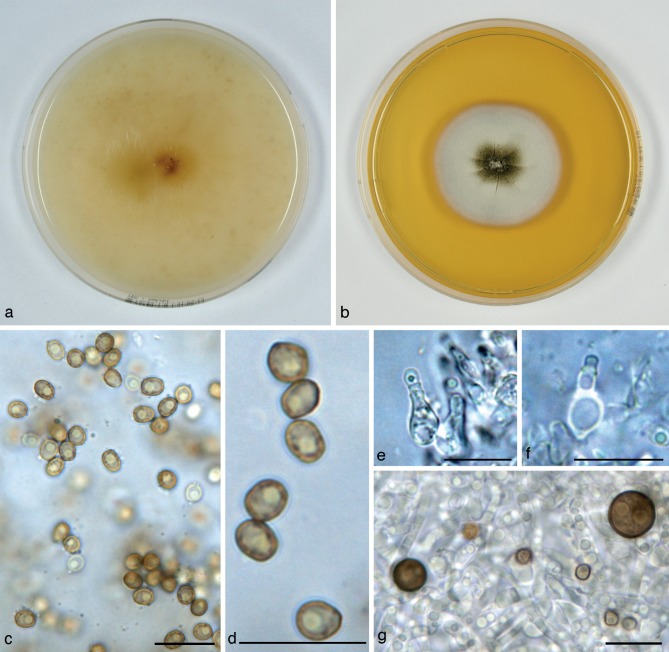
Alloconiothyrium aptrootii (CBS 980.95T, ex-type culture). a. Colony on OA; b. colony on MEA; c, d. conidia on OA; e, f. conidiogenous cells showing distinct annellations; g. chlamydospores. — Scale bars = 10 μm.
Etymology. Named after André Aptroot, who collected the soil sample from which the species was isolated.
Conidiomata pycnidial, 300–450 μm diam and with a single cavity, or eustromatic and consisting of complexes reaching 1 mm diam, with several cavities, the outer surface black, glabrous or covered by grey mycelium. Conidiomatal wall composed of an outer layer of brown, thick-walled textura angularis and an inner layer of hyaline, thick-walled textura angularis-globulosa, the outer surface sometimes covered by a diffuse web of brown hyphae. Conidiogenous cells discrete, often positioned on clumps of cells that protrude into the cavity, broadly ampulliform, holoblastic, annellidic, often with an elongated neck showing several distinct percurrent proliferations, 4–9 × 3–4 μm. Conidia globose to irregularly ellipsoid, initially hyaline, after secession olivaceous-brown, mature conidial wall orange-brown, the outer surface verruculose giving the conidium an irregular outline, with 1 large oil-droplet 1–1.5 μm diam, 0-septate, 3–4(–5) × 2.5–3(–3.5) μm, average L/W ratio 1.2 ± 0.2. Chlamydospores formed in the mycelium, terminal or intercalary, usually solitary, globose, mostly 6–8.5 μm diam, with a smooth brown wall and 1–2 large oil-droplets. Sexual morph unknown.
Colonies on OA reaching 37–40 mm diam in 10 d, with an even, glabrous, colourless margin. Immersed mycelium mostly colourless, but with some faint buff to honey in the centre after 10 d. Aerial mycelium very diffuse, white or absent. Reverse concolourous. Conidiomata developing after 10–15 d. Colonies on MEA reaching 36–40 mm diam in 10 d, with an even, buff margin. Immersed mycelium greenish olivaceous to olivaceous, fading to buff at margin, mostly covered by a moderately dense layer of woolly to floccose grey to white aerial mycelium. Reverse in the centre isabelline, fading over cinnamon to buff at the margin.
Specimens examined. PAPUA NEW GUINEA, Central Province, Varirata Nat. Park near Port Moresby, isolated by A. van Iperen from a soil sample, Oct. 1995, A. Aptroot, holotype CBS H-21035, living ex-type culture CBS 980.95; isolated from the same soil sample CBS 981.95.
Notes — The fungus is only known from a soil sample collected in Papua New Guinea, and all other coniothyrium-like fungi studied here are relatively distantly related. The annellidic conidiogenous cells and the verruculose conidia remind of Coniothyrium palmarum, the type species of the genus, but that species is characterised by 2-celled conidia and is also genetically distinct, and belongs in the Leptosphaeriaceae (de Gruyter et al. 2009).
Dendrothyrium Verkley, Göker & Stielow, gen. nov. — MycoBank MB800758
Type species. Dendrothyrium variisporum Verkley, Göker & Stielow.
Etymology. Named after the branched, tree (= dendron)-like conidiophores occurring in the conidiomata of the type species.
Conidiomata pycnidial or eustromatic. Conidiogenous cells discrete or integrated in conidiophores that are branched at the base, phialidic, terminal cells of the conidiophore occasionally also percurrently proliferating. Conidia 1-celled, olivaceous-brown, thin- and smooth-walled. Sexual morph unknown.
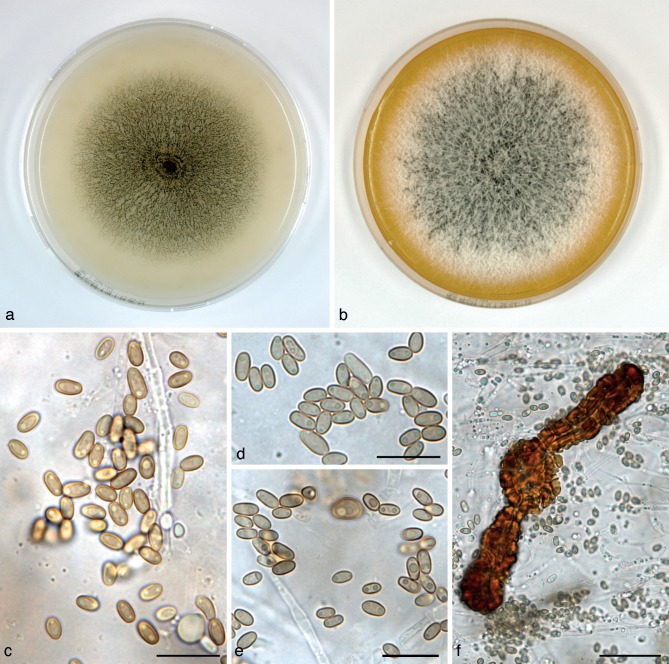
Dendrothyrium longisporum Verkley, Göker & Stielow, sp. nov. — MycoBank MB800759; Fig. 4
Fig. 4.
Dendrothyrium longisporum (CBS 582.83T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c. conidia on OA; d. conidiogenous cells on OA; e. conidia on OA. — Scale bars = 10 μm.
Etymology. Named after the comparatively long conidia of this species.
Conidiomata pycnidial, globose, 140–170 μm diam, with a single, central ostiolum 10–20 μm. Conidiomatal wall composed of textura angularis with pale yellowish brown cells and darker cells around the ostiolum, sometimes overlaid with a diffuse web of thin-walled brown hyphae. Conidiogenous cells discrete or integrated in simple, 1–2-septate, 10–17 μm long conidiophores, phialidic, doliiform to ampulliform, with a distinct periclinal thickening, 3.5–6(–8) × 2–3 μm. Conidia consistently cylindrical-ellipsoid, initially hyaline, soon after secession with a olivaceous-brown, thin, smooth wall, with minute granules and no oil-droplets, 0-septate, (3.5–)4–5(–6) × 1.5–2 μm, average L/W ratio 2.8 ± 0.4. Sexual morph unknown.
Colonies on OA reaching 28–32 mm diam in 10 d, with a smooth, glabrous margin. Immersed mycelium colourless, faintly yellowish to ochreous in the centre where scattered pycnidia emerge after 5–7 d. Aerial mycelium only in the centre, fluffy, white. Reverse concolourous. Colonies on MEA reaching 23–25 mm diam in 10 d, with an even to slightly undulating buff margin; immersed mycelium buff to ochreous in the centre, where also numerous densely aggregated pycnidia are formed after 5–7 d, colony surface mostly hidden under a mat of pure white, woolly-tufty aerial mycelium. Reverse in the centre chestnut, fading over fulvous to ochreous or buff near the margin.
Specimens examined. CANADA, Manitoba, Grand Beach, isolated from Arceuthobium pusillum, 25 July 1981, J. Reid, holotype CBS H-10965, living ex-type culture CBS 582.83. – GERMANY, Monheim, from leaf spot in Triticum aestivum, June 1984, M. Hossfeld 111, living culture CBS 824.84 (preserved as Coniothyrium cerealis).
Notes – See following species.
Dendrothyrium variisporum Verkley, Göker & Stielow, sp. nov. — MycoBank MB800760; Fig. 5
Fig. 5.
Dendrothyrium variisporum (CBS 121517T, ex-type culture). a. Colony on OA; b. colony on MEA; c–e. conidia and conidiogenous cells on OA. — Scale bars = 10 μm.
Etymology. Named after the variation in the shape of the conidia.
Conidiomata eustromatic, often merged to complexes reaching 400–500 μm diam with several discrete or fused cavities, dark brown to black; sporocarps on the agar surface appearing grey due to numerous colourless hyphal outgrowths. Conidiomatal wall relatively thick, composed of a single layer of textura angularis with hyaline to pale yellow, relatively thick-walled cells 4–7 μm diam. Outer surface sometimes overgrown by a diffuse web of brown, glabrous hyphae oriented parallel to the wall surface. Conidiogenous cells integrated in 1–4-septate acropleurogenous conidiophores that are simple or branched at the base, 10–18(–25) × 2.5–4 μm, phialidic, terminal cells cylindrical and slightly attenuating to the apex where sometimes one or more percurrent proliferations can be seen. Conidia variable in shape, subglobose, ellipsoid or obovoid, sometimes curved or with a broad, blunt end, initially hyaline, soon after secession with an olivaceous-brown, thin, smooth wall, contents with 1–3 minute oil-droplets, 0-septate, 3–4(–4.5) × 1.5–2.5(–3) μm, average L/W ratio 1.6 ± 0.3. Sexual morph unknown.
Colonies on OA reaching 35–38 mm diam in 10 d, with an even, glabrous and colourless margin. Immersed mycelium colourless, aerial mycelium absent. Reverse concolourous. Pycnidia formed after 7–10 d in concentrical zones. Colonies on MEA reaching 26–28 mm diam in 10 d, with an even to slightly ruffled, colourless margin. Immersed mycelium buff to ochreous but mostly hidden under a dense mat of woolly-floccose, pure white to buff, later in the centre greyish aerial mycelium. Reverse in the centre umber, fading over sienna to luteous to buff near the margin. Pycnidia formed after 10–14 d.
Specimens examined. SWITZERLAND, Zürich, isolated as endophyte of Erica carnea, July 1981, O. Petrini, living culture CBS 197.82, CBS H-10964 (dried culture). – SYRIA, isolate from declined grape vine, K.A. Halim 35, holotype CBS H-21036, living ex-type culture CBS 121517.
Notes — The branched, acropleurogenous conidiophores (Fig. 5d) that can be provided with annellidic terminal apertures are the most distinctive feature of this species. Dendrothyrium longisporum is a close relative, but morphologically quite distinct from D. variisporum by the pycnidial sporocarps with a well-developed ostiolum, more consistently cylindrical-ellipsoid conidia (average L/W ratio 2.8 vs 1.6 in D. variisporum) and the absence of branched conidiophores or annellidic conidiogenesis. Despite these differences, the multi-locus phylogenetic analysis supports the placement of the two fungi in a single genus. In contrast to D. longisporum, the two strains assigned to D. variisporum do not group in a monophyletic cluster in the multi-locus phylogeny. CBS 197.82 is nonetheless considered to be conspecific with the ex-type strain, mainly based on good agreement in phenotypic characters and because there is no support for the non-monophyly of D. variisporum (Fig. 2). Based on the material available it can be postulated that the genus Dendrothyrium is a widely dispersed genus of endophytes and (weak) plant pathogens with a wide host spectrum.
Paraconiothyrium Verkley, Stud. Mycol. 50: 327. 2004
Type species. Paraconiothyrium estuarinum Verkley & Manuela Silva, Stud. Mycol. 50: 327. 2004.
A description of the type species was provided by Verkley et al. (2004). Main features of this species are summarised in Table 3.
Table 3.
Overview of morphological characters of investigated asexual species in Montagnulaceae.
| Species | Conidiogenous cells | Conidial septa and sizes (in μm) | Average L/W ratio conidia | Conidium wall surface | Growth rate on OA (colony diam in mm after 10 d) | Reference |
|---|---|---|---|---|---|---|
| Alloconiothyrium aptrootii | Annellidic, discrete | 0-septate, 3–4(–5) × 2.5–3(–3.5) Chlamydospores globose, 6–8.5 diam | 1.2 ± 0.2 | Verruculose | 37–40 | This study |
| Dendrothyrium longisporum | Phialidic, discrete or in simple, 1–2-septate conidiophores | 0-septate, (3.5–)4–5(–6) × 1.5–2 | 2.8 ± 0.4 | Smooth | 28–32 | This study |
| D. variisporum | Phialidic, integrated in 1–4-septate acropleurogenous conidiophores | 0-septate, 3–4(–4.5) × 1.5–2.5(–3) | 1.6 ± 0.3 | Smooth | 35–38 | This study |
| Paraconiothyrium africanum | Phialidic, also proliferating percurrently, discrete | (0–)1(–3)-septate, (4–)6.5–9.5(–12) × (2.5–)3–4(–5) | 2.3 | Verruculose | 44 (7 d) | Damm et al. (2008) |
| Parac. archidendri | Holoblastic, occasionally annellidic, discrete | 0-septate, 3.5–6 × 2.5–3.5(–4) | 1.5 ± 0.2 | Smooth, or very minutely verruculose | 50–55 | This study |
| Parac. babiogorense | Phialidic, discrete | 0(–1)-septate, (7–)8–9(–10) × 1–2(–3) | – | Smooth | 5 (on PDA after 7 d, darkness, 17 °C) | Budziszewska et al. (2011) |
| Parac. brasiliense | Phialidic, discrete | 0-septate, (3–)3.4–4.6(–5) × (1.8–)2–2.3(–2.5) | 1.9 ± 0.2 | Smooth | 60–68 | Verkley et al. (2004) |
| Parac. cyclothyrioides | Phialidic, occassionally with 1–2 percurrent proliferations, integrated in compact conidiophores, rarely discrete | 0-septate, (2.5–)3–4.2(–5) × (1–)1.2–1.5(–1.8) | 2.9 ± 0.3 | Smooth | 60–68 | Verkley et al. (2004) |
| Parac. estuarinum | Phialidic, occassionally with a percurrent proliferation, discrete, sometimes integrated in compact conidiophores | 0-septate, (3–)3.2–4(–6) × 1.4–1.7(–2) | 2.4 ± 0.4 | Smooth | 60–68 | Verkley et al. (2004) |
| Parac. flavescens | phialidic, discrete | 0-septate, | – | smooth | 15 (7 d), 25 (14 d) | Boerema et al. (2004) |
| Parac. fuckelii (syn. Coniothyrium fuckelii) | Annellidic, discrete or integrated in short, simple 1–2-septate conidiophores | 0-septate, 3–4 × 2–3(–3.5) | 1.4 ± 0.2 | Smooth | 70–75 | This study |
| Parac. fungicola | Phialidic, occassionally with 1–3 percurrent proliferations, discrete | 0–1-septate, (4.2–)4.4–6.2(–7) × (2.7–)3–3.4(–3.6) | 1.7 ± 0.2 | Smooth | 30–35 | Verkley et al. (2004) |
| Parac. hawaiiense | Phialidic, also proliferating percurrently several times near apex, ocassionally polyphialidic, discrete | 1(–2)-septate, (10–)12–13 × (4–)5(–5.5) | – | Verruculose | 45 (on PDA after 2 wk, 25 °C) | Crous & Groenewald (2006) |
| Parac. lini | phialidic, discrete | 0-septate, 3.5–5.5 × 1.5–2 | – | smooth | 65 (7 d) | Boerema et al. (2004) |
| Parac. maculicutis | Phialidic, discrete | 0-septate, 1.5–2.5 × 0.5–1.5 | 1.5–3.2 | Smooth | 50–52 (7 d) | Gruyter et al. (2012) |
| Parac. variabile | Phialidic, occassionally with 1–2 percurrent proliferations, integrated in 1–3-celled conidiophores | 0-septate, (2.5–)3–4(–5) × 1–2(–2.5) | 2.2 | Smooth to fine verruculose | 43 (7 d) | Damm et al. (2008) |
| Paraconiothyrium sp.1 Microsphaeropsis pseudaspera’) | Phialidic, discrete | 0-septate, 3–4.5(–5) × 2–3 | 1.3 ± 0.2 | Smooth | 41–46 | This study |
| Paraphaeosphaeria angularis | Phialidic, discrete | 0-septate, 4.5–7(–8) × 3–4, occasionally 1-septate, 8 × 5 | 1.9 ± 0.2 (0-septate conidia) | Smooth | 53–56 | This study |
| Paraph. arecacearum | Phialidic, discrete | 0-septate, (3–)3.5–6(–8.5) × 2–3 | 2.0 ± 0.4 | Smooth | 70–75 | This study |
| Paraph. michotii1 | Phialidic, discrete (?) | 0-septate, 4–8 × 2.4–4.4 | – | Smooth, with a wrinkled sheath on mature conidia | 28 (on CMA after 7 d) | Câmara et al. (2001) |
| Paraph. minitans (syn. Paraconiothyrium minitans) | Phialidic, discrete | 0-septate, 4.5–7 × 3.5–4.5(–5) | 1.4 ± 0.4 | Verrucose | 38–45 | This study |
| Paraph. neglecta | Phialidic, discrete | 0-septate, (3–)3.5–6(–8.5) × 2–3 | 1.7 ± 0.4 | Smooth to minutely verruculose | 45–50 | This study |
| Paraph. pilleata1 | Phialidic, discrete (?) | 0-septate, 3.5–7 × 2–4 | – | Smooth | 24 (on CMA after 7 d) | Câmara et al. (2001) |
| Paraph. sardoa | Phialidic, discrete or integrated in short, simple 1–2-septate conidiophores | 0-septate, (4.5–)5–6(–7) × (3–)3.5–4.5(–5) | 1.4 ± 0.2 | Verruculose | 40–44 | This study |
| Paraph. sporulosa | Phialidic, occassionally proliferating percurrently, discrete | 0-septate, 3.5–5(–6) × 3–4 | 1.5 ± 0.2 | Smooth | 42–50 | This study |
| Paraph. verruculosa | Phialidic, discrete | 0-septate, (3–)4–5(–6) × (2.5–)3–3.5(–5) | 1.3 ± 0.2 | Verruculose | 50–54 | This study |
| Paraph. viridescens | Phialidic, occassionally proliferating percurrently, discrete | 0-septate, (3–)4–4.5(–5) × 1.8–2.2 | 2.0 ± 0.2 | Smooth | 52–55 | This study |
1 For a description of the sexual morph see Câmara et al. (2001) .
Conidiomata eustromatic, simple or complex, or pycnidial. Conidiogenous cells discrete or integrated, phialidic or holoblastic, annellidic. Conidia aseptate, sometimes 1-septate, thin- to relatively thick-walled, smooth-walled or verruculose, hyaline when liberated, later brown.
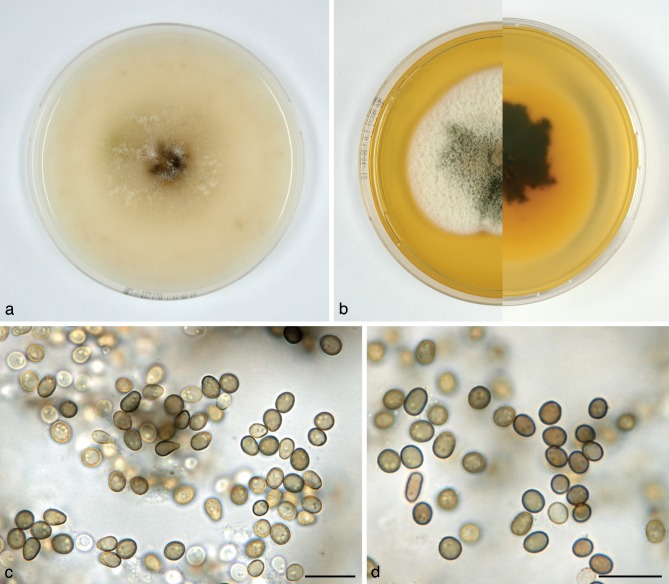
Paraconiothyrium archidendri Verkley, Göker & Stielow, sp. nov. — MycoBank MB800761; Fig. 6
Fig. 6.
Paraconiothyrium archidendri (CBS 168.77T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c–e. conidia on OA. — Scale bars = 10 μm.
Etymology. Named after the host genus, Archidendron, from which the species was isolated.
Conidiomata pycnidial, globose, with a single ostiolum 10–30 μm diam, initially glabrous and pale brown, or pilose and appearing grey, later black due to conidia produced inside, 250–350(–400) μm diam, the surface of the wall provided with hyaline to pale brown hyphal outgrowths. Conidiomatal wall composed of single layer of relatively thick-walled, pale yellowish textura angularis with cells mostly 5–10 μm diam. Conidiogenous cells discrete, globose to doliiform, holoblastic, occasionally annellidic with 1–3 percurrent proliferations, 3.5–5(–6.5) × 2.5–4 μm. Conidia variable in shape, subglobose or ellipsoid, more rarely obovoid, ends rounded, sometimes one end more or less blunt, initially hyaline, soon after secession olivaceous-brown, contents with several small oil-droplets (< 0.5 μm diam) near each end, conidial wall at maturity relatively thick, smooth, sometimes minutely verruculose, 0-septate, 3.5–6 × 2.5–3.5(–4) μm, average L/W ratio 1.5 ± 0.2. Sexual morph unknown.
Colonies on OA reaching 50–55 mm diam in 10 d, with an even, glabrous and colourless margin; immersed mycelium ochreous to cinnamon, aerial mycelium absent. Reverse concolourous. Conidiomata developing after 20–25 d. Colonies on MEA reaching 50–53 mm diam in 10 d, with an even, colourless to buff margin; immersed mycelium not visible from above, entirely hidden under a dense moderately high mat of woolly-floccose, white to greyish, in the centre weakly citrine to hazel aerial mycelium; conidiomata not observed. Reverse predominantly ochreous to fulvous, in the centre olivaceous-black with rust patches or circular zones. Conidiomata developing after 20–25 d.
Specimen examined. BURMA, E. of Yezin, Kyaukthanbut Village, on leaf spot in Pithecellobium bigeminum (= Archidendron bigeminum), Oct. 1976, M.M. Thaung, isolated by H.A. van der Aa 5654B, holotype CBS H-21037, living ex-type culture CBS 168.77.
Notes — The only strain available of this species sporulated tardily on OA and MEA with small numbers of sporocarps. It was isolated from leaf spots on the leguminose tree Archidendron bigeminum in Burma, and more material needs to be collected in order to assess its ecology and geographic distribution. In the multi-locus phylogeny, Parac. archidendri clusters with Parac. fuckelii, but this grouping is not supported by bootstrapping. The two taxa do share annellidic conidiogenesis (not seen in the other Paraconiothyrium species) and a relatively low conidium L/W ratio (1.3–1.5) compared to other members of Paraconiothyrium (≥ 1.7).
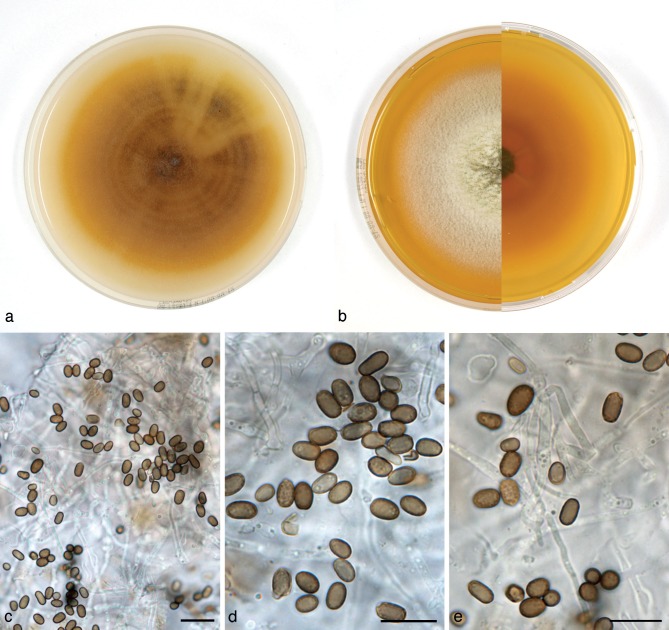
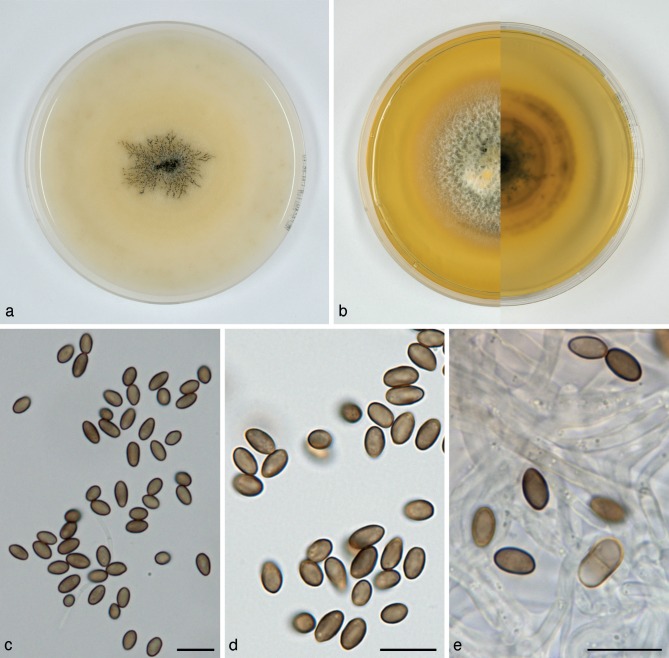
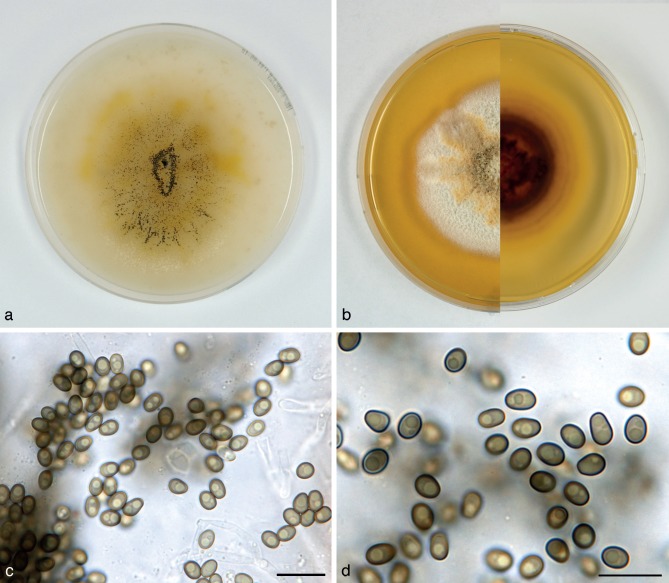
Paraconiothyrium fuckelii (Fuckel) Verkley & Gruyter, Stud. Mycol. 75: 25. 2012. — Fig. 7
Fig. 7.
Paraconiothyrium fuckelii (CBS 797.95). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c, d. conidia on OA; e. conidiogenous cells and conidia on OA. — Scale bars = 10 μm.
Basionym. Coniothyrium fuckelii Sacc., Nuovo Giorn. Bot. Ital. 7: 318. 1875 (asexual morph).
=Sphaeria coniothyrium Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 115. 1870 (sexual morph).
≡ Leptosphaeria coniothyrium (Fuckel) Sacc., Nuovo Giorn. Bot. Ital. 7: 317. 1875.
More synonyms are provided in Domsch et al. (2007), who described the sexual morph. Below only a description of the asexual morph in vitro is given.
Conidiomata pycnidial 300–400 μm diam and with a single cavity, more often eustromatic and consisting of complexes up to 1.2 mm diam, with several cavities, the outer surface black, glabrous, but often covered by a diffuse web of white to greyish hyphae. Conidiomatal wall composed of an outer layer of textura angularis with somewhat thickened, brown walls, and an inner layer of textura angularis-globulosa with somewhat thickened, hyaline walls. Conidiogenous cells discrete or integrated in short, simple, 1–2-septate conidiophores, broadly ampulliform to globose, holoblastic, often annellidic with 1 or 2 percurrent proliferations noticeable by the distinct scars on a somewhat elongated neck, hyaline, 4–10(–13) × 3–5 μm. Conidia variable in shape, subglobose to ellipsoid or obovoid, rarely more cylindrical, initially hyaline with mostly 1–3(–5) small oil-droplets (< 1 μm diam), soon after secession olivaceous-brown, conidial wall smooth, orange-brown, 0-septate, 3–4 × 2–3(–3.5) μm, average L/W ratio 1.4 ± 0.2.
Colonies on OA reaching 70–75 mm diam in 10 d, with an even, glabrous and colourless margin. Immersed mycelium in the centre faintly hazel or ochreous, aerial mycelium absent or diffuse, pure white. Reverse concolourous. Colonies on MEA reaching 60–65 mm diam in 10 d, with an even to slightly ruffled colourless margin mostly covered under the aerial mycelium. Immersed mycelium completely hidden under a dense but not high mat of woolly to woolly-floccose, glaucous grey to pale grey-olivaceous aerial mycelium. Reverse bay, fading over sienna to luteous at the margin.
Specimens examined. DENMARK, loc. unknown, isolated from root of gymnosperm, May 1969, D.S. Malla S 7(45), living culture CBS 584.69; Geelskov, on canes of Rubus sp., A.M. Dahl-Jensen, Dec. 1995, isol. G. Verkley 338, living culture CBS 797.95. – GERMANY, München, Feldberg, isolated from Picea abies with cankers, Oct. 1985, O. Kandler, living culture 653.85. – THE NETHERLANDS, from sputum of man, Nov. 1971, isolated by M. Luykx, living culture CBS 764.71B.
Notes — Wollenweber & Hochapfel (1937) included the pathogens on Rosaceae in their concept of Coniothyrium fuckelii, and this is the core of the phylogenetic species here recognised under the name Paraconiothyrium fuckelii. In the literature the name for the sexual morph Leptosphaeria coniothyrium has mostly been used, but as has been established in previous molecular studies, the species is not congeneric with the type species of Leptosphaeria, L. doliolum, which resides in the Leptosphaeriaceae (Verkley et al. 2004, de Gruyter et al. 2009). According to Domsch et al. (2007) this species has a world-wide distribution.
Paraconiothyrium sp. 1 (‘Microsphaeropsis pseudaspera’?) — Fig. 8
Fig. 8.
Paraconiothyrium sp. (‘Microsphaeropsis pseudaspera’?) (CBS 113682). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c–e. conidia on OA. — Scale bars = 10 μm.
Conidiomata pycnidial, globose to elliptical in surface view, with one or two ostioli, 8–12 μm diam, dark olivaceous-brown to black, pilose, 200–350(–400) μm diam, the surface provided with brown hyphal outgrowths emerging from a dense web of hyaline to dark brown hyphae growing parallel over the wall surface. Conidiomatal wall composed of an outer layer of relatively thin-walled brown textura angularis with cells mostly 4–6 μm diam, and an inner layer of similar but smaller, hyaline cells. Conidiogenous cells discrete, doliiform to broadly ampulliform, phialidic, with an indistinct periclinal thickening, 4–5(–6) × 3–4 μm. Conidia variable in shape, globose to subglobose or ellipsoid, more rarely obovoid, initially hyaline, contents with mostly 1–3(–5) small oil-droplets (< 1 μm diam), soon after secession olivaceous-brown, conidial wall smooth, orange-brown, 0-septate, 3–4.5(–5) × 2–3 μm, average L/W ratio 1.3 ± 0.2. Sexual morph unknown.
Colonies (CBS 113682) on OA reaching 41–46 mm diam in 10 d, with an even, glabrous colourless margin. Immersed mycelium colourless, with numerous pycnidia formed in distinct concentrical zones after 4–5 d. Reverse concolourous, appearing grey-olivaceous where pycnidia develop. Colonies on MEA reaching 32–36 mm diam in 10 d, with an even, glabrous, buff margin. Immersed mycelium in the centre olivaceous to olivaceous-black, buff in a submarginal zone, covered in the centre by olivaceous to olivaceous buff, woolly-floccose aerial mycelium, in a submarginal zone abruptly changing to pure white. Reverse in the centre umber to sienna, with dull luteous areas, fading to pale luteous at the margin.
Specimens examined. SPAIN, Santiago de Compostela, isolated from air sample, 15 Mar. 2002, M.J. Aira, deposited by A.M. Stchigel, living culture CBS 113682 (preserved as Microsphaeropsis pseudaspera). – THE NETHERLANDS, from nail of human, Apr. 1987, living culture CBS 251.87.
Notes — CBS 113682 was identified as Microsphaeropsis pseudaspera, a fungus described by Sutton (1974) from dead branches of Eucalyptus in Portugal. The conidiogenous cells and conidia of this isolate agree well with those described for this coelomycete based on material in planta. The clinical strain from the Netherlands is very similar in colony characters and other phenotypic traits, as are the sequences of CBS 251.87 generated in this study. Whether the name M. pseudaspera definitively applies to this material can only be confirmed by sequencing of the type material or recollecting from Eucalyptus.
ParaphaeosphaeriaO.E. Erikss.
Type species. Paraphaeosphaeria michotii (Westend.) O.E. Erikss., Ark. Bot., ser. 2, 6: 406. 1967.
Câmara et al. (2001) provide descriptions of sexual and asexual morphs of Paraph. michotii and Paraph. pilleata, while other species treated there under Paraphaeosphaeria were transferred later to Neophaeosphaeria and Phaeosphaeriopsis (Câmara et al. 2003). None of the amerosporic coniothyrium-like fungi associated with these sexual morphs has been assigned a formal name.
Asexual morphs classified in Paraphaeosphaeria can be described as follows:
Conidiomata eustromatic or pycnidial. Conidiogenous cells discrete or integrated, phialidic, or annellidic with one or two percurrent proliferations. Conidia aseptate or 1-septate, smooth to verrucose.
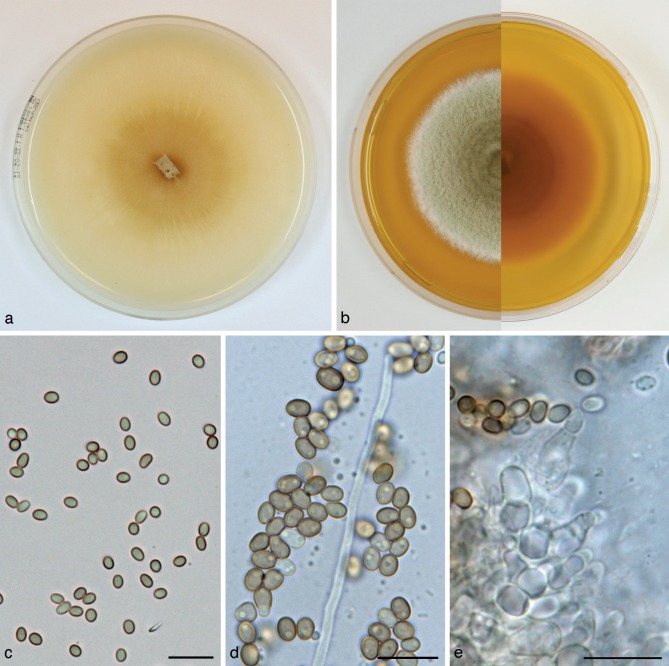
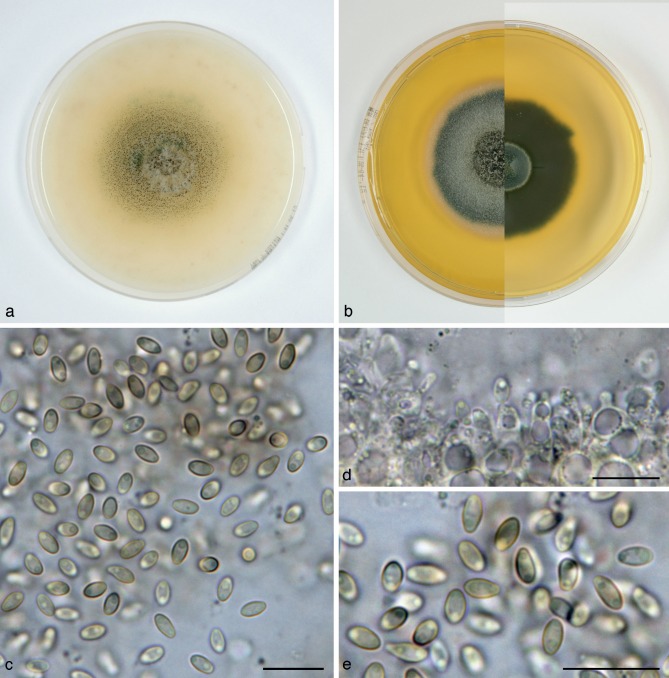
Paraphaeosphaeria angularis Verkley & Aa, sp. nov. — MycoBank MB800765; Fig. 9
Fig. 9.
Paraphaeosphaeria angularis (CBS 167.70T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c–e. conidia on OA. — Scale bars = 10 μm.
Etymology. Named after the angular shape of the conidia.
Conidiomata pycnidial, globose, ostiolum absent or with a single undifferentiated ostiolum 15–20 μm diam, pale olivaceous-brown, black due to mature conidia inside, 150–350(–450) μm diam. Conidiomatal wall composed of an outer layer of relatively thick-walled, yellowish brown textura angularis with cells 5–12 μm diam, and an inner layer of similar structure with hyaline and smaller cells. Conidiogenous cells discrete, globose to doliiform, phialidic, with an indistinct periclinal thickening, 4–7.5 × 4–6 μm. Conidia ellipsoid or elongated-ellipsoid, with a more or less clear angular outline, initially hyaline with 2–5 small oil-droplets (1–1.5 μm diam), then smoky greyish brown with relative dark, amorphous contents mostly showing no oil-droplets, often a brighter longitudinal band can be seen, conidial wall glabrous and moderately thick, 0-septate, 4.5–7(–8) × 3–4 μm, average L/W ratio 1.9 ± 0.2. Occasionally 2-celled conidia 8 × 5 μm are observed. Sexual morph unknown.
Colonies on OA reaching 53–56 mm diam in 10 d, with an even, glabrous and colourless margin. Immersed mycelium colourless, fully covered by a very diffuse mat of pure white finely felted aerial mycelium, and pycnidia developing after 3–5 d in a pattern of radiating and branching rows. Reverse concolourous, greyish where pycnidia are formed. Colonies on MEA reaching 43–46 mm diam in 10 d, with an even to slightly undulating, glabrous margin. Immersed mycelium buff, appearing darker and olivaceous or greyish in the centre where pycnidia develop, colony largely covered by a high, tufty to woolly-floccose mat of dirty white, in the centre more greyish, aerial mycelium. Reverse cinnamon to ochreous, darker where pycnidia are formed.
Specimen examined. BRAZIL, Bahia, Salvador, isolated from Saccharum officinarum, Oct. 1969, C. Ram, isol. H.A. van der Aa no. 1870, holotype CBS H-11085, living ex-type culture CBS 167.70.
Notes — A relatively high average conidial L/W ratio and especially the peculiar bright longitudinal band that can be observed over the mature conidium wall (difficult to record in photomicrographs) characterize this unique species, which is only known from a strain isolated from Saccharum officinarum in Brazil. The sexual morph is currently unknown, but since the species groups in a well-supported cluster with the pleomorphic species, Paraph. michotii and Paraph. pilleata, it would not be unlikely that it exists. The asexual morphs of these two close relatives of Paraph. angularis are otherwise highly similar (summarised in Table 3). All three species are associated with monocots.
Paraphaeosphaeria arecacearum Verkley, Göker & Stielow, sp. nov. — MycoBank MB800762; Fig. 10
Fig. 10.
Paraconiothyrium arecacearum (CBS 158.75T, ex-type culture). a. Colony on OA; b. colony on MEA; c–e. conidia on OA; f. amorphous hyphal exsudate on OA. — Scale bars = 10 μm.
Etymology. Named after the occurrence in association with genera of the family Arecaceae (= Palmae).
Conidiomata pycnidial, globose, glabrous, mostly with a rather undifferentiated single ostiolum, pale olivaceous or greenish, soon black due to mature conidia inside, 130–350 μm diam. Conidiomatal wall composed of an outer layer of relatively thick-walled, pale yellow to olivaceous textura angularis with cells mostly 4–7.5 μm diam, and an inner layer of hyaline thin-walled textura angularis-globulosa. Conidiogenous cells discrete, globose, doliiform to broadly ampulliform, phialidic with a distinct periclinal thickening, 4–6.5 × 3–4.5 μm. Conidia ellipsoid to obovoid-pyriform, initially hyaline, soon after secession olivaceous-brown, predominantly with two persistent polar oil-droplets (1.5–2 μm diam), conidial wall glabrous, 0-septate, (3–)3.5–6(–8.5) × 2–3 μm, average L/W ratio 2.0 ± 0.4. Sexual morph unknown.
Colonies on OA reaching 70–75 mm diam in 10 d, with an even to slightly ruffled, glabrous and colourless margin. Immersed mycelium colourless, aerial mycelium absent or very scanty, felted, pure white, vegetative hyphae exudating orange-brown material in amorphous masses (up to 25 μm wide) over variable length along the hyphae. Pycnidia developing after 3 d in a pattern of branching and radiating rows (but evenly distributed and numerous), in the centre also concentrated in concentrical zones after 10 d. Reverse concolourous, but appearing grey due to pycnidial development. Colonies on MEA reaching 65–69 mm diam in 10 d, with a somewhat ruffled, colourless glabrous margin. Immersed mycelium buff, appearing grey due to developing pycnidia that are completely covered by a dense, woolly-floccose to tufty, pure white to faintly greyish or luteous mat of aerial mycelium. Reverse buff to pale luteous, with greyish brown concentrical zones where the pycnidia develop.
Specimens examined. IVORY COAST, isolated from Cocos nucifera, Dec. 1975, J. Mouchacca, living culture CBS 614.75. – SURINAM, isolated from soil under Elaeis guineensis, Mar. 1974, J.H. van Emden, holotype CBS H-11048, living ex-type culture CBS 158.75.
Notes — This species is notable for its rapid growth rate and sporulation. The two cultures that are known thus far are both associated with tropical palms. Coniothyrium palmarum, the type species of Coniothyrium (Leptosphaeriaceae) is frequently found on palms as well, but that species can easily be distinguished from Paraph. arecacearum by the annellidic conidiogenesis and verrucose and 0–1-septate conidia 6–8.5 × 4–5 μm (Sutton 1980).
Paraphaeosphaeria minitans (W.A. Campb.) Verkley, Göker & Stielow, comb. nov. — MycoBank MB800766
Basionym. Coniothyrium minitans W.A. Campb., Mycologia 39: 191. 1947.
≡ Paraconiothyrium minitans (W.A. Campb.) Verkley, Stud. Mycol. 50: 332. 2004.
A detailed description of the fungus, of which the sexual morph is unknown, is provided by Domsch et al. (2007). In the past a number of CBS strains have been identified as Coniothyrium minitans (Table 1, Fig. 1), but the sequence data indicate they belong to a number of different taxa. Most of these strains were obtained from soil samples, and critical characteristics of this species like infective capability of sclerotia of Sclerotinia, were not documented.
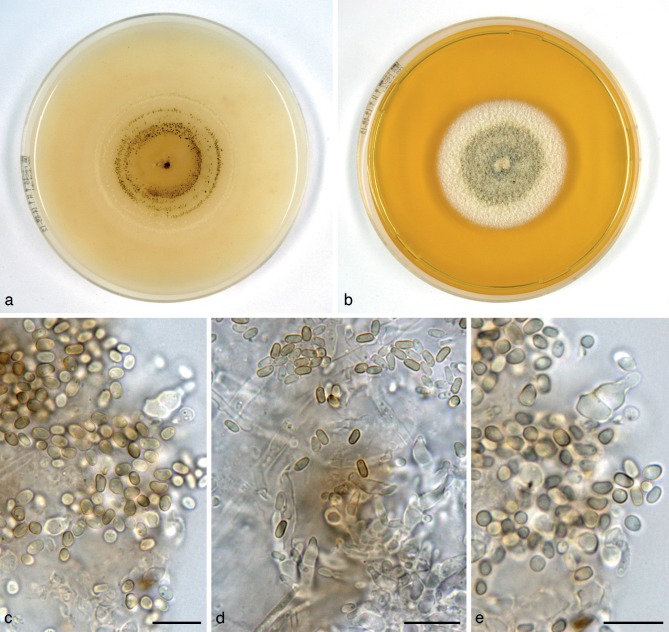
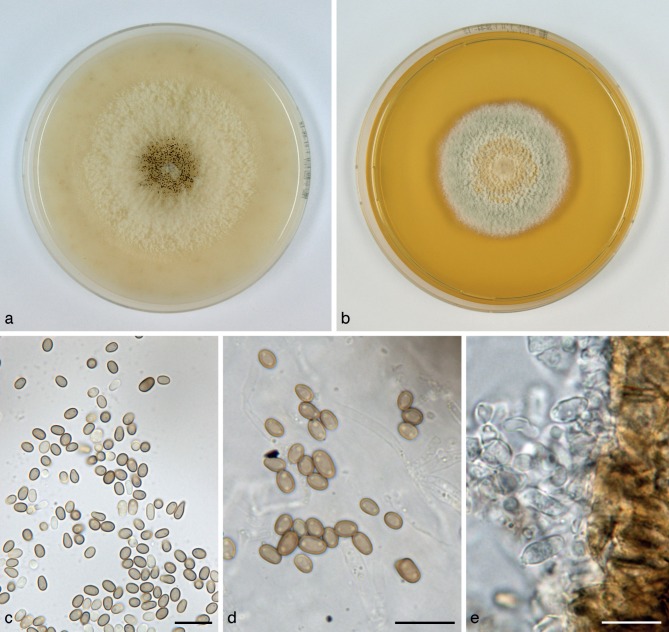
Paraphaeosphaeria neglecta Verkley, Riccioni & Stielow, sp. nov. — MycoBank MB800767; Fig. 11
Fig. 11.
Paraphaeosphaeria neglecta (CBS 124078T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse of CBS 124077 on the right; c, d. conidia on OA; e. conidiogenous cells on OA. — Scale bars = 10 μm.
Etymology. Named for the fact that this fungus was not recognised as distinct within the Paraphaeosphaeria (Paraconiothyrium) sporulosum complex.
Conidiomata pycnidial, globose, glabrous, with a single ostiolum 20–30(–50) μm diam, black due to mature conidia inside, the wall yellowish brown but cells surrounding the ostiolum darker, conidiomata 240–350 μm diam. Conidiomatal wall composed of an outer layer of yellow-brown, relatively thick-walled textura angularis, and an inner layer of similar structure but with hyaline, thin-walled cells. Conidiogenous cells discrete or positioned on clumps of cells that protrude into the cavity, globose, doliiform to broadly ampulliform, phialidic with a distinct periclinal thickening, 4–6 × 3–5 μm. Conidia highly variable in shape, subglobose, ellipsoid to obovoid-pyriform, or more cylindrical, initially hyaline, soon after secession olivaceous-brown, mostly with two polar oil-droplets (1.5–2 μm diam), and rarely with a few additional smaller ones, conidial wall glabrous or minutely roughened, 0-septate, (3–)3.5–6(–8.5) × 2–3 μm, average L/W ratio 1.7 ± 0.4 (CBS 124078T; 1.5 ± 0.3 for CBS 303.77). Sexual morph unknown.
Colonies on OA reaching 45–50 mm diam in 10 d, with an even, glabrous and colourless margin. Immersed mycelium initially colourless, then luteous sometimes with sienna centre, with rather diffuse but high, tufty, pure white aerial mycelium. Pycnidia developing in discontinuous concentrical zones or scattered after 7–10 d. Reverse concolourous. Colonies on MEA reaching 34–39 mm diam in 10 d, with an even to undulating, glabrous margin. Immersed mycelium entirely hidden under a dense mat of woolly-floccose, white to pale luteous, sometimes also glaucous to glaucous grey aerial mycelium. Reverse mostly sienna, fading to pale luteous at the margin. Pycnidia absent or developing after 12–15 d.
Specimens examined. CHILE, Valdivia, South Chilean Forest, isolated from rotten wood, June 1978, A.E. Gonzáles, living cultures CBS 335.78 and CBS 337.78 (CBS H-10913, H-10923). – FRANCE, Brest, from cankered Juniperus sp., July 1975, M. Morelet, living culture CBS 359.75; isolated from Taxus baccata, 5 Nov. 1975, I. Vegh, living culture CBS 303.77; same substrate, 14 May 1976, I. Vegh 9793, living culture CBS 305.77; isolated from Cupressocyparis leylandii, 1 Apr. 1977, I. Vegh 10145, living culture CBS 307.77. – GERMANY, Ülzen, from Erica carnea, Aug. 1970, L. Kiewnick, living culture CBS 434.71A; Freiburg, H. Courtois, living culture 431.77 (CBS H-10916, dried culture); Bavendorf, Ravensburg, on dead branches of Pyrus malus, Aug. 1981, R. Weiler (CBS H-10915, CBS H-10926), living culture CBS 452.81 isolated by H.A. van der Aa 7867. – ITALY, Latina, from wood of Actinidia chinensis var. hort. 16A, L. Riccioni, holotype CBS H-21039, living ex-type culture CBS 124078 (ER 1503); isolated from the same material CBS 124077 (ER 1501). – THE NETHERLANDS, Oostvoorne, on Pyrola rotundifolia, 3 Apr. 1971, H.A. van der Aa 2526 (CBS H-10734), living culture CBS 434.71B; Baarn, on leaf of Azalea sp., Mar. 1972, H.A. van der Aa 3012, living culture CBS 300.72; Wageningen, on Azalea sp., Feb. 1972, H. van Kesteren, living culture CBS 302.72 isolated by H.A. van der Aa 2998; loc. unknown, J.C. Went 1021a, isolated from acid mull soil, with very well decomposed leaves, Feb. 1961, living culture CBS 180.61 (VKM F-2659); Eese Estate near Steenwijk, on seed of Quercus robur, 13 Oct. 1983, H.A. van der Aa 8895, living culture CBS 683.83; Utrecht, ear of human, 15 Dec. 2005, living culture CBS 119637 isolated by J. Vlooswijk.
Notes — Nine of the isolates formerly identified as Parac. sporulosum in CBS belong to a new species, for which the name Paraph. neglecta is proposed. Paraphaeosphaeria neglecta and Paraph. sporulosa are not sister taxa and material available in this study suggests that, although both species are ubiquitous fungi occurring in soil and on various plants in different habitats, only Paraph. neglecta may be truly cosmopolitan, at least besides Europe also occurring in the Americas. Records of Paraph. sporulosa from outside Europe should be confirmed by sequence studies. Paraphaeosphaeria neglecta may have a preference for plants, as only one soil isolate belonged to that species. One isolate originated from the human ear. The colonies of Paraph. sporulosa and Paraph. neglecta look very similar especially on OA, and there is also overlap in conidial sizes. In Paraph. sporulosa conidia are 3.5–5(–6) × 3–4 μm, average L/W ratio 1.5 ± 0.2, and in Paraph. neglecta (3–)3.5–6(–8.5) × 2–3 μm, average L/W ratio 1.7 ± 0.4. Conidia for some strains of Paraph. neglecta show a minutely roughened outer wall surface, a feature not observed in Paraph. sporulosa. A further difference pertains to percurrent proliferation in the conidiogenous cells, which was observed in Paraph. sporulosa and not in Paraph. neglecta, but it should be noted that this character has proven not to be very reliable in coelomycetes.
Paraphaeosphaeria sardoa Verkley, W. Gams & Aa, sp. nov. — MycoBank MB800769; Fig. 12
Fig. 12.
Paraphaeosphaeria sardoa (CBS 501.71T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c–e. conidiogenous cells on OA; f, g. conidia on OA. — Scale bars = 10 μm.
Etymology. Named after Sardinia, where this fungus was collected.
Conidiomata pycnidial, single, or eustromatic and more complex, globose, glabrous, superficial or immersed in the agar, 300–450(–600) μm diam, initially pale but soon appearing dark brown to black due to mature conidia inside. Conidiomatal wall composed of hyaline to very pale yellowish textura angularis with moderately thickened walls, lined by a layer of globose hyaline thin-walled cells. Conidiogenous cells globose to broadly ampulliform, hyaline, discrete, or integrated in short, simple, 1–2-septate conidiophores, but more often positioned on clumps of cells that protrude into the cavity, phialidic, with a distinct periclinal thickening, 5–10 × 4.5–6 μm. Conidia variable in shape, subglobose, ellipsoid, obovoid, or pyriform, often irregular in outline, initially hyaline, after secession olivaceous-brown, verruculose, with mostly 6–12 oil-droplets up to 1 μm diam, 0-septate, (4.5–)5–6(–7) × (3–)3.5–4.5(–5) μm, average L/W ratio 1.4 ± 0.2. Sexual morph unknown.
Colonies on OA reaching 40–44 mm diam in 10 d, with an even, glabrous and colourless margin; immersed mycelium colourless, forming a glaucous pigmentation in sectors or irregular patches, or locally faintly olivaceous after 10–14 d; the surface covered by a diffuse layer of woolly to tufty, first pure white, later locally glaucous aerial mycelium. Reverse concolourous. Conidiomata developing scattered or in dense clusters mostly colourless sectors after 5–7 d. Colonies on MEA reaching 38–40 mm diam in 10 d, with an even, colourless margin; immersed mycelium not visible from above, entirely hidden under a dense but relatively low, woolly-floccose mat of aerial mycelium that shows glaucous and rosy buff to salmon areas; conidiomata developing on the agar surface underneath the aerial mycelium, scattered or in dense clusters. Reverse predominantly ochreous, locally with rust patches, olivaceous black where pycnidia are numerous.
Specimen examined. ITALY, Sardinia, on dead leaves of Smilax aspera, 18 May 1971, W. Gams, isolated by H.A. van der Aa 2568, holotype CBS H-21040, living ex-type strain CBS 501.71.
Notes — Only a single isolate was available of this species. More isolates need to become available in order to assess its ecology and geographic distribution.
Paraphaeosphaeria sporulosa (W. Gams & Domsch) Verkley, Göker & Stielow, comb. nov. — MycoBank MB800768; Fig. 13
Fig. 13.
Paraphaeosphaeria sporulosa (CBS 218.68, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c, d. conidia on OA. — Scale bars = 10 μm.
Basionym. Coniothyrium fuckelii var. sporulosum W. Gams & Domsch, Nova Hedwigia 18: 9. 1969.
≡ Coniothyrium sporulosum (W. Gams & Domsch) Aa, Verh. Kon. Ned. Akad. Wetensch., tweede sect., 68: 3. 1977.
≡ Paraconiothyrium sporulosum (W. Gams & Domsch) Verkley, Stud. Mycol. 50: 332. 2004.
Conidiomata pycnidial, single, or eustromatic and more complex, globose, superficial or immersed in the agar, glabrous, 120–250(–350) μm diam, initially pale but soon appearing dark brown to black due to mature conidia inside. Conidiomatal wall composed of hyaline to pale yellowish brown textura angularis, lined by a thin layer of globose hyaline cells. Conidia released through one, rarely two, well-developed ostioli 15–25 μm diam that are somewhat darker then the surrounding wall and lined with hyaline short clavate periphyses. Conidiogenous cells globose to ampulliform, hyaline, discrete or positioned on aggregated clumps of cells that protrude into the cavity, phialidic or with 1–2 percurrent proliferations, mostly 4.5–7 × 3.5–4.5(–5) μm. Conidia subglobose, ellipsoid or obovoid-pyriform, initially hyaline, after secession olivaceous-brown, glabrous, with one large (1.5–2 μm diam) and often also 1–2 additional smaller oil-droplets, 0-septate, 3.5–5(–6) × 3–4 μm, average L/W ratio 1.5 ± 0.2. Sexual morph unknown.
Colonies on OA reaching 42–50 mm diam in 10 d, with an even, glabrous and colourless margin. Immersed mycelium colourless but in most strains soon showing a pure yellow to pale luteous, more rarely brownish pigmentation, the surface mostly glabrous but in the centre and sometimes also elsewhere with tufts of pure white aerial mycelium. Reverse concolourous, appearing olivaceous-brown where numerous pycnidia are formed. Conidiomata developing after 7–10 d. Colonies on MEA reaching 30–35 mm diam in 10 d, with an even to slightly ruffled, colourless to buff margin. Immersed mycelium barely visible from above, appearing olivaceous in the centre, covered by a dense mat of woolly-floccose, dirty white to rosy buff or ochreous aerial mycelium. Reverse mostly pale luteous to ochreous, in the centre with darker areas of umber and chestnut.
Specimens examined. GERMANY, Kitzeberg, isolated from wheat field soil, 1963, W. Gams C353, living culture ex-isotype CBS 218.68 (CBS H-6956, isotype of Coniothyrium fuckelii var. sporulosum). Other isolates examined are listed in Table 1.
Notes — In the literature Paraph. sporulosa has been reported as a cosmopolitan soil fungus (Domsch et al. 2007), but this study sheds doubt on whether those records were based on correct identifications. Isolates in CBS from outside Europe all proved to belong to Paraph. neglecta or to other species outside the Montagnulaceae. For morphological differences with the closely related Paraph. neglecta, see the note under that species.
Paraphaeosphaeria verruculosa Verkley, Göker & Stielow, sp. nov. — MycoBank MB800770; Fig. 14
Fig. 14.
Paraphaeosphaeria verruculosa (CBS 263.85T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c, d. conidia on OA. — Scale bars = 10 μm.
Etymology. Named after the moderately roughened outer wall of the conidia.
Conidiomata pycnidial, globose, ostiolum 10–15 μm diam or absent, black due to mature conidia inside, 140–200 μm diam, predominantly formed in the aerial mycelium (on OA). Conidiomatal wall composed of an outer layer of a relatively thick-walled, orange-brown textura angularis (cells 4–8 μm diam) and an inner layer of hyaline, thin-walled textura angularis. Conidiogenous cells discrete, globose to broadly ampulliform, phialidic, 4–6 × 3–4 μm. Conidia globose, subglobose to ellipsoid, initially hyaline with mostly 3–7 small oil-droplets (< 1 μm diam), after secession olivaceous-brown and mostly with fewer but larger oil-droplets (1.5–2 μm diam), mature conidial wall orange-brown, verruculose, 0-septate, (3–)4–5(–6) × (2.5–)3–3.5(–5) μm, average L/W ratio 1.3 ± 0.2. Sexual morph unknown.
Colonies on OA reaching 50–54 mm diam in 10 d, with an even, glabrous and colourless margin. Immersed mycelium first colourless, becoming chestnut, often with a greenish haze, in the centre. Aerial mycelium very diffuse, felty, locally also with long, grey tufts. Reverse concolourous. Pycnidia developing after 12–18 d. Colonies on MEA reaching 40–44 mm diam in 10 d, with an even, colourless margin. Immersed mycelium entirely hidden under a dense, high mat of floccose aerial mycelium which is for the most pure white, but in an irregularly outlined central area grey to grey-olivaceous. Reverse chestnut in the centre (irregular outline), surrounded by umber fading to ochreous, margin pale luteous.
Specimens examined. CHILE, Valdivia, isolated from wood logs of Pinus radiata stored outdoors for 1–8 months, Nov. 1984, H.L. Peredo, living culture CBS 682.84. – COLOMBIA, Cundinamarca, Monserrate, isolated from páramo soil, after burning, Mar. 1980, W. Gams, living culture CBS 354.80. – GERMANY, Bayerischer Wald, from needle of Picea abies, Mar. 1985, H. Butin, holotype CBS H-21041, living ex-type culture CBS 263.85.
Notes — This species is characteristic for having globose to subglobose conidia at maturity with an elegant verruculose ornamentation of the outer conidium wall. It may be widely distributed in soils, and shows a preference for conifers. More material needs to become available in order to better understand its ecology.
Paraphaeosphaeria viridescens Verkley, Göker & Stielow, sp. nov. — MycoBank MB800764; Fig. 15
Fig. 15.
Paraconiothyrium viridescens (CBS 854.73T, ex-type culture). a. Colony on OA; b. colony on MEA, also showing reverse on the right; c. conidia on OA; d. conidiogenous cells on OA; e. conidia on OA. — Scale bars = 10 μm.
Etymology. Named after the green pigment this fungus produces in culture.
Conidiomata pycnidial, with a single cavity but lacking a differentiated ostiolum, black due to mature conidia inside, 250–450 μm diam. Conidiomatal wall composed of an outer layer of yellow to very pale orange-brown and thin-walled textura angularis with relatively large cells 5–12 μm diam, and an inner layer of hyaline, thin-walled textura angularis-globosa. Conidiogenous cells discrete, globose, doliiform to broadly ampulliform, phialidic with a distinct periclinal thickening, occasionally percurrently proliferating to form a neck-like protrusion, 5–7.5 × 4–5 μm. Conidia consistently ellipsoid, initially hyaline, soon after secession with a greenish yellow, thin, smooth wall, contents with 1–2 oil-droplets (< 1 μm diam) at each end, 0-septate, (3–)4–4.5(–5) × 1.8–2.2 μm, average L/W ratio 2.0 ± 0.2. Sexual morph unknown.
Colonies on OA reaching 52–55 mm diam in 10 d, with an even, glabrous colourless margin. Immersed mycelium colourless to very faintly yellow, later with a green pigment and with numerous pycnidia developing in distinct concentrical zones after 3–5 d. Reverse concolourous. Colonies on MEA reaching 40–43 mm diam in 10 d, with an even to slightly ruffled, buff margin. Immersed mycelium in the centre dark herbage green (often with bluish tinges), darkening to dull green, covered by an appressed, diffuse to more dense mat of finely felted to floccose, greyish aerial mycelium. Reverse in the centre greenish olivaceous to olivaceous, quite abruptly changing to buff at the margin.
Specimen examined. MONTENEGRO, Lake of Skadar, isolated from freshwater, Oct. 1973, M. Muntañola-Cvetkovic, No. SK 1-32, holotype CBS H-21038, living ex-type culture CBS 854.73.
Notes — The species is noted for producing a green pigment diffusing in the agar and conidia with a consistently ellipsoid shape and relatively high L/W ratio (2.0 ± 0.2), and a relatively faintly green yellowish wall at maturity. CBS 854.73T originates from fresh water and it remains unclear if the fungus also occurs in soils or plants, as do most of its close relatives.
DISCUSSION
By combining multi-locus DNA sequencing with detailed morphological analyses, we were able to delimit and formally propose nine new species and two new genera among the fungi in the Montagnulaceae formerly recognisable as coniothyrium-like asexual morphs. The genus Paraconiothyrium as accepted here appears paraphyletic, but because the branches that conflict with its monophyly are insufficiently supported, it was decided not to split it up into further genera. We furthermore demonstrated that the diversity of soil-borne fungi in this family is considerable, as 12 out of 24 species studied here are found in soils. Four of these soil-borne fungi are novel species proposed here, viz. Paraph. arecacearum, Paraph. neglecta, Paraph. verruculosa and Alloconiothyrium aptrootii, while well-known species such as Paraph. sporulosa (Coniothyrium sporulosum) and Paraconiothyrium fuckelii are now more accurately delimited and described compared to manuals available for identifying soil fungi (Domsch et al. 2007). Many isolates deposited in the CBS collection under these two older names needed to be re-identified (Table 1), including a number of strains proven not to belong in Montagnulaceae by LSU and ITS sequencing. These will be treated in forthcoming publications focussing on other families of the Pleosporales.
Morphological characters traditionally used to delimit genera in coelomycetes include conidiomatal structure, structure of the conidiophores, conidiogenesis and conidial characters such as pigmentation, septal structure and number, and conidial appendages (Sutton 1980, Nag Raj 1993). Recent molecular studies have shown that these features are not always suitable to delimit genera as natural entities, and they may vary even between sibling species (Crous et al. 2012). Generic boundaries drawn in the present study were based primarily on statistically well-supported branches in the multi-locus phylogeny. Some of the characters mentioned above are thus overlapping between the accepted genera. For example, phialidic and annellidic conidiogenesis occur both in Paraconiothyrium and Paraphaeosphaeria (Verkley et al. 2004, Damm et al. 2008). Furthermore, in the new genus Dendrothyrium the conidiogenous cells are phialidic in both species, but the conidiophores of the type species are acropleurogenous, while those of the other species are acrogenous, a difference that in earlier coelomycete taxonomy would normally not be acceptable in the same genus.
For an accurate species identification of coniothyrium-like fungi, a molecular evaluation has been long overdue, as some species are morphologically very similar and difficult to distinguish based on available literature. Also, with reference to the taxa treated in the present work, it is required to first ascertain the phylogenetic position of the fungus in the Montagnulaceae, as similar fungi occur in other Pleosporales as well; LSU can be used to determine the order and mostly also the family to which the fungus belongs. ITS alone might suffice for an accurate identification of most species, as it is sufficiently variable among most closely related taxa in Montagnulaceae, but it fails to distinguish all species. Better suited for this purpose are, in principle, more variable (Table 2) genes such as ACT and TUB. Whereas ACT did not result that well in Montagnulaceae molecular taxonomy, optimised TUB sequence clustering yielded exactly the proposed classification into species with the exception of a single species complex. But when careful morphological analyses are conducted on fresh isolates under standard conditions and media, most species with (almost) identical ITS sequences can also be distinguished by colony features and conidial characters such as ornamentation of the wall, contents and L/W ratio. The main characters are summarised in Table 3.
Apart from occurring in soils, most taxa also colonise tissues of plants that are evolutionary diverse. This study is just one more to show that the host-based taxonomy commonly practised by many (coelomycete) mycologists in the last century was inadequate. Highly host-specific species do exist, but this should be corroborated by infection studies including multiple plant species. It is unlikely that they are more common than generalists.
When Damm et al. (2008) introduced Parac. variabile, the species was known from wood necroses and pycnidia on the bark of Prunus persica and wood necroses in P. salicina in South Africa, leaves of Laurus nobilis in Turkey, and wood of decaying trunks and vines of Actinidia in Italy (Riccioni et al. 2007). In the present study it was shown that this fungus occurs on more matrices in various habitats, viz. on various distantly related plants in Europe and Africa, including Chamaerops, Spartium, Dianthus, Platanus (in pruning wounds) and on sori of Puccinia rusts. It also occurs in soils (Egypt, The Netherlands) and sandstone (France). CBS 680.83 was isolated from a toenail of man, but other clinical records are unknown. The closely related Parac. brasiliense was introduced by Verkley et al. (2004) for CBS 100299 isolated from coffee fruit in Brazil, but this species has later been reported from various habitats on other continents as well. Most records pertain to woody and herbaceous host plants, especially Prunus spp. in South Africa (Damm et al. 2008). Near-identical ITS sequences have been deposited in GenBank for endophytes isolated from trees like Ginkgo biloba (DQ094168), Juniperus virginiana (Hoffman & Arnold 2008), and Ulmus davidiana var. japonica (AB665311), and also from the herb Alliaria petiolata (EF432267). In the present study, also CBS 395.87 from soil sampled in Italy could be identified as Parac. brasiliense. The ACT and TUB sequences of strains available in the present study were more variable than in other related species, suggesting that Parac. brasiliense could be a species complex. However, the internal branches of the multilocus phylogeny were insufficiently supported to split it up.
Acknowledgments
We thank the technical staff of the CBS Collections (cultures), Marjan Vermaas (photographic plates) and Hans de Gruyter and Joyce Woudenberg (primers). This work was supported through funding from the European Community’s Seventh Framework Programme (FP7, 2007-2013), Research Infrastructures action, under the grant agreement No. FP7-228310 (EMbaRC project).
REFERENCES
- Aveskamp MM, Gruyter J de, Woudenberg JHC, Verkley GJM, Crous PW. 2010. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp MM, Verkley GJM, Gruyter J de, Murace MA, Perelló A, et al. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellea and multiple taxonomic novelties. Mycologia 101: 363–382 [DOI] [PubMed] [Google Scholar]
- Baker RH, DeSalle R. 1997. Multiple sources of character information and the phylogeny of Hawaiian Drosophilids. Systematic Biology 46: 654–673 [DOI] [PubMed] [Google Scholar]
- Baker RH, Yu XB, DeSalle R. 1998. Assessing the relative contribution of molecular and morphological characters in simultaneous analysis trees. Molecular Phylogenetics and Evolution 9: 427–436 [DOI] [PubMed] [Google Scholar]
- Balajee SA, Sigler L, Brandt ME. 2007. DNA and the classical way: Identification of medically important molds in the 21st century. Medical Mycology 45: 475–490 [DOI] [PubMed] [Google Scholar]
- Bestagno Biga ML, Ciferri R, Bestagno G. 1958. Ordinamento artificiale delle specie del genere Coniothyrium Corda. Sydowia, Annales Mycologici ser. II, 12: 258–320 [Google Scholar]
- Boerema GH, Gruyter J de, Noordeloos ME, Hamers MEC. 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI Publishing, Wallingford, United Kingdom [Google Scholar]
- Budziszewska J, Szypula W, Wilk M, Wrzosek M. 2011. Paraconiothyrium babiogorense sp. nov., a new endophyte from fir club moss Huperzia selago (L.) Bernh. ex Schrank & Mart. (Huperziaceae). Mycotaxon 115: 457–468 [Google Scholar]
- Câmara MPS, Palm ME, Berkum P van, Stewart EL. 2001. Systematics of Paraphaeosphaeria: a molecular and morphological approach. Mycological Research 105: 41–56 [Google Scholar]
- Câmara MPS, Ramaley AW, Castlebury LA, Palm ME. 2003. Neophaeosphaeria and Phaeosphaeriopsis, segregates of Paraphaeosphaeria. Mycological Research 107: 516–522 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Carisse O, Bernier J. 2002a. Effect of environmental factors on growth, pycnidial production and spore germination of Microsphaeropsis isolates with biocontrol potential against apple scab. Mycological Research 106: 1455–1462 [Google Scholar]
- Carisse O, Bernier J. 2002b. Microsphaeropsis ochracea sp. nov. associated with dead apple leaves. Mycologia 94: 297–301 [PubMed] [Google Scholar]
- Carisse O, El-Bassam S, Benhamou N. 2001. Effect of Microsphaeropsis sp. strain P130A on germination and production of sclerotia of Rhizoctonia solani and interaction between the antagonist and the pathogen. Phytopathology 91: 782–791 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ. 2006. Microdiplodia hawaiiensis. Fungal Planet, no. 7. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands [Google Scholar]
- Crous PW, Summerell BA, Swart L, Denman S, Taylor JE, et al. 2011. Fungal pathogens of Proteaceae. Persoonia 27: 20–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Christensen M, Castañeda-Ruiz RF, Groenewald JZ. 2012. How important are conidial appendages? Persoonia 28: 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. 2009. Fungal biodiversity. CBS Laboratory Manuals Series 1. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands [Google Scholar]
- Damm U, Verkley GJM, Crous PW, Fourie PH, Haegi A, Riccioni L. 2008. Novel Paraconiothyrium species on stone fruit trees and other woody hosts. Persoonia 20: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsch KH, Gams W, Anderson T-H. 2007. Compendium of soil fungi. Second edition IHW Verlag, Eching [Google Scholar]
- El-Bassam S, Benhamou N, Carisse O. 2002. The role of melanin in the antagonistic interaction between the apple scab pathogen Venturia inaequalis and Microsphaeropsis ochracea. Canadian Journal of Microbiology 48: 349–358 [DOI] [PubMed] [Google Scholar]
- Farris J. 1972. Estimating phylogenetic trees from distance matrices. The American Naturalist 106: 645–667 [Google Scholar]
- Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. Journal of Molecular Evolution 17: 368–376 [DOI] [PubMed] [Google Scholar]
- Fitch WM. 1971. Towards defining the course of evolution: minimal change for a specified tree topology. Systematic Zoology 20: 406–416 [Google Scholar]
- Göker M, García-Blázquez G, Voglmayr H, Tellería MT, Martín MP. 2009a. Molecular taxonomy of phytopathogenic fungi: a case study in Peronospora. PLoS ONE 4: e6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göker M, Voglmayr H, García-Blázquez G, Oberwinkler F. 2009b. Species delimitation in downy mildews: the case of Hyaloperonospora in the light of nuclear ribosomal internal transcribed spacer and large subunit sequences. Mycological Research 113: 308–325 [DOI] [PubMed] [Google Scholar]
- Gordon RA, Sutton DA, Thompson EH, Shrikanth V, Verkley GJM, et al. 2012. Cutaneous phaeohyphomycosis caused by Paraconiothyrium cyclothyrioides. Journal of Clinical Microbiology 50: 3795–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW. 2009. Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research 113: 508–519 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. 2010. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. 2012, ‘2013’. Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess PN, Moraes Russo CA de. 2007. An empirical test of the midpoint rooting method. Biological Journal of the Linnean Society 92: 669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MT, Arnold AE. 2008. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycological Research 112: 331–344 [DOI] [PubMed] [Google Scholar]
- Ivanova NV, deWaard J, Hebert PDN. 2006. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes 6: 998–1002 [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario [Google Scholar]
- Pattengale ND, Alipour M, Bininda–Emonds ORP, Moret BME, Stamatakis A. 2009. How many bootstrap replicates are necessary? Lecture Notes in Computer Science 5541: 184–200 [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, Barreto RW, Alfenas AC, et al. 2013. Sizing up Septoria. Studies in Mycology 75: 307–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew, UK [Google Scholar]
- Riccioni L, Manning M, Valvassori M, Haegi A, Casanato S, Spinelli R. 2007. A new disease: leader die-back in Actinidia chinensis Hort16A in Italy. Acta Horticulturae 753: 669–676 [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences 109: 6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M da, Cerniglia CE, Pothuluri JV, Canhos VP, Esposito E. 2003a. Screening filamentous fungi isolated from estuarine sediments for the ability to oxidize polycyclic aromatic hydrocarbons. World Journal of Microbiology and Biotechnology 19: 399–405 [Google Scholar]
- Silva M da, Umbuzeiro GA, Pfenning LH, Canhos VP, Esposito E. 2003b. Filamentous fungi isolated from estuarine sediments contaminated with industrial discharges. Soil and Sediment Contamination 12: 345–356 [Google Scholar]
- Someya A, Yaguchi T, Udagawa S-I. 1997. Microsphaeropsis rugospora, a new species from Japanese soil. Mycoscience 38: 429–431 [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 75: 758–771 [DOI] [PubMed] [Google Scholar]
- Stielow B, Bratek Z, Orczán KA, Rudnoy S, Hensel G, et al. 2011. Species delimitation in taxonomically difficult fungi: the case of Hymenogaster. PLoS ONE 6: e15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stielow B, Bubner B, Hensel G, Munzenberger B, Hoffmann P, et al. 2010. The neglected hypogeous fungus Hydnotrya bailii Soehner (1959) is a widespread sister taxon of Hydnotrya tulasnei (Berk.) Berk. & Broome (1846). Mycological Progress 9: 195–203 [Google Scholar]
- Sutton BC. 1974. Miscellaneous coelomycetes on Eucalyptus. Nova Hedwigia 25: 161–172 [Google Scholar]
- Sutton BC. 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. CMI, Kew, UK [Google Scholar]
- Swofford DL. 2002. PAUP*: Phylogenetic analysis using parsimony (*and other methods), Version 4. Sinauer Associates, Sunderland, Massachusetts [Google Scholar]
- Vaas LAI, Sikorski J, Michael V, Göker M, Klenk H-P. 2012. Visualization and curve-parameter estimation strategies for efficient exploration of Phenotype Microarray kinetics. PLoS ONE 7: e34846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin HD, Crous PW. 2013. A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJM, Silva M da, Wicklow DT, Crous PW. 2004. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323–335 [Google Scholar]
- Vu TD, Eberhardt U, Szöke S, Groenewald M, Robert V. 2012. A laboratory information management system for DNA barcoding workflows. Integrative Biology 4: 744–755 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322. Academic Press, New York, USA [Google Scholar]
- Wollenweber HW, Hochapfel H. 1937. Beitrage zur Kenntnis parasitarer und saprophtischer Pilze. IV. Z. Parasitenkunde 9: 600–638 [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. 2012. Pleosporales. Fungal Diversity 53: 1–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de, et al. 2009. Multilocus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102 [DOI] [PMC free article] [PubMed] [Google Scholar]