Abstract
Species of Diaporthe are important plant pathogens of a wide range of hosts worldwide. In the present study the species causing melanose and stem end rot diseases of Citrus spp. are revised. Three species of Diaporthe occurring on Citrus are characterised, including D. citri, D. cytosporella and D. foeniculina. Morphology and phylogenetic analyses of the complete nuclear ribosomal internal transcribed spacer regions and partial sequences of actin, beta-tubulin, calmodulin and translation elongation factor 1-α were used to resolve species on Citrus and related Diaporthe species. Diaporthe citri occurs on Citrus throughout the Citrus-growing regions of the world. Diaporthe cytosporella is found on Citrus in Europe and California (USA). Diaporthe foeniculina, including the synonym D. neotheicola, is recognised as a species with an extensive host range including Citrus. Diaporthe medusaea, a name widely used for D. citri, was determined to be a synonym of D. rudis, a species with a broad host range. Diaporthe citri is delimited based on molecular phylogenetic analysis with the inclusion of the conserved ex-type and additional collections from different geographic locations worldwide. Diaporthe cytosporella, D. foeniculina and D. rudis are epitypified, fully described and illustrated with a review of all synonyms based on molecular data and morphological studies. Newly designed primers are introduced to optimise the amplification and sequencing of calmodulin and actin genes in Diaporthe. A discussion is provided of the utility of genes and the need for multi-gene phylogenies when distinguishing species of Diaporthe or describing new species.
Keywords: epitypification, genealogical sorting index, melanose, multi-gene phylogeny, new primers, Phomopsis, species recognition, stem end rot, systematics
INTRODUCTION
The genus Diaporthe is an economically important group of plant pathogenic fungi causing diseases on a wide range of crops, ornamentals and forest trees (Farr et al. 2002a, b, Crous 2005, Udayanga et al. 2011). Accurate species identification is vital for controlling the diseases caused by these fungi as well as for implementing quarantine regulations (Rossman & Palm-Hernández 2008, Cai et al. 2011, Shivas & Cai 2012). Until recently, species of Diaporthe have been defined based on morphology and host association. However, patterns of host association and speciation have yet to be fully understood within Diaporthe. Multiple species of Diaporthe can often be found on a single host and a single species of Diaporthe can be associated with many different hosts (Crous 2005, van Niekerk et al. 2005, Santos & Phillips 2009, Diogo et al. 2010, Gomes et al. 2013). Using molecular data, much progress has been made towards identifying and characterising emerging pathogens, prevalent endophytes and saprobes in the genus Diaporthe (Santos & Phillips 2009, Diogo et al. 2010, Luongo et al. 2011, Udayanga et al. 2012a, b, Thomidis et al. 2013).
Modern systematic accounts of Diaporthe have used DNA sequence data as the most accurate means to circumscribe species within this genus (Rehner & Uecker 1994, Castlebury et al. 2003, van Rensburg et al. 2006). Markers used in contemporary phylogenetic revisions include the complete nuclear ribosomal internal transcribed spacer regions (ITS) and more recently partial sequences of actin (ACT), beta-tubulin (TUB), calmodulin (CAL), histone H3 (HIS), mating type genes (MAT 1-1-1 and MAT 1-2-1) and translation elongation factor 1-alpha (EF1-α) (van Niekerk et al. 2005, Diogo et al. 2010, Santos et al. 2010, Udayanga et al. 2012a, b, Gomes et al. 2013). Multi-gene phylogenetic species delineation has become the most effective tool for taxonomic studies of fungi compared to traditional mating experiments and morphology (Taylor et al. 2000, Dettman et al. 2003). Although the ITS region is often useful for identification of Diaporthe species, multi-gene phylogenetic analyses are required for accurate reconstruction of species boundaries and relationships (Udayanga et al. 2012a, Gomes et al. 2013). Intraspecific variation observed in ITS sequences in several species of Diaporthe can cause confusion in species recognition when used alone (Farr et al. 2002a, b, Santos et al. 2010).
Diaporthe citri is a pathogen that causes melanose and stem end rot disease of Citrus spp. throughout the world (Whiteside & Timmer 2000a, Mondal et al. 2007). Melanose disease can affect young leaves and fruits of different species and varieties of Citrus causing black blemishes on fruit rind and small, black, raised lesions often surrounded by yellow necrotic halos (Timmer & Kucharek 2001). Symptoms of the disease may vary with host variety, geographic location, seasonal occurrence, ecophysiological factors and severity of infection (Timmer & Fucik 1976, Whiteside 1977, Kucharek et al. 1983). The range of symptoms varies from small spots, scab lesions and mudcake to star melanose on different tissues of Citrus spp. (Timmer 2000, Whiteside & Timmer 2000a, Agostini et al. 2003). Perithecia and pycnidia are only produced on dead and dying twigs and on fruit affected by stem end rot. Because perithecia are rarely formed, conidia produced by pycnidia are the primary source of inoculum (Bach & Wolf 1928, Kuhara 1999).
Although the biology and epidemiology of melanose are well studied, the phylogenetic relationships of the causal organisms, genetic variability and population structure have not been investigated (Burnett 1962, Moherek 1970, Mondal et al. 2004, 2007). Diaporthe pathogens of Citrus are usually identified as D. citri in taxonomic and plant pathological studies and regional checklists (Timmer & Kucharek 2001, Udayanga et al. 2011). In addition to D. citri, several other species of Diaporthe have been reported from Citrus, often as Phomopsis. These include D. citrincola described from the Philippines, Phomopsis californica from California, P. caribaea from Cuba and P. cytosporella (as Phoma cytosporella) from Italy, which have all previously been considered synonyms of D. citri (Rehm 1914, Fawcett 1922, 1936, Horne 1922). Yamato (1976) recognised four unidentified morphological species on Citrus spp. in Japan. Diaporthe citri was also considered a synonym of D. medusaea by Wehmeyer (1933) who also listed D. californica, P. citri and P. citrincola as host or ecological forms of D. medusaea. Others followed this synonymy including Punithalingam & Holliday (1973) and Whiteside & Timmer (2000a). The name D. medusaea is used in several articles and checklists for the fungus causing melanose and stem end rot, therefore, the true host range and geographic distribution of D. citri are difficult to determine (Kobayashi 1970, Pantidou 1973, French 1987).
Given the vague species concept of D. citri and its broad application, a modern taxonomic and phylogenetic reappraisal of D. citri and other Diaporthe species on Citrus is necessary. In this study, we analyse DNA sequence data from recent collections of Diaporthe isolated from Citrus and other hosts in Asia, Europe and the United States to accurately identify the taxa associated with Citrus. The objectives of this study are: 1) to define the species of Diaporthe on Citrus worldwide based on phylogenetic analysis of multi-gene sequence data, the genealogical sorting index and morphological characters; 2) to resolve taxonomic and nomenclatural uncertainty by providing modern descriptions for D. citri and designating epitypes for D. cytosporella, D. foeniculina and D. rudis and their synonyms; 3) to evaluate their host range and geographic distribution; and 4) to assess the utility of individual genes for accurate circumscription of these species.
MATERIALS AND METHODS
Isolates and morphology
Strains of Diaporthe from Citrus hosts were obtained from China, Korea, New Zealand, Spain and the United States (California, Florida and Texas). These strains have been isolated from specimens with typical symptoms of Citrus melanose and stem end rot as well as saprobes on twigs and branches. Isolates from other hosts were obtained from culture collections including CBS (The Netherlands), Fawcett Laboratory, University of California, Riverside (CA, USA), ICMP (New Zealand), MFLUCC (Thailand) and the SMML, USDA-ARS (MD, USA) and various contributors listed in Table 1. Morphological descriptions are based on sporulating pycnidia from inoculated alfalfa stems placed on 1.5 % water agar (WA) for living cultures as well as type and other specimens. Digital images of fruiting bodies were captured using a Discovery V20 stereomicroscope and AxioCam digital camera (Carl Zeiss Microscopy, Thornwood, NY, USA) imaging system. Whenever possible, 20–30 measurements were made of the structures mounted in 5 % KOH using a Carl Zeiss Axioplan2 compound light microscope using the 40× or 100× objectives. The extreme measurements are given in parentheses with mean and standard deviation. Three sets of duplicate cultures of each isolate were used for determining colony characters on potato-dextrose agar (PDA, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 25 °C in the dark following the methods of Brayford (1990). Colony diameters on PDA were recorded at intervals of 24 h for 1 wk and used to calculate the growth rate of eight replicates per isolate. After 1 wk, colony size and colour of the colonies (Rayner 1970) and zonation were recorded.
Table 1.
Isolates used and genes sequenced in this study.
| Species | Isolate no.1 | Host | Origin | Collector/contributor | GenBank accession no. |
||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | EF 1-α | TUB | ACT | CAL | |||||
| D. alleghaniensis | CBS 495.72* | Betula alleghaniensis | Canada: Ontario | R. Arnold | FJ889444 | GQ250298 | KC843228 | JQ807299 | KC343249 |
| D. australafricana | CBS 113487* | Vitis vinifera | South Africa | L. Mostert | KC343039 | KC843099 | JX275457 | KC843265 | JX197448 |
| CBS 111886 | Vitis vinifera | Australia | R.W.A. Schepers | KC343038 | KC343764 | KC344006 | – | KC343280 | |
| AR5209 = CBS 135771 | Persea americana | USA: California | Akif Eskalen | KF199875 | KF199877 | KF199879 | KF199883 | KF199881 | |
| AR5210 = CBS 135772 | Persea americana | USA: California | Akif Eskalen | KF199876 | KF199878 | KF199880 | KF199884 | KF199882 | |
| D. canthii | CBS 132533* | Canthium inerme | South Africa | P.W. Crous | JX069864 | KC843120 | KC843230 | KC843291 | KC843174 |
| D. citri | AR3405* = CBS 135422 | Citrus sp. | USA: Florida | L.W. Timmer | KC843311 | KC843071 | KC843187 | KC843234 | KC843157 |
| AR3404 | Citrus sp. | USA: Florida | L.W. Timmer | KC843316 | KC843076 | KC843192 | KC843239 | KC843162 | |
| AR3406 | Citrus sp. | USA: Florida | L.W. Timmer | KC843320 | KC843080 | KC843196 | KC843243 | KC843166 | |
| AR4469 = CBS 135423 | Citrus sp. | USA: Florida | L.W. Timmer | KC843321 | KC843081 | KC843197 | KC843244 | KC843167 | |
| AR4470 | Citrus sp. | USA: Florida | L.W. Timmer | KC843318 | KC843078 | KC843194 | KC843241 | KC843164 | |
| AR4471 | Citrus sp. | USA: Florida | L.W. Timmer | KC843317 | KC843077 | KC843193 | KC843240 | KC843163 | |
| FAU583 = CBS 135424 | Citrus paradisi | USA: Florida | F.A. Uecker | KC843327 | KC843087 | KC843203 | KC843250 | KC843173 | |
| AR3403 | Citrus sp. | USA: Florida | L.W. Timmer | KC843310 | KC843070 | KC843186 | KC843233 | KC843156 | |
| AR4473 | Citrus sp. | USA: Florida | L.W. Timmer | KC843319 | KC843079 | KC843195 | KC843242 | KC843165 | |
| AR3407 | Citrus sp. | USA: Florida | L.W. Timmer | KC843313 | KC843073 | KC843189 | KC843236 | KC843159 | |
| AR4472 | Citrus sp. | USA: Florida | L.W. Timmer | KC843312 | KC843072 | KC843188 | KC843235 | KC843158 | |
| AR4364 = CBS 135425 | Citrus unshiu cv. juwadeun | Korea: Odeung-dong | S.K. Hong | KC843326 | KC843086 | KC843202 | KC843249 | KC843172 | |
| AR4370 = CBS 135426 | Citrus unshiu cv. juwadeun | Korea: Odeung-dong | S.K. Hong | KC843324 | KC843084 | KC843200 | KC843247 | KC843170 | |
| AR4350 | Citrus unshiu cv. juwadeun | Korea: Odeung-dong | S.K. Hong | KC843325 | KC843085 | KC843201 | KC843248 | KC843171 | |
| CBS 135767 | Citrus reticulata | China | D. Udayanga | KC843322 | KC843082 | KC843198 | KC843245 | KC843168 | |
| DA103 = CBS 135427 | Citrus reticulata | China | D. Udayanga | KC843323 | KC843083 | KC843199 | KC843246 | KC843169 | |
| ICMP 10355 | Citrus reticulata | New Zealand: Kerikeri | G.J. Samuels | KC843314 | KC843074 | KC843190 | KC843237 | KC843160 | |
| ICMP 6981 | Citrus sp. | USA: Texas | G.J. Samuels | KC843315 | KC843075 | KC843191 | KC843238 | KC843161 | |
| D. cotoneastri | CBS 439.82* | Cotoneaster sp. | UK: Scotland | H. Butin | FJ889450 | GQ250341 | JX275437 | KC843231 | JX197429 |
| DP0667 = CBS 135428 | Juglans cinerea | USA: North Carolina | S. Anagnostakis | KC843328 | KC843121 | KC843229 | KC843232 | KC843155 | |
| D. cynaroidis | CBS 122676* | Protea cynaroidis | South Africa | P.W. Crous | EU552122 | EU552093 | KC344026 | – | KC343300 |
| D. cytosporella | FAU461 = CBS 137020 | Citrus limon | Spain | M. Palm | KC843307 | KC843116 | KC843221 | KC843285 | KC843141 |
| AR5148 | Citrus sinensis | USA: California | A. Eskalen | KC843308 | KC843117 | KC843222 | KC843286 | KC843142 | |
| AR5149* | Citrus sinensis | USA: California | A. Eskalen | KC843309 | KC843118 | KC843222 | KC843287 | KC843143 | |
| D. foeniculina | FAU460 | Citrus limon | Spain | M. Palm | KC843304 | KC843113 | KC843218 | KC843282 | KC843138 |
| MEP1289-1 | Citrus limon | Spain | D. Grenier | KC843305 | KC843114 | KC843219 | KC843283 | KC843139 | |
| FAU462 = CBS 135429 | Citrus limon | Spain | M. Palm | KC843292 | KC843101 | KC843206 | KC843270 | KC843126 | |
| ICMP 6986 | Citrus limon | New Zealand: Hope | – | KC145897 | KC145989 | – | – | – | |
| ICMP 6970 | Acacia sp. | New Zealand: Auckland | G.J. Samuels | KC145896 | KC145984 | – | – | – | |
| ICMP 12285 | Juglans regia | New Zealand | K. Knight | KC145853 | KC145937 | – | – | – | |
| ICMP 6987 | Malus domestica | New Zealand: Nelson | G.J. Samuels | KC145894 | KC145990 | – | – | – | |
| ICMP 17058 | Paraserianthes lophantha | New Zealand: Auckland | C.F. Hill | KC145842 | KC145977 | – | – | – | |
| ICMP 11892 | Fuchsia excorticata | New Zealand: Taupo | J.M. Young | KC145898 | KC145931 | – | – | – | |
| DP0454 | Ribes nigrum | New Zealand: Nelson | C.F. Hill | KC843297 | KC843106 | KC843211 | KC843275 | KC843131 | |
| AR3607 = STE-U2654 | Vitis vinifera | South Africa | L. Mostert | AF230743 | JQ807419 | KC843204 | JQ807344 | KC843123 | |
| DP0392 = CBS 111554 | Foeniculum vulgare | Portugal: Marcos | A.J.L. Phillips | KC843296 | KC843105 | KC843210 | KC843274 | KC843130 | |
| DP0391 = CBS 111553* | Foeniculum vulgare | Portugal: Marcos | A.J.L. Phillips | KC843295 | KC843104 | KC843209 | KC843273 | KC843129 | |
| AR5151 | Citrus latifolia | USA: California | A. Eskalen | KC843303 | KC843112 | KC843217 | KC843281 | KC843137 | |
| D. foeniculina (cont.) | AR5142 = CBS 135430 | Citrus limon | USA: California | A. Eskalen | KC843301 | KC843110 | KC843215 | KC843279 | KC843135 |
| AR5145 | Citrus limon | USA: California | A. Eskalen | KC843306 | KC843115 | KC843220 | KC843284 | KC843140 | |
| AR5147 | Citrus limon | USA: California | A. Eskalen | KC843299 | KC843108 | KC843213 | KC843277 | KC843133 | |
| AR5144 | Citrus limon | USA: California | A. Eskalen | KC843302 | KC843111 | KC843216 | KC843280 | KC843136 | |
| AR5143 | Citrus limon | USA: California | A. Eskalen | KC843294 | KC843103 | KC843208 | KC843294 | KC843128 | |
| AR5146 | Citrus limon | USA: California | A. Eskalen | KC843298 | KC843107 | KC843212 | KC843298 | KC843132 | |
| AR5150 = CBS 135431 | Citrus latifolia | USA: California | A. Eskalen | KC843293 | KC843102 | KC843207 | KC843293 | KC843127 | |
| AR5152 | Citrus latifolia | USA: California | A. Eskalen | KC843300 | KC843109 | KC843214 | KC843300 | KC843134 | |
| D. foeniculina (syn. P. theicola) | CBS 187.27* | Camellia sinesis | Italy | M. Curzi | DQ286287 | DQ286261 | JX275463 | JQ807298 | KC843122 |
| D. foeniculina (syn. D. neotheicola) | CBS 123208* | Foeniculum valgare | Portugal | A.J.L. Phillips | EU814480 | GQ250315 | JX275464 | KC843269 | KC843125 |
| D. foeniculina (syn. D. rhusicola) | CBS 129528* | Rhus pendulina | South Africa | P.W. Crous | JF951146 | KC843100 | KC843205 | KC843268 | KC843124 |
| D. helianthi | CBS 592.81 | Helianthus annuus | Serbia | M Muntanola-Cvetkovic | AY705842 | GQ250308 | JX275465 | KF199885 | JX197454 |
| D. pterocarpi | MFLUCC10-588 | Magnolia sp. | Thailand: Chiang Rai | D. Udayanga | JQ619900 | JX275417 | JX275461 | KC843289 | JX197452 |
| MFLUCC10-575 = CBS 137021 | Pterocarpus indicus | Thailand: Chiang Rai | N.F. Wulandari | JQ619901 | JX275418 | JX275462 | KC843288 | JX197453 | |
| MFLUCC10-571* = CBS 135768 | Pterocarpus indicus | Thailand: Chiang Rai | D. Udayanga | JQ619899 | JX275416 | JX275460 | KC843290 | JX197451 | |
| D. pterocarpicola | MFLUCC10-580a* = CBS 135432 | Pterocarpus indicus | Thailand: Chiang Rai | D. Udayanga | JQ619887 | JX275403 | JX275441 | KF214779 | JX197433 |
| MFLUCC10-580b | Pterocarpus indicus | Thailand: Chiang Rai | N.F. Wulandari | JQ619889 | JX275405 | JX275443 | KF214780 | JX197435 | |
| D. rudis | AR3654 = CBS 135433 | Rosa canina | Austria | W. Jaklitsch | KC843338 | KC843097 | KC843184 | KC843262 | KC843153 |
| DP0423 | Pyrus sp. | New Zealand | W. Kandula | KC843335 | KC843094 | KC843181 | KC843258 | KC843150 | |
| DP0350 = CBS 135434 | Castanea sp. | New Zealand: Churchill | H. Smith | KC843338 | KC843098 | KC843185 | KC843264 | KC843154 | |
| ICMP 12522 | Ileostylis micranthus | New Zealand: Central Otago | P.R. Johnston | KC145906 | KC145940 | – | – | – | |
| ICMP 15267 | Alnus sp. | New Zealand: Mid Canterbury | K. Eade | KC145839 | KC145998 | – | – | – | |
| ICMP 16419 | Castanea sativa | New Zealand: Mid Canterbury | H.C. Smith | KC145904 | KC145976 | – | – | – | |
| ICMP 7025 | Vaccinium corymbosum | New Zealand: Waikato | P.R. Johnston | KC145885 | KC145995 | – | – | – | |
| AR3422 = CBS 109292* | Laburnum anagyroides | Austria | W. Jaklitsch | KC843331 | KC843090 | KC843177 | KC843254 | KC843146 | |
| AR3646 | Epilobium angustifolium | Canada: British Columbia | M. Barr | KC843330 | KC843089 | KC843176 | KC843253 | KC843145 | |
| AR3478 = CBS 109768 | Epilobium angustifolium | Canada: British Columbia | M. Barr | KC843329 | KC843088 | KC843175 | KC843252 | KC843144 | |
| DA243 = CBS 135435 | Brugmansia sp. | Germany | R. Schumacher | KC843332 | KC843091 | KC843178 | KC843255 | KC843147 | |
| DA244 | Brugmansia sp. | Germany | R. Schumacher | KC843334 | KC843093 | KC843180 | KC843257 | KC843149 | |
| ER285A = CBS 135437 | Acer opalus | Italy | E. Camporesi | KC843336 | KC843095 | KC843182 | KC843259 | KC843151 | |
| ER286C | Acer opalus | Italy | E. Camporesi | KC843337 | KC843096 | KC843183 | KC843260 | KC843152 | |
| ER286D | Acer opalus | Italy | E. Camporesi | KC843333 | KC843092 | KC843179 | KC843256 | KC843148 | |
| DPG01 | Vitis viniferra | Italy | X.Z. Liu | JQ619896 | JX275412 | JX27545 | KC843261 | JX197446 | |
| DPG02 | Vitis viniferra | Italy | X.Z. Liu | JQ619897 | JX275413 | JX275456 | KC843263 | JX197447 | |
| D. rudis (syn. D. viticola) | CBS 113201* | Vitis vinifera | Portugal | A.J.L. Phillips | AY485750 | GQ250327 | JX275454 | KC843251 | JX197445 |
| D. thunbergii | MFLUCC10-576a* = CBS 135769 | Thunbergia laurifolia | Thailand: Chiang Mai | D.S. Manamgoda | JQ619893 | JX275409 | JX275449 | KF199886 | JX197440 |
| MFLUCC10-576b | Thunbergia laurifolia | Thailand: Chiang Mai | S.C. Karunaratne | JQ619894 | JX275410 | JX275450 | KF199887 | JX197441 | |
| MFLUCC10-576c | Thunbergia laurifolia | Thailand: Chiang Mai | D. Udayanga | JQ619895 | JX275411 | JX275451 | KF199888 | JX197442 | |
| D. vaccinii | CBS 160.32* | Vaccinium macrocarpon | USA: Massachusetts | C. Shear | AF317578 | GQ250326 | JX275436 | JQ807297 | KC343470 |
| (as Oxycoccus macrocarpos) | |||||||||
| FAU446 = CBS 122113 | Vaccinium macrocarpon | USA: Massachusetts | F. Caruso | U11317,U11367 | JQ807398 | KC843224 | JQ807322 | KC849455 | |
| DF5032 = CBS 135436 | Vaccinium corymbosum | USA: North Carolina | D. Farr | AF317570 | JQ807380 | KC843225 | JQ807303 | KC849456 | |
| FAU633 | Vaccinium sp. | USA: Michigan | – | U11360,U11414 | JQ807413 | KC843226 | JQ807338 | KC849457 | |
| FAU468 | Vaccinium macrocarpon | USA: New Jersey | – | U113327,U11377 | JQ807399 | KC843227 | JQ807323 | KC849458 | |
1 AR, DA, DF, DLR, DP, DPG, ER, FAU, MEP: isolates in SMML culture collection, USDA-ARS, Beltsville, MD, USA; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; ICMP: International Collection of Microorganisms from Plants, Landcare Research, New Zealand; MFLUCC: Mae Fah Luang University Culture Collection; STE-U: University of Stellenbosch culture collection, Stellenbosch, South Africa;
* = ex-type cultures.
DNA extraction, PCR and sequencing
Mycelial scrapings (50–60 mg) from the leading edge of cultures on PDA, incubated for 4–5 d at 25 °C were harvested and lysed in tubes containing 500 μm garnet media and a 6 mm zirconium bead (OPS Diagnostics, Lebanon, NJ, USA) with the Fast Prep FP120 (Fischer Scientific Inc, Waltham, MA, USA) for 20 s. Genomic DNA was extracted with the DNeasy Plant Mini Kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s instructions. The ACT, CAL, EF1-α, ITS and TUB gene regions were amplified following the conditions outlined in Table 2 on a Bio-Rad Dyad Peltier thermal cycler in a 25 μL reaction volume: 10–15 ng genomic DNA, 12.5 μL Quick load Taq 2x Master Mix (New England BioLabs, Ipswich, MA, USA), 1 μL 10 mM of each primer and 1 % DMSO with volumes adjusted to 25 μL with nuclease-free water.
Table 2.
Primers used and alternative new primers designed for current study and optimised PCR protocols.
| Locus | Primers | Optimised PCR protocols | Approximate sizes of the PCR amplicons obtained | References for primersa & protocolsb |
|---|---|---|---|---|
| ACT | ACT-512F: | (95 °C: 30 s, 55 °C: 50 s, 72 °C: 1 min) | 280bp (ACT512F/ACT783R) | Carbone & Kohn 1999a |
| ATGTGCAAGGCCGGTTTCGC | ×39 cycles for ACT512F/ACT783R | |||
| ACT-783R | (95 °C: 30 s, 58 °C: 50 s, 72 °C: 1 min) | 380bp (ACT512F/ACT878R) | This studya,b | |
| (5’-TACGAGTCCTTCTGGCCCAT-3’) | ×39 cycles for ACT512F/ACT878R | |||
| ACT878R (new): | ||||
| ATCTTCTCC ATGTCGTCCCAG | ||||
| TUB | Bt-2a: | (95 °C: 30 s, 58 °C: 50 s, 72 °C: 1 min) | 500bp | Glass & Donaldson 1995a |
| GGTAACCAAATCGGTGCTGCTTTC | ×39 cycles | Udayanga et al. 2012ab | ||
| BT-2b: | ||||
| ACCCTCAGTGTAGTGACCCTTGGC | ||||
| CAL | CAL-228F: | (95 °C: 30 s, 55 °C: 50 s, 72 °C: 1 min) | 500bp (CAL228F.CAL737R) | Carbone & Kohn 1999a |
| GAGTTCAAGGAGGCCTTCTCCC | ×39 cycles for CAL228F/CAL737R | |||
| CAL-737R: | (95 °C: 30 s, 52 °C: 50 s, 72 °C: 1 min) | 800bp (CL1/CL2A) | O’Donnell et al. 2000a; | |
| CATCTTCTGGCCATCATGG | ×39 cycles for CL1/CL2A | Udayanga et al. 2012ab | ||
| CL1F: | (95 °C: 30 s, 51 °C: 50 s, 72 °C: 1 min) | 570bp (CAL563F/CL2A) | This studya,b | |
| GARTWCAAGGAGGCCTTCTC | ×39 cycles for CAL563F/CL2A | |||
| CL2A : | ||||
| TTTTTGCATCATGAGTTGGAC | ||||
| CAL563F (new): | ||||
| GACAAATCA CCACCAARGAGC | ||||
| EF1-α | EF1-728F: | (95 °C: 30 s, 58 °C: 50 s, 72 °C: 1 min) | 350bp | Carbone & Kohn 1999a |
| CATCGAGAAGTTCGAGAAGG | ×39 cycles | Udayanga et al. 2012ab | ||
| EF1-986R: | ||||
| TACTTGAAGGAACCCTTACC | ||||
| ITS | ITS1: | (95 °C: 30 s, 55 °C: 50 s, 72 °C: 1 min) | 600bp | White et al. 1990a |
| TCCGTAGGTGAACCTGCGG | ×39 cycles | Udayanga et al. 2012ab | ||
| ITS4: | ||||
| TCCTCCGCTTATTGATATGC |
PCR products were visualised by electrophoresis in 1 % agarose gels stained with SYBR Safe DNA Gel Stain (Invitrogen, Eugene, OR, USA). Excess primers and dNTPs were removed from PCR amplification mixtures with ExoSAP-IT (USB Corp., Cleveland, OH, USA) according to the manufacturer’s instructions. Amplicons were sequenced using the BigDye Terminator v. 3.1 cycle sequencing kit (Life Technologies, Grand Island, NY, USA) on an Applied Biosystems 3130xl Genetic Analyzer using the primers used to amplify each of the gene regions (Table 2).
New primer design and PCR optimisation
Complete failure of amplification of the isolates in the D. foeniculina clade (Fig. 1) and evidence of non-specific priming in the sequences of the CAL gene region was observed when using the CAL-228F/CAL-737R (Carbone & Kohn 1999) or CL1/CL2A (O’Donnell et al. 2000) primer sets. Additionally frequent failures of sequencing when using ACT-512F/ACT-783R (Carbone & Kohn 1999) were encountered in this clade. On closer inspection of ACT and CAL multiple sequence alignments for Diaporthe, non-specific binding sites were observed for both ACT-783R and CAL-228F primers (Carbone & Kohn 1999).
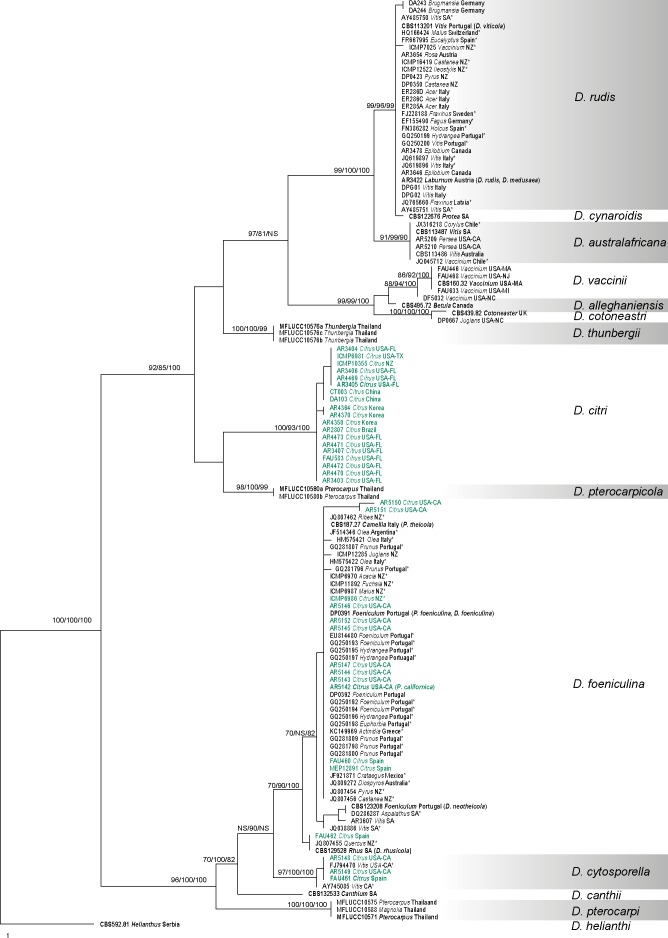
Fig. 1.
One of the 45 equally parsimonious trees generated from the analysis of the ITS sequence alignment. MP/RAxML bootstrap values/Bayesian posterior probabilities ≥ 70 % are displayed above or below each branch. Ex-type and ex-epitype culture numbers are in bold. GenBank accessions are given for downloaded sequences and isolate codes for the newly generated sequences annotated with host and location. Isolates from Citrus are indicated in green. ITS sequences obtained from GenBank verified as D. cytosporella, D. foeniculina and D. rudis are indicated with an asterisk. The tree is rooted with D. helianthi (CBS 592.81).
A sequence alignment consisting of both complete and partial sequences of CAL from Neurospora crassa (L02964), Pyricularia grisea (AF089808), Apiognomonia errabunda (DQ313615, DQ313596), Ophiognomonia clavigignenti-juglandacearum (GU993756), Diaporthe lusitanicae (JX197416), D. melonis (JX197417), D. ampelina (as Phomopsis viticola in GenBank) (AY745032), D. phaseolorum (JX197418, JX197419), D. rudis (JX197447), D. sclerotiodes (JX197420) and D. eres (as Phomopsis sp. OH-48 in GenBank, AY745025) was generated to design a new internal forward primer (CAL563F) located in the region corresponding to exon 4 in the N. crassa calmodulin gene (Table 2).
A sequence alignment of both complete and partial sequences of the actin gene from Neurospora crassa (U78026), Gaeumannomyces graminis (AY424309), Hypocrea orientalis (JQ238613), Magnaporthe oryzae (XM003719823), Fusarium oxysporum f. cubense (JQ965663), Thielavia terrestris (XM003649706), Nectria haematococca (XM003050001), Colletotrichum gloeosporioides f. sp. malvae (AF112537), Cleistogenes songorica (FJ972820), Verticillium alboatrum (XM003008431), Phaeosphaeria nodorum (XM001791742), Pyrenophora teres f. teres (XM003298028), Gibberella zeae (XM387511), Diaporthe neotheicola (JQ807344), D. vaccinii (JQ807322) and D. ampelina (as Phomopsis viticola in GenBank, JN230390) revealed that non-specific binding sites for the ACT-783R (Carbone & Kohn 1999) primer exist in Diaporthe resulting in the frequent failures of amplification and sequencing. To eliminate these problems a new reverse primer (ACT-878R) was designed. The primer combination of ACT-512F/ACT-878R was used for amplification with isolates in which amplification failed with the primer combination ACT-512F/783R in this study (Table 1).
Gradient PCR and reagent optimisations were used to develop the standard protocols for amplification of ACT and CAL genes (Table 2). Twelve reactions across an annealing temperature gradient of 50–65 °C for each of the test isolates were performed in three replicates. Optimal annealing temperatures were determined by the intensity of the amplicons visualized by agarose gel electrophoresis. Also the addition of 1 % DMSO to the PCR mix was used to enhance the reaction. Existing and newly designed primers used to amplify ACT and CAL were evaluated for thermal properties, hairpin formation and self-complementarities using the online platforms of OligoCalc (http://www.basic.northwestern.edu/biotools/oligocalc.html) and the Sequence Manipulation Suite (http://www.bioinformatics.org/sms2/pcr_primer_stats.html).
Sequence alignment and phylogenetic analysis
Raw sequences were assembled with Sequencer v. 4.9 for Windows (Gene Codes Corp., Ann Arbor, MI, USA). The assembled consensus sequences were initially aligned with Clustal W and optimised with MAFFT v. 7 using default settings (http://mafft.cbrc.jp/alignment/server/) and adjusted manually where necessary (Katoh & Standley 2013). Newly generated ITS sequences were analysed with all available type-derived sequences listed in Udayanga et al. (2011, 2012a) to determine the preliminary identifications of the isolates. Sequences from isolates recognised as D. citri, D. cytosporella, D. foeniculina and D. rudis were analysed with a selected set of additional ITS sequences available in GenBank identified using the NCBIs BLAST search and authenticated by the publications where sequences were reported. To more fully resolve closely related species, single gene phylogenies were inferred for ACT, CAL, EF1-α, ITS and TUB and a selected set of isolates were subjected to a multi-gene combined analysis. Trees were rooted with D. helianthi (CBS 592.81), which was determined to fall outside of the clades included in this study (trees not shown).
PAUP v. 4.0b10 (Swofford 2002) was used to perform maximum parsimony analyses. Trees were inferred using the heuristic search option with 1 000 random sequence additions. Maxtrees were unlimited, branches of zero length were collapsed and all multiple equally parsimonious trees were saved. Descriptive tree statistics for parsimony (Tree Length (TL), Consistency Index (CI), Retention Index (RI), Related Consistency Index (RC) and Homoplasy Index (HI)) were calculated for trees generated in a parsimony analysis. Evolutionary models for phylogenetic analyses were selected independently for each locus using MrModeltest v. 2.3 (Nylander 2004) under the Akaike Information Criterion (AIC) implemented in both PAUP v. 4.0b10 and MrBayes v. 3. Phylogenetic reconstructions of concatenated and individual gene-trees were performed using both Bayesian Inference (BI) Markov Chain Monte Carlo and Maximum Likelihood (ML) criteria. Bayesian reconstructions were performed using MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001). Two simultaneous analyses, each consisting of six Markov chains, were run for 1 000 000 generations with trees sampled every 100 generations resulting in 20 000 total trees. The first 2 000 trees, representing the burn-in phase of the analyses were discarded from each run and the 16 000 remaining trees were used for calculating posterior probabilities (PP).
Maximum likelihood trees were generated using the software RAxML v 7.4.2 Black Box (Stamatakis 2006, Stamatakis et al. 2008) in the CIPRES Science Gateway platform (Miller et al. 2010). For the combined dataset all free modal parameters were estimated by RAxML with ML estimate of 25 per site rate categories. The combined five-gene dataset was partitioned by gene region. The RAxML software accommodated the GTR model of nucleotide substitution with the additional options of modelling rate heterogeneity (Γ) and proportion invariable sites (I). These analyses utilised the rapid bootstrapping algorithm in RAxML in XSEDE high performance online computing service. Phylogenetic trees and data files were viewed in MEGA v. 5 (Tamura et al. 2011), TreeView v. 1.6.6 (Page 1996) and FigTree v. 1.4 (Rambaut & Drummond 2008).
Genealogical sorting index
The rooted gene genealogies resulting from each of the single gene analyses of ACT, CAL, EF1-α, ITS and TUB were submitted to the genealogical sorting index (gsi) parallel computing resource (http://www.genealogicalsorting.org/) for analysis. The gsi estimates the degree of exclusive ancestry of individuals in labelled predefined groups in a rooted tree (Cummings et al. 2008). Values range from 0 to 1 with 0 corresponding to a lack of genealogical divergence from other groups and 1 corresponding to monophyly for the predetermined clade (or species). Each isolate was assigned to a predetermined species based on Genealogical Concordance Phylogenetic Species Recognition (GCPSR) and the gsi was calculated for the best tree selected in parsimony analysis and for all trees using 10 000 permutations (Cummings et al. 2008). The assignment of each tip to groups representing the recognised species was identical for the EF1-α and combined phylogenetic trees (Fig. 2, 3). Taxa in the ITS tree that were not present in EF1-α and combined trees were not included in the calculation of gsi. The gsi and each of the probability values (P) corresponding to the species represented by more than one isolate were tabulated (Table 3). Species with one representative isolate including the outgroup were not subjected to gsi analysis. The ensemble genealogical sorting index (gsiT) is the sum of the gsi values calculated for all individual gene trees (Table 3).
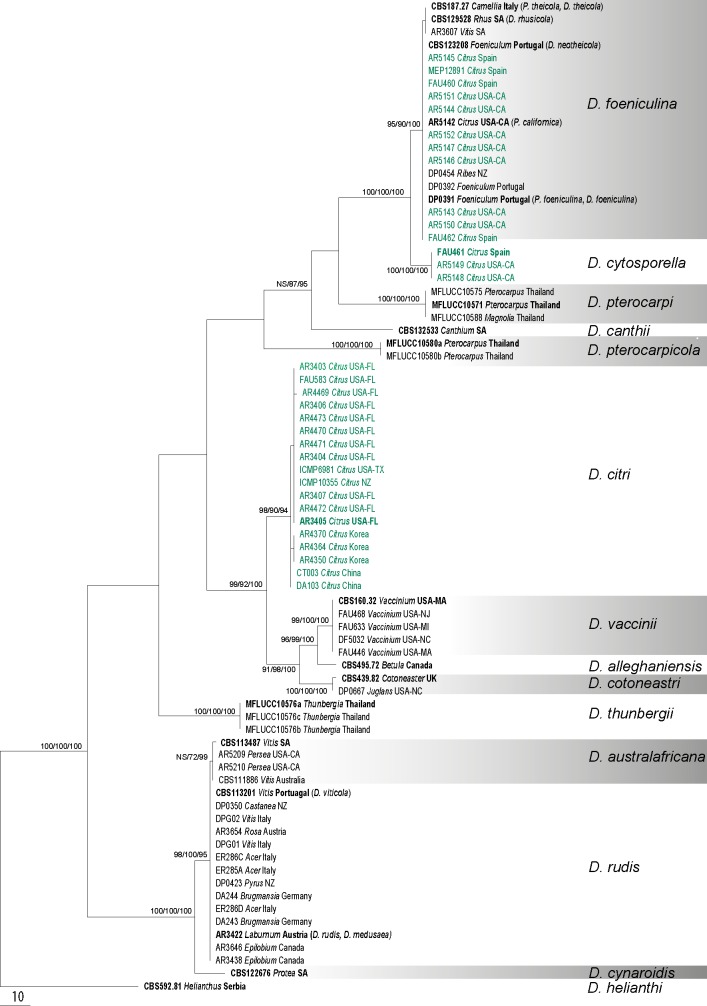
Fig. 2.
The single most parsimonious tree generated from the analysis of the EF1-α sequence alignment. MP/RAxML bootstrap values/Bayesian posterior probabilities ≥ 70 % are displayed above or below each branch. Ex-type and ex-epitype culture numbers are in bold. GenBank accessions are given for downloaded sequences and isolate codes for the newly generated sequences annotated with host and location. Isolates from Citrus are indicated in green. The tree is rooted with D. helianthi (CBS 592.81)
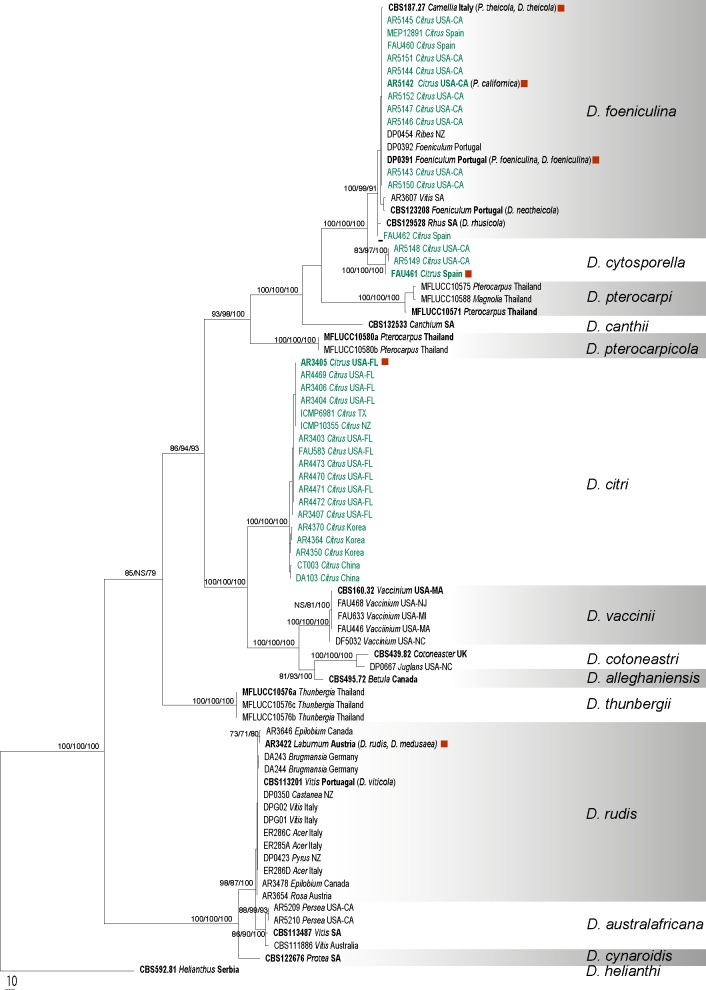
Fig. 3.
The single most parsimonious tree generated from the analysis of the combined ACT, CAL, EF1-α, ITS and TUB sequence alignment. MP/RAxML bootstrap values/Bayesian posterior probabilities ≥ 70 % are displayed above or below each branch. Ex-type and ex-epitype culture numbers are in bold. GenBank accessions are given for the downloaded sequences and isolate codes for the newly generated sequences annotated with host and location. Isolates from Citrus are indicated in green. Red squares indicate the epitypes designated in this study including the conserved ex-type of Diaporthe citri in Rossman et al. (2013). The tree is rooted with D. helianthi (CBS 592.81)
Table 3.
Comparison of alignment properties in parsimony analysis of genes and nucleotide substitution models used in phylogenetic analysis.
| Genes/loci | ITS | EF1-α | TUB* | ACT* | CAL* | Combined ITS / EF / ACT / CAL |
|---|---|---|---|---|---|---|
| Characters included (with gaps) | 508 | 347 | 454 | 279 | 232 | 2033 |
| Invariable characters | 360 | 155 | 318 | 205 | 129 | 1337 |
| Parsimony informative characters (%) | 97 (20 %) | 189 (55 %) | 132 (29 %) | 68 (24 %) | 95 (40 %) | 659 (32 %) |
| Uninformative polymorphic characters | 14 | 3 | 4 | 6 | 8 | 37 |
| Alignment strategy (MAFFT v6) | FFT-NS-I+manual | FFT-NS-I+manual | FFT-NS-I | FFT-NS-I | FFT-NS-I+manual | – |
| Number of branches > 70 % bootstrap MP/BI and ML analysis | 18 | 17 | 15 | 11 | 11 | 20 |
| Nucleotide substitution models for Bayesian analysis (determined by MrModeltest) | SYM+I+G | GTR+I+G | HKY+G | GTR+G | HKY+G | GTR+I+G |
* Phylogenetic trees not shown.
All the novel sequences were deposited in GenBank and the sequence alignments were submitted to TreeBASE (www.treebase.org) as S14141 (ITS), S14146 (EF1) and S14147 (combined alignment). Taxonomic novelties (MB) and typifications (MBT175959–MBT175968) were registered in MycoBank (www.mycobank.org) (Crous et al. 2004).
RESULTS
Phylogenetic analysis
Three hundred new sequences were generated in this study from 77 cultures (Table 1). Other available sequences were obtained from GenBank. Six alignments were analysed corresponding to single gene analyses of ACT, CAL, EF1-α, ITS and TUB and a combined alignment of the five genes. Comparison of the alignment properties and nucleotide substitution models are provided in Table 3. Phylogenetic trees inferred from EF1-α and ITS to show the phylogenetic placement of species and a combined alignment of five genes are presented with annotations for species, host and geographic origin (Fig. 1, 2, 3). Individual gene trees for ACT, CAL and TUB did not markedly differ from the EF1-α and ITS gene trees and are not shown.
ITS phylogenetic analysis
The ITS sequence alignment contained 126 sequences including the outgroup taxon (Table 3). Maximum parsimony analysis resulted in 45 equally most parsimonious trees (TL = 209, CI = 0.684, RI = 0.977, RC = 0.668, HI = 0.316). BI and ML trees were identical to the MP tree presented in Fig. 1. A total of 12 clades were resolved corresponding to the species recognised as D. alleghaniensis, D. australafricana, D. canthii, D. citri, D. cotoneastri, D. cytosporella, D. foeniculina, D. pterocarpi, D. pterocarpicola, D. rudis, D. thunbergii and D. vaccinii. Diaporthe cynaroidis was not resolved as distinct from D. rudis. Among the major clades of interest in this study, the D. foeniculina clade consists of 48 isolates derived from 21 different hosts in ten countries representing the geographic regions of Australia, Europe, New Zealand, northern South America and South Africa. The isolates from Citrus in this clade originated from California (USA), Spain and New Zealand. The ex-type of D. rhusicola is also placed within the D. foeniculina clade. Diaporthe cytosporella is represented by four isolates from Citrus in Spain and Citrus and Vitis in California (USA). Diaporthe rudis comprises 34 isolates derived from 18 different hosts from 13 countries representing the geographic regions of Canada, Europe, New Zealand, South America and South Africa, including the epitype culture of D. viticola. No isolates of D. rudis were reported from Citrus. Isolates identified here as D. citri include 19 from various Citrus spp. in China, Korea, New Zealand and the United States (Florida and Texas).
EF1-α phylogenetic analysis
The EF1-α data matrix contained 77 sequences including the outgroup and consisted of 429 characters including gaps (Table 3). Maximum parsimony analysis yielded a single most parsimonious tree and is presented here as Fig. 2 (TL = 442, CI = 0.742, RI = 0.964, RC = 0.715, HI = 0.258). The MP, BI and ML trees generated were identical. The closely related taxa D. foeniculina and D. cytosporella were clearly distinguished and D. rhusicola was placed within D. foeniculina. Isolates of D. australafricana including the ex-type isolate were placed within the D. rudis clade, whereas D. cynaroidis, represented by the ex-type isolate, formed a distinct branch. Inspection of EF1-α sequences of D. australafricana vs D. rudis isolates revealed two base changes including one insertion and one transversion between the two taxa.
Combined analysis of five genes
The combined data matrix consisted of 74 isolates including the outgroup with 2 033 characters included in the maximum parsimony analysis (Table 3). The maximum parsimony analysis of the alignment yielded a single most parsimonious tree presented here as Fig. 3 (TL = 1302, CI = 0.720, RI = 0.961, RC = 0.692, HI = 0.280). The MP, BI and ML trees generated were identical. A total of 13 clades were resolved in the combined phylogenetic tree. Diaporthe citri forms a sister clade to a clade containing D. cotoneastri and D. vaccinii. Diaporthe citri occurs only on Citrus in the United States and elsewhere while D. vaccinii occurs only on Vaccinium in North America. The D. rudis clade includes the taxon previously known as D. viticola represented by an ex-epitype culture (CBS 113201) and several authentic isolates previously known as D. medusaea. Diaporthe australafricana forms a well-supported clade closely related to D. rudis. The multi-gene phylogenetic tree resolves the closely related taxa D. canthii, D. cytosporella, D. foeniculina and D. pterocarpi. The ex-type of D. rhusicola is placed within the D. foeniculina clade. Diaporthe cytosporella, D. foeniculina and D. pterocarpi are all found to occur on multiple, unrelated hosts. Diaporthe canthii, represented by a single isolate, is known only from its type host.
Analysis of gsi data
All gsi values were in range of 0.5–1.0 with the exception of TUB (0.4482) for D. cytosporella and TUB (0.4051), CAL (0.2712) and ACT (0.1187) for D. australafricana (Table 4). Despite minor variation within the ITS1 region in both D. foeniculina and D. rudis, the gsi recognised each as monophyletic for each of the genes, confirming the placement of the ex-type culture of D. rhusicola with D. foeniculina. Therefore, the observed variation in the ITS regions of these two species is not considered meaningful in terms of species distinction and does not conflict with the other gene regions. Individually, the ITS and EF1-α genes estimated significant measures of exclusive ancestry for all the species including D. australafricana and D. cytosporella. The ACT gene resolved all species as monophyletic except D. australafricana. The ensemble gsi value (gsiT) for all species included indicated significant genealogical divergence from all other species in spite of the conflict observed among genes for D. australafricana and D. cytosporella. All other species resolved in the combined phylogeny were supported without conflict.
Table 4.
Genealogical Sorting Index (gsi) and probability values (P) for gene trees of species resolved in this study.
| Species | ITS gsi1 P | EF1-α gsi1 P | TUB gsi1 P | CAL gsi1 P | ACT gsi1 P | ALL gsi1T PT |
|---|---|---|---|---|---|---|
| D. australafricana | 1* | 1* | 0.4051* | 0.2712* | 0.1187 | 0.7353* |
| 0.0004 | 0.0001 | 0.001 | 0.0078 | 0.0483 | 0.0001 | |
| D. citri | 1* | 1* | 1* | 1* | 1* | 1* |
| 0.0004 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| D. cotoneastri | 1* | 1* | 1* | 1* | 1* | 1* |
| 0.0005 | 0.0019 | 0.0008 | 0.0079 | 0.0007 | 0.0001 | |
| D. cytosporella | 1* | 1* | 0.4492* | 1* | 1* | 1* |
| 0.0001 | 0.0002 | 0.0003 | 0.0003 | 0.0004 | 0.0001 | |
| D. foeniculina | 0.869* | 1* | 0.9476* | 1* | 0.9301 | 0.9598* |
| 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| D. pterocarpi | 1* | 1* | 1* | 1* | 1* | 1* |
| 0.0001 | 0.0002 | 0.0001 | 0.0003 | 0.0002 | 0.0001 | |
| D. pterocarpicola | 1* | 1* | 1* | 1* | 1* | 1* |
| 0.0014 | 0.0020 | 0.0010 | 0.0088 | 0.0002 | 0.0001 | |
| D. rudis | 1* | 1* | 0.9138* | 0.8392 | 0.7719 | 0.9332* |
| 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| D. thunbergii | 1* | 1* | 1* | 1* | 1* | 1* |
| 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0001 | 0.0001 | |
| D. vaccinii | 1* | 1 | 1* | 1* | 1* | 1* |
| 0.0004 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
1 The gsi statistic is based on a continuum of 0–1, with 0 = lack of genealogical divergence from other groups and 1 = monophyly; (*) = statistically significant P-value ≤ 0.05. The gsi is calculated under the null hypothesis that the gene copies labeled as each species assigned are a single group of mixed genealogical ancestry. gsiT = ensemble gsi of 5 gene trees. Species represented by single isolate are excluded in calculation of gsi.
New primers for Diaporthe and protocols for amplification
The evaluation of the thermal properties of the primers by OligoCalc and Sequence Manipulation Suite revealed that the forward CAL primers, CAL-228F (Carbone & Kohn 1999) and CL1 (O’Donnell et al. 2000), showed potential for self-annealing in each of the tests in addition to issues with non-specific binding sites in the targeted gene region. The newly designed CAL-563F primer and the existing CL2A reverse primer (O’Donnell et al. 2000) were determined to be a suitable primer pair under the criteria given including percentage GC, self-annealing, GC clamp, hairpin formation and length. They were used to eliminate the problems in amplification and sequencing encountered in this study. Use of this primer pair resulted in an amplicon overlapping c. 300 bp of the 500-bp CAL-228F/CAL-737R fragment. However, two additional introns, each c. 60–100 bp in length, are found in the extended sequence obtained using the primers CAL-563F/CL2A. One of these informative introns in Diaporthe is not found in either of the N. crassa or P. grisea reference sequences used as references for primer design.
The newly designed reverse primer for actin (ACT-878R) worked well in combination with ACT-512F for isolates that failed with the ACT-512F/ACT-783R primer combination and resulted in an amplicon of c. 350 bp in length. The extended 3’ region of the newly generated amplicons was not included in the analyses as the majority of the sequences were generated with primer pairs ACT-512F/ACT-783R and it consisted entirely of exon sequence with little variation among isolates.
TAXONOMY
In this section we provide modern descriptions and illustrations of the species resolved here based on multi-gene phylogenetic analyses and morphological characters. Diaporthe citri occurs only on Citrus while D. cytosporella and D. foeniculina occur on Citrus and other woody and herbaceous hosts including high value crops. Diaporthe rudis is not known from Citrus but was previously confused with those species especially as D. medusaea and has a broad host range. Each species is described based on type and other specimens as well as ex-epitype cultures. Synonymous names of Diaporthe or Phomopsis are reviewed based on protologues, type and other specimens and cultures. When specimens with cultures from similar substrates and localities are available, epitype specimens with ex-epitype cultures are designated for both accepted and synonymous names.
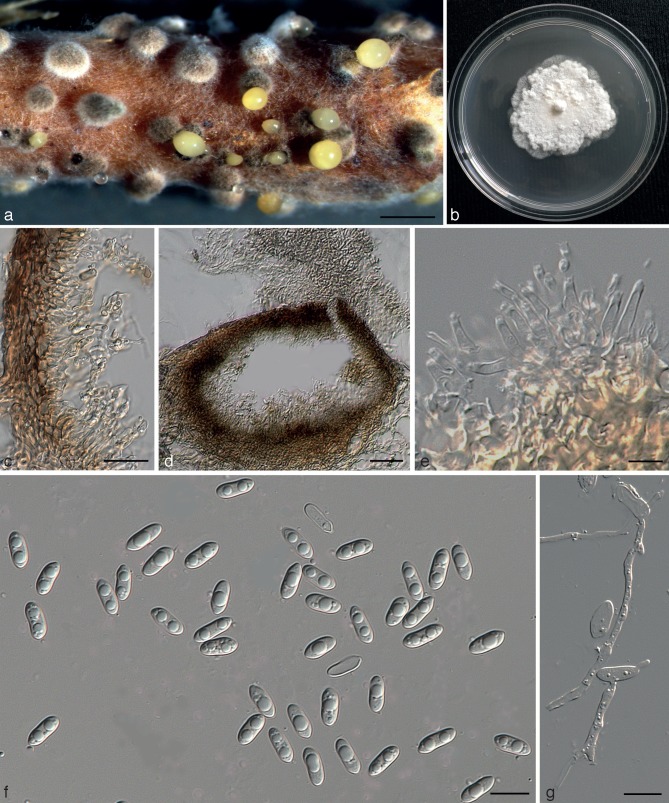
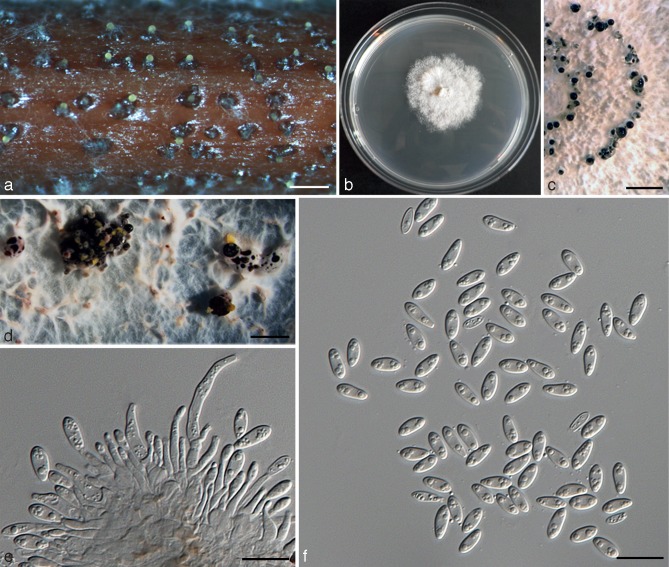
Diaporthe citri (H.S. Fawc.) F.A. Wolf, J. Agric. Res. 33: 625. 1926. — Fig. 4
Fig. 4.
Diaporthe citri (ex-epitype culture AR3405 = CBS 135422). a. Sporulation on alfalfa stem in WA; b. culture on PDA (25 °C, dark, 7 d); c. pycnidial walls lined with paraphyses and conidiophores; d. section through conidiomata; e. conidiophores; f. alpha conidia; g. germinating conidia on a slide. — Scale bars: a = 500 μm; c = 20 μm; d–g = 10 μm.
Basionym. Phomopsis citri H.S. Fawc., Phytopathology 2: 109. 1912 nom. conserv. prop. non Phomopsis citri (Sacc.) Traverso & Spessa 1910.
= Diaporthe citrincola Rehm, Leafl. Philipp. Bot. 6: 2269. 1914.
= Phomopsis caribaea W.T. Horne, Phytopathology 12: 417. 1922.
Perithecia on decaying twigs, black, globose to conical, 130–200 μm diam, scattered, solitary or in groups, immersed deep in bark with tapering perithecial necks 190–700 μm long. Asci unitunicate, 8-spored, sessile, elongate to clavate, (37.3–)40.5–50.5(–55) × (9–)10.5–12(–12.2) μm. Ascospores hyaline, 2-celled, often 4-guttulate, with larger guttules at centre and smaller one at ends, elongated to elliptical, (12–)12.4–14(–14.2) × 3.2–3.6(–3.8) μm (av. ± SD = 13.2 ± 0.8 × 3.3 ± 0.2, n = 30). Pycnidia on alfalfa twigs on WA: globose, 200–250 μm diam, later conical, embedded in tissue, erumpent at maturity, up to 450 μm diam, 65–100 μm high, with an elongated black neck, often with a yellowish, spiral conidial cirrus extruding from ostiole; walls parenchymatous, consisting of 3–4 layers of medium brown textura angularis. Conidiophores hyaline, smooth, unbranched, ampulliform, straight to sinuous, 10–15 × 1–2 μm. Conidiogenous cells phialidic, cylindrical, terminal, slight tapering towards apex, 0.5–1 μm diam. Paraphyses abundant among conidiophores, 20–40 × 1–2 μm. Alpha conidia aseptate, hyaline, smooth, ovate to ellipsoidal, mono- to biguttulate, rarely 3-guttulate, base subtruncate, (7.6–)8–9(–10.2) × 3–4.2 μm (av. ± SD = 8.5 ± 0.8 × 3.7 ± 0.2 , n = 30). Beta and gamma conidia not observed on alfalfa twigs or in culture.
Culture characteristics — In dark at 25 °C for 1 wk, colonies on PDA slow growing, 4.2 ± 0.2 mm/day (n = 8), white, fluffy aerial mycelium, reverse centre greenish yellow pigmentation developing in centre.
Host range — Causing melanose and stem end rot disease, associated with dying or dead twigs of Citrus spp. and closely related hosts including C. aurantiifolia, C. aurantium, C. maxima (= C. grandis), C. nobilis, C. paradisi, C. reticulata, C. sinensis, C. sudachi, C. unshiu, × Citrofortunella microcarpa (= × C. mitis), Fortunella japonica (Kobayashi 2007), F. margarita, Poncirus trifoliata.
Distribution — Probably throughout Citrus-growing regions of the world. Reported from Brazil, China, Cuba, Japan, Korea, New Zealand, The Philippines, Puerto Rico and the United States (Florida, Texas).
Type specimens examined. USA, Florida, Lake Alfred, Ana, on twigs of Citrus sp, 26 Apr. 2000, L.W. Timmer, dried specimen from culture sporulating on alfalfa stem (type of Phomopsis citri proposed for conservation in Rossman et al. (2013) (BPI 892456, ex-type culture AR 3405 = CBS 135422). – PHILIPPINES, Los Baños, on dead twigs of Citrus nobilis, Oct. 1913, coll. M.B. Raimundo, comm. C.F. Baker, no. 1875 (holotype of Diaporthe citrincola S-F52860). – CUBA, Isle of Pines, on Citrus paradisi, 30 Oct. 1917, Fredrick Maskew, intercepted in San Francisco, derived culture sporulating on Citrus twig (lectotype of Phomopsis caribaea designated here BPI 358328, isolectotype NY01097305; MBT175959).
Additional materials examined. BRAZIL, Escola Agr., Vicosa, Minas Gerais, on peel of Citrus sp., 17 May 1932, P.H. Rolfs (BPI 615855); intercepted New York #pi 7163, on fruit of Citrus sinensis, 22 June 1924, A.C. Hill, det. A.J. Bruman (BPI 358408). – JAPAN, Yokohama, intercepted Seattle Washington #pi 4780, on fruit of Citrus sinensis, 14 Jan. 1940, A.G. Webb, det. J.A. Stevenson (BPI 358405). – MEXICO, intercepted Brownsville Texas #692229, on leaves of Citrus sp., 30 Jan. 1930, Mueller, det. D.J. Smith, A.E. Jenkins, J.A. Watson (BPI 615856); intercepted Laredo Texas #50818, on leaves of Citrus sp., 23 Jan. 1951, Trotter, det. A.H. Lewis, J.A. Watson (BPI 615857). – PUERTO RICO, Bayamon, on leaves of Citrus grandis, 22 Aug. 1933, C.G. Anderson (BPI 358392). – USA, Florida, Orlando, on dead stems of Citrus aurantifolia, July 1925, F.A. Wolf (BPI 615860); Florida, Orlando, on dead stems of Citrus sp., Jan. 1926, F.A. Wolf (BPI 615959); Florida, Orlando, on leaves of Citrus sinensis, Mar. 1922, J.R. Winston (BPI358409); Florida, Eustis, on leaves of Citrus grandis, 8 Jan. 1932, H.S. Fawcett (BPI 358391); Florida, St. Nicholas, on stem of Citrus sinensis, 28 Nov. 1895, det. F. Albert, W.W. Diehl (BPI 358404); Florida, Fort Myers, on stems of Citrus sinensis, 16 Feb. 1924, J.A. Stevenson (BPI 358407); Florida, Gainesville, Florida Agricultural Experiment Station, on stems of Citrus sinensis, 16 Mar. 1910, H.S. Fawcett (BPI 358406); Florida, Winter Park, on stems of Citrus grandis, 21 Feb. 1923, C.L. Shear (BPI 615868); Florida, Winter Park, on stems of Citrus grandis, 20 Jan. 1925, H.E. Stevens, det. C.L. Shear (BPI 615869); Florida, on dead stem of Citrus sp., 1913, J.G. Grossenbacker, det. C.L. Shear (BPI 615858); Florida, on dead stem of Citrus sp., 8 July 1929, F.A. Wolf, det. C.L. Shear (BPI 615854); Florida, on leaves of Citrus grandis, 6 Jan. 1932, H.S. Fawcett (BPI 358393).
Notes — The name D. citri is based on Phomopsis citri H.S. Fawc. 1912, a later homonym of Phomopsis citri (Sacc.) Traverso & Spessa 1910. A conservation proposal has been published to continue the use of the widely used name for the species associated with melanose or stem end rot of Citrus as D. citri (Rossman et al. 2013). Diaporthe citri based on the basionym Phomopsis citri H.S. Fawc. has priority over the later synonyms D. citrincola and P. caribaea. This is also in accordance with the change to unit nomenclature with the older genus Diaporthe serving as the correct name for all species in Diaporthe-Phomopsis (McNeill et al. 2012). No type specimen for P. citri could be located at BPI or FLAS, leaving only an illustration (Fawcett 1912) as a potential, but unsatisfactory, iconotype, thus P. citri is proposed for conservation with a new type specimen (Rossman et al. 2013). The type specimens of D. citrincola and P. caribaea were examined and contributed to the conclusions that these names are synonyms of D. citri. A lectotype of P. caribaea is designated.
The fruiting structures of D. citri are found on dead twigs, stems and fruits of Citrus affected by melanose and stem end rot (Wolf 1926, Fawcett 1932, Whiteside & Timmer 2000b). The fungus generally propagates itself on dead twigs of Citrus. A few days after infecting leaf tissue or fruit, the melanose symptoms appear as small, brown, discrete or confluent, sunken spots. A few epidermal cell layers on infected tissue are killed and become impregnated with reddish brown gum that later become raised black pustules (Timmer 2000). Although pustules on leaves are initially surrounded by yellow halos, they recover and become green again and corky pustules are often the only symptoms (Bach & Wolf 1928, Nelson 2008). Fungal structures such as pycnidia or perithecia are never visible in these melanose lesions, therefore, the fungus cannot be observed in the infected leaves or fruit. When the fruiting structures are present on dead twigs or bark of the stems, the pycnidia or ascomata are abundant deep in the tissue.
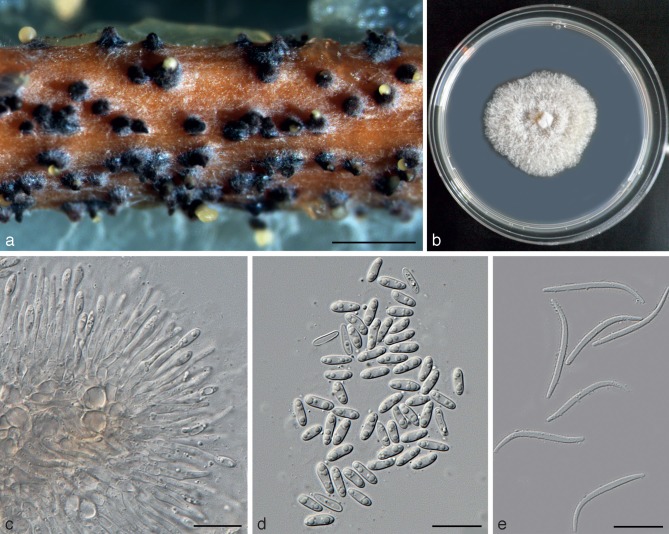
Diaporthe cytosporella (Penz. & Sacc.) Udayanga & Castl., comb. nov. — MycoBank MB803986; Fig. 5, 6
Fig. 5.
Diaporthe cytosporella (AR5149). a. Sporulating pycnidia on alfalfa stem; b. culture on PDA (25 °C, dark, 7 d); c. concentric pycnidial in rings on culture; d. pycnidia on culture; e. conidiophores; f. alpha conidia. — Scale bars: a, c = 2 000 μm; d = 3 000 μm; e, f = 20 μm.
Fig. 6.
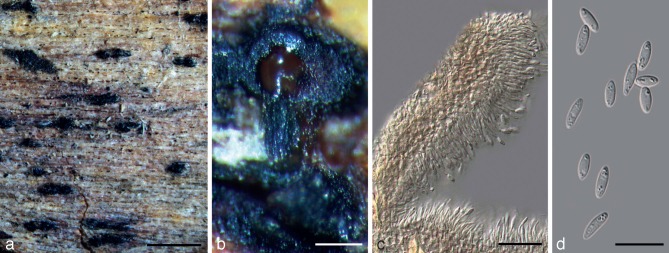
Holotype specimen of Diaporthe cytosporella (BPI 798526). a. Pycnidia-bearing bark of Citrus sp.; b. branched stroma and sporulating pycnidia; c. section through pycnidia with pycnidial wall and conidiophores; d. alpha conidia. — Scale bars: a = 1 000 μm; b = 50 μm; c, d = 15 μm.
Basionym. Phoma cytosporella Penz. & Sacc., Fung. Agron.: 361. 1887.
≡ Phomopsis cytosporella (Penz. & Sacc.) H.S. Fawc. & H.A. Lee, Citrus diseases and their control, Ed. 1: 407. 1926.
Perithecia unknown. Pycnidia on alfalfa twigs on WA: globose, 150–200 μm diam, mostly embedded in tissue and erumpent at maturity, up to 450 μm diam, 65–100 μm high, with an elongated black neck, often with a yellowish conidial cirrus extruding from ostiole; walls parenchymatous consisting of 3–4 layers of medium brown textura angularis. Paraphyses lacking. Conidiophores 7–18 × 1–2 μm, hyaline, smooth, branched or unbranched, ampulliform, cylindrical, wider at base, occurring in dense clusters. Conidiogenous cells phialidic, cylindrical, terminal, with slight tapering towards apex, 0.5–1 μm diam. Alpha conidia (6.9–)8–9(–12.6) × (2.3–)2.6–3.2 μm (av. ± SD = 8.8 ± 0.9 × 3.0 ± 0.3, n = 30), aseptate, hyaline, smooth, ovate to ellipsoidal, biguttulate or multi-guttulate, base subtruncate, occasionally larger alpha conidia present in culture and on alfalfa stems. Beta and gamma conidia not observed on alfalfa twigs or in culture.
Culture characteristics — In dark at 25 °C for 1 wk, colonies on PDA relatively slow growing, 4.0 ± 0.2 mm/day (n = 8). On PDA white, fluffy aerial mycelium, reverse with ash colour pigmentation developing in centre. In a 2-wk-old culture, clusters of black, branched stromata occurring in concentric rings with sporulating pycnidia.
Host range — Citrus limon, C. sinensis and Vitis vinifera.
Distribution — Europe (Spain, Italy), United States (California).
Type specimens examined. ITALY, Rome, Modena, on Citrus limonia, Jan. 1886 (holotype of Phoma cytosporella BPI 798526). – SPAIN, on Citrus limon, M.E. Palm, dried culture (epitype of Phoma cytosporella designated here: BPI 892459, living culture FAU461 = CBS 137020; MBT 175960).
Additional material examined. USA, California, on twigs of Citrus sinensis, 4 Oct. 2011, A. Eskalen UCR1751, dried culture with pycnidia sporulating on alfalfa stems (BPI 892457, living culture AR5149); ibid., UCR1750, dried culture with pycnidia sporulating on alfalfa stems (BPI 892458, living culture AR5148).
Notes — Diaporthe cytosporella is phylogenetically closely related to D. foeniculina but clearly distinguished based on ITS and EF1-α sequences (Fig. 1, 2). The species was first described from Italy and later synonymised under D. citri. Although in this study this species is primarily recognized using isolates from Citrus limon in Europe (Spain) and the United States (California), two ITS sequences (FJ94470, AY745085) from GenBank are 100 % identical suggesting that this species may also occur on Vitis and other host species in California (retrieved on 1 Feb. 2013). At maturity cultures of D. cytosporella (AR5148 and AR5149) on PDA produce distinctive black, branched stromata. Perithecia were not observed in culture. Morphological characters were highly similar among the type specimen (Fig. 6) and the isolates used in epitype and genetically similar additional materials examined.
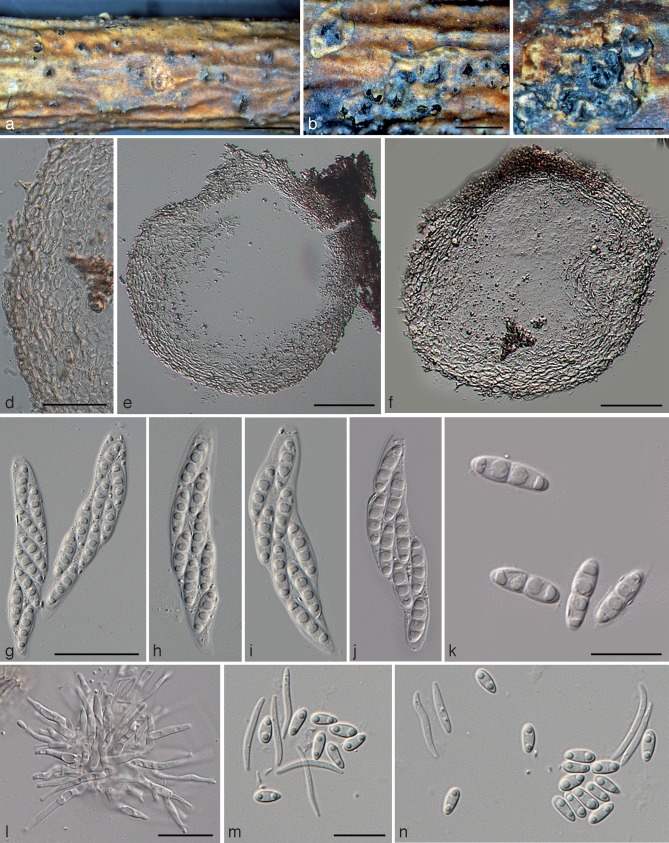
Diaporthe foeniculina (Sacc.) Udayanga & Castl., comb. nov. — MycoBank MB803929; Fig. 7
Fig. 7.
Diaporthe foeniculina (ex-epitype culture DP0391 = CBS 111553). a. Sporulation on alfalfa stem in WA; b. culture on PDA (25 °C, dark, 7 d); c. conidiophores; d. alpha conidia; e. beta conidia. — Scale bars: a = 2 000 μm; all others = 10 μm.
Basionym. Phoma foeniculina Sacc., Michelia 2: 95. 1880.
≡ Phomopsis foeniculina (Sacc.) Sousa da Câmara, Agron. Lusit. 9: 104. 1947.
= Diaporthe theicola Curzi, Atti Ist. Bot. Lab. Crittog. Univ. Pavia 3 Sér. 3: 60. 1927.
= Phomopsis theicola Curzi, Atti Ist. Bot. Lab. Crittog. Univ. Pavia 3 Sér. 3: 64. 1927.
= Phomopsis californica H.S. Fawc., Phytopathology 12: 419. 1922.
= Diaporthe neotheicola A.J.L. Phillips & J.M. Santos, Fung. Diversity 34: 120. 2009.
= Diaporthe rhusicola Crous, Persoonia 26: 135. 2011.
Perithecia on decaying twigs black, globose to subglobose (200–)360 × 200 μm, scattered, solitary or in groups, with tapering perithecial necks barely protruding through epidermis. Asci unitunicate, 8-spored, sessile, cylindrical to clavate, (40–)50.5–60.5(–65) × 8–10(–12.2) μm. Ascospores hyaline, 2-celled, often with four guttules, larger guttules near centre and smaller ones at ends, elongated to elliptical, (9.0–)12.4–14(–15.2) × (3.2–)3.4–3.6(–5.2) μm (av. ± SD = 13.2 ± 0.8 × 3.5 ± 0.1, n = 30). Pycnidia on alfalfa twigs on WA: globose to subglobose 400–700 μm diam, erumpent at maturity, (300–)500–800(–930) μm high, with an elongated, black neck, mostly embedded in tissue, often with a yellowish, drop-like conidial cirrus extruding from ostiole; walls parenchymatous, consisting of 2–3 layers of medium brown textura angularis. Paraphyses lacking. Conidiophores hyaline, smooth, unbranched, cylindrical, straight to sinuous, 9–15(–18) × 1–2 μm. Conidiogenous cells phialidic, cylindrical, terminal, with slight taper towards apex, 0.5–1 μm diam. Alpha conidia aseptate, hyaline, smooth, ellipsoidal or fusiform, with none, two or many guttules, rarely with subtruncate base, (7.5–)8.5–9(–9.2) × (2–)2.3–2.5(–2.7) μm (av. ± SD = 8.8 ± 0.3 × 2.4 ± 0.1 μm, n = 30). Beta conidia hyaline, aseptate, eguttulate, hamate or slightly curved, abundant, base subtruncate, acute apex, (20–)22–28(–29) × (1.1–)1.4–1.6(–2) μm (av. ± SD = 25.1 ± 3.3 × 1.5 ± 0.1 μm, n = 30).
Culture characteristics — In dark at 25 °C for 1 wk, colonies on PDA slow growing, 5.2 ± 0.2 mm/day (n = 8), white, sparse aerial mycelium, greenish yellow pigmentation developing in reverse centre.
Host range — Acacia, Acer, Actinidia deliciosa, Aspalathus linearis, Bougainvillea spectabilis, Camellia sinensis, Castanea, Citrus limon, C. limonia, Crataegus, Diospyros, Foeniculum vulgare, Fuchsia, Hydrangea, Juglans, Malus, Olea, Prunus, Pyrus, Quercus, Rhus, Ribes, Vitis vinifera and Wisteria sinensis. In addition to the hosts on the specimens listed below, these hosts are represented in Fig. 1 based on the ITS phylogeny and Gomes et al. (2013) as D. foeniculacea.
Distribution — Argentina, Australia, Europe (Greece, Portugal, Spain, Italy), New Zealand, South Africa and USA (California).
Type specimens examined. FRANCE, on stems of Foeniculum ‘arvensis’, Brunaud, cited in Phillips (2003) with illustration (holotype of Phoma foeniculina PAD 281 – unavailable, not examined). – PORTUGAL, Madeira, Serra da Agua, at base of 2-yr-old stem of Foeniculum vulgare, Aug. 2001, A.J.L. Phillips (epitype of Phoma foeniculina designated here LISE 94791, ex-epitype culture from single ascospores CBS 111553 = DP0391; MBT175961). – USA, California, Santa Barbara County, on dead outer bark and decaying fruit of Citrus limonia, 3 Mar. 1922, H.S. Fawcett (holotype of Phomopsis californica BPI0358313); California, San Diego, on branch of Citrus limonia, 16 Nov. 2012, Akif Eskalen (epitype of Phomopsis californica designated here BPI 892460, ex-epitype culture AR5142 = CBS135430; MBT 175962). – ITALY, on Camellia sinensis, Curzi, dried culture specimen (epitype of Diaporthe theicola designated here BPI 892462, ex-epitype culture CBS 187.27, same as ex-isotype culture of Phomopsis theicola; MBT175963); Illustration in Atti dell’Istituto Botanica della Universita e Laboratoria Crittogamico di Pavia 3 Sér. 3: 60 (1926) [1927] (lectotype of Phomopsis theicola designated here; MBT175964). – PORTUGAL, Évora, on Foeniculum vulgare, Nov. 2007, A.J.L. Phillips (holotype of Diaporthe neotheicola CBS-H 20131, ex-holotype culture (Di-C004/5 = CBS 123208).
Additional specimens examined. PORTUGAL, Madeira, Serra da Agua, at base of 2-yr-old stem of Foeniculum vulgarae, Aug. 2001, A.J.L. Phillips (LISE 94792 as Diaporthe foeniculacea, culture from single ascospores DP0392 = CBS 111554. – SPAIN, on fruit of Citrus limon, intercepted at Elizabeth, New Jersey, 20 Mar. 1987, C. Markham 001514, M.E. Palm (BPI 1107900, living culture MEP1289); ibid. (BPI 747926); ibid. (BPI 747927); on peel of Citrus limonia, intercepted New York #87452, 13 Nov. 1940, Hodson, E.A. Jenkins (BPI 615878); ibid., on fruit of Citrus limon, M.E. Palm (BPI 892461, culture FAU460 = CBS).
Notes — Diaporthe foeniculina is known to occur on Citrus and many other woody plants hosts in temperate and tropical regions. This species causes a stem end rot of lemandarin (Citrus limonia) in Europe and the United States (California) and was observed as a saprobe on branches of this host. As D. neotheicola, this species has been reported to cause diseases of temperate and tropical fruits from Australia, Europe and South Africa. Our results indicate that isolates from Citrus in Spain are conspecific with the type isolate of the recently described D. neotheicola from Foeniculum as well as isolates from other hosts now considered to be D. foeniculina. We reviewed the possible synonyms of this species based on available molecular data, living cultures and type specimens. The specimen deposited in LISE 94792 corresponding to the living culture CBS 111554 was selected as the epitype specimen for Phoma foeniculina, now recognised as D. foeniculina. Molecular data derived from the epitype of Phoma foeniculina, now D. foeniculina, and additional isolates show that this taxon is conspecific with the ex-type isolates of D. neotheicola and P. theicola (Phillips 2003, Santos & Phillips 2009, Gomes et al. 2013) as well as isolates from Citrus in Spain. The name D. neotheicola has been widely used for this taxon (Santos & Phillips 2009, Udayanga et al. 2012a, Thomidis et al. 2013).
Phomopsis foeniculina (syn. Phoma foeniculina) was considered a synonym of D. foeniculacea (syn. Sphaeria foeniculacea) by Phillips (2003). We examined the type specimen of Sphaeria foeniculacea and agree with von Arx & Müller (1954) who recognised this species as Guignardia foeniculacea (Mont.) Arx & E. Müll. (as G. foeniculata). Gomes et al. (2013) used the name D. foeniculacea to refer to this species based on Phillips (2003). However, observation of type specimens of Sphaeria foeniculacea confirmed that this name cannot be applied to a species of Diaporthe. This is further explained under the excluded species.
Although type specimens of Phomopsis theicola and D. theicola could not be located, an ex-type culture of P. theicola exists as mentioned by Santos & Phillips (2009). They stated that P. theicola was not the same as D. theicola based on the illustrations in the protologues of these taxa and described the name D. neotheicola for the sexual state of P. theicola, with an ex-holotype culture from Foeniculum. However, measurements of asci and ascospores in Curzi’s (1927) original description of D. theicola are consistent with those of D. foeniculina specimens examined in this study. We agree with the opinion of Curzi (1927) that D. theicola and P. theicola are known sexual and asexual states of the same fungus, simultaneously described from the same specimen and therefore here we epitypify the name D. theicola with Curzi’s ex-type culture of P. theicola.
Diaporthe rudis (Fr.) Nitschke, Pyrenomycetes Germanici 2: 282. 1870. — Fig. 8
Fig. 8.
Diaporthe rudis. a, b. Ectostroma and perithecia on Laburnum anagyroides; c. perithecia in transverse section; d. perithecial wall in longitudinal section; e, f. perithecia in longitudinal section; g–j. asci; k. ascospores; l. conidiophores developing on alfalfa stem in culture; m. alpha and beta conidia developing on alfalfa stem in culture (a–k. Epitype specimen BPI 748231; l–n. ex-epitype culture AR3422 = CBS 109292). — Scale bars: a = 2 000 μm; b, c = 1 000 μm; d = 50 μm; e, f = 100 μm; g–k = 25 μm; l–n = 15 μm.
Basionym. Sphaeria rudis Fr., Elench. Fung. (Griefswald) 2: 98. 1828.
≡ Rabenhorstia rudis (Fr.) Fr., Summa Veg. Scand., Section Post. (Stockholm): 410. 1849.
≡ Aglaospora rudis (Fr.) Tul. & C. Tul., Select. Fung. Carpol. (Paris) 2: 165. 1863.
= Phoma rudis Sacc., Michelia 1: 257. 1878.
≡Phomopsis rudis (Sacc.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1, 115: 680. 1906.
= Diaporthe faginea Sacc., Syll. Fung. 1: 619. 1882.
= Diaporthe macrostoma Nitschke, Pyrenomycetes Germanici 2: 284. 1870.
= Diaporthe medusaea Nitschke, Pyrenomycetes Germanici 2: 251. 1870.
= Diaporthe viticola Nitschke, Pyrenomycetes Germanici 2: 264. 1870.
=Diaporthe silvestris Sacc. & Berl., Atti Rev. Instit. Venet. Ser. II, 6: 737. 1885.
Perithecia black, clustered, globose, 300–350 μm, with tapering perithecial necks, 200–700 μm long. Asci unitunicate, sessile, elongate to clavate, (50.3–)53.5–58.5(–59.6) × (8.9–)10.6–12(–12.3) μm. Ascospores hyaline, 2-celled, often tetra-guttulate, with larger guttules at centre and smaller at ends, elongated to clavate, (11.6–)12–14.2(–15) × (2.8–)3.5–3.7(–3.8) μm (av. ± SD = 13.2 ± 1.1 × 3.6 ± 0.1, n = 30). Pycnidia on alfalfa twigs on WA: globose 200–250 μm diam, erumpent at maturity, up to 400–500 μm diam; walls 60–150 μm diam, parenchymatous, consisting of 3–4 layers of medium brown textura angularis. Conidiophores cylindrical, hyaline, smooth, branched, ampulliform, straight to sinuous, 20–45 × 2–2.4 μm. Conidiogenous cells phialidic, cylindrical, terminal, with slight tapering towards apex, 0.5–1 μm diam. Paraphyses abundant among conidiophores 20–40 × 1–2 μm. Alpha conidia aseptate, hyaline, smooth, ovate to ellipsoidal, biguttulate, base subtruncate (6.3–)7–8(8.7) × 2–2.5 μm (av. ± SD = 7.5 ± 0.4 × 2.2 ± 0.2, n = 30). Beta conidia aseptate, hyaline, smooth, fusiform or hooked, base subtruncate, 27–31(–35.2) × (3–)3.4–3.8(–4.2) μm (av. ± SD = 29.5 ± 2 ×3.6 ± 2, n = 30). Gamma conidia aseptate, hyaline, smooth, fusiform, mostly biguttulate, base subtruncate (10–)14–15 × 1–2 μm (av. ± SD = 14.4 ± 0.2 × 1.7 ± 0.24, n = 30).
Culture characteristics — In dark at 25 °C for 1 wk, colonies on PDA relatively slow growing, 4.2 mm/day, white, fluffy aerial mycelium, reverse with yellow pigmentation developing in centre.
Host range — Acer, Asphodelus albus, Aucuba japonica, Brugmansia, Castanea, Corylus, Dipsacus fullonum, Epilobium, Eucalyptus, Fagus, Fraxinus, Holcus, Hydrangea, Ileostylis, Laburnum, Lupinus, Malus, Protea, Pyrus, Rosa, Sambucus, Salix, Vaccinium and Vitis vinifera. In addition to the hosts on the specimens listed below, these hosts are represented in Fig. 1 based on ITS phylogeny and Gomes et al. (2013) as D. viticola.
Distribution — Australia, Canada, Chile, Europe (Austria, Germany, Italy, Latvia, Portugal, Spain, Sweden, Switzerland), New Zealand and South Africa.
Type specimens examined. FRANCE, on a dead branch of Laburnum anagyroides (as Cytisus laburnum), ex herb. Guépin no. 163 (holotype of Sphaeria rudis UPS F-004948). – AUSTRIA, Vienna, 19. 7763/2, Reisenbergbach-Weg, on stem of Laburnum anagyroides, 8 Apr. 2000, W. Jaklitsch (epitype of Sphaeria rudis designated here BPI 748231, ex-epitype culture AR 3422 = CBS 109292; MBT175965). – GERMANY, Nordrhein-Westfalen, Landkreis Unna, Cappenberg, Schloßgarten zu Cappenberg, on twigs of Laburnum anagyroides (syn. Cytisus laburnum), 18 Aug. 1866, T. Nitschke (holotype of Diaporthe medusaea B 70 0009168). – AUSTRIA, Vienna, 19. 7763/2, Reisenbergbach-Weg, on stem of Laburnum anagyroides, 8 Apr. 2000, W. Jaklitsch (epitype of Diaporthe medusaea designated here BPI 748231, ex epitype culture AR3422 = CBS 109292; MBT175966). – GERMANY, Nordrhein-Westfalen, Munsterland, Munster Botanischer Garten, on thin branch of Fagus sylvatica, 18 May 1866, T. Nitschke (holotype of Diaporthe macrostoma B 70 0009167). – GERMANY, Westfalen, Munster, bei der Wienburg, on Vitis vinifera, Feb. 1866, Nitschke (holotype of Diaporthe viticola B: not seen), ibid. (isotype BPI 797316). – PORTUGAL, Santo Tirso, Burgaes, on Vitis vinifera,16 Feb. 1998, A.J.L. Phillips (epitype of D. viticola designated in van Niekerk et al. (2005) CBS-H 7950 not seen, ex-epitype culture STE-U 5683 = CBS 113201). – ITALY, “In sarmentis Vitis viniferae silvestris, Cervarese” (holotype of Diaporthe silvestris: PAD 228 not seen). The synonymy of this name is based on van Niekerk et al. (2005) in which the holotype specimen was observed and considered to be D. viticola.
Additional specimens examined. AUSTRIA, Vienna, stem of Rosa canina, 13 May 2001, W. Jaklitsch (BPI 840948, living culture AR3654); Vienna, stem of Acer pseudoplatanus, 31 Mar. 2001, W. Jaklitsch (BPI840940, living culture AR3634). – GERMANY, urban residential area, container plant, dead stem of Brugmansia sp., 31 Oct. 2012, R. Schumacher (BPI 892463, living culture DA243 = CBS135435). – ITALY, on dead stem of Acer opalus, 2 May 2012, E. Camporesi ER285 (BPI 892464, living culture ER285A = CBS 135437); ibid., (ER 286, BPI 892465, living culture ER286D).
Notes — The name D. rudis is based on the oldest epithet of the many synonyms for this species including D. medusaea. Diaporthe medusaea, originally described from Laburnum anagyroides in Germany, has been used as the name for the fungus causing melanose and stem end rot of Citrus in North America. We observed holotype material as well as isolates on the same host from Austria in order to recognise the similarities or differences as discussed herein. Wehmeyer (1933) listed a number of synonyms for D. medusaea including D. citri, D. citrincola, D. faginea, D. rudis and D. viticola. Diaporthe citrincola is here recognised as a synonym of D. citri. Diaporthe faginea was established as a legitimate name for Sphaeria faginea Curr., Trans. Linn. Soc. London 22: 281. 1859 nom. illeg. non S. faginea Pers. 1794. No specimen as D. faginea exists in PAD. Based on an ITS sequence (EF155490) of the isolate from Fagus in Germany and a morphological comparison of the protologue, this name is accepted as a synonym of D. rudis. Although D. viticola was recognised as a distinct taxon and characterised and epitypified using a specimen on Vitis by van Niekerk et al. (2005), it is here determined to be a synonym of D. rudis.
EXCLUDED SPECIES
Phoma citri Sacc., Nuovo Giorn. Bot. Ital. 8: 200. 1876.
≡ Phomopsis citri (Sacc.) Traverso & Spessa, Bol. Soc. Brot. 25: 100. 1910.
ITALY, Traviso, a Vittorio, on branches of Citrus limon, Oct. 1873 (lectotype specimen of Phoma citri designated here, Mycotheca Veneto no. 332, FH labelled Diplodia citri; MBT175967).
Notes — Phoma citri has been confused with Phomopsis citri, now regarded as Diaporthe citri. Examination of type material of Phoma citri confirms that this taxon is not a Phomopsis and should be treated as a distinct taxon in the genus Phoma.
Diaporthe foeniculacea (Mont.) Niessl, in Thüm., Inst. Rev. Sci. Litt. Coimbra 27: 250. 1879.
Basionym. Sphaeria foeniculacea Mont., Ann. Sci. Nat., Bot., sér. 3, 11: 40. 1849.
≡ Physalospora foeniculacea (Mont.) Sacc. (as ‘foeniculata’), Syll. Fung. (Abellini) 1: 445. 1882.
≡ Sphaerella foeniculacea (Mont.) Cooke (as ‘foeniculata’), J. Bot. London 21: 70. 1883.
≡ Guignardia foeniculacea (Mont.) Arx & E. Müll. (as ‘foeniculata’), Beitr. Kryptogamenfl. Schweiz 11 (no. 1): 48. 1954.
PORTUGAL, Coimbra, on stem of Foeniculum officinalis, June 1881, (lectotype specimen of Sphaeria foeniculacea designated here: in Thümen, Mycotheca Universalis 2260, bound collection in BPI; MBT175968). Isolectotypes: ibid. (BPI 616247, BPI 797288 Shear Types and Rarities).
Notes — One of the names mentioned in Phillips (2003) is Diaporthe foeniculacea (Mont.) Niessl (basionym Sphaeria foeniculacea Mont.), a name that has been confused with Phomopsis foeniculina (basionym Phoma foeniculina). We observed three isotype specimens of D. foeniculacea in BPI and confirmed the status of this species as a Guignardia (sexual morph of Phyllosticta) as suggested by von Arx & Müller (1954), unrelated to Diaporthe. One of these specimens is here designated the lectotype.
DISCUSSION
Melanose and stem end rot of Citrus have been reported from the United States since the late 18th century killing twigs and causing a minor form of gummosis by latent infection (Floyd & Stevens 1912, Fawcett 1936). A disease of Citrus to which the common name melanose is applied was first recognised near Citra, Florida, by Swingle & Webber in 1892 (Floyd & Stevens 1912). The stem end rot disease was investigated in Florida by Burger (1923) and Winston et al. (1923). Phomopsis citri was first described from the United States (Florida) as a pycnidial fungus on dead branches and decayed fruits of Citrus aurantium, C. decumana and C. nobilis (Fawcett 1912). This name is a later homonym of Phomopsis citri (Sacc.) Traverso & Spessa (1910) based on Phoma citri Sacc. (1876) originally described from Citrus limonia in Italy and now considered to belong in Phoma. Diaporthe citri (H.S. Fawc.) F.A. Wolf, described as sexual morph of Phomopsis citri H.S. Fawc., was originally collected from the United States (Florida) and has since been reported as saprobic or parasitic on leaves, stems and fruits of Citrus spp. throughout the world. Fisher (1972) used the concept of Wehmeyer (1933) and considered Phomopsis cytosporella as the valid name for D. citri based on the chronology of names. However, this interpretation was not adopted by plant pathologists or taxonomists. In our study P. cytosporella is determined to be a distinct taxon in Diaporthe, D. cytosporella, and epitypified based on fresh collections from Europe.
In addition to the association with Citrus in the United States, Diaporthe citri is confirmed here in Brazil, China, Korea and New Zealand and appears to be widespread in Asia, Australasia and South America. Based on sampling in this study, we did not find D. citri to occur in Europe or any sequences in public databases corresponding to D. citri from Europe. However, our results suggest that this species may be pantropical. The genetic similarities of the isolates of D. citri from Asia where Citrus originated with those found worldwide suggest a long standing co-existence and the probable widespread movement of the pathogen with its host. Diaporthe citri was the dominant species causing melanose and stem end rot symptoms among the recent collections from Citrus spp. throughout China (Huang et al. 2013). Two newly described species discovered in north central China (Shaanxi Province) in the same study, Diaporthe citiasiana and D. citrichinensis, were primarily associated with dead wood of Citrus unshiu (satsuma mandarin) and not with melanose and stem end rot diseases. Gomes et al. (2013) included two species from Citrus from Suriname in their analysis, Diaporthe arecae and an unidentified species. None of these species were encountered in this study. This indicates that numerous species are associated with Citrus worldwide and it is likely that more will be discovered.
Diaporthe foeniculina (referred to as D. foeniculacea in Gomes et al. 2013, see taxonomy section) is found to be a pathogen of diverse hosts ranging from crops to temperate woody plants and fruit trees. The recent reports of D. foeniculina (as D. neotheicola) causing diseases of temperate cultivated fruit trees including shoot blight of persimmon in Australia and kiwifruit disease in Greece suggest potential for this species to infect a wide range of fruits as an opportunistic pathogen (Golzar et al. 2012, Thomidis et al. 2013). Although a number of isolates from Citrus in California are identified in this study as D. foeniculina, its pathogenicity on Citrus in California is unknown. Herbarium specimens previously identified as D. citri intercepted at ports in the United States on the fruits of Citrus limonia from Spain were identified morphologically as D. foeniculina (BPI 615878: intercepted in New York, 1940; BPI 747926, BPI 747927, BPI 1107900: intercepted in New Jersey, 1987). A living culture (MEP1289-1) from BPI 1107900 was used in the phylogenetic analyses in this study and is confirmed as D. foeniculina.
Phylogenetic analysis of ITS sequence data was able to resolve the closely related species D. canthii, D. cytosporella, D. foeniculina and D. pterocarpi. Although the ITS sequence analysis resolved a clade corresponding to the recently described D. rhusicola, this clade was not supported by the ACT, CAL, EF1-α or TUB gene regions, individually or in the multi-gene phylogeny. Inspection of the ITS sequences for this group of isolates (D. foeniculina and D. rhusicola) indicated that the ITS differences consisted of two deletions and one transition in the ITS1 and two transitions in the ITS2 region. Alternatively, in the case of D. cynaroidis and D. rudis, ITS sequences do not definitively distinguish the two species although EF1-α, CAL, HIS and TUB do distinguish the two species.
In the case of D. rudis (referred to as D. viticola in van Niekerk et al. 2005 and Gomes et al. 2013) and D. australafricana, the ITS1 region differs by a single transition while the ITS2 region shows minor differences over a span of c. 7 bp consisting of three transitions, one transversion and one deletion. The CAL gene differs by one transition and isolates of D. rudis share a 9 bp insertion in ACT not found in D. australafricana. Data from Gomes et al. (2013) show a 3 bp insertion in isolates of D. australafricana not shared by D. rudis in HIS. Isolates with ITS sequences matching that of D. australafricana were previously only known from Africa and Australia on Vitis but have recently been found on Vaccinium and Corylus in Chile and Persea from California in the United States (Latorre et al. 2012, Elfar et al. 2013). Although closely related, the low but consistent variation found in the sampled genes is considered sufficient to recognise D. australafricana as a distinct phylogenetic species in agreement with van Niekerk et al. (2005), Udayanga et al. (2012a) and Gomes et al. (2013). However, additional isolates of both taxa would be desirable in order to further investigate population structure and species boundaries.
Much confusion in the literature exists in how to interpret the ITS sequences of closely related species in Diaporthe and authors treat observed variation in ITS sequences in different ways (Farr et al. 2002a, b, Murali et al. 2006, Santos et al. 2010). This can result in superfluous, multiple terminal branches in combined gene analyses, even when other gene regions do not support these distinctions. Additionally Santos et al. (2010) showed the occurrence of two phylogenetically distinct ITS populations within an unidentified species of Diaporthe based on the sequencing of single ascospore-derived isolates from the same perithecium. Sequence differences were confined to the ITS1 region over a span of c. 40 bp and are more extensive than those differences noted among isolates of a single species in this study. Sequence differences were not noted in the EF1-α and mating type genes in their analyses and the isolates were fully reproductively compatible (Santos et al. 2010).
While we consider ITS rDNA sequences to be useful as barcodes for identification of known, circumscribed Diaporthe species, we suggest that caution is warranted when differences are noted in the absence of other data and that at the minimum EF1-α should be used to confirm identities of Diaporthe species. The EF1-α gene region amplified by primers EF1-728F/EF1-986R has given the most consistent results when analysing available ex-type sequences of reliably identified and vouchered species (Castlebury 2005, Santos et al. 2010, 2011, Udayanga et al. 2012a). Phylogenetic signals observed in protein coding genes are generally considered superior to rRNA genes, although there is less standardisation in terms of universal primers, genes or even regions of genes sequenced for fungi (Schoch et al. 2012). However, results from this study suggest that use of CAL and TUB genes can be problematic with the potential for incorrect species identification in Diaporthe due to non-specific priming and the resulting poor sequence quality (CAL) or potential paralogy (TUB), necessitating critical evaluation of sources of conflict in gene trees.
Epitypification is recognised as the best approach to resolve long standing taxonomic and phylogenetic issues of known taxa that are poorly circumscribed, thus providing a modern interpretive type (Hyde & Zhang 2008). There is an unprecedented need for mycologists to return to the field to recollect species, fully characterise taxa with DNA sequences and morphological descriptions and epitypify species in Diaporthe, which includes a large number of names not linked to DNA sequence data or ex-type cultures (Hyde et al. 2010a, b, Ko-Ko et al. 2011, Udayanga et al. 2011). Several modern studies aimed at epitypification of important pathogens in Diaporthe provide clarification and knowledge of the phylogeny and species boundaries within the genus (Castlebury et al. 2003, van Niekerk et al. 2005, Rensburg et al. 2006, Diogo et al. 2010, Udayanga et al. 2012b, Gomes et al. 2013). Application of genealogical concordance and/or gsi in combination with epitypification can provide critical insights into speciation processes, ecology and host associations (Sakalidis et al. 2011, Gazis et al. 2011, Mejia et al. 2011, Silva et al. 2012, Walker et al. 2012).
Species circumscription should only be undertaken in conjunction with rigorous application of multi-gene analyses and genealogical concordance. In addition best practices for introduction of new names should require a thorough investigation of existing names and type specimens. This is particularly important to prevent additional superfluous names in a genus such as Diaporthe with an abundance of existing names including those in Phomopsis. A total of 2 453 results are returned when searching for Diaporthe and Phomopsis ITS sequences in the NCBI GenBank databases. A search using the terms ‘Phomopsis sp.’ (Organism) AND ‘internal transcribed’ (All Fields) returned 922 sequences; substituting ‘Diaporthe sp.’ returned 341 sequences (http://www.ncbi.nlm.nih.gov, retrieved 3 April 2013), which suggests that c. 51 % of all Diaporthe and Phomopsis ITS sequences in GenBank are unidentified. Of the 49 % that do have species names, many do not include culture or specimen vouchers and it is likely that the majority are misidentified or named without a proper taxonomic revision. As sequence data accumulate in public databases, the need for correctly identifying and vouchering sequences, particularly from ex-type or epitype cultures, becomes pressing as these data are being used for identification of pathogens by plant pathologists and in public health and quarantine situations.
Acknowledgments
The following individuals are thanked for sharing cultures, specimens and unpublished sequence data: Aaron Kennedy, John McKemy and Mary Palm (USDA-APHIS); Akif Eskalen and Joey Mayorquin (Fawcett Laboratory of University of California Riverside, CA); Peter Johnston and Bevan Weir (Landcare Research, New Zealand); Erio Camporesi (Italy, Asco-France); Sung Kee Hong (Korea); Rene Schumacher (Germany); and Walter Jaklitsch (University of Vienna, Austria). Herbarium curators and managers of B, BPI, FH, LISE, NY and S are gratefully acknowledged for loan of specimens. The technical assistance of Tunesha Phipps is greatly appreciated.
REFERENCES
- Agostini JP, Bushong PM, Bhatia A, Timmer LW. 2003. Effects of environmental factors on the severity of citrus scab and melanose. Plant Disease 87: 69–74 [DOI] [PubMed] [Google Scholar]
- Arx JA von, Müller E. 1954. Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11, 1: 1–434 [Google Scholar]
- Bach WJ, Wolf FA. 1928. The isolation of the fungus that causes citrus melanose and the pathological anatomy of the host. Journal of Agricultural Research 37: 243–252 [Google Scholar]
- Brayford D. 1990. Variation in Phomopsis isolates from Ulmus species in the British Isles and Italy. Mycological Research 94: 691–697 [Google Scholar]
- Burger OF. 1923. Citrus stem-end rot. Citrus Industry 4: 14 [Google Scholar]
- Burnett HC. 1962. Melanose: Diaporthe citri (Fawc) Wolf. Plant pathology circular No 2. Florida Department of Agriculture, Division of Plant Industry [Google Scholar]
- Cai L, Giraud T, Zhang N, Begerow D, Cai GH, Shivas RG. 2011. The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Diversity 50: 121–133 [Google Scholar]
- Carbone I, Kohn L. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Castlebury LA. 2005. The Diaporthe vaccinii complex of fruit pathogens. Inoculum 56: 12 [Google Scholar]
- Castlebury LA, Farr DF, Rossman AY, Jaklitsch WJ. 2003. Diaporthe angelicae comb. nov., a modern description and placement of Diaporthopsis in Diaporthe. Mycoscience 44: 203–208 [Google Scholar]
- Crous PW. 2005. Impact of molecular phylogenetics on the taxonomy and diagnostics of fungi. EPPO Bulletin 35: 47–51 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Cummings MP, Neel MC, Shaw KL. 2008. A genealogical approach to quantifying lineage divergence. Evolution 62: 2411–2422 [DOI] [PubMed] [Google Scholar]
- Curzi M. 1927. De novis Theae micromycetibus pathogenis. Atti dell’Istituto Botanico della Università e Laboratorio Crittogamico di Pavia 3: 59–72 [Google Scholar]
- Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW. 2003. Reproductive isolation and phylogenetic divergence in Neurospora: comparing methods of species recognition in a model eukaryote. Evolution 57: 2721–2741 [DOI] [PubMed] [Google Scholar]
- Diogo ELF, Santos JM, Phillips AJL. 2010. Phylogeny, morphology and pathogenicity of Diaporthe and Phomopsis species on almond in Portugal. Fungal Diversity 44: 107–115 [Google Scholar]
- Elfar K, Torres R, Diaz GA, Latorre BA. 2013. Characterization of Diaporthe australafricana and Diaporthe spp. associated with stem canker of blueberry in Chile. Plant Disease 97: 1042–1050 [DOI] [PubMed] [Google Scholar]
- Farr DF, Castlebury LA, Rossman AY. 2002a. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States. Mycologia 94: 494–504 [PubMed] [Google Scholar]
- Farr DF, Castlebury LA, Rossman AY, Putnam ML. 2002b. A new species of Phomopsis causing twig dieback of Vaccinium vitisidaea (lingonberry). Mycological Research 106: 745–752 [Google Scholar]
- Fawcett HS. 1912. The cause of stem-end rot of Citrus fruits (Phomopsis citri n. sp.). Phytopathology 2: 109–113 [Google Scholar]
- Fawcett HS. 1922. A Phomopsis of citrus in California (abstract). Phytopathology 12: 107 [Google Scholar]
- Fawcett HS. 1932. Diaporthe citri (Fawc.) Wolf, the perfect stage of Phomopsis citri and P. californica. Phytopathology 22: 928 [Google Scholar]
- Fawcett HS. 1936. Citrus diseases and their control. McGraw Hill, New York, USA [Google Scholar]
- Fisher FE. 1972. Diaporthe citri and Phomopsis citri: A correction. Mycologia. 64: 422 [Google Scholar]
- Floyd BF, Stevens HE. 1912. Melanose and stem-end rot. Agricultural Experiment Station Bulletin 111: 1–16. Florida [Google Scholar]
- French AM. 1987. California plant disease host index. Part 1: Fruit and nuts. California Department of Food and Agriculture, Sacramento [Google Scholar]
- Gazis R, Rehner S, Chaverri P. 2011. Species delimitation in fungal endophyte diversity studies and its implications in ecological and biogeographic inferences. Molecular Ecology 20: 3001–3013 [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzar H, Tan YP, Shivas RG, Wang C. 2012. First report of shoot blight of persimmon caused by Diaporthe neotheicola in Australia. Australasian Plant Disease Notes 7: 115–117 [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, Lombard L, Groenewald JZ, Crous PW. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne WT. 1922. A Phomopsis in grape fruit from the isle of Pines W. I., with notes on Diplodia natalensis. Phytopathology 12: 414–418 [Google Scholar]
- Huang F, Hou X, Dewdney MM, Fu Y, Chen G, Hyde KD, Li H. 2013. Diaporthe species occurring on citrus in China. Fungal Diversity 61: 237–250 [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Abd-Elsalam K, Cai L. 2010a. Morphology: still essential in a molecular world. Mycotaxon 114: 439–451 [Google Scholar]
- Hyde KD, Chomnunti P, Crous PW, Groenewald JZ, Damm U, et al. 2010b. A case for re-inventory of Australia’s plant pathogens. Persoonia 25: 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KD, Zhang Y. 2008. Epitypification: should we epitypify? Journal of Zhejiang University-Science B 9: 842–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko-Ko TW, McKenzie EHC, Bahkali AH, To-Anun C, Chukeatirote E, et al. 2011. The need for re-inventory of Thai phytopathogens. Chiang Mai Journal of Science 38: 1–13 [Google Scholar]
- Kobayashi T. 1970. Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bulletin of the Government Forest Experiment Station, Tokyo 226: 1–242 [Google Scholar]
- Kobayashi T. 2007. Index of fungi inhabiting woody plants in Japan. Host, distribution and literature. Zenkoku-Noson-Kyoiku Kyokai Publishing Co., Ltd. [Google Scholar]
- Kucharek T, Whiteside J, Brown E. 1983. Melanose and stem end rot of citrus. Plant pathology fact sheet. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainsville [Google Scholar]
- Kuhara S. 1999. The application of the epidemiologic simulation model ‘MELAN’ to control citrus melanose caused by Diaporthe citri (Faw.) Wolf. Food and Fertilizer Technology Center Extension Bulletins, National Fruit Research Institute, Japan [Google Scholar]
- Latorre BA, Elfar K, Espinoza JG, Torres R, Díaz GA. 2012. First report of Diaporthe australafricana associated with stem canker on blueberry in Chile. Plant Disease 96: 768. [DOI] [PubMed] [Google Scholar]
- Li HL. 1970. The origin of cultivated plants in Southeast Asia. Economic Botany 24, 1: 3–19 [Google Scholar]
- Luongo L, Santori A, Riccioni L, Belisario A. 2011. Phomopsis sp. associated with post-harvest fruit rot of kiwifruit in Italy. Journal of Plant Pathology 93: 205–209 [Google Scholar]
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, et al. 2012. International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Regnum Vegetabile 154. Koeltz Scientific Books, Germany [Google Scholar]
- Mejia LC, Rossman AY, Castlebury LA, White JF., Jr 2011. New species, phylogeny, host-associations, and geographic distribution of the genus Cryptosporella (Gnomoniaceae, Diaporthales). Mycologia 103: 379–399 [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, Louisiana: 1–8 [Google Scholar]
- Moherek EA. 1970. Disease control in Florida citrus with Difolatan fungicide. Proceedings of the 83rd Annual Meeting of the Florida State Horticultural Society 83: 59–65 [Google Scholar]
- Mondal SN, Agostini JP, Zhang L, Timmer LW. 2004. Factors affecting pycnidium production of Diaporthe citri on detached citrus twigs. Plant Disease 88: 379–382 [DOI] [PubMed] [Google Scholar]
- Mondal SN, Vincent A, Reis RF, Timmer LW. 2007. Saprophytic colonization of citrus twigs by Diaporthe citri and factors affecting pycnidial production and conidial survival. Plant Disease 91: 387–392 [DOI] [PubMed] [Google Scholar]
- Murali TS, Suryanarayanan TS, Geeta R. 2006. Endophytic Phomopsis species: host range and implications for diversity estimates. Canadian Journal Microbiology 52: 673–680 [DOI] [PubMed] [Google Scholar]
- Nelson S. 2008. Citrus melanose. Online document of Cooparative extension service, College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa. Plant Disease 59: 1–5 http://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-59.pdf [Google Scholar]
- Niekerk JM van, Groenewald JZ, Farr DF, Fourie PH, Halleen F, Crous PW. 2005. Reassessment of Phomopsis species on grapevines. Australasian Plant Pathology 34: 27–39 [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University [Google Scholar]
- O’Donnell K, Nirenberg HI, Aoki T, Cigelnik E. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 41: 61–78 [Google Scholar]
- Page RDM. 1996. TreeView: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358 [DOI] [PubMed] [Google Scholar]
- Pantidou ME. 1973. Fungus-host index for Greece. Benaki Phytopathology Institute, Kiphissia, Athens [Google Scholar]
- Phillips AJL. 2003. Morphological characterization of Diaporthe foeniculacea and its Phomopsis anamorph on Foeniculum vulgare. Sydowia 55: 274–285 [Google Scholar]
- Punithalingam E, Holliday P. 1973. Diaporthe citri CMI descriptions of pathogenic fungi and bacteria, No. 396. Commonwealth Mycological Institute, Kew, Surrey, England [Google Scholar]
- Rambaut A, Drummond A. 2008. FigTree: Tree figure drawing tool, version 1.2. 2. Institute of Evolutionary Biology, University of Edinburgh [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Kew, Surrey, UK: CMI and British Mycological Society [Google Scholar]
- Rehm H. 1914. Ascomycetes Philippinenses VI. Leaflets of Philippine Botany 6: 2258–2281 [Google Scholar]
- Rehner SA, Uecker FA. 1994. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Canadian Journal of Botany 72: 166–167 [Google Scholar]
- Rensburg JCJ van, Lamprecht SC, Groenewald JZ, Castlebury LA, Crous PW. 2006. Characterization of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Studies in Mycology 55: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Palm-Hernández ME. 2008. Systematics of plant pathogenic fungi: why it matters. Plant Disease 92: 1376–1386 [DOI] [PubMed] [Google Scholar]
- Rossman AY, Udayanga D, Castlebury LA, Hyde KD. 2013. Proposal to conserve the name Diaporthe citri (H.S. Fawc.) Wolf based on Phomopsis citri H.S. Fawc. against Phomopsis citri (Sacc.) Traverso & Spessa. Taxon 62: 627 [Google Scholar]
- Sakalidis ML, Hardy GE, Burgess TI. 2011. Use of the Genealogical Sorting Index (GSI) to delineate species boundaries in the Neofusicoccum parvum-Neofusicoccum ribis species complex. Molecular Phylogenetics and Evolution 60: 333–344 [DOI] [PubMed] [Google Scholar]
- Santos JM, Correia VG, Phillips AJL. 2010. Primers for mating-type diagnosis in Diaporthe and Phomopsis: their use in teleomorph induction in vitro and biological species definition. Fungal Biology 114: 255–270 [DOI] [PubMed] [Google Scholar]
- Santos JM, Phillips AJL. 2009. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Diversity 34: 111–125 [Google Scholar]
- Santos JM, Vrandečić K, Ćosić J, Duvnjak T, Phillips AJL. 2011. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 27: 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas RG, Cai L. 2012. Cryptic fungal species unmasked. Microbiology Australia 33: 36–37 [Google Scholar]
- Silva DN, Talhinhas P, Cai L, Manuel L, Gichuru EK, et al. 2012. Host-jump drives rapid and recent ecological speciation the emergent fungal pathogen Colletotrichum kahawae. Molecular Ecology 21: 2655–2670 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771 [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002. PAUP 4.0b10: phylogenetic analysis using parsimony. Sinauer Associates, Sunderland, Massachusetts [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32 [DOI] [PubMed] [Google Scholar]
- Thomidis T, Exadaktylou E, Chen S. 2013. Diaporthe neotheicola, a new threat for kiwi fruit in Greece. Crop Protection 47: 35–40 [Google Scholar]
- Timmer LW. 2000. Scab diseases. In: Whiteside JO Garnsey SM Timmer LW (eds), Compendium of citrus diseases, revised edition: 31–32. American Phytopathological Society, St. Paul, Minnesota [Google Scholar]
- Timmer LW, Fucik JE. 1976. The relationship of rainfall distribution, fruit growth, and fungicide application to the incidence of melanose on grapefruit in Texas. Plant Disease Reporter 60: 565–568 [Google Scholar]
- Timmer LW, Kucharek TA. 2001. Melanose (revised). Plant Pathology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Fact Sheet PP-150. [Google Scholar]
- Traverso GB, Spessa C. 1910. La flora micologica del Portugallo. Saggio. Boletim da Sociedade Broteriana 25: 26–187 [Google Scholar]
- Udayanga D, Liu X, Crous PW, McKenzie EHC, Chukeatirote E, Hyde KD. 2012a. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Diversity 56: 157–171 [Google Scholar]
- Udayanga D, Liu X, McKenzie EHC, Chukeatirote E, Bahkali AHA, Hyde KD. 2011. The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Diversity 50: 189–225 [Google Scholar]
- Udayanga D, Liu X, Mckenzie EHC, Chukeatirote E, Hyde KD. 2012b. Multi-locus phylogeny reveals three new species of Diaporthe from Thailand. Cryptogamie Mycologie 33: 295–309 [Google Scholar]
- Walker DM, Castlebury LA, Rossman AY, Mejía LC, White JF. 2012. Phylogeny and taxonomy of Ophiognomonia (Gnomoniaceae, Diaporthales), including twenty-five new species in this highly diverse genus. Fungal Diversity 57: 85–147 [Google Scholar]
- Wehmeyer LE. 1933. The genus Diaporthe Nitschke and its segregates. University of Michigan Studies, Science Series 9: 1–349 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. 1990Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA Gelfand DH Sninsky JJ White TJ (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego [Google Scholar]
- Whiteside JO. 1977. Sites of action of fungicides in the control of Citrus melanose. Phytopathology 67: 1067–1072 [Google Scholar]
- Whiteside JO, Timmer LW. 2000a. Citrus diseases: general concepts. In: Whiteside JO Garnsey SM Timmer LW (eds), Compendium of citrus diseases, revised edition: 3–4. American Phytopathological Society, St. Paul, Minnesota [Google Scholar]
- Whiteside JO, Timmer LW. 2000b. Melanose. In: Whiteside JO Garnsey MS Timmer LW (eds), Compendium of citrus diseases, revised edition: 20–21. American Phytopathological Society, St. Paul, Minnesota [Google Scholar]
- Winston JR, Fluton HR, Bowman JJ. 1923. Commercial control of Citrus stem end rot. United States Department of Agriculture Circular 193: 1–10 [Google Scholar]
- Wolf FA. 1926. The perfect stage of the fungus which causes melanose of citrus. Journal of Agricultural Research 33: 621–625 [Google Scholar]
- Yamato H. 1976. A species of Diaporthe pathogenic to citrus. Annals of the Phytopathological Society Japan 42: 56–59 [Google Scholar]