Abstract
Almost a decade has passed since first STIM, and later Orai, proteins were identified as the molecular constituents of store-operated calcium entry (SOCE). Whereas their roles in immune function have been intensely investigated, the roles of STIM and Orai in neuronal cells have been much less clear. Lalonde et al. show that when neurons are hyperpolarized or “at rest”, constitutive endoplasmic reticulum (ER) Ca2+ release leads to SOCE-mediated activation of neuronal transcription factors. Precisely why ER Ca2+ release is constitutive in neurons remains an important question. Irrespective of the answer, this observation provides an intriguing new perspective into why a relatively low-abundance, small-conductance channel such as Orai1 would be important in neurons, which contain a relative abundance of voltage-operated Ca2+ channels.
Cellular Ca2+ homeostasis is a complex, energy-consuming process that involves multiple players and subcellular compartments. Whereas the calcium concentration ([Ca2+]) in the extracellular milieu is typically ~2 mM; at rest, cytosolic [Ca2+] is ~100 nM and in the ER [Ca2+] is close to 500 µM. Due to the existence of poorly defined ‘leak’ channels, maintaining high [Ca2+] in the ER requires the constant activity of sarcoplamic and endoplasmic reticulum Ca2+-ATPase (SERCA) pumps. In most cells, this arrangement produces a stable ER [Ca2+] unless SERCA function is blocked. However, papers by Lalonde et al. (1) and Hartmann et al. (2) reveal that in neurons, ER [Ca2+] cannot be maintained unless there is constant Ca2+ influx across the plasma membrane (Fig. 1).
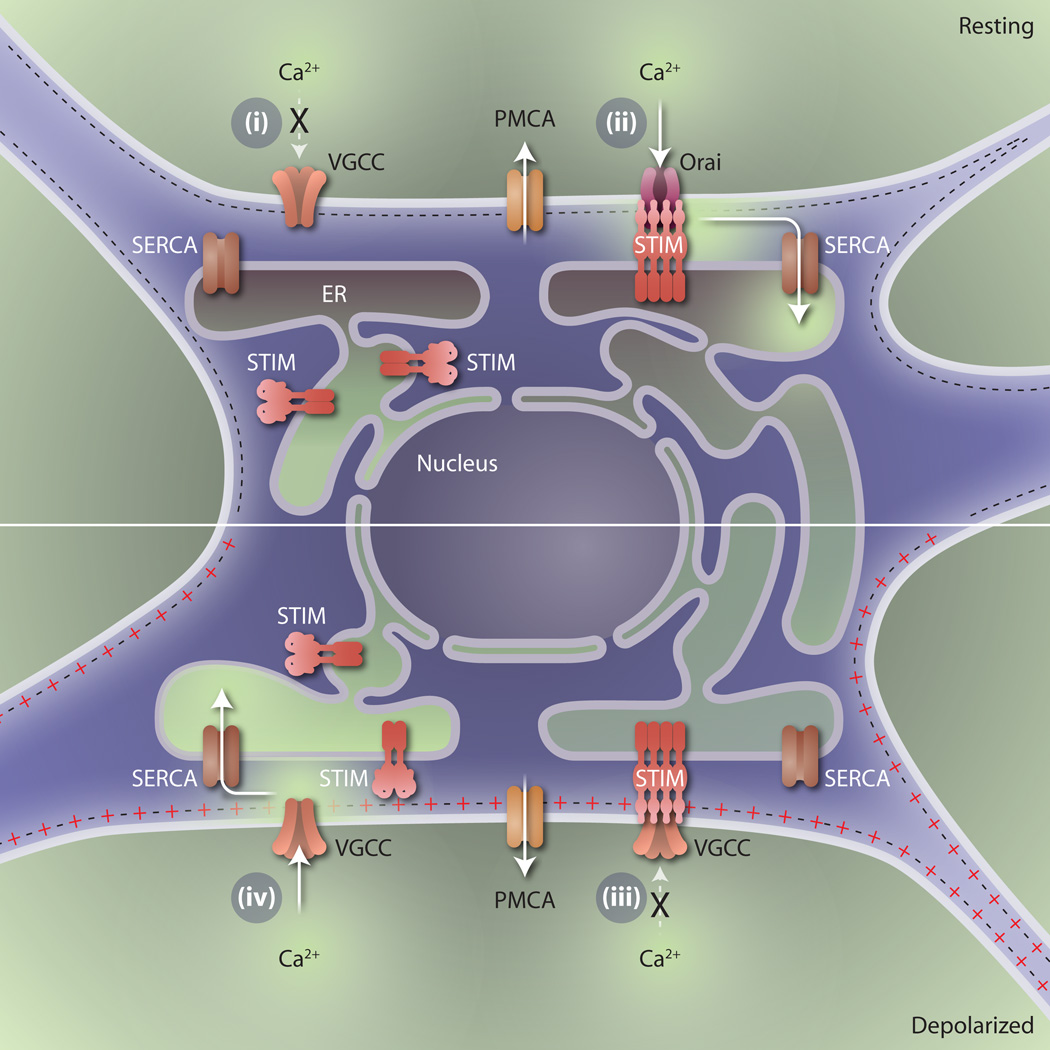
Fig. 1. Control of ER Ca2+ content in neurons.
The proteins and pathways pertinent to Ca2+ homeostasis in neurons are shown, with relative localized Ca2+ concentration indicated by blue shading. The plasma membrane Ca2+-ATPase (PMCA) is responsible for extrusion of Ca2+ from the cell. The neuron can be considered in four parts. In the first part, the neuron has hyperpolarized, blocking VGCC activity and causing ER Ca2+ depletion. In the second part, ER Ca2+ depletion activates STIM and engages Orai, enabling SERCA activity and refilling the ER Ca2+ content. In the third part, the resting neuron with some amount of ER Ca2+ depletion becomes depolarized, but the activated STIM inhibits VGCC activation. In the fourth part, the ER Ca2+ content of the activated neuron has recovered, leading to VGCC activity, disengagement of STIM, and normal ER Ca2+ content.
Whereas voltage-gated calcium channels (VGCCs) are the most abundant Ca2+ channels in neurons, store-operated calcium entry (SOCE) is a universal process that occurs in all cells, including neurons. SOCE is the process whereby ER Ca2+ depletion leads to Ca2+ influx across the plasma membrane. In most cells, ER Ca2+ depletion can be attributed to the activation of either inositol-1,4,5-trisphosphate or ryanodine receptor channels (InsP3Rs or RyRs, respectively) on the ER membrane and subsequent release of ER Ca2+ through these channels. In principle, any event leading to ER Ca2+ depletion should induce SOCE; in neurons, this event may simply be the absence of Ca2+ influx through VGCCs when the neuron is silent (that is near the resting membrane potential). When VGCC activity is low, the inability of neurons to maintain ER Ca2+ content leads to the activation of STIM1 and STIM2, proteins present in the ER membrane, through dissociation of Ca2+ from their luminal calcium-binding domains called EF hands, leading to facilitation of Orai-mediated Ca2+ entry (3). In both cultured cerebellar granule neurons (1) and acutely isolated Purkinje neurons (2), the STIM moiety serving as the primary mediator of SOCE is STIM1. However, other studies reported that STIM2 was shown to be the primary STIM expressed in hippocampal (4) and cortical (5) neurons. Further, genetic ablation of STIM2 in mice reduced life expectancy, caused memory defects, and protected neurons from ischemia-reperfusion (4). Consequently, it is surprising that both cerebellar granule neurons and Purkinje neurons exhibited predominantly STIM1-mediated SOCE. Future investigations focused on STIM expression patterns in specific neuronal cell types may lead to a better understanding of differential ER Ca2+ handling in neuronal subtypes and the context-specific roles of the different STIM moieties.
Given that Ca2+ entry through either SOCE or VGCC activity is required to maintain neuronal ER Ca2+ content, it is interesting to speculate about whether STIM1 inhibits VGCC (6, 7). Although the initial observations were made in vascular smooth muscle and T cells and focused on Cav1.2 (6, 7), STIM1-mediated suppression of VGCCs was implicated in the differentiation of neurons from mouse ES cells (8). Inhibition of VGCCs by STIM1 and STIM2 could have profound implications for excitability in neurons, because constitutive STIM activation in resting neurons would presumably lead to VGCC inhibition. In this scenario, STIMs would create resistance to activation that would need to be overcome in the transition from resting to excitation.
Lalonde et al. (1) link SOCE--distinct from depolarization-induced Ca2+ entry through VGCCs or NMDA-type glutamate receptors-- as directly responsible for Sp4 activity. Sp4 is a neuron-specific transcription factor that is primarily controlled via ubiquitin-proteasome-mediated degradation. SP4 regulates the expression of both tissue-specific genes and housekeeping genes;; and has been previously shown to be degraded under depolarizing conditions, with a profound impact on memory and synaptic plasticity (9). Precisely why there would be a unique link between Ca2+ entering cells through Orai1, and not VGCCs, and Sp4 is not known. However, Orai1 activity does not create equivalent cytosolic [Ca2+] in resting neurons to those observed in stimulated ones. These differences presumably lead to the engagement of distinct effectors and the initiation of alternate transcriptional programs.
The authors speculate that their findings may provide a new link between Ca2+ dysregulation and neuronal pathologies, such as schizophrenia, in which Sp4 abundance inversely correlates with disease symptoms (9, 10). However, the findings of the current studies (1, 2) also have implications to Alzheimer’s disease. Mutations in the ER membrane proteins presenilin 1 and 2 lead to Familial Alzheimer’s disease and these mutations have been shown to reduce ER Ca2+ leak (11–13). Neurons are presumably uniquely sensitive to loss of ER Ca2+ leak since this would decrease engagement of SOCE and potentially alter downstream signaling, such as Sp4 activation. Future investigations of STIM function in neurons expressing presenilin mutants may improve our understanding of this relationship.
In another recent study, Hartmann et al (2) use Purkinje neuron-specific STIM1 deletion to reveal that STIM1 is critical for metabotropic glutamergic synaptic transmission and cerebellar motor function. Interestingly, these defects were linked primarily to a STIM1 requirement for TRPC3-mediated excitatory postsynaptic currents. The ability of STIM1 to modulate TRPC function has been a highly controversial topic for many years. It is tempting to speculate that this controversy might stem from internal factors in Purkinje neurons (and some, but not all other cell types) that support STIM-TRPC interactions. Defining these conditions would go a long way towards addressing this controversy.
In conclusion, Lalonde et al. have provided an intriguing new perspective on the roles of STIM and Orai in neurons. STIM and Orai provide a crucial source of Ca2+ when neurons are at rest to maintain ER Ca2+ concentration (Fig. 1). In addition to this homeostatic function, STIM activation in neurons also regulates gene transcription through Sp4 (1) and, likely, other transcription factors. Given the defects in both memory (4) and motor control (2) observed in animals with neuron-specific STIM knockout, these STIM-dependent effects on Ca2+ handling can have profound effects in the brain. Considering the multifaceted roles of STIM1 in the control of Ca2+ signals (14), future investigations will be required to define the STIM targets responsible for these unique functions.
References
- 1.Lalonde J, Saia G, Gill G. Store-Operated Calcium Entry Promotes the Degradation of the Transcription Factor Sp4 in Resting Neurons. Sci Signal. 2014;7 doi: 10.1126/scisignal.2005242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartmann J, Karl RM, Alexander RP, Adelsberger H, Brill MS, Ruhlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, Misgeld T, Konnerth A. STIM1 Controls Neuronal Ca(2+) Signaling, mGluR1-Dependent Synaptic Transmission, and Cerebellar Motor Behavior. Neuron. 2014;82:635–644. doi: 10.1016/j.neuron.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- 5.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J. Differential Roles for STIM1 and STIM2 in Store-Operated Calcium Entry in Rat Neurons. PloS one. 2011;6:e19285. doi: 10.1371/journal.pone.0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 8.Hao B, Lu Y, Wang Q, Guo W, Cheung KH, Yue J. Role of STIM1 in survival and neural differentiation of mouse embryonic stem cells independent of Orai1-mediated Ca2+ entry. Stem cell research. 2014;12:452–466. doi: 10.1016/j.scr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Pinacho R, Villalmanzo N, Lalonde J, Haro JM, Meana JJ, Gill G, Ramos B. The transcription factor SP4 is reduced in postmortem cerebellum of bipolar disorder subjects: control by depolarization and lithium. Bipolar disorders. 2011;13:474–485. doi: 10.1111/j.1399-5618.2011.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Tang W, Greenwood TA, Guo S, He L, Geyer MA, Kelsoe JR. Transcription factor SP4 is a susceptibility gene for bipolar disorder. PloS one. 2009;4:e5196. doi: 10.1371/journal.pone.0005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper R, Samakai E, Kedra J, Soboloff J. Multifaceted roles of STIM proteins. Pflugers Arch. 2013;465:1383–1396. doi: 10.1007/s00424-013-1270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]