Abstract
Introduction
Although ex vivo lung perfusion (EVLP) is increasingly being used to evaluate and manipulate potential donor lungs prior to lung transplantation (LTx), data on the biochemistry of lungs during EVLP is limited. In this study, we examined the physiology and biochemistry of human lungs on an EVLP circuit.
Methods
Unallocated double lungs were recovered in standard fashion and split into single lungs. All lungs received a nebulized arginase inhibitor, 2-S-amino-6-boronohexanoic acid (ABH), at either the onset (n=6) or after 3 hours (n=8) of EVLP. Serial biochemical analysis included levels of arginase, endogenous nitric oxide synthase (eNOS), cyclic guanosine monophosphate, and reactive oxygen species. Lungs were considered transplantable if they sustained a PaO2:FiO2≥350 in addition to stable pulmonary function during EVLP.
Results
A total of 14 single lungs were recovered. 3 single lungs from different donors were deemed transplantable after EVLP. These lungs had superior oxygenation, lower carbon dioxide, and more stable pulmonary artery pressures. Transplantable lungs had higher baseline levels of eNOS and higher final levels of cGMP than non-transplantable lungs. Early ABH administration was associated with a transient increase in dynamic compliance.
Conclusion
In this biochemical characterization of lungs deemed unsuitable for LTx, early levels of eNOS and late levels of cGMP appear to be associated with improved allograft function during EVLP. Additionally, nebulized ABH is associated with a significant increase in dynamic compliance. These data suggest that biochemical markers during EVLP may predict acceptable allograft function and that this platform can be used to biochemically manipulate donor lungs prior to LTx.
Keywords: Lung transplantation, Ex Vivo Lung Perfusion
INTRODUCTION
Lung transplantation (LTx) remains the standard of care for end-stage lung disease; however, the number of patients awaiting transplantation greatly exceeds the available organs. [1] Most potential donor lungs are injured either by the donor cause of death, the brain death process, or from ICU-related complications. [2] Thus, only 15–25% of potential donor lungs are ultimately transplanted. [3–5] Although static hypothermia is a common and effective means of preserving lung allografts, hypothermia slows metabolism, preventing cellular repair and functional evaluation during allograft storage. [3,6,7] Therefore, an alternative strategy that allows for assessment and potentially repair of injured lungs prior to transplantation could increase the number of transplantable lungs. [2]
Normothermic ex vivo lung perfusion (EVLP) is a novel platform for the evaluation and manipulation of potential donor lungs prior to transplantation. Recently, EVLP has been used to successfully screen and recondition previously unacceptable donor lungs for LTx with acceptable outcomes. [3,8] Although the early clinical results of EVLP are encouraging, the biochemical and physiologic changes that occur with EVLP, particularly in human lungs, remain poorly understood. Therefore, we undertook this study to evaluate the physiologic and biochemical profile of human lungs undergoing EVLP with special emphasis on our ability to biochemically manipulate potential donor lungs.
METHODS
Human Lungs
Human lungs determined to be unacceptable for clinical LTx were recovered from donors (n=7) in standard fashion. Donor lungs were flushed with 6 L of cold (4°C) Perfadex (Vitrolife, Inc., Englewood, CO), including 4 L antegrade through the pulmonary artery (PA) and 500 mL retrograde through each pulmonary vein. The lungs were then cold-stored (4°C) in Perfadex until reperfusion. We received an institutional review board waiver from our institution. Our protocol was approved by the Living Legacy Foundation of Maryland (Shore Health System Institutional Review Board).
Donor Lung Preparation
Our EVLP procedure was adapted from the protocol of the Toronto Lung Transplant Program. [2,3] In preparation for reperfusion, the donor lungs were split into single lungs. The main bronchus of each single lung was intubated with an endotracheal tube. The lungs were then gently inflated and the endotracheal tube clamped. A cone cannula (XVIVO Lung Cannula Pack; Vitrolife, Inc.) was sewn to each atrial cuff with 4-0 prolene and a straight cannula (Vitrolife, Inc.) was inserted into each PA and secured with umbilical tape. Each single lung was again retrograde flushed through the left atrium (LA) cannula with 250 mL of Perfadex under gravity drainage at 30 cm. One lung was randomly chosen for immediate EVLP while the other was cold stored for 4 additional hours for delayed EVLP.
Initiation of Ex Vivo Lung Perfusion
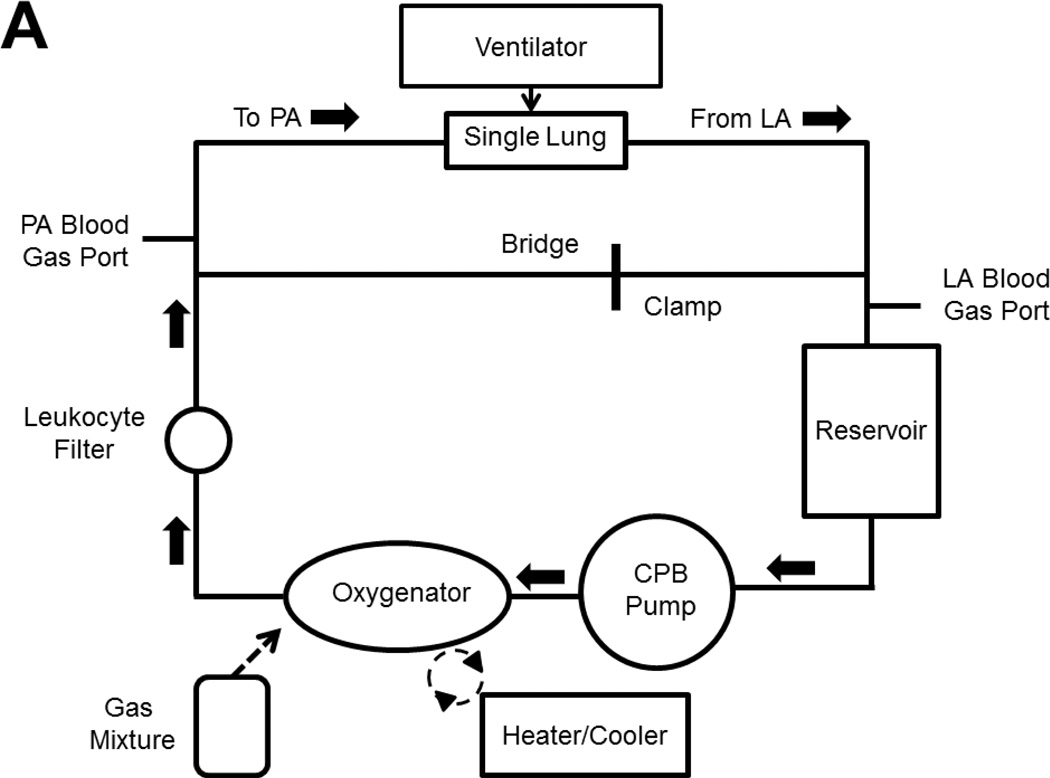
The EVLP circuit is shown in Figure 1. The circuit is primed with 1.5 L of Steen’s solution (Vitrolife, Inc.), 10,000 IU of heparin, and 500 mg of methylprednisolone. In an XVIVO chamber (Vitrolife, Inc.), the lung undergoes a slow retrograde flush (150 mL/min) through the LA cannula to de-air the PA cannula which is then connected to the circuit. Antegrade flow begins at 150 mL/min at 20°C using a Sarns 5000 roller head pump (Sarnes Inc., Ann Arbor, MI). [2] Table 1 and Table 2 show the perfusion and ventilation protocols, respectively, based on an average male donor (weight: 75 kg; height: 5 feet, 9.5 inches).
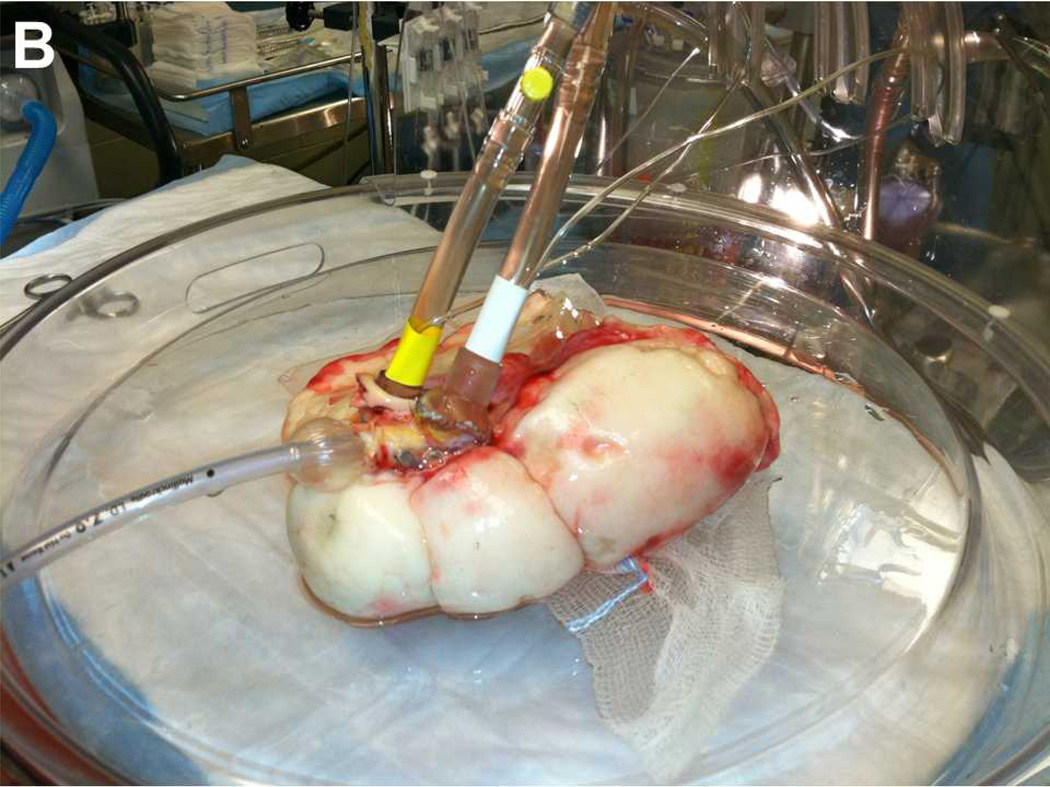
Figure 1.
(A) Schematic representation of the ex vivo lung perfusion circuit. Bridge tubing is clamped to facilitate antegrade flow. The bridge can be unclamped and the pulmonary artery line can be clamped to facilitate retrograde flow. (B) Picture of single lung attached to ex vivo circuit sitting in XVIVO chamber. Endotracheal tube is in main bronchus. Cannula with yellow tape is entering the pulmonary artery. Cannula with white tape is sutured to the left atrium. Abbreviations: PA, pulmonary artery; LA, left atrium, CPB, cardiopulmonary bypass.
Table 1.
Protocol for Ex Vivo Lung Perfusion during the First Hour
| Perfusion Time (minutes) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| Perfusion Temperature (°C) | 20 | 30 | 32–35 | 37 | 37 | 37 | 37 |
| Flow (%)a | 10 | 10 | 20 | 30 | 50 | 80 | 100 |
| Double lung flow (mL/min) | 230 | 230 | 460 | 689 | 1149 | 1839 | 2298 |
| Single lung flow (mL/min) | 115 | 115 | 230 | 345 | 575 | 919 | 1149 |
| Ventilation | None | None | Start | ||||
| Gas Exchanger | None | None | Start | ||||
| LA Pressure (mmHg) | 3–5 | 3–5 | 3–5 | 3–5 | 3–5 | 3–5 | 3–5 |
Percent of target maintenance perfusion rate.
Abbreviations: LA, left atrium.
Table 2.
Ex Vivo Lung Ventilation Protocol
| Variable | Setting |
|---|---|
| Double Lung Tidal Volume | 7 mL/kg = 525 mL |
| Single Lung Tidal Volume | 3.5 mL/kg = 262.5 mL |
| Respiratory Rate | 7 breaths/minute |
| FiO2 | 21% |
| PEEP | 5 cm H2O |
| Recruitment maneuver | Peak airway pressure of 25 cm H2O × 15 seconds × 2 |
Abbreviations: FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure.
Sweep gas flow into the oxygenator is used to de-oxygenate the perfusate returning from the LA. The gas mixture consists of 6% oxygen, 8% carbon dioxide, and 86% nitrogen (Airgas, Salem, NH). Gas flow is titrated to maintain a PA PCO2 of 35–45 mmHg to simulate venous blood.
PA and LA pressures are transduced constantly. The LA pressure is maintained between 3–5 mmHg.
Maintenance of Ex Vivo Lung Perfusion
Each lung undergoes EVLP for a total of 4 hours. LA and PA blood gases are initially obtained at 30 minutes. Additional measurements are taken as needed to titrate gas flow and pH. Sodium bicarbonate is given throughout EVLP to maintain a pH≥7.3. Every 30 minutes, LA and PA blood gases are obtained on an FiO2 of 21%, and every hour, on both 21% and 100% FiO2. Every hour, recruitment maneuvers are performed during which the peak airway pressures are twice sustained to 25 mm H2O for 15 seconds.
Experimental Groups
A total of 7 double lung blocks were split, yielding 14 single lungs for experimentation. Half of the lungs (n=7) were subjected to immediate EVLP while half (n=7) underwent delayed EVLP.
A novel arginase inhibitor, 2(S)-amino-6-boronohexanoic acid (ABH), was dissolved in normal saline to a concentration of 25 uM and nebulized (Aeroneb Pro, Phillips Respironics, Andover, MA) in all lungs. In the early ABH group (n=6), the lungs received nebulized ABH over 10 minutes beginning at the commencement of ventilation. In the late ABH group (n=8), the lungs received nebulized ABH after 3 hours of EVLP.
Suitability for Transplantation
Based on physiologic parameters, a post hoc assessment was undertaken to determine suitability for clinical transplantation using previously established EVLP criteria. [3] Lungs were considered suitable for transplantation if the LA PaO2:FiO2 ratio during EVLP was ≥350 mmHg and if deterioration from baseline levels of PA pressure, dynamic compliance, and peak inspiratory pressure was less than 15%.
Lung Samples
Lung samples were taken from each lung at baseline (prior to EVLP) and at each hour during EVLP. All lung samples were removed with a 15 mm Endo GIA stapler (Covidien, Mansfield, MA), flash frozen in liquid nitrogen, and stored at −80°C for biochemical analysis.
Arginase Activity
Arginase activity was measured colorimetrically. 50 uL of protein solution was incubated with 75 uL of 10 mM MnCl2 in 50 mM Tris-HCl, pH 7.5 buffer to activate the arginase. 50 uL of 500 mM L-arginine (prepared in 50 mMTris-HCl pH 9.7) was added and shaken at 37°C for 1 hour. The urea produced was assayed using 25 uL of α-isonitrosopropiophenone (9% in neat ethanol) followed by heating at 100°C for 1 hour. Absorbance was measured at 544 nm.
Nitric Oxide Synthase Levels
Lung tissue levels of endothelial nitric oxide synthase (eNOS) expression were evaluated by western blotting. 25 ug of total protein was resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, electro-transferred to a nitrocellulose membrane, and blotted using mouse monoclonal antibody to eNOS (BD Biosciences).
Cyclic GMP Levels
Cyclic guanosine monophosphate (cGMP) concentrations were determined by a commercially available enzyme immunoassay (Amersham cGMP Enzymeimmunoassay, GE Healthcare Life Sciences, Piscataway, NJ). Tissue samples were homogenized in 500 uL of 6% trichloroacetic acid, centrifuged, and the supernatants were washed five times in water-saturated ether. The aqueous layer was recovered and dried to recover a pellet which was re-suspended in assay buffer. The acetylation assay was performed according to vendor specifications.
Reactive Oxygen Species
Reactive oxygen species (ROS) levels were examined in lung samples using a green fluorescence assay (OxiSelect In Vitro ROS Assay Kit, Cell Biolabs, San Diego, CA). The lung samples were homogenized on ice in phosphate buffered solution, centrifuged, and re-suspended in assay buffer. The cell permeable 2’,7’-dichlorohydrofluorescin diacetate fluorogenic probe was used to assess ROS levels.
Statistical Analysis
Serial physiologic and biochemical data were evaluated by repeated-measures analysis of variance (RM-ANOVA) testing. Post hoc comparisons at specific time points were evaluated using the Tukey-Honest significant differences test. Data are presented as means ± standard deviation. P-values<0.05 (two-tailed) were considered statistically significant. Statistical analysis was performed using Stata 12.0 (Stata Corporation LP, College Station, TX).
RESULTS
A total of 7 human lungs were determined to be clinically unacceptable for LTx and were subsequently split to yield 14 single lungs (Table 3). The average total ischemic time was 5.3 (±2.5) hours. 6 of the lungs experienced a mean of 1.3 (±0.7) hours of warm ischemic time after donor cardiac death. 7 single lungs were subjected to immediate EVLP and 7 were subjected to delayed EVLP. Additionally, 6 lungs received early nebulized ABH and 8 lungs received late nebulized ABH. The ischemic times were not different between these groups (p=0.6).
Table 3.
Characteristics of Donor Lungs
| Lung | Cause of Death | Reason for Discard |
Age | Gender | Total Ischemic Time (hours) |
Warm Ischemic Time (hours) |
ABH | Transplantable |
|---|---|---|---|---|---|---|---|---|
| 1 | CVA | Tobacco use | 59 | Male | 2.95 | 0 | Late | No |
| 2 | CVA | Tobacco use | 59 | Male | 7.75 | 0 | Late | Yes |
| 3 | Anoxic brain injury | Active infection | 59 | Female | 2.1 | 0 | Late | Yes |
| 4 | Anoxic brain injury | Active infection | 59 | Female | 6.95 | 0 | Late | No |
| 5 | CVA | Active infection | 37 | Male | 3.75 | 0 | Late | No |
| 6 | CVA | Active infection | 37 | Male | 8.5 | 0 | Late | No |
| 7 | CVA | Aspiration PNA | 18 | Male | 4 | 0 | Late | No |
| 8 | CVA | Aspiration PNA | 18 | Male | 9 | 0 | Late | No |
| 9 | Aortic dissection | Prolonged hypoxia | 49 | Male | 2.5 | 1.5 | Early | No |
| 10 | Aortic dissection | Prolonged hypoxia | 49 | Male | 7 | 1.5 | Early | No |
| 11 | CVA | Bilateral infiltrates | 27 | Male | 3 | 2 | Early | No |
| 12 | CVA | Bilateral infiltrates | 27 | Male | 7 | 2 | Early | No |
| 13 | DCD | DCD | 47 | Male | 2.5 | 0.5 | Early | Yes |
| 14 | DCD | DCD | 47 | Male | 7 | 0.5 | Early | No |
Abbreviations: CVA, cerebrovascular accident; DCD, donor after cardiac death; PNA, pneumonia; ABH, 2(S)-amino-6-boronohexanoic acid
According to our predetermined criteria, 3 single lungs from different donors were considered clinically transplantable after EVLP. Their average ischemic time was similar to those not considered transplantable (5.6 (±2.4) vs. 4.1 (±1.8) hours, p=0.4) and one of these lungs experienced 30 minutes of warm ischemic time after donor cardiac death. One of these lungs was subjected to delayed EVLP and one was given ABH immediately at the time of ventilation.
Physiology
Pressures
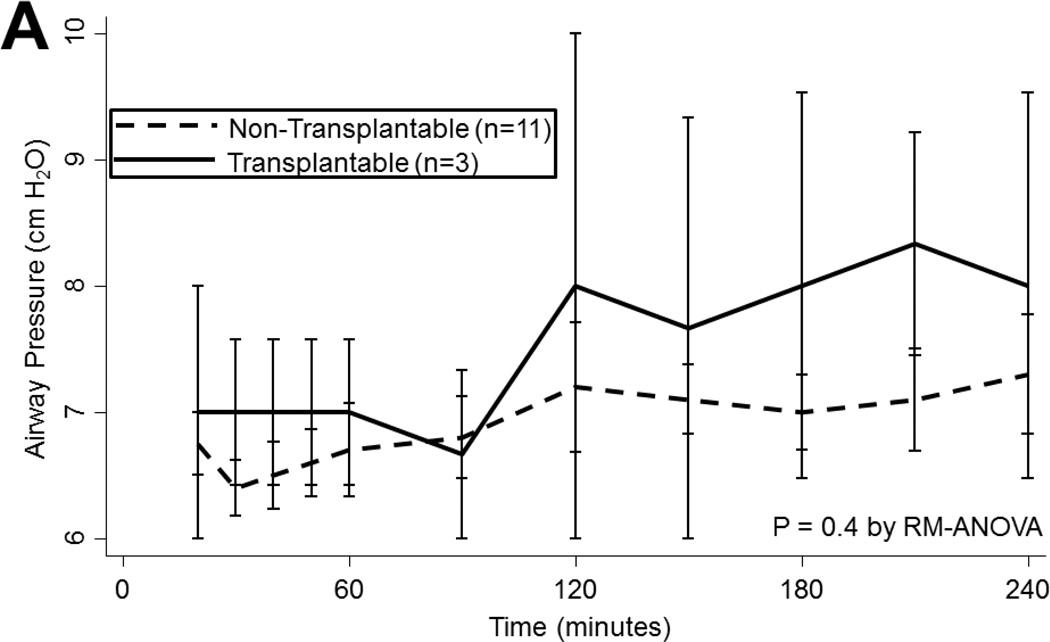
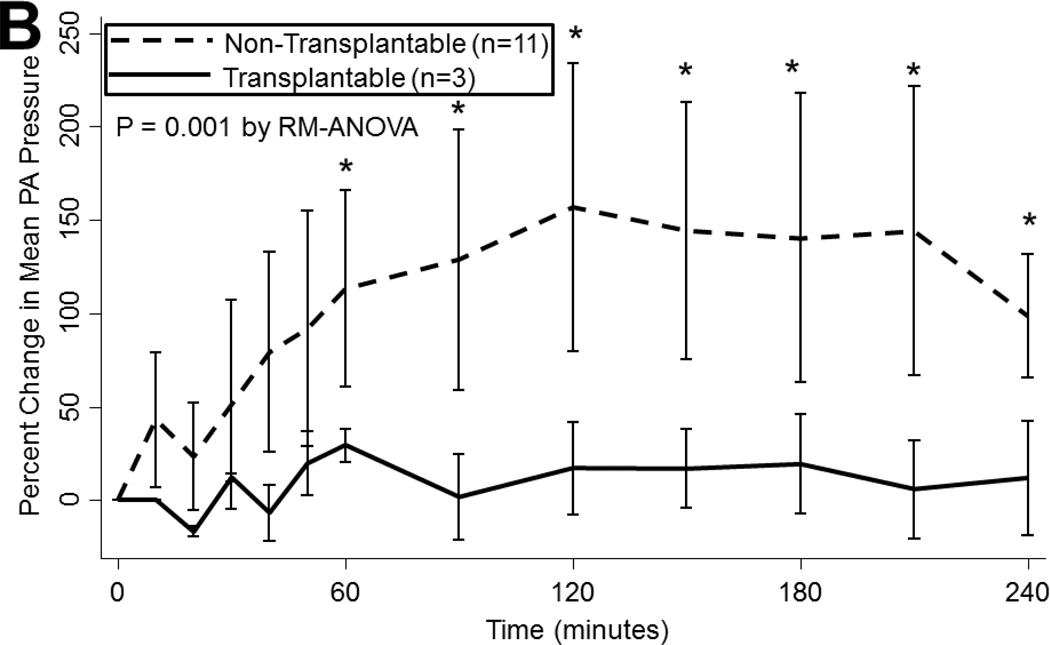
During EVLP, airway pressures tended to increase over time (p<0.001), regardless of ischemic time (p=0.8) or ABH treatment (p=0.5). Lungs ultimately considered to be transplantable also had similar airway pressures as non-transplantable lungs (p=0.4; Figure 2a). Mean PA pressures also tended to increase during EVLP, particularly during the first hour when flow is increasing (p<0.001). Lungs subjected to prolonged ischemia had higher PA pressures throughout reperfusion (p<0.001) and a greater increase in mean PA pressure at 4 hours compared to baseline (100[IQR: 57–125] vs. 59[IQR: −29–67]% increase, p<0.05). Mean PA pressures did not vary with ABH administration (p=0.4). Transplantable lungs tended to experience less change in mean PA pressure (p=0.001; Figure 2b).
Figure 2.
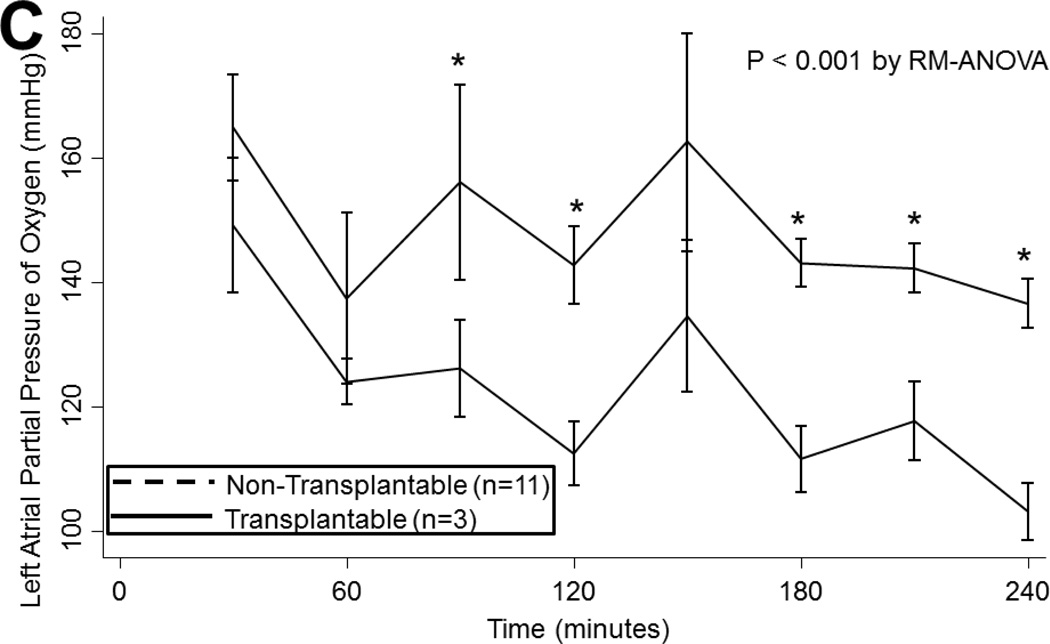
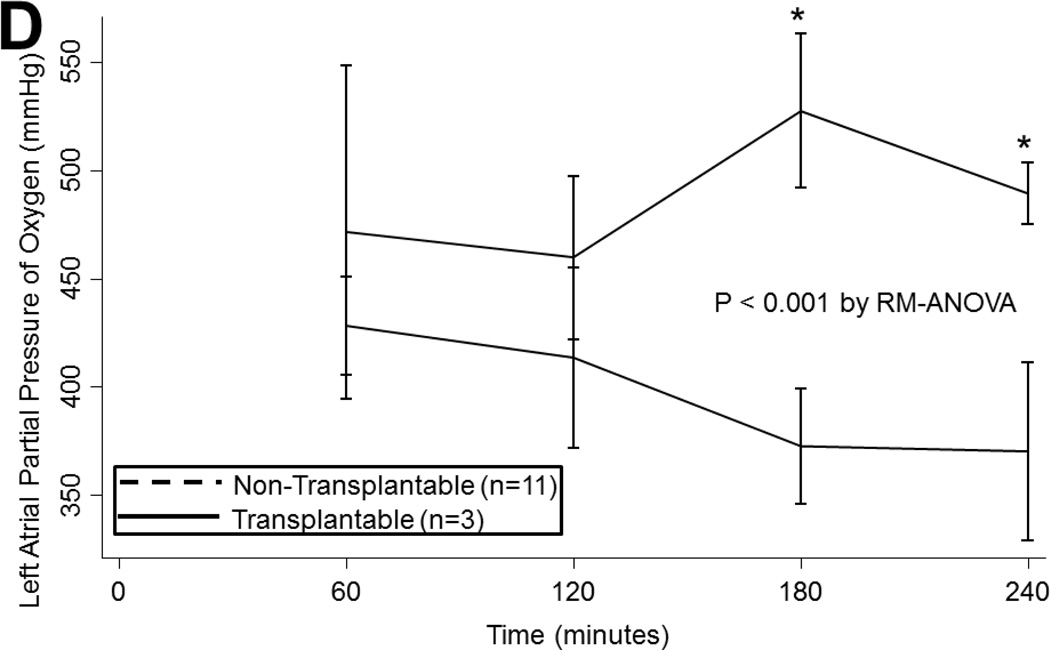
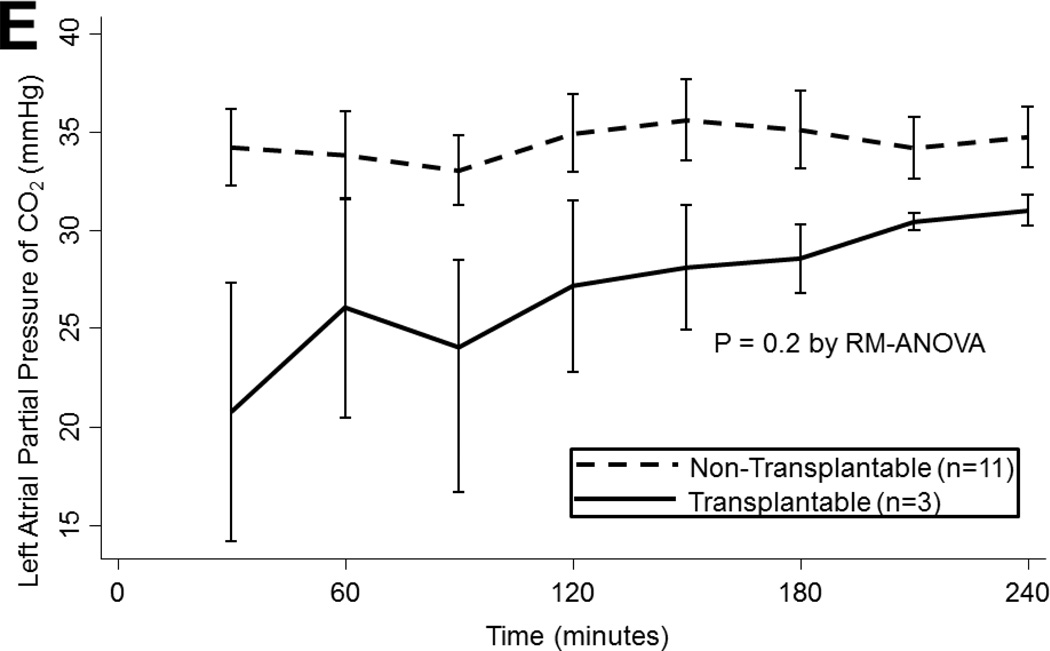
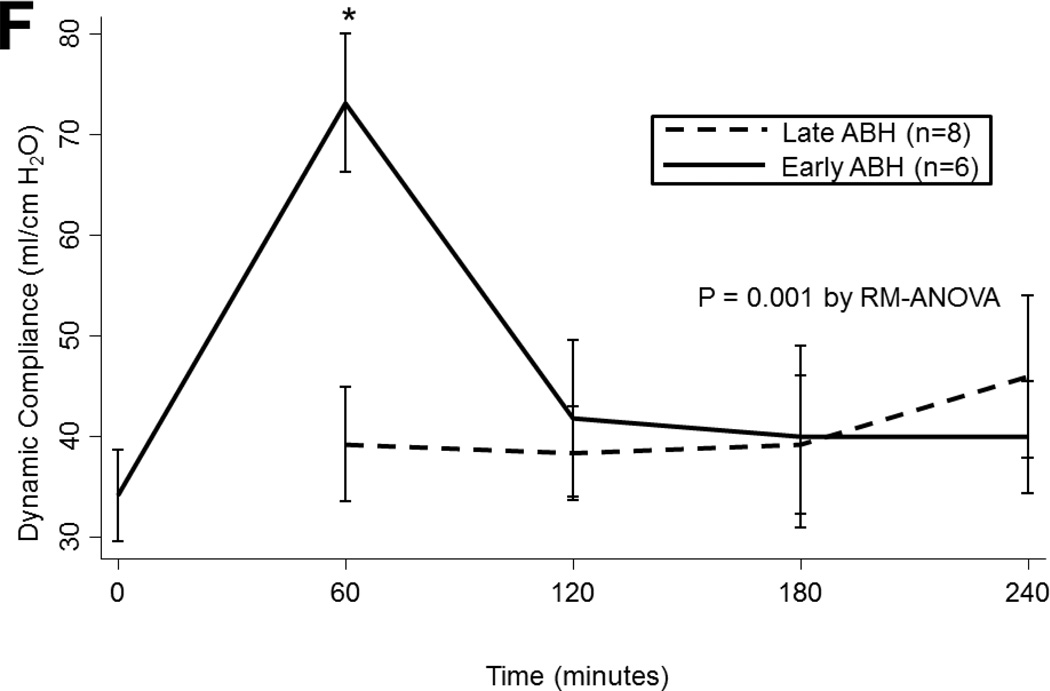
(A) Airway pressures, (B) pulmonary artery pressures, (C) left atrial PO2 on an FiO2 of 21%, (D) 100%, (E) left atrial PCO2, and (F) dynamic compliance during ex vivo lung perfusion stratified by transplantability. Asterisks denote post hoc significant differences. Error bars denote standard error. Abbreviations: RM-ANOVA, repeated-measures analysis of variance; PA, pulmonary artery.
Gas Exchange
LA PO2 tended to decline overtime both with an FiO2 of 21% (p<0.001) and 100% (p<0.001). Oxygenation did not differ by ischemic time or by ABH treatment on either 21% or 100% (p>0.05). However, transplantable lungs tended to have better oxygenation for a more sustained period than non-transplantable lungs on 21% (p<0.001; Figure 2c) and on 100% (p=0.06; Figure 2d). Transplantable lungs tended to have lower CO2 levels at the beginning of EVLP and demonstrated a trend toward better ventilation throughout perfusion (p=0.2; Figure 2e). CO2 levels did not vary by ischemic time (p=0.4) or by ABH treatment (p=0.4).
Compliance
During EVLP, dynamic compliance changed dramatically over time with notable peaks in compliance at 1 hour and at 4 hours, following administration of nebulized ABH (p=0.004). Compliance did not vary by ischemic time (p=0.2) or by transplantability of the lungs (p=0.3). In lungs that received early nebulized ABH, compliance increased from 34 (±11) at baseline to 73 (±17) ml/cm H2O at 1 hour (p=0.001; figure 2f). This effect was transient, however, as compliance in this group returned to 41 (±19) ml/cm H2O by 2 hours. In the lungs that received late ABH, compliance increased modestly from 39 (±19) to 46 (±23) ml/cm H2O though this difference did not reach statistical significance (p=0.8).
Biochemistry
Arginase Activity
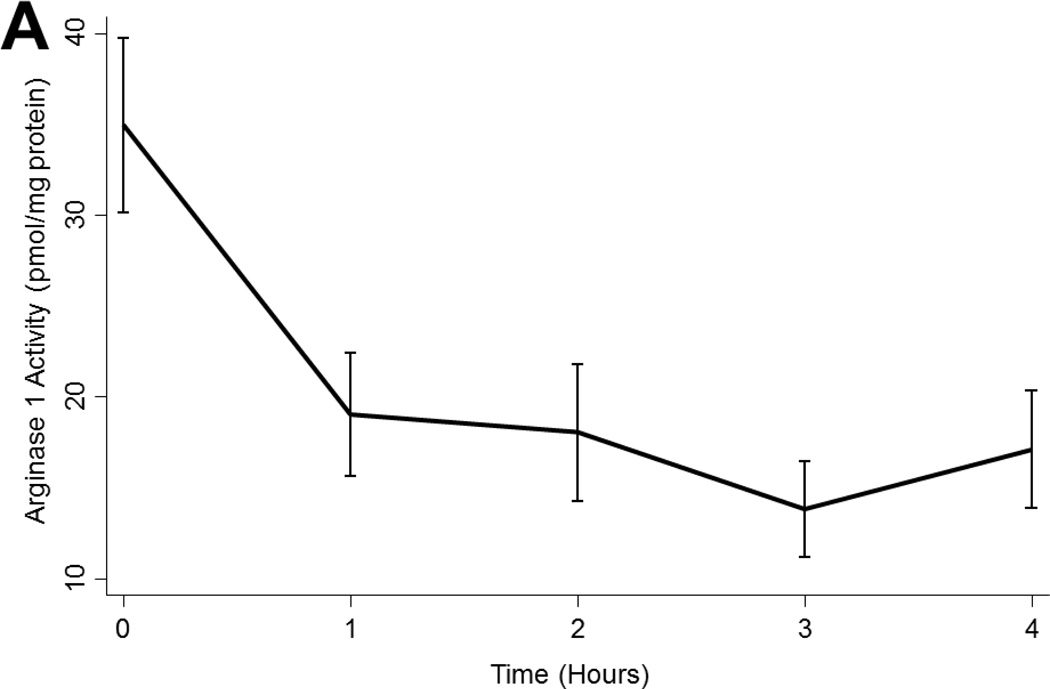
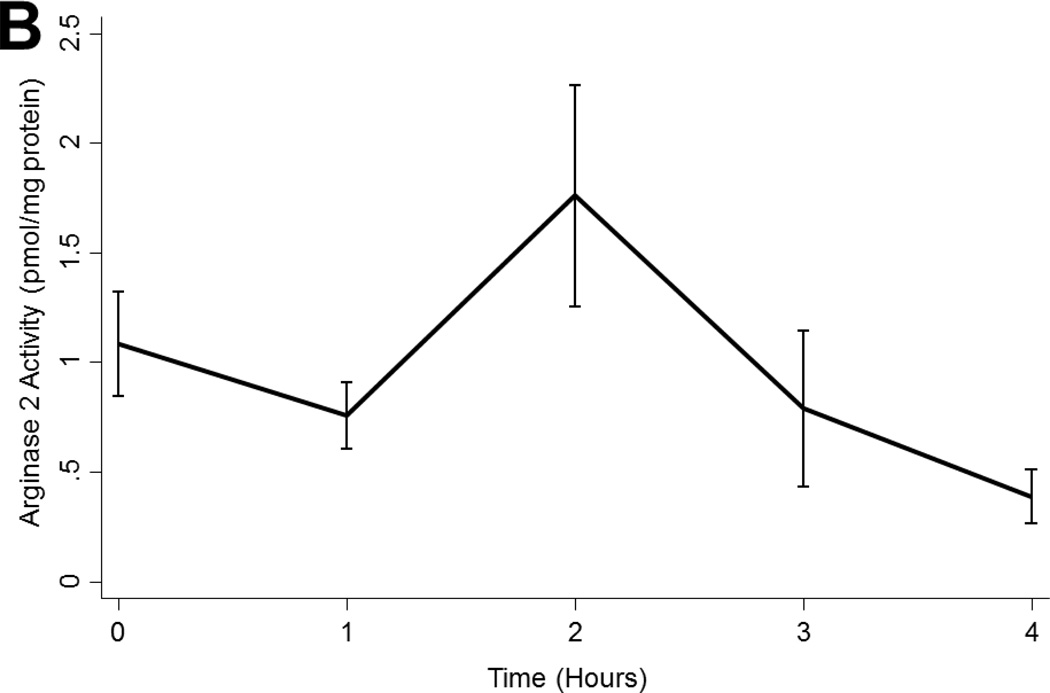
Arginase 1 levels tended to decline during EVLP perfusion (p<0.001; Figure 3a) while arginase 2 levels were relatively stable with a non-significant peak at 2 hours (p=0.054; Figure 3b). Neither arginase 1 nor arginase 2 levels varied by ischemic time, timing of ABH administration, or by transplantability (p>0.05 in all cases; Figure 3a).
Figure 3.
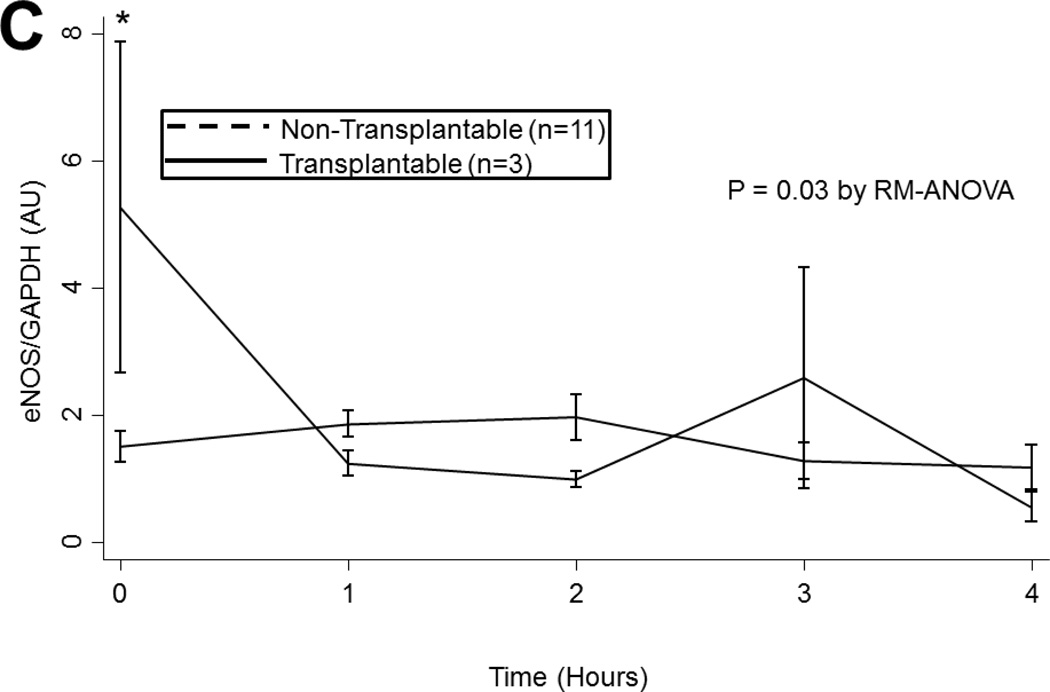
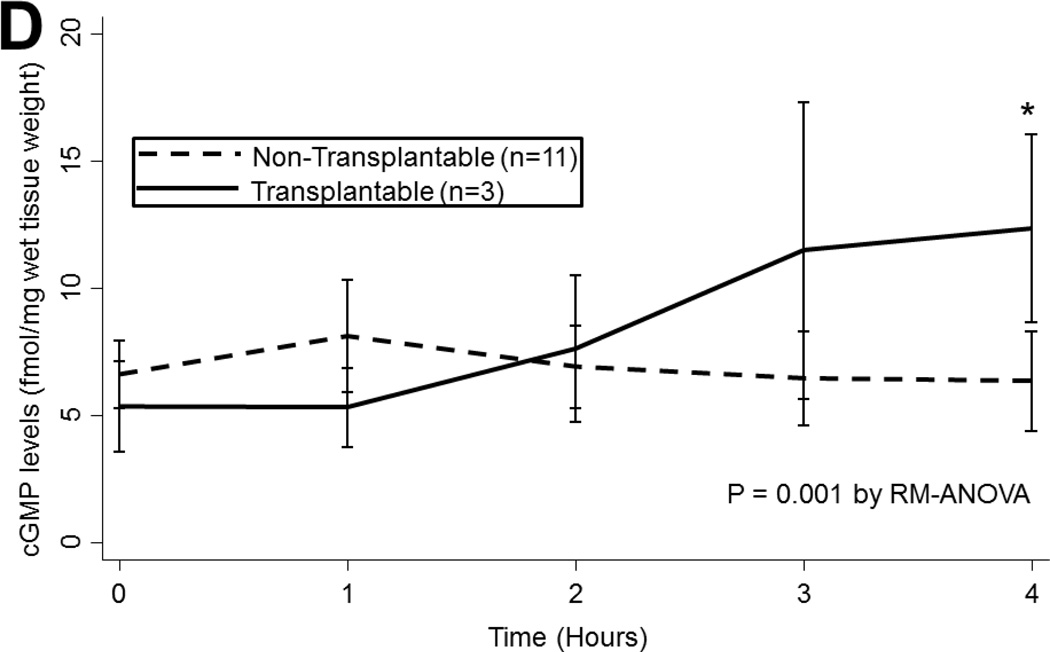
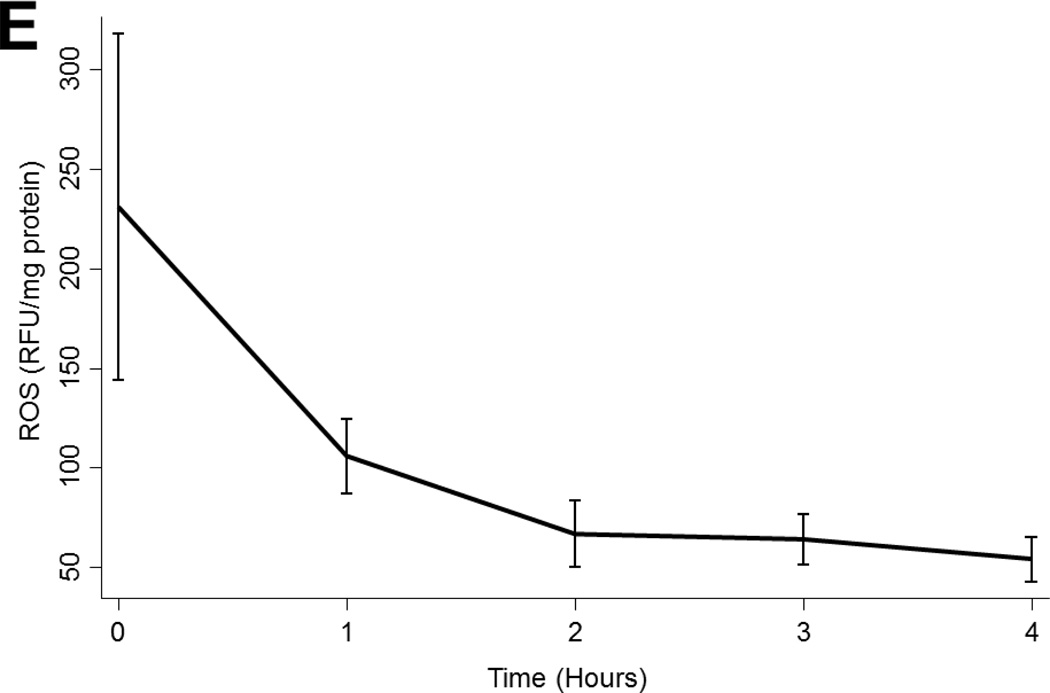
(A) Arginase 1 and (B) Arginase 2 levels in all lungs during EVLP. (C) eNOS and (D) cGMP levels in all lungs stratified by transplantability. (E) ROS levels in all lungs over time. Asterisks denote post hoc significant differences. Error bars denote standard error. eNOS levels shown relative to GAPDH loading control. Abbreviations: RM-ANOVA, repeated-measures analysis of variance; eNOS, endothelial nitric oxide synthase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; AU, arbitrary units; cGMP, cyclic guanosine monophosate; ROS, reactive oxygen species; RFU, relative fluorescence units.
Nitric Oxide Synthase
eNOS levels tended to decline during EVLP though this decline did not reach statistical significance (p=0.3). eNOS levels did not vary by ischemic time (p=0.9). However, in lungs deemed to be transplantable, eNOS levels were significantly higher at baseline compared to non-transplantable lungs (1.50 (±0.80) vs. 5.27 (±4.51) AU, p=0.03; Figure 3c). This difference abated after 1 hour of reperfusion (p>0.05).
cGMP
cGMP levels were relatively stable throughout EVLP (p=0.9) and did not vary by ischemic time (p=0.9) or by ABH treatment (p=0.9). Although lungs exhibited similar levels of cGMP at baseline regardless of their transplantability, transplantable lungs had slightly higher levels of cGMP at the end of EVLP (6.33 (±6.53) vs. 12.32 (±6.41) fmol/mg protein, p=0.001; Figure 3d).
ROS
ROS levels declined significantly during EVLP (p=0.02). ROS levels did not vary by ischemic time (p=0.1), by ABH treatment (p=0.1), or by transplantability (p=0.5; Figure 3e).
DISCUSSION
To our knowledge, this study represents the first biochemical characterization of human lungs deemed unsuitable for LTx during reconditioning on an EVLP platform. In this preliminary study, lungs ultimately determined to be clinically transplantable demonstrated higher levels of eNOS at baseline and higher levels of cGMP after 4 hours of EVLP. Moreover, early administration of a novel, nebulized arginase inhibitor resulted in a significant but transient increase in dynamic compliance, despite the absence of changes in arginase tissue levels.
The shortage of acceptable donor lungs has lead clinicians to consider the use of extended criteria donors and donors after cardiac death. [5,9] However, as donor criteria are expanded, the risk of adverse outcomes may increase. Thus, an ex vivo platform that allows for functional evaluation without increasing cold ischemic time could increase the number of donor organs and the precision with which they are chosen. [3] Moreover, such an ex vivo platform may allow for physical and biochemical allograft manipulation without the risk of systemic side effects.
In recent years, several centers have developed normothermic EVLP platforms to evaluate and manipulate high-risk donor lungs for transplantation. [3,8,10] Early results with these protocols have been encouraging. Cypel et al. [3] recently reported the Toronto experience with EVLP for clinical transplantation. In their study, high-risk EVLP lungs demonstrated a similar rate of primary graft dysfunction, 30-day mortality, and other complications compared to standard donor lungs. Other clinical studies of EVLP have demonstrated similar results. [8,11–14]
In our study, we evaluated biochemical patterns of unallocated lungs undergoing EVLP. In selecting biomarkers for evaluation, we chose several biochemicals of known importance to LTx. eNOS is an important regulator of nitric oxide (NO) synthesis and is known to be modulated in human and experimental models of LTx. [15–19] cGMP, a downstream by-product of eNOS and NO, activates protein kinase G which is responsible for the phosphorylation of numerous downstream targets involved in the alteration of vascular tone and other protective effects. [20–22] Finally, ROS are thought to be important mediators of ischemia-reperfusion injury and primary graft dysfunction, particularly after LTx. [23,24]
In general, eNOS and ROS levels declined during reperfusion while cGMP levels remain relatively stable. Our findings confirm previous reports that suggest that overall NOS levels are suppressed during the reperfusion phase of LTx. [16,17] The decline in NOS activity may be detrimental as NOS is an important mediator of NO up-regulation, which has important vasodilatory and anti-inflammatory effects. [19] The high levels of ROS at the outset of reperfusion followed by the progressive decline during reperfusion is also consistent with previous experimental research suggesting that ROS are produced during reperfusion when oxygen supply is rapidly increased in cells primed for the low oxygen environment of cold storage. [25,26]
In addition to establishing the overall pattern of these biochemicals during EVLP, when stratified by transplantability, we found that transplantable lungs were associated with higher eNOS levels at the onset of EVLP and higher levels of cGMP at the conclusion of EVLP. These findings support previous experimental findings suggesting that eNOS is protective of lung ischemia-reperfusion injury. [15,18] It is possible that while ischemia-reperfusion inhibits NOS expression overall, a relative up-regulation of eNOS is protective. Although the role of cGMP is complicated, its up-regulation is generally associated with healthy lungs. In the broad sense of using EVLP as a platform for evaluating potentially injured lungs for transplantability, these findings suggest that biomarkers may be able to predict acceptable allograft function alone or in combination with the physiologic profile demonstrated on EVLP.
In addition to using EVLP to evaluate potential lung allografts, EVLP may serve as a platform to repair organs prior to transplantation. Although cold storage inhibits metabolism, slowing or preventing cellular repair mechanisms after donor injury, [2] normothermic EVLP, allows cellular metabolism to resume and intrinsic reparative processes to commence. Evidence of ongoing cellular metabolism during EVLP is evident from reports of significant lactate production during EVLP. [27] In addition to reparative processes intrinsic to the lungs, Steen’s solution, the most commonly used EVLP perfusate, has been specifically designed with a high colloid oncotic pressure in an effort to remove both interstitial and alveolar fluid from damaged lungs. [11] The EVLP platform also allows for physical manipulation with the removal of blood and secretions from the lungs; [10] biochemical manipulation with anti-inflammatory cytokines and vasodilatory agonists; [28–31] and cellular manipulation with mesenchymal stem cells. [32]
In our study, we utilized the novel arginase inhibitor, ABH, in an effort to manipulate human lungs during EVLP. In normal lung tissue, NOS metabolizes L-arginine to NO and L-citrulline; however, arginase competitively converts L-arginine to L-ornithine and urea, thus decreasing NO. [33] As a potent vasodilator and anti-inflammatory molecule, NO is thought to be protective in the inflamed airway. [34] Interestingly, in our study, early nebulized ABH resulted in a significant though transient increase in dynamic airway compliance. The increase in airway compliance supports prior work that found ABH effective at decreasing airway hyper-responsiveness in animal models of asthma. [34] However, in our study, tissue levels of arginase were not affected. The lack of change in arginase levels may represent a sampling error as tissue levels were determined by more peripheral lung biopsies and arginase levels may be most affected in the more central airways. Alternatively, the current preliminary study with its wide variation in lung quality may be of inadequate sample size to detect a difference. Regardless, the change in compliance elicited by the nebulized ABH suggests arginase activity is a critical regulator of lung compliance and provides further evidence that human lungs can be biochemically manipulated on the EVLP platform.
Limitations
Although our study produced several significant findings, it is limited by a small sample size of variably injured lungs. Furthermore, the baseline lung variability is complicated by the variable ischemic times and treatment modalities. Despite our best efforts to compare lungs during EVLP to baseline function so that each lung would serve as its own control, this remains a potential limitation. Additionally, although biochemical findings on EVLP may reflect changes that occur during in vivo LTx, the in vivo environment is different in several ways. Thus, generalization from our findings to clinical LTx should be approached with caution.
CONCLUSION
In this preliminary study, lungs exhibiting favorable EVLP physiology demonstrated increased levels of eNOS and cGMP. Additionally, early administration of a novel arginase inhibitor is associated with a significant but transient increase in dynamic compliance in human lungs, suggesting that arginase activity is a critical regulator of lung compliance. In conclusion, early biochemical markers obtained during EVLP may be used in concert with the lung’s physiologic profile to help predict acceptable allograft function. Additionally, EVLP can be used as a platform to biochemically manipulate lungs prior to LTx.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Jeffrey Braun and Ms. Melissa Jones for their technical assistance.
Footnotes
Conflicts: Dr. Berkowitz is a scientific founder of and consultant for Arginetix, a biotechnology company that develops arginase inhibitors.
Presentation: The contents of this manuscript were presented at the 32nd Annual Meeting of the International Society for Heart and Lung Transplantation in Prague, Czech Republic.
REFERENCES
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report-2010. J Heart Lung Transplant. 2010;29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 4.Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS. Organ donation and utilization in the United States, 1996–2005. Am J Transplant. 2007;7(5 Pt 2):1327–1338. doi: 10.1111/j.1600-6143.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 5.Pomfret EA, Sung RS, Allan J, et al. Solving the organ shortage crisis: the 7th annual American Society of Transplant Surgeons' State-of-the-Art Winter Symposium. Am J Transplant. 2008;8(4):745–752. doi: 10.1111/j.1600-6143.2007.02146.x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer S, Matte-Martyn A, De Perrot M, et al. Low-potassium dextran preservation solution improves lung function after human lung transplantation. J Thorac Cardiovasc Surg. 2001;121(3):594–596. doi: 10.1067/mtc.2001.109703. [DOI] [PubMed] [Google Scholar]
- 7.Arnaoutakis GJ, Allen JG, Merlo CA, et al. Low potassium dextran is superior to University of Wisconsin solution in high-risk lung transplant recipients. J Heart Lung Transplant. 2010;29(12):1380–1387. doi: 10.1016/j.healun.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindstedt S, Hlebowicz J, Koul B, et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg. 2011;12(2):162–165. doi: 10.1510/icvts.2010.244830. [DOI] [PubMed] [Google Scholar]
- 9.Yeung JC, Cypel M, Waddell TK, van Raemdonck D, Keshavjee S. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19(2):261–274. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83(6):2191–2194. doi: 10.1016/j.athoracsur.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Wierup P, Haraldsson A, Nilsson F, et al. Ex vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg. 2006;81(2):460–466. doi: 10.1016/j.athoracsur.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Pego-Fernandes PM, de Medeiros IL, Mariani AW, et al. Ex vivo lung perfusion: early report of Brazilian experience. Transplant Proc. 2010;42(2):440–443. doi: 10.1016/j.transproceed.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Steen S, Liao Q, Wierup PN, et al. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–252. doi: 10.1016/s0003-4975(03)00191-7. discussion 52. [DOI] [PubMed] [Google Scholar]
- 14.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009;87(1):255–260. doi: 10.1016/j.athoracsur.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Weiss ES, Champion HC, Williams JA, Baumgartner WA, Shah AS. Long-acting oral phosphodiesterase inhibition preconditions against reperfusion injury in an experimental lung transplantation model. J Thorac Cardiovasc Surg. 2009;137(5):1249–1257. doi: 10.1016/j.jtcvs.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Cardella JA, Keshavjee SH, Bai XH, et al. Increased expression of nitric oxide synthase in human lung transplants after nitric oxide inhalation. Transplantation. 2004;77(6):886–890. doi: 10.1097/01.tp.0000118477.11722.a2. [DOI] [PubMed] [Google Scholar]
- 17.Marczin N, Riedel B, Gal J, Polak J, Yacoub M. Exhaled nitric oxide during lung transplantation. Lancet. 1997;350(9092):1681–1682. doi: 10.1016/S0140-6736(05)64281-X. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Tremblay L, Cassivi SD, et al. Alterations of nitric oxide synthase expression and activity during rat lung transplantation. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L1071–L1081. doi: 10.1152/ajplung.2000.278.5.L1071. [DOI] [PubMed] [Google Scholar]
- 19.Suda T, Mora BN, D'Ovidio F, et al. In vivo adenovirus-mediated endothelial nitric oxide synthase gene transfer ameliorates lung allograft ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2000;119(2):297–304. doi: 10.1016/S0022-5223(00)70185-1. [DOI] [PubMed] [Google Scholar]
- 20.Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75(2):303–314. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Dong BM, Abano JB, Egan TM. Nitric oxide ventilation of rat lungs from non-heart-beating donors improves posttransplant function. Am J Transplant. 2009;9(12):2707–2715. doi: 10.1111/j.1600-6143.2009.02840.x. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky DJ, Naka Y, Chowdhury NC, et al. The nitric oxide/cyclic GMP pathway in organ transplantation: critical role in successful lung preservation. Proc Natl Acad Sci U S A. 1994;91(25):12086–12090. doi: 10.1073/pnas.91.25.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299(5):H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 24.Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med. 2011;32(2):279–293. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 25.George TJ, Arnaoutakis GJ, Beaty CA, et al. Hydrogen sulfide decreases reactive oxygen in a model of lung transplantation. J Surg Res. 2012 doi: 10.1016/j.jss.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 27.Koike T, Yeung JC, Cypel M, et al. Kinetics of lactate metabolism during acellular normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2011;30(12):1312–1319. doi: 10.1016/j.healun.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1(4):4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 29.Yeung JC, Wagnetz D, Cypel M, et al. Ex Vivo Adenoviral Vector Gene Delivery Results in Decreased Vector-associated Inflammation Pre- and Post-lung Transplantation in the Pig. Mol Ther. 2012 doi: 10.1038/mt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama S, Sato M, Liu M, et al. Local long-term expression of lentivirally delivered IL-10 in the lung attenuates obliteration of intrapulmonary allograft airways. Hum Gene Ther. 2011;22(11):1453–1460. doi: 10.1089/hum.2010.225. [DOI] [PubMed] [Google Scholar]
- 31.Emaminia A, Lapar DJ, Zhao Y, et al. Adenosine AA agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92(5):1840–1846. doi: 10.1016/j.athoracsur.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratt JM, Franzi LM, Linderholm AL, et al. Arginase inhibition in airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2010;242(1):1–8. doi: 10.1016/j.taap.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maarsingh H, Zuidhof AB, Bos IS, et al. Arginase inhibition protects against allergen-induced airway obstruction, hyperresponsiveness, and inflammation. Am J Respir Crit Care Med. 2008;178(6):565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]