Abstract
Background
Connective tissue pulleys determine extraocular muscle force directions and pulley heterotopy can induce strabismus. The etiology and type of pulley abnormalities vary with patient age, resulting in different but predictable types presentations of strabismus.
Methods
Magnetic resonance imaging (MRI) was obtained in 95 patients with pulley heterotopy, of whom 56 had childhood-onset pattern strabismus, and was compared with published data on 28 patients aged 69 ± 12 years who had sagging eye syndrome. Control data were from age-matched with no strabismus.
Results
Patients with childhood-onset strabismus had intact lateral rectus–superior rectus band ligaments and straight extraocular muscle paths but exhibited pulley array A pattern–associated incyclorotation or V pattern–associated excyclorotation. Rectus transposition surgery collapsed patterns. Patients with sagging eye syndrome exhibited blepharoptosis, superior sulcus defect, and inferolateral displacement of rectus pulleys with elongation of extraocular muscles that followed curved paths. Symmetrical lateral rectus pulley sag was associated with divergence paralysis esotropia; asymmetrical sag >1 mm, with cyclovertical strabismus. Both lateral rectus resection and medial rectus recession treated divergence paralysis esotropia. Partial vertical rectus tenotomy treated cyclovertical strabismus.
Conclusions
Childhood onset pulley abnormalities are associated with A or V pattern strabismus and external anatomical features suggest that these pulley defects are probably congenital. Adult onset pulley defects commonly result from age-related tissue involution and external features such as adnexal laxity are also helpful in recognizing involution as a possible etiology of strabismus.
In the late 20th century, pioneering work of Joel M. Miller indicated that extraocular muscle paths must be constrained by connective tissue pulleys that thus control pulling directions.1 Subsequent investigations revealed that pulleys for rectus and inferior oblique muscles are condensations of connective tissue located where these muscles penetrate posterior Tenon’s fascia near the globe equator.2–4 Moreover, magnetic resonance imaging (MRI) demonstrated that pulleys are actively shifted by the extraocular muscle orbital layers to modulate pulling directions systematically with gaze direction.5,6 The conclusion that these active pulley shifts, rather than explicit cyclovertical neural commands, underlie Listing’s law of ocular torsion has been supported by electrophysiological7 and behavioral8,9 findings in monkeys. Taken together, these data indicate that extraocular muscle pulling direction is under anatomical constraint so stringent that innervation cannot always overcome them.10,11 It was therefore proposed that some pattern strabismus might arise, not from abnormal contractility of oblique extraocular muscles but rather from abnormal rectus pulley locations.12
Incomitant strabismus is indeed associated with heterotopic (malpositioned) rectus pulleys13–15 and with unstable pulleys shifting abnormally with gaze.16,17 Directions and amounts of pulley heterotopy in Crouzon syndrome correlate strongly with A and V patterns,15 but age of strabismus onset is unclear. In contrast, onset of acquired strabismus in sagging eye syndrome is readily established by history. In 2009 it was proposed that horizontal and vertical strabismus could result from inferior displacement of the lateral rectus pulley due to degeneration of the ligament—the lateral rectus–superior rectus (LR-SR) band—that suspends the lateral rectus pulley from the superior rectus pulley.18 Indeed, Kono and colleagues19 had earlier documented a profound loss of connective tissue in the ligament over the human life span. The 2013 Apt Lecture compares features and treatment of strabismus due to childhood-onset pulley heterotopy, with strabismus in sagging eye syndrome due to later acquired heterotopy.
Subjects and Methods
From a prospective MRI and computed x-ray tomography (CT) study20 that included 577 cases of strabismus and 157 confirmed normal control subjects, consecutive strabismic cases were identified in whom at least one rectus pulley was obviously displaced from normal position when imaged in the quasicoronal plane. The study received approval of the University of California Los Angeles Institutional Review Board approval and was compliant with the US Health Insurance Portability and Accountability Act of 1996. Subjects with axial high myopia were excluded.
The imaging techniques employed have been described elsewhere.21,22 The LR-SR band was identified in quasicoronal image planes.19 Subjects underwent complete ophthalmic and motility evaluations, including heterophorias in diagnostic positions by prism cover testing, except when immaturity or noncooperation precluded such testing, and external photography to document eyelid configuration. Age of strabismus onset was recorded as stated if the reporting person was confident of the age but was otherwise conservatively recorded as age at presentation. Indications of variability represent standard deviation.
Comparison was made with recently published data on sagging eye syndrome23 and with recently reported surgical outcomes.24,25 Additional example cases are included here for illustration.
Results
Childhood-Onset Pulley Heterotopy
A total of 34 subjects with presumed childhood-onset pulley heterotopy were identified with an average age of 6.6 ± 9.0 years. This age distribution, while skewed, includes birth and is clearly well below middle age.
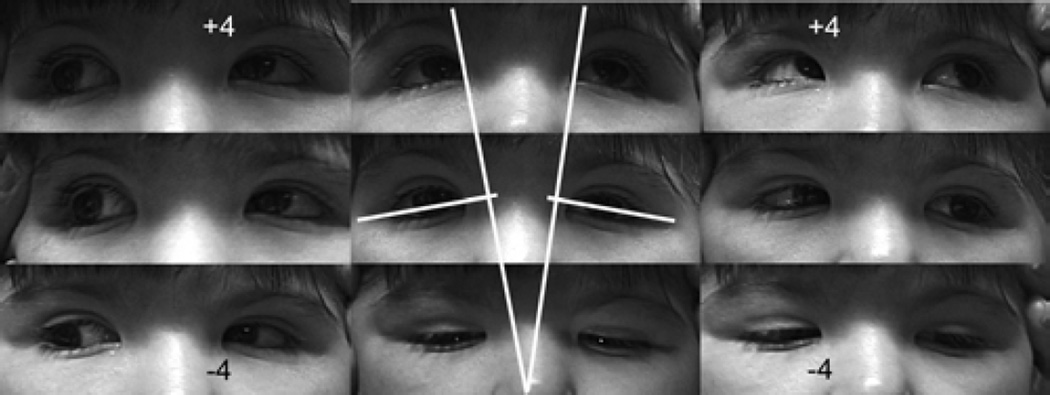
A-pattern strabismus, associated with overdepression and underelevation in adduction, was present in 15 subjects (Figure 1). Figure 2A illustrates the typical anatomical findings for the subject in Figure 1: the LR-SR band ligament is robust, but the rectus pulley array is bilaterally intorted, causing superior displacement of the lateral rectus pulley relative to medial rectus pulley and lateral displacement of the inferior rectus relative to superior rectus pulley. Temporally elevated inclination of the palpebral canthal fissure typically signaled pulley array intorsion (Figure 1). When perpendiculars are drawn to the intercanthal lines, these perpendiculars intersect superiorly, inscribing the letter A.
FIG. 1.
Versions in subject with A-pattern exotropia demonstrate marked (+4) overdepression in adduction and marked (−4) underelevation in adduction. In central gaze panel, white lines join the medial and lateral canthi; perpendiculars added to these lines inscribe the letter A, correlating with the incomitance.
FIG. 2.
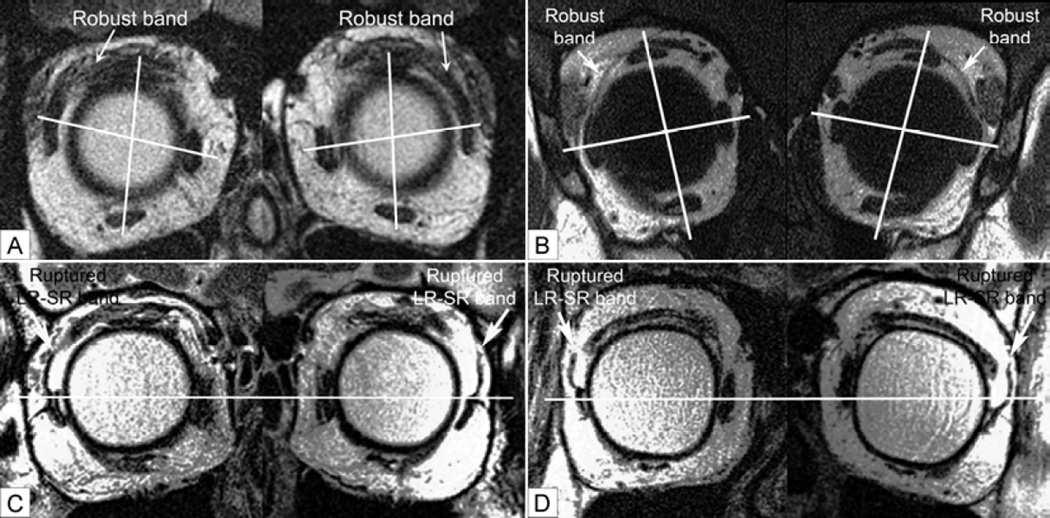
Quasi-coronal plane MRI. A, Subject of Figure 1 with A-pattern exotropia demonstrating robust LR-SR band bilaterally, with rectus pulley heterotopy. Lines connect centers of the horizontal and vertical rectus pairs at roughly the pulley locations, showing bilateral superior displacement of the lateral relative to the medial rectus pulley, and lateral displacement of the inferior relative to the superior rectus pulley. B, Same subject of Figure 3 with V-pattern exotropia showing robust LR-SR band bilaterally, with rectus pulley heterotopy. Lines connect centers of the horizontal and vertical rectus pairs at roughly the locations of their pulleys, showing bilateral inferior displacement of the lateral relative to the medial rectus pulley, and medial displacement of the inferior relative to the superior rectus pulley. C, Subject with adult-onset divergence paralysis esotropia. Note bilaterally symmetric infraplacement of the lateral rectus pulleys with bilateral discontinuity of the LR-SR band ligaments. Horizontal line indicates centers of the medial rectus muscles. D, Subject with adult-onset cyclovertical strabismus causing left hypotropia. Note asymmetric infraplacement of the left lateral rectus pulley and bilateral discontinuity of the LR-SR band ligaments. Line indicates centers of the left horizontal rectus muscles.
V-pattern strabismus, associated with underdepression and overelevation in adduction, was observed in 10 subjects (Figure 3). Figure 4 illustrates typical anatomical findings for the subject illustrated in Figure 3: the LR-SR band ligament is robust, but the rectus pulley array is bilaterally extorted so that there is inferior displacement of the lateral rectus relative to medial rectus pulley, and medial displacement of the inferior rectus relative to superior rectus pulley. Temporally downward inclination of the palpebral canthal fissure was typical, forming the letter V.
FIG. 3.
Versions in subject with V-pattern exotropia demonstrate marked (−4) underdepression in adduction and marked (+4) overelevation in adduction. In central gaze panel, white lines join the medial and lateral canthi; perpendiculars added to these lines inscribe the letter V, correlating with the incomitance.
FIG. 4.
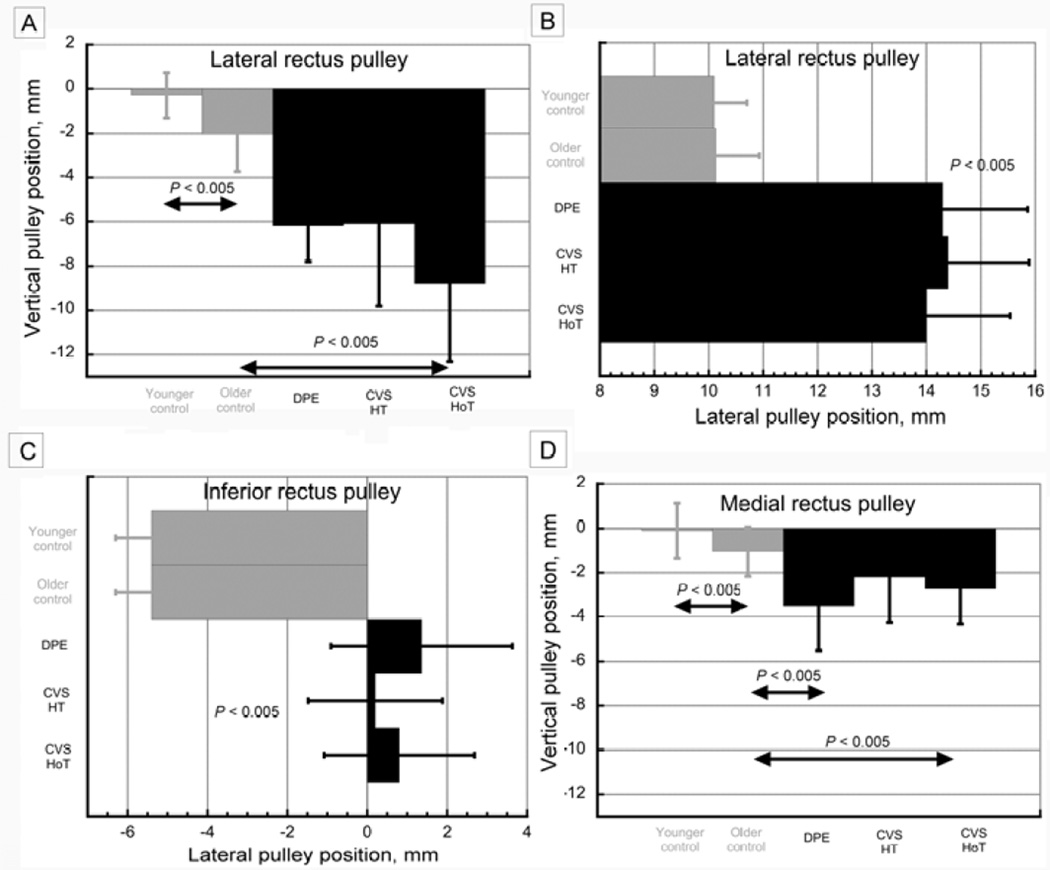
Rectus pulley positions from quasi-coronal plane MRI. A, Vertical position of lateral rectus pulley in control subjects and in subjects with sagging eye syndrome, replotted from published data23. CVS, cyclovertical strabismus; DPE, divergence paralysis esotropia; HoT, hypotropic orbit in cyclovertical strabismus; HT, hypertropic orbit in cyclovertical strabismus. B, Lateral position of lateral rectus pulley in control subjects and in subjects with sagging eye syndrome, replotted from published data.23 C, Lateral position of inferior rectus pulley in control subjects and in subjects with sagging eye syndrome, replotted from published data.23 D, Vertical position of medial rectus pulley in control subjects and in subjects with sagging eye syndrome, replotted from published data.23
The author finds it surgically challenging to correct pattern strabismus associated with rectus pulley heterotopy as illustrated in Figure 2. It has typically been necessary to perform insertional transpositions, with or without scleral posterior fixation, of two rectus muscles in each orbit. Although such surgery collapses the A or V incomitance, Kushner has noted that this is at the cost of inducing adverse torsion.26 In fusing patients, correction of torsion may necessitate surgery on oblique muscles, which are not the underlying causes of the patterns.
Adult-Onset Pulley Heterotopy
We have recently published23 an analysis of MRI and clinical characteristics in 56 orbits of 28 patients of mean age 69 ± 12 years who had sagging eye syndrome. This age range is significantly older than observed in subjects with childhood-onset pulley heterotopy. Control data were obtained from 25 orbits of 14 age-matched normal subjects and 52 orbits of 28 normal younger subjects (mean age, 23 ± 5 years). Our findings are summarized below.
Subjects with sagging eye syndrome characteristically exhibit external signs of adnexal laxity, including blepharoptosis (29%), deep superior lid sulcus defect (64%), and floppy lower lid tissues.23 There was a history of blepharoplasty, brow lift, or facelift surgery in 29% of cases. These changes are consistent with corresponding connective tissue degeneration demonstrated by quantitative postmortem histology.18,19 In contrast to subjects with childhood-onset pulley heterotopy, palpebral fissure inclination was not evident.
MRI demonstrated striking findings in sagging eye syndrome. Although lateral rectus pulley sag averaged 2 mm more in orthotropic older controls than in orthotropic young adults (P < 0.005), sag was ≥6 mm in sagging eye syndrome (P < 0.005, Figure 4A). Lateral rectus sag was symmetrical to ≤0.5 mm in divergence paralysis esotropia but was always asymmetrical by ≥1 mm in cyclovertical strabismus. The greater inferior lateral rectus sag was in the hypotropic eye. There was about 4 mm lateral displacement of the lateral rectus pulley in all subjects with sagging eye syndrome but not in the orthotropic older controls (P < 0.005, Figure 4B). There was also about 6 mm lateral displacement of the inferior rectus pulley in all subjects with sagging eye syndrome but not in the orthotropic older controls (P < 0.005, Figure 4C). The inferior rectus pulley was also displaced inferiorly in sagging eye syndrome. Whereas 1 mm average inferior sag of the medial rectus pulley in orthotropic controls was statistically significant (P < 0.005), medial rectus sag of 2–3.5 mm in sagging eye syndrome was significantly greater than in older controls (P < 0.005, Figure 4D); the medial rectus pulley was also displaced laterally.
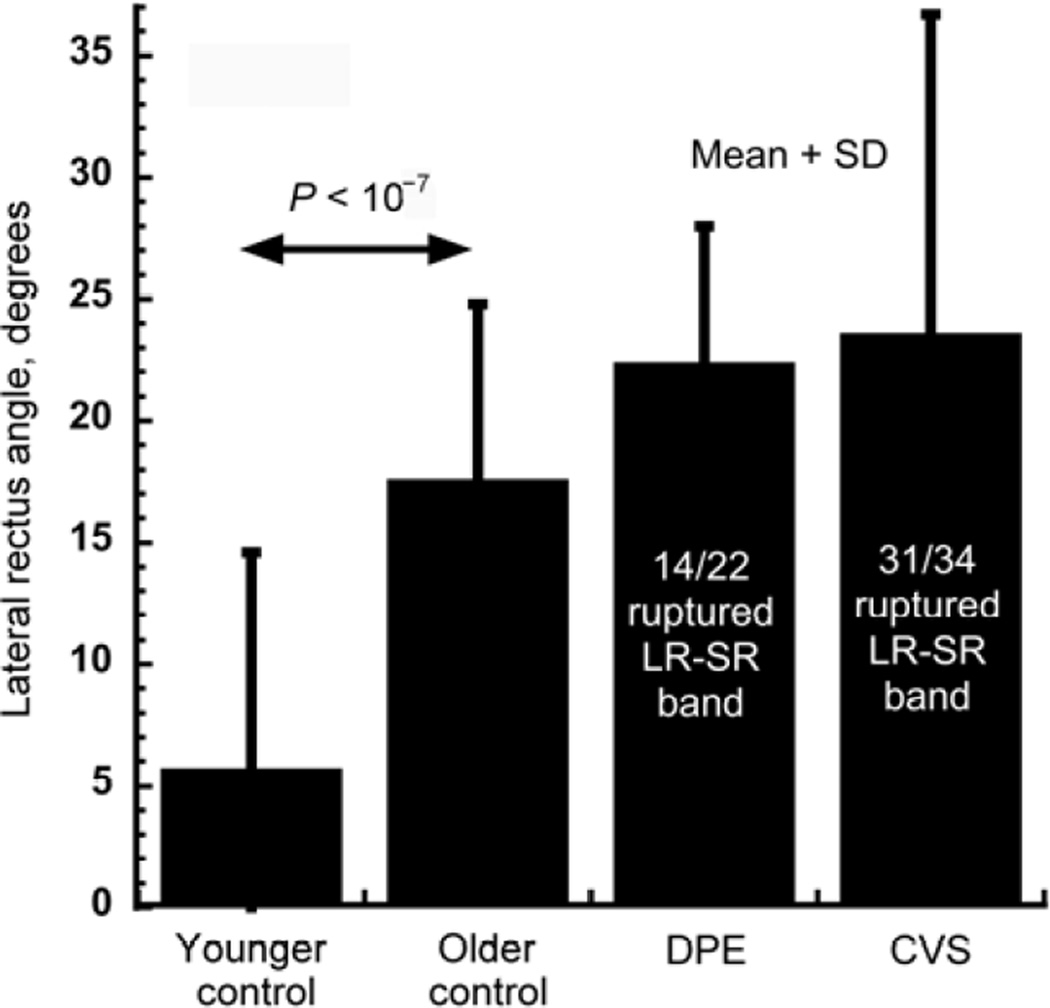
Degeneration of the LR-SR band in sagging eye syndrome frequently led to temporal tilting of the normally vertically oriented lateral rectus muscle (Figure 2C, D). This angle of temporal lateral rectus tilting averaged about 5° in younger orthotropic subjects and increased significantly to about 17° in orthotropic older controls (P < 10−7, Figure 5). Tilting of the lateral rectus muscle increased insignificantly to about 23° in sagging eye syndrome, but the LR-SR band was ruptured in 14 of 22 orbits with divergence paralysis esotropia and in 31 of 34 orbits with cyclovertical strabismus (Figure 5).
FIG. 5.
Temporal tilt of lateral rectus muscle in control subjects and in subjects with sagging eye syndrome, replotted from published data.23 DPE, divergence paralysis esotropia. CVS, cyclovertical strabismus.
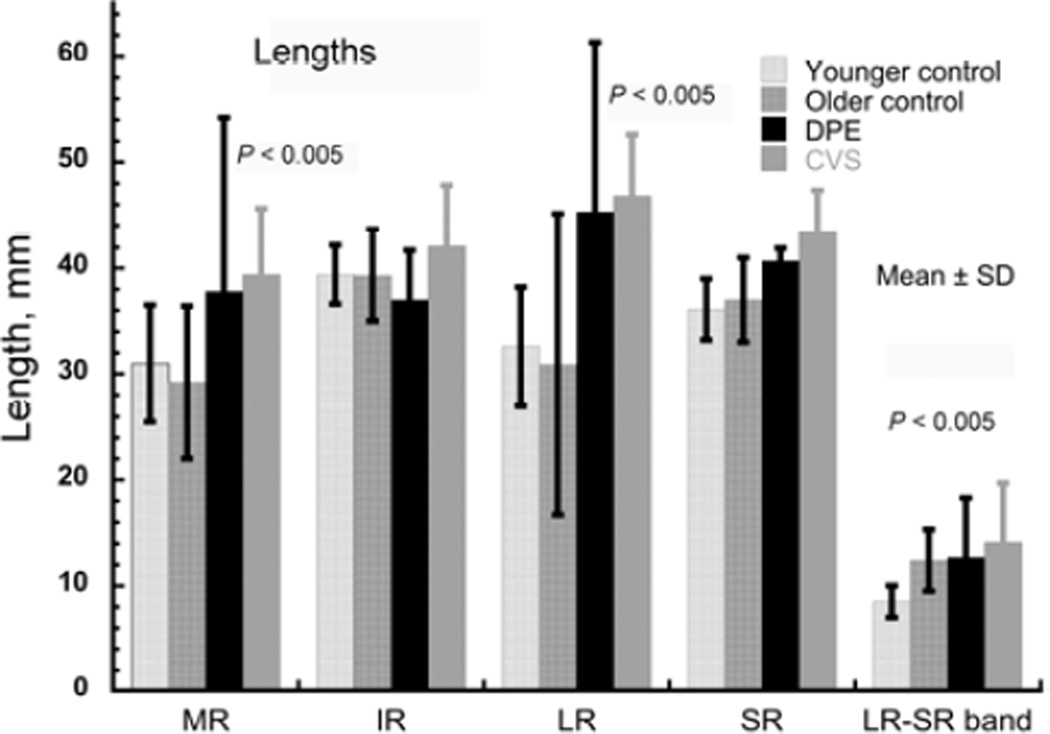
The foregoing pulley position data indicate displacement of the lateral, inferior, and medial rectus pulleys away from normal extraocular muscle paths, which approximate the shortest distances from origin over the globe to scleral insertion. Therefore, the displaced extraocular muscle path lengths in sagging eye syndrome necessarily exceed normal for the medial rectus and lateral rectus (P < 0.005, Figure 2). In particular, lateral rectus path length in sagging eye syndrome increased by about 50% from the normal value of about 30–45 mm. Could this marked horizontal rectus elongation simply represent a stretching of otherwise unchanged extraocular muscles? Computational simulation using the Orbit 1.8 model27 limited to the changes in pulley location observed in sagging eye syndrome predicts marked exotropia, inconsistent with the esotropia observed. Realistic simulation of binocular alignment additionally requires increase in rest lengths of the rectus muscles.
Many cases of divergence paralysis esotropia are appropriately treated using base-out spectacle prisms. Older patients made spectacle-independent by refractive or multifocal intraocular lens surgery are typically dissatisfied with prisms. Perhaps because of horizontal rectus muscle elongation the response of divergence paralysis esotropia to surgery differs from other forms of esotropia. Whereas divergence paralysis esotropia may be treated by either lateral rectus resection or medial rectus recession, the latter requires dose augmentation.24 Convenience and patient comfort are advantages of medial rectus recession, but it is recommended that recession dose be targeted from standard tables to twice the maximum distance esotropia measured in central or lateral gaze.24 A variety of surgical techniques may address cyclovertical strabismus. Small-angle cyclovertical strabismus may be conveniently treated by partial vertical rectus tenotomy at the scleral insertion under topical anesthesia.25 Surgeons may expect about 2Δ from 40% tenotomy, increasing to about 6Δ with 80% to 90% tenotomy. Incomitant surgical effects may be targeted by selection of vertical rectus muscles according to preoperative incomitance and torsional effects according to whether the medial or lateral vertical rectus tendon margin is tenotomized.
Clinical features distinguish sagging eye syndrome from neurological strabismus. Patients with divergence paralysis esotropia have full abduction and normal abducting saccades. In contrast, lateral rectus weakness due to abducens paresis limits abduction and slows abducting saccades. None of the foregoing subject with sagging eye syndrome had MRI evidence of superior oblique atrophy suggestive of superior oblique palsy. Although there may be some effect of head tilt on hypertropia in cyclovertical strabismus due to sagging eye syndrome, patients with cyclovertical strabismus exhibit excyclotropia in the hypotropic rather than the hypertropic eye as expected in superior oblique palsy. In cyclovertical strabismus, mean fundus excycloposition in the hypotropic eye was 12° but only 7° in the hypertropic eye (P = 0.01).23
Discussion
Childhood-onset pulley heterotopy can occur in infancy and presumably reflects effects of congenital or early developmental anatomic factors on rectus muscle paths. Extraocular muscle forces are both readily amenable to physiologic neural adjustment as well as to surgical manipulation. In contrast, extraocular muscle paths determine pulling directions and represent “hard constraints” against which compensatory changes in innervation apparently have little recourse. It appears that binocularity requires that the rectus pulley arrays of both orbits to be aligned so that the ipsilateral medial rectus and lateral rectus are not only purely horizontal antagonists in the same orbit, but also that the contralateral medial rectus and lateral rectus are also horizontal coagonists between the orbits. Such anatomical alignment greatly simplifies neurophysiological implementation of Hering’s law of equal innervational effort at the brainstem level, reducing it to a single scalar dimension for horizontal versions. It is now well established that the orbital pulleys implement mechanical simplification, relieving the brain of the enormous computational burden of kinematics for each eye considered separately.10,11,28 This concept likely extends to a pivotal role for the pulley system in facilitating binocular alignment. Perhaps this constraint dooms children with dystopic orbits and pulley heterotopy to pattern strabismus, as has been demonstrated predictably in Crouzon syndrome.15 It is now clear that pulley heterotopy acquired much later in life from age-related degeneration of pulley connective tissues is also associated with strabismus in neurologically normal people.23 Although absence of strabismus in older people who have mild, symmetrical medial and lateral rectus sag argues for existence of at least limited compensatory mechanisms for age-related degeneration,29 larger and especially asymmetrical lateral rectus sag is quantitatively correlated with predictable strabismus patterns.23 A longitudinal study has provided evidence that divergence paralysis esotropia becomes symptomatic only when progressively increasing distance esophoria exceeds the compensatory mechanism of fusional divergence.30
Early childhood pulley heterotopy may be suspected clinically from eyelid configuration. Upward lateral canthal fissure slanting typically reflects rectus pulley array incyclorotation strongly associated with A-pattern strabismus and fundus incyclotorsion. Because the medial rectus muscle is inferior to the lateral rectus bilaterally, the medial rectus muscle develops abnormal infraduction and the lateral rectus abnormal supraduction. Vertical actions of contralateral agonist medial and lateral rectus muscles are opposite, compounding vertical imbalance. Because the superior rectus muscle is nasal to the inferior rectus, it develops abnormal adduction while the inferior rectus muscle develops abnormal abduction. Horizontal actions of contralateral agonist inferior and superior rectus muscles are opposite, compounding horizontal imbalance to produce exoshift in infraversion and esoshift in supraversion, characteristic of A pattern. None of these imbalances involve oblique muscle abnormality , and surgeries limited to the oblique muscles in such cases are often unrewarding.31
A downward lateral canthal fissure slanting typically reflects rectus pulley array excyclorotation strongly associated with V-pattern strabismus and fundus excyclotorsion. Because the medial rectus muscle is superior to the lateral rectus bilaterally, it develops abnormal supraduction while the lateral rectus muscle develops abnormal infraduction. Vertical actions of the contralateral agonist medial and lateral rectus muscles are opposed, compounding the vertical imbalance. Because the superior rectus muscle is temporal to the inferior rectus, the superior rectus develops abnormal abduction while the inferior rectus muscle develops abnormal adduction. Horizontal actions of contralateral agonist inferior and superior rectus muscles are opposite, compounding the horizontal imbalance to produce esoshift in infraversion and exoshift in supraversion characteristic of V pattern. Again, none of these imbalances involve abnormality of the oblique muscles.
Orbital imaging, by coronal plane MRI or CT, can often be helpful in characterizing unilateral or asymmetrical rotation of the entire orbit, distinguishing this from unilateral or bilateral malpositioning of only one or several rectus pulleys and detecting possible coexisting hypoplastic,32 dysplastic,33, 34 or supernumerary extraocular muscles35 that may be clinically unsuspected. Imaging is particularly important in young children suspected of plagiocephaly, which is not only a cause of pulley heterotopy but also an independent indication for imaging.
Adnexal signs also assist in diagnosis of strabismus due to adult acquired pulley heterotopy. The great majority of patients with divergence paralysis esotropia and cyclovertical strabismus exhibit adnexal sag, manifested by blepharoptosis, superior sulcus defect, and floppy lower lids, or have previously undergone corrective surgery for these conditions. This association is unsurprising because the pulley system is composed of the same tissue constituents as the eyelids and the levator tendon; these tissues age and stretch together. However, even delicate manipulation during cataract, refractive, or eyelid surgery may be the final trigger in rupturing a tenuous LR-SR band. This phenomenon may explain new onset of strabismus after apparently uncomplicated ophthalmic surgeries and occasional complaints of spontaneous acute, painful onset of strabismus in sagging eye syndrome.
What is the role of orbital imaging in strabismus suspected secondary to sagging eye syndrome? Imaging is probably unnecessary if adequate clinical history and examination are obtained. Full abduction and normal abducting saccadic velocities are characteristic of divergence paralysis esotropia36 but inconsistent with neurogenic lateral rectus weakness. Imaging of the brain or orbits is generally uninformative in older patients with distance esotropia, fusion at near, null and brisk abduction, and no neurological symptoms or signs. Considerations are more complex in small-angle hypertropia lacking other potentially neurological symptoms or signs and in whom the strabismus is atypical for superior oblique palsy. Fundus torsion may be particularly useful because in superior oblique palsy the hypertropic eye should exhibit excycloposition, whereas in sagging eye syndrome the hypotropic eye exhibits excycloposition.
Sagging eye syndrome must be distinguished from the heavy eye syndrome, which is the clinical association of unilateral or bilateral axial high myopia with ipsilateral esotropia and hypotropia.37 Imaging consistently demonstrates inferior lateral rectus shift and nasal shift of the inferior and superior rectus muscles in heavy eye syndrome, so that the globe shifts superotemporally from the pulley array, converting the lateral rectus muscle from abductor to infraductor. In contrast, in sagging eye syndrome there is no axial high myopia, but temporal shift of the inferior rectus and lateral rectus without appreciable superior rectus shift. Heavy eye syndrome responds poorly to lateral rectus tightening or medial rectus recession alone but instead to loop myopexy uniting adjacent borders of the lateral rectus and superior rectus.38
Dr. Leonard Apt, pediatrician and ophthalmologist, understood that important ocular changes occur as we mature and was a careful student of ocular anatomy39 and its relevance to strabismus.40 There is now evidence that some forms of strabismus are caused, perhaps inevitably, by abnormalities of orbital connective tissues that either arise in early development or as a result of later involutional changes. Strabismologists can honor the legacy of Leonard Apt by alertness to the adnexal signs of connective tissue–related strabismus, judicious diagnostic testing, and appropriately targeted therapy for these distinctive conditions.
FIG. 6.
Lengths of rectus extraocular muscles and LR-SR band. Subjects with divergence paralysis esotropia (DPE) and cyclovertical strabismus (CVS) exhibited significant elongations of the medial rectus (MR) and lateral rectus (LR) muscles, and the LR-SR band, in comparison with younger orthotropic controls.
Acknowledgments
Supported by U.S. Public Health Service, National Eye Institute: grants EY08313 and EY0331. J. Demer is Leonard Apt Professor of Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This lecture memorializes Dr. Leonard Apt, a towering figure born in Philadelphia on June 28, 1922. Apt trained in hematology, pediatrics, pathology, and ophthalmology at Harvard and the University of Cincinnati. He was the inaugural National Institutes of Health fellow in pediatric ophthalmology, and the first US citizen board certified in both pediatrics and ophthalmology. Dr. Apt was active on the University of California California–Los Angeles faculty from 1961 until his death on February 1, 2013. Leonard Apt is widely known for the “Apt test” to distinguishing fetal from maternal blood in newborn stool, developed in the 1950s, while he was a Harvard pediatrician and hematologist. Collaborating with Sherwin J. Isenberg, MD, Apt extensively studied prevention and treatment of ophthalmic infections using povidone-iodine. Apt published over 300 papers, and received numerous awards, including the American Academy of Pediatrics Lifetime Achievement Award and the 2010 National Physician of the Year. In his honor, the American Academy of Pediatrics created the biennial “Leonard Apt Lecture,” given here in 2013.
References
- 1.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 2.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 3.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 4.Miller JM. Understanding and misunderstanding extraocular muscle pulleys. J Vision. 2007;7:1–15. doi: 10.1167/7.11.10. [DOI] [PubMed] [Google Scholar]
- 5.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 6.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 7.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Klier EM, Meng H, Angelaki DE. Three-dimensional kinematics at the level of the oculomotor plant. J Neurosci. 2006;26:2732–2737. doi: 10.1523/JNEUROSCI.3610-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klier EM, Meng H, Angelaki D. Revealing the kinematics of the oculomotor plant with tertiary eye positions and ocular counterroll. J Neurophysiol. 2011;105:640–649. doi: 10.1152/jn.00737.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157. doi: 10.1159/0000100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Cur Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–759. doi: 10.1016/s0002-9394(01)01099-6. [DOI] [PubMed] [Google Scholar]
- 13.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 14.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Buren (Netherlands): Aeolus Press; 1999. pp. 91–94. [Google Scholar]
- 15.Weiss AH, Phillips JO, Kelly JP. Crouzon syndrome: relationship of rectus muscle pulley location to pattern strabismus. Inv Ophtalmol Vis Sci. 2014;55:310–317. doi: 10.1167/iovs.13-13069. [DOI] [PubMed] [Google Scholar]
- 16.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–2178. [PubMed] [Google Scholar]
- 17.Bhola R, Rosenbaum AL, Ortube MC, Demer JL. High-resolution magnetic resonance imaging demonstrates varied anatomic abnormalities in Brown syndrome. J AAPOS. 2004;9:438–448. doi: 10.1016/j.jaapos.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Rutar T, Demer JL. “Heavy eye syndrome” in the absence of high myopia: a connective tissue degeneration in elderly strabismic patients. J AAPOS. 2009;13:36–44. doi: 10.1016/j.jaapos.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 20.Demer JL. A 12-year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–347. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 21.Demer JL, Dusyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23. doi: 10.1016/j.jaapos.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999. pp. 84–98. [Google Scholar]
- 23.Chaudhuri Z, Demer JL. Sagging eye syndrome: connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013;131:619–625. doi: 10.1001/jamaophthalmol.2013.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhuri Z, Demer JL. Medial rectus recession is as effective as lateral rectus resection in divergence paralysis esotropia. Arch Ophthalmol. 2012;130:1280–1284. doi: 10.1001/archophthalmol.2012.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri Z, Demer JL. Graded rectus tenotomy in small angle hypertropia due to sagging eye syndrome (SES) Abstr of Ann Mtg of Am Assn Pediatr Ophthalmol Strabismus. 2013:32. [Google Scholar]
- 26.Kushner BJ. Torsion and pattern strabismus: potential conflicts in treatment. JAMA Ophthalmol. 2013;131:190–193. doi: 10.1001/2013.jamaophthalmol.199. [DOI] [PubMed] [Google Scholar]
- 27.Miller JM, Pavlovski DS, Shaemeva I. Orbit 1.8 Gaze Mechanics Simulation. San Francisco: Eidactics; 1999. [Google Scholar]
- 28.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 29.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872–878. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 30.Godts D, Mathysen DG. Distance esotropia in the elderly. Br J Ophthalmol. 2013;97:1415–1419. doi: 10.1136/bjophthalmol-2013-303139. [DOI] [PubMed] [Google Scholar]
- 31.Coats DK, Paysse EA, Stager DR. Surgical management of V-pattern strabismus and oblique dysfunction in craniofacial dysostosis. J AAPOS. 2000;4:338–342. doi: 10.1067/mpa.2000.110337. [DOI] [PubMed] [Google Scholar]
- 32.Demer JL, Clark RA, Kung J. Functional imaging of human extraocular muscles in head tilt dependent hypertropia. Inv Ophtalmol Vis Sci. 2011;52:3023–3031. doi: 10.1167/iovs.10-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demer JL, Clark RA, Tischfield MA, Engle EC. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest Ophthalmol Vis Sci. 2010;51:4600–4611. doi: 10.1167/iovs.10-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane’s retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202. doi: 10.1167/iovs.06-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khitri MR, Demer JL. Magnetic resonance imaging of tissues compatible with supernumerary extraocular muscles. Am J Ophthalmol. 2010;150:925–931. doi: 10.1016/j.ajo.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim L, Rosenbaum AL, Demer JL. Saccadic velocity analysis in patients with digergence paralysis. J Pediatr Ophthalmol Strabismus. 1995;32:76–81. doi: 10.3928/0191-3913-19950301-04. [DOI] [PubMed] [Google Scholar]
- 37.Krzizok T, Wagner D, Kaufmann H. Elucidation of restrictive motility in high myopia by magnetic resonance imaging. Arch Ophthalmol. 1996;115:1019–1027. doi: 10.1001/archopht.1997.01100160189008. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi M, Yokoyama T, Shiraki K. Surgical procedure for correcting globe dislocation in highly myopic strabismus. Am J Ophthalmol. 2010;149:341–346. doi: 10.1016/j.ajo.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Apt L, Call NB. An anatomical reevaluation of rectus muscle insertions. Ophthalmic Surg. 1980;12:108–112. [PubMed] [Google Scholar]
- 40.Apt L, Axelrod N. Generalized fibrosis of the extraocular muscles. Am J Ophthalmol. 1978;85:822–829. doi: 10.1016/s0002-9394(14)78112-7. [DOI] [PubMed] [Google Scholar]