Abstract
The appearance of dengue-specific plasma membrane (DSPM) antigens in infected LLC-MK2 cell cultures was studied by 51Cr release in immune cytolysis and at an ultrastructural level using peroxidase-labeled antibodies. DSPM antigen was first detected at 36 h with electron microscopy in approximately 30% of the cells, and this percentage did not increase with time. However, both surface staining with peroxidase-labeled antibodies and 51Cr release indicated that the amount of DSPM antigen per cell increases with time. The appearance of 51Cr release in immune cytolysis experiments with dengue-infected cells occurred much later than the peak of infectious virus release. This was in sharp contrast to immune cytolysis with a group A arbovirus, Eastern equine encephalitis, in which the kinetics of release of infectious virus and 51Cr release were identical. This suggests different mechanisms of insertion of viral plasma membrane antigens in Eastern equine encephalitis and dengue-infected LLC-MK2 cells.
Full text
PDF



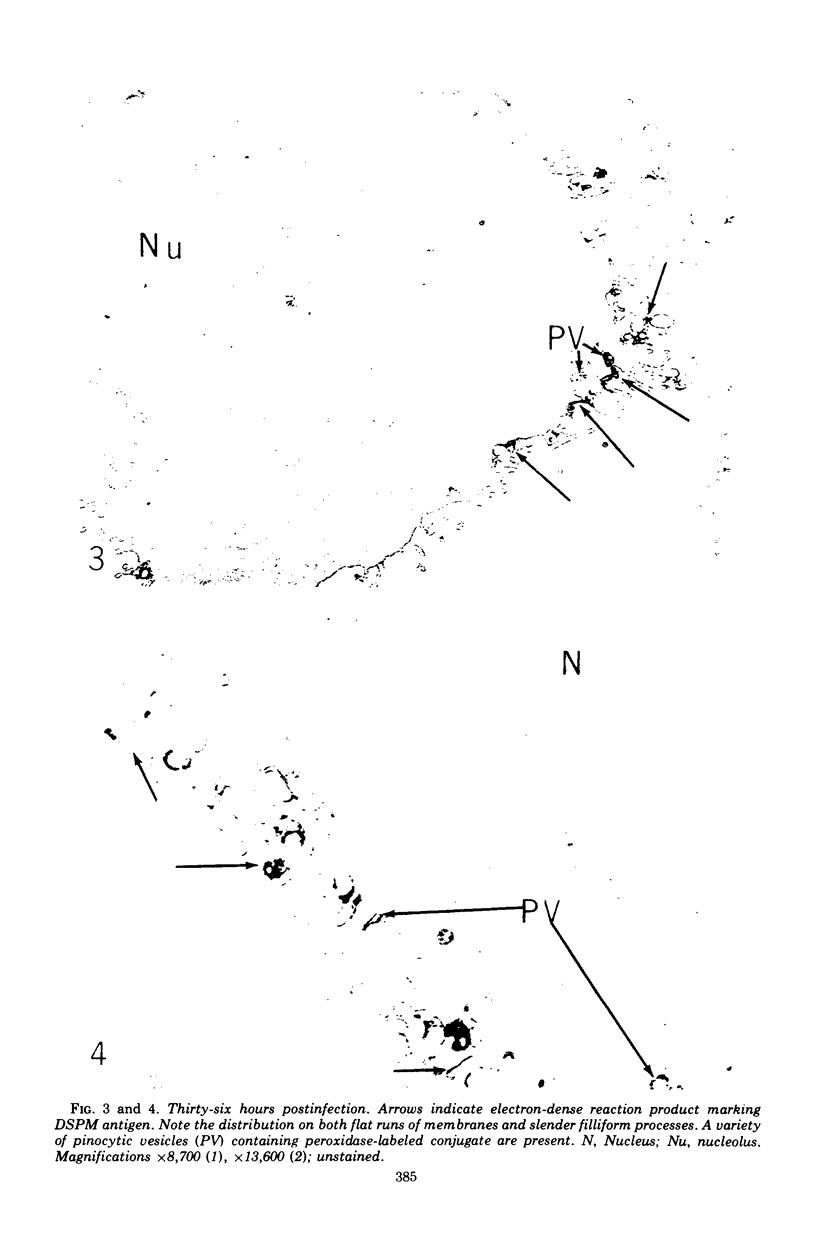
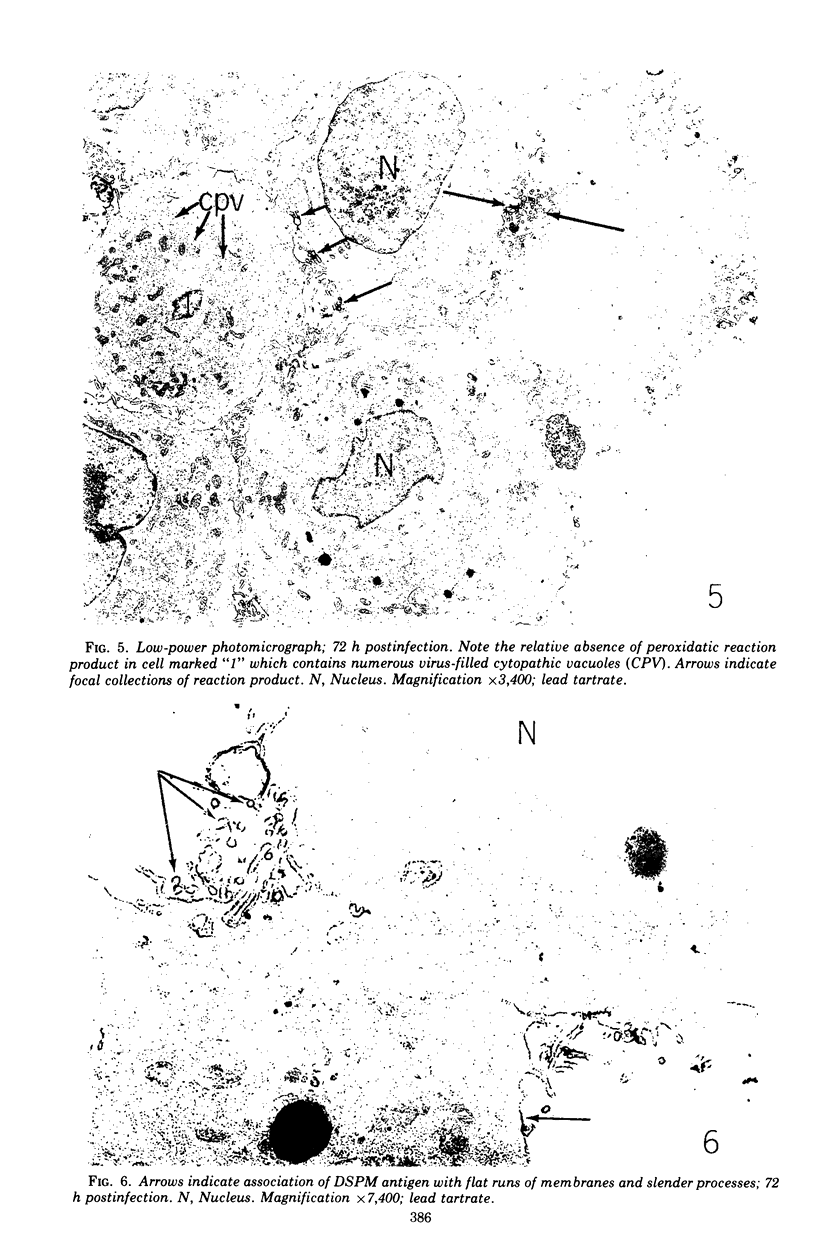
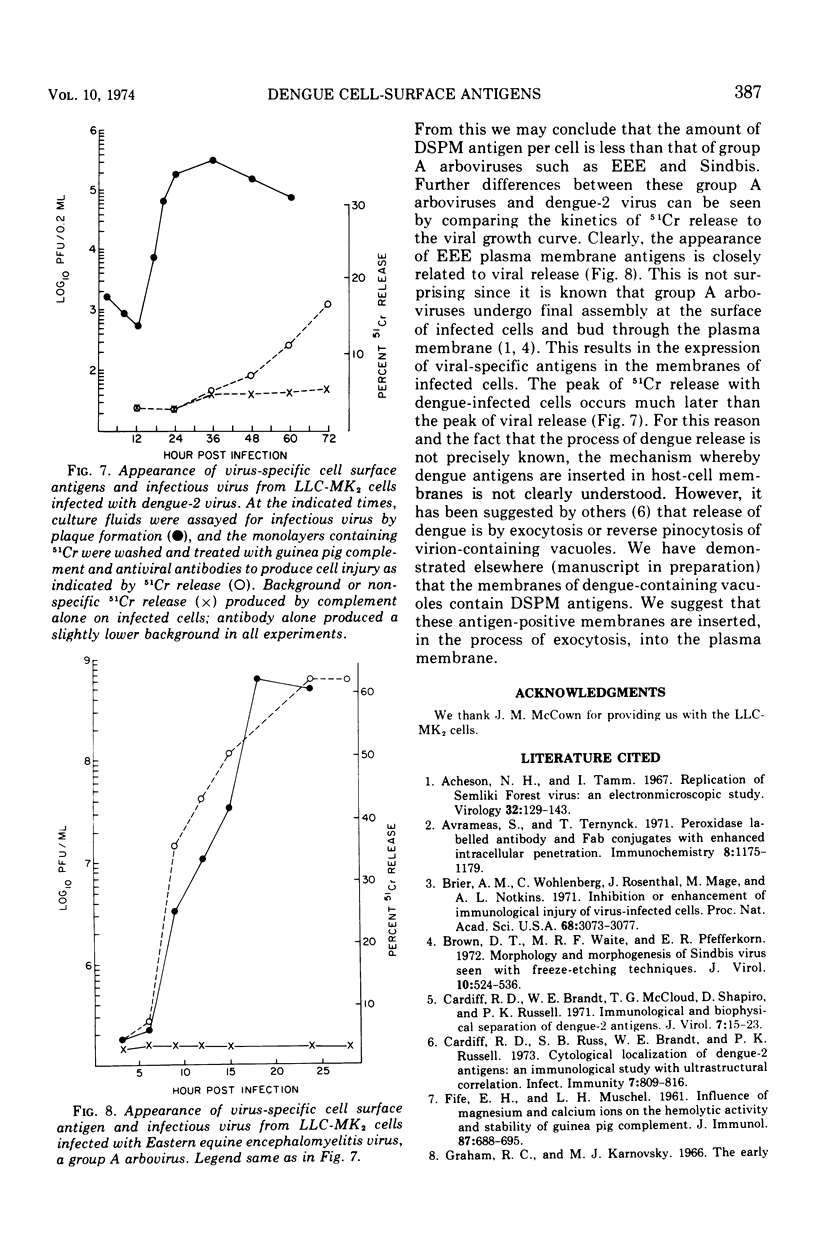

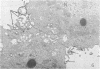
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Brandt W. E., McCloud T. G., Shapiro D., Russell P. K. Immunological and biophysical separation of dengue-2 antigens. J Virol. 1971 Jan;7(1):15–23. doi: 10.1128/jvi.7.1.15-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Russ S. B., Brandt W. E., Russell P. K. Cytological localization of Dengue-2 antigens: an immunological study with ultrastructural correlation. Infect Immun. 1973 May;7(5):809–816. doi: 10.1128/iai.7.5.809-816.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIFE E. H., Jr, MUSCHEL L. H. Influence of magnesium and calcium ions on the hemolytic activity and stability of guinea pig complement. J Immunol. 1961 Dec;87:688–695. [PubMed] [Google Scholar]
- Russell P. K., Nisalak A., Sukhavachana P., Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967 Aug;99(2):285–290. [PubMed] [Google Scholar]