Abstract
Aim
To study the influence of segment width on plan quality for volumetric modulated arc based stereotactic body radiotherapy.
Background
The redundancy of modulation for regularly shaped small volume tumors results in creation of many small segments and an increase of monitor units, with a consequent prolongation of treatment and uncertainty in treatment delivery.
Materials and methods
Six cases each in lung, abdomen and liver were taken for the study. For each case, three VMAT SBRT plans were generated with different penalties on minimum segment width of 0.5, 1.0 and 1.5 cm. A comparison was made on the metrics of dose volume histogram, dosimetric indices, monitor units (MUs) and delivery accuracy.
Results
The mean reduction of total MUs when compared with 0.5 cm plan was observed as 12.7 ± 6.0% and 17.5 ± 7.2% for 1.0 cm and 1.5 cm of minimum segment width, respectively. The p value showed a significant degradation in dosimetric indices for 1.5 cm plans when compared with 0.5 cm and 1.0 cm plans. The average deviation of measured dose with TPS calculated was 3.0 ± 1.1%, 2.1 ± 0.84% and 1.8 ± 0.9% for 0.5, 1.0 and 1.5 cm, respectively. The calculated gamma index with pass criteria of 2% dose difference and 2 mm distance to agreement was 95.9 ± 2.8%, 96.5 ± 2.6% and 97.8 ± 1.6% as calculated for 0.5, 1.0 and 1.5 cm of penalties, respectively. In view of the trade off between delivery efficiency and plan quality, 1 cm minimum segment width plans showed an improvement.
Conclusions
VMAT SBRT plans with increased optimal value of minimum segment width showed better plan quality and delivery efficiency for stereotactic body radiotherapy.
Keywords: VMAT SBRT, Optimization, Minimum segment width, Small field measurements
1. Background
Intensity modulated arc therapy is a widely accepted mode of delivery for all regimens of radiotherapy. This novel radiation technique, named as Volumetric Modulated Arc Therapy (VMAT), allows a simultaneous variation of three parameters during treatment delivery, i.e. gantry rotation speed, treatment aperture shape via movement of MLC leaves and dose rate. VMAT is a time efficient treatment delivery platform capable of producing highly conformal dose distributions with a single 360° arc.1 There is a significant increase in delivery of stereotactic body radiotherapy (SBRT), a kind of ablative treatment, with volumetric modulated arc delivery, because of better reduction of doses to surrounding critical organs and normal structures with a short delivery time.2 SBRT has emerged as an alternative treatment option to surgical resection for patients who are medically inoperable; giving excellent local control rates (up to 95%).3 Delivering SBRT with the technique of intensity modulation can improve dose conformity compared with conventional radiotherapy4 by creating many beamlets that are differently weighted in the treatment planning optimizing software. Several planning studies have evaluated the performance of VMAT in delivering SBRT.5–8 Generally, VMAT planning has a two-stage optimization in which fluence-map-based optimization algorithm calculates optimized fluence maps as a series of discrete beam angles and subsequently, an arc sequencer algorithm converts the fluence maps for multiple fixed-angle delivery to those for arc delivery while optimizing an MLC leaf shape sequence.9 VMAT uses sweeping leaf sequencer to create an arc treatment plan using an MLC to shape a radiation field so that the delivered dose volume conforms well to a given prescribed dose volume.
SBRT delivers a very high dose per fraction of radiation for five or fewer fractions. Most tumors planned for SBRT are early in stage, more localized, regular and smaller in dimension. Moreover, it has the major challenges in patient immobilization, contouring, and respiratory organ motion management and delivery accuracy. For SBRT, a crucial patient specific planning target volume is required to improve a targeting and delivery accuracy as well as to potentially reduce the dose to surrounding normal tissues and critical organs. Besides, organ motion has become an important consideration for SBRT treatment planning for tumors in the thorax, abdomen and prostate that are known to move with breathing motion.10 The management of motion control is established with the setup of motion encompassing methods such as respiratory gated imaging, breath hold technique, forced shallow breathing with abdominal compression, etc. Also, a stringent tolerance limit is required in MLC leaf position to deliver the dose in small and highly complex apertures created by the VMAT optimization. However, the redundancy of modulation for regularly shaped small volume tumors creates a large number of smaller segments with a consequent increase in monitor units, treatment time and dosimetric uncertainties. The dose calculation algorithm has a difficulty in predicting the dose accurately for these segments because of the lack of charged particle equilibrium and requires precise modelling of lateral electron scatter.11 Unlike conventional radiotherapy, any small uncertainty in the dose calculation for the small, narrow and irregular segments will have a notable impact on the accuracy of delivering the requisite high dose per fraction. Young et al. have discussed the use of edge penalty during optimization that reduces the required MUs up to 30% by avoiding the creation of complex apertures and increases accuracy of dose delivery with gamma passing rate from 79.5% to about 95.4%.12 Hence the creation of such smaller segments can be controlled with sequencing parameters by the segmentation of theoretical fluence into deliverable MLC segments. The minimum segment width parameter in the segmentation process has a significant role in the creation of segments with different sizes and shapes. This study investigates the plan delivery and quality with different penalty on segment width in volumetric modulated arc (VMAT) delivery for SBRT cases.
2. Methods and materials
2.1. Patient selection and simulation
A total of 18 patients treated for SBRT were taken for this study. This included six cases each of lung, abdomen and liver sites. For the lung, stage I/II non-small-cell lung cancer and for the liver, hepatocellular carcinoma and metastatic tumors were taken, while abdominal cases studied were pancreas, lymph nodes and adrenal gland tumors. The average PTV volume for lung, liver and abdomen cases were 38.8 cm3 (range 18.6–58.6 cm3), 120.1 cm3 (range 30.9–280.3 cm3) and 154.6 cm3 (range 30.9–280.3 cm3), respectively. All these patients were simulated with the vac-lac immobilization system with hands above the head position for both simulation and treatment procedure. For all the patients, simulation study were done with computed tomography imaging on 64 slice Biograph mCT 15 cms above and below the upper and lower limit of the target to encompass all organs at risk and obtain geometric and dosimetric information for the treatment setup. The patients were scanned in a continuous mode with gantry rotation time of 0.5 s and reconstructed in 2 mm slice thickness for precise target delineation. Since tumors of the lung, liver, and abdomen are prone to move with respiratory motion, Active Breathing Coordinator system was applied for patients undergoing treatment for lung and liver cancers to manage the respiratory motion. For patients with other intra-abdominal tumors, a stringent immobilization was done using BodyFix system from Elekta.
2.2. Contouring and dose prescription
Based on multimodality imaging process, gross tumor volume (GTV) for all the sites were defined on primary CT image by an experienced radiation oncologist and checked with radiologist as per the multidisciplinary protocol of the institution. Using CMS FocalSim 4.6v (Elekta, Maryland Heights, MO, USA) virtual simulation workstation patient-specific margins were applied for the GTV to the planning target volume (PTV) expansion with superior/inferior marginal range of 7–10 mm and axial margin of 5–7 mm. In addition, organs at risk (OARs), such as the lung, liver, spinal cord, heart, kidneys and, if relevant, bowel, esophagus were delineated corresponding to the tumor location. Each patient for lung, liver and abdomen cancer were planned on the RTOG 0915 protocol that delivers the prescription dose in the range of 7 Gy/fx to 20 Gy/fx. The stated goals for the tumors were to deliver 90–99% of prescription dose to cover 95% of its volume while allowing the maximum doses into GTV by up to 120–140% of prescription dose. For critical structures, the maximum dose for serial organs and volume constrains for parallel structures were respected based on total dose and dose per fraction using departmental protocol. The total number of fractions ranged from 1 to 5 and it is shown in Table 1.
Table 1.
Metrics of DVH showing difference in Target Coverage (PTV D95) for different Minimum Segment Width (MSW) while maintaining the similarity on critical organ doses as the mean ± SD for three different MSW plans.
| (a) Lung cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor vol (cc) | Rx Gy × Fr | PTV D95 (Gy) |
Mean + SD for 3 different MSW plans |
|||||||
| 0.5 cm | 1.0 cm | 1.5 cm | IpLung-PTV (cc) | ConLung D20 (Gy) | Heart D20 (Gy) | Ribs Max (Gy) | Spine Max (Gy) | |||
| Pt 1 | 52.8 | 15 × 4 | 60.8 | 60.5 | 59.5 | 332 ± 6 (V20) 648 ± 9 (V10) |
2.8 ± 0.8 | 9.6 ± 0.8 | 57.5 ± 0.05 | 12.0 ± 0.3 |
| Pt 2 | 24.8 | 15 × 4 | 60.2 | 60.3 | 59.4 | 156 ± 2 (V20) 219 ± 5 (V10) |
1.8 ± 0.09 | NCR | 66.5 ± 0.09 | 13.8 ± 0.3 |
| Pt 3 | 33.2 | 15 × 3 | 44.7 | 45.1 | 44.1 | 123 ± 5 (V20) 283 ± 7 (V10) |
2.4 ± 0.3 | NCR | 46.3 ± 0.4 | 17.8 ± 0.3 |
| Pt 4 | 18.6 | 15 × 3 | 59.5 | 59.3 | 58.3 | 192 ± 6 (V20) 308 ± 5 (V10) |
8.2 ± 0.5 | 3.6 ± 0.2 | NCR | 21.4 ± 0.5 |
| Pt 5 | 31.8 | 20 × 3 | 60.6 | 60.2 | 59.2 | 188 ± 4 (V20) 501 ± 12 (V10) |
2.5 ± 0.06 | 7.0 ± 0.2 | 52.5 ± 0.4 | 5.6 ± 0.2 |
| Pt 6 | 32.6 | 15 × 3 | 44.8 | 44.5 | 44.1 | 163 ± 8 (V20) 535 ± 18 (V10) |
2.7 ± 0.2 | NCR | 48.4 ± 0.5 | 6.9 ± 0.1 |
| (b) Liver cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor vol (cc) | Rx Gy × Fr | PTV D95 (Gy) |
Mean + SD for 3 different MSW plans |
|||||||
| 0.5 cm | 1.0 cm | 1.5 cm | Liver (cc) | Rt Kidney D20 (Gy) | Lt Kidney D20 (Gy) | Bowel Max (Gy) | Spine Max (Gy) | |||
| Pt 7 | 241.8 | 8 × 5 | 38.5 | 37.7 | 36.9 | 371 ± 7 (V20) 687 ± 11 (V10) |
24.2 ± 0.1 | 6.3 ± 0.02 | 36.9 ± 0.3 | 25.6 ± 0.1 |
| Pt 8 | 30.1 | 15 × 4 | 61.7 | 61.7 | 61.0 | 170 ± 3 (V20) 433 ± 9 (V10) |
NCR | NCR | NCR | NCR |
| Pt 9 | 18.4 | 8 × 5 | 39.9 | 39.8 | 38.3 | 54 ± 3 (V20) 174 ± 7 (V10) |
NCR | NCR | NCR | NCR |
| Pt 10 | 101.9 | 15 × 3 | 42.2 | 42.6 | 41.3 | 297 ± 6 (V20) 822 ± 11 (V10) |
NCR | NCR | 23.7 ± 0.3 | NCR |
| Pt 11 | 61.3 | 15 × 4 | 61.0 | 60.6 | 59.6 | 396 ± 8 (V20) 746 ± 12 (V10) |
3.8 ± 0.3 | 2.5 ± 0.1 | NCR | 16.8 ± 0.2 |
| Pt 12 | 146.8 | 8 × 5 | 40.5 | 40.9 | 39.8 | 144 ± 3 (V20) 372 ± 6 (V10) |
4.4 ± 0.2 | NCR | 22.1 ± 0.5 | 8.6 ± 0.2 |
| (c) Abdomen cases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor vol (cc) | Rx Gy × Fr | PTV D95(Gy) |
Mean + SD for 3different MSW plans |
||||||||
| 0.5 cm | 1.0 cm | 1.5 cm | Rt Kidney D20 (Gy) | Lt Kidney D20 (Gy) | Liver D20 (Gy) | Bowel Max (Gy) | Stomach Max (Gy) | Esophagus Max (Gy) | |||
| Pt 13 | 115.2 | 20 × 3 | 37.1 | 36.6 | 36.0 | 7.4 ± 0.6 | 18.5 | 1.3 ± 0.03 | 24.9 ± 0.3 | NCR | NCR |
| Pt 14 | 171.3 | 12 × 4 | 48.8 | 49.2 | 48.1 | 7.2 ± 0.04 | NCR | NCR | 41.4 ± 0.2 | NCR | NCR |
| Pt 15 | 30.9 | 12 × 3 | 33.9 | 34.0 | 32.8 | 18.3 ± 0.3 | 17.6 ± 0.3 | 9.7 ± 0.3 | 36.2 ± 0.6 | NCR | NCR |
| Pt 16 | 280.3 | 7 × 5 | 35.4 | 35.4 | 34.1 | 4.3 ± 0.5 | 3.3 ± 0.4 | 11.0 ± 0.09 | NCR | 20.4 ± 0.2 | NCR |
| Pt 17 | 60.8 | 7 × 5 | 59.3 | 59.7 | 59.5 | 8.9 ± 0.4 | 8.2 ± 0.5 | 18.5 ± 0.05 | NCR | 52.2 ± 0.1 | 34.8 ± 0.1 |
| Pt 18 | 114.4 | 7 × 5 | 34.2 | 34.1 | 32.6 | 7.1 ± 0.1 | 6.9 ± 0.2 | 8.0 ± 0.08 | – | – | – |
NCR, Not Clinically Relevant.
2.3. Treatment planning
2.3.1. General parameters
For all patients, VMAT SBRT plans were developed using CMS Monaco 3.1 (Elekta, Maryland Heights, Missouri, USA) treatment planning system and the plans were delivered using Elekta Synergy® (Elekta, Crawley, UK) machine equipped with a Beam Modulator head assembly consisting of 40 paired leaves, each measuring 4 mm in width at the isocenter and Cone-Beam Computed Tomography (CBCT) device (XVI®) for volumetric imaging. The treatment planning in Monaco TPS is a two step process where theoretical fluence is created based on given dose constraints and followed by segmentation of theoretical fluence into deliverable MLC segments. Each case was planned with a single arc either complete or a partial coplanar arc depending upon the location of tumor to avoid the dose to the contralateral organs. The gantry of an accelerator cannot pass from 180° to −180° and vice versa, hence the ideal partial arcs often have to be delivered by splitting into two partial arcs. Thus, the two partial arcs were disjointed and delivered as part of the same gantry rotation. The arc length was in the range of 200–360° with an increment of 10–20° and fluence smoothing parameters at medium mode. The number of control points per arc was kept in the range of 180–300, depending on the length of arc. Monte Carlo was chosen as a secondary algorithm for segmentation and final dose was calculated with a calculation grid resolution of 2.5 mm. The physical parameters, like number of arcs, arc length, increment, number of control points/arc, fluence smoothing parameters were all kept the same for all three different plans with different minimum segment width in each case. Homogeneity correction was used in all the plans.
2.3.2. Minimum segment width
During plan optimization, sequencing algorithm may create a higher number of monitor units and segments to generate intensity maps by creation of small and narrow segments. In Monaco VMAT, sequencing is based on an alternative Sliding Window pattern in which all the leaves move from start to end position in a continuous, unidirectional manner. The leaf assembly moves first one way and then the other way alternating between sectors of the full arc and varying the leaf speeds and the gaps are produced between opposing leaves, while the system modulates intensity of the delivered fluence. The parameter minimum segment width (MSW) was used in the sequencing algorithm to determine the minimum leaf separation between two opposing leaves within the segmented field of any given segment. Thus, the parameter MSW was devised to generate a sequence with limited narrow segments for plan delivery. The range of values allowable in the optimizer is 0.5–2.0 cm. Three VMAT SBRT plans were generated with different penalties on MSW of 0.5, 1.0 and 1.5 cm for each case in the study.
2.4. Plan evaluation
For lung cases, the OARs were the healthy ipsilateral lung, contralateral lung, heart and spinal cord. The OARs for the liver and abdomen cases were the healthy liver, kidneys, bowel, spinal cord, esophagus and stomach subject to involvement in the beam path. The qualitative analysis for the differently penalized plans were performed based on the comparison of dosimetric indices (DIs) such as
(i) Target coverage (TC), which is defined as the ratio of total target volume receiving at least the prescription dose to the total target volume;
TC = TVPI/TV, where TV is the target volume;
(ii) Conformity index (CI), which is the ratio of the total volume receiving the prescription dose VPI to the target volume receiving at least the prescription TVPI;
CI = VPI/TVPI
(iii) Conformity number (CN) which is the ratio of the target coverage to CI; and
CN = TC/CI or (TVPI)2/(TV × VPI),
(iv) Gradient index (GI) which is the ratio of the volume of 50% of the prescription isodose to the volume of the prescription isodose;
GI = V50PI/VPI, where V50PI is the volume of 50% of the prescription isodose.
The monitor units (MUs) and delivery accuracy were analyzed for all differently penalized plans.
2.5. Delivery analysis
The accuracy of the dose calculation algorithm on all VMAT SBRT plans with different penalties of MSW was evaluated from the measured fluence by a Matrixx 2D detector ion chamber for all arc segments with the beam central axis oriented perpendicular to the plane of the detector. In order to do the measured versus calculated dose analysis, the treatment planning system was used to calculate a separate dose distribution to a flat solid-water phantom for the VMAT SBRT arcs and dose planes of individual arcs at a specified SSD and depth in phantom. In this study, the dose was calculated for a plane at 11 cm depth, which corresponds to the plane of ionchambers of the Matrixx with 11 cm build-up, and SSD of 89 cm. The effect of the MSWs on delivery accuracy was quantitatively analyzed using Omnipro IMRT software. The measured and computed doses were analyzed by both point dose verification and planar dose measurements with gamma analysis using a 2% of dose difference and 2 mm of distance to agreement criteria. For collaboration, three more cases were measured for dose calculation verification using a small PMMA phantom with A16 in perceptiveness of matrix measurement as point dose verification with computed dose.
3. Results
To have an accurate comparison, each plan with different penalties of MSW was made in a way to achieve the same dose distribution and effect on dosimetric indices, plan efficiency in terms of MUs and delivery accuracy was analyzed. For doing so, the plans with 0.5 cm of MSW (MSW) were taken as reference and compared with other two plans. Fig. 1 shows the difference in total MUs for different segments width optimization schemes. For all the cases, plan with 0.5 cm of MSW showed a higher value of total MUs. When compared with the reference plan, the mean reduction of total MUs for the plan with 1.0 cm and 1.5 cm of MSW was observed as 12.7 ± 6.0% and 17.5 ± 7.2%, respectively. The maximum variation of 23.2% and 32.5% of decrease in total MUs was observed for plans with 1.0 cm and 1.5 cm of MSW, respectively. In all the figures, patient Pt1–Pt6, Pt7–Pt12, and Pt13–Pt18 represented lung, liver and abdomen cases, respectively.
Fig. 1.
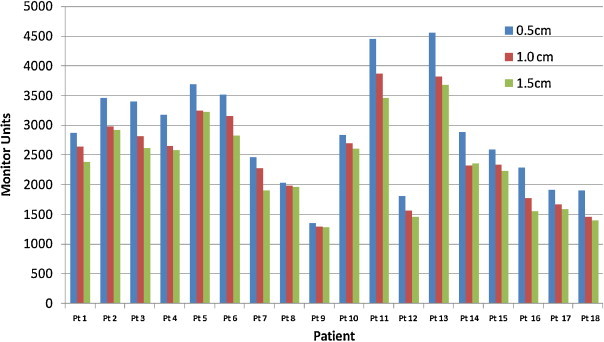
TPS calculated monitor units for plans with 0.5 cm, 1.0 cm and 1.5 cm of minimum segment width.
The metrics of DVHs planned for different penalties of MSW are shown in Table 1. For the lung cases, the percentage of normal ipsilateral lung volume exceeding 20 Gy (V20), 10 Gy (V10) and dose to 20% of contra lateral lung were compared. For liver and abdominal tumors, the percentage of normal liver volume exceeding 20 Gy (V20), 10 Gy (V10) and dose to 20% (D20) of kidneys were taken for the assessment. The dose maximum (0.01%) to the bowel, spine, stomach, esophagus and organs receiving relatively low dose were also analyzed as shown in Table 1. For most of the patients, metrics of critical organs from DVH for differently optimized plans were well within ±3%. For a few cases, the OAR dose values had a deviation outside 3% that was not clinically significant. For all the treatment plans, 90–99% of prescription dose covered 95% of tumor volume. Fig. 2 shows the deviation in target coverage (TC), conformity index (CI), conformity number (CN) and gradient index (GI) for three different optimization scenarios. Table 2 lists the statistical data of paired two tailed t-tests which demonstrates the significance of TC, CI, CN and GI for the plans with 1.0 cm and 1.5 cm of MSW. The p-value (p > 0.05) of mean and standard deviation for TC, CI, CN and GI demonstrated no significance for plans with 1.0 cm of MSW when compared with the 0.5 cm MSW plans. The t-test demonstrated a significant difference (p < 0.001) in TC, CI and CN for the plans with 1.5 cm of MSW when compared with the reference plan.
Fig. 2.

Comparison of normalized dosimetric indices like target coverage (TC), conformity index (CI), conformity number (CN) and gradient index (GI) for plans with different minimum segment width.
Table 2.
Deviation of dosimetric indices for plans with 1.0 cm and 1.5 cm of MSW w.r.t. plan with 0.5 cm of MSW. The significance were calculated using Student's t-test.
| MSW 1.0 cm |
MSW 1.5 cm |
|||||
|---|---|---|---|---|---|---|
| Mean Diff. | Std. Dev. | p-Value | Mean Diff. | Std. Dev. | p-Value | |
| TC | 0.00 | 0.0091 | 0.984† | 0.03 | 0.0167 | <0.0001* |
| CI | −0.02 | 0.0454 | 0.1318† | −0.06 | 0.0669 | 0.0005* |
| CN | 0.01 | 0.0308 | 0.1504† | 0.06 | 0.0440 | <0.0001* |
| GI | 0.04 | 0.2301 | 0.420† | −0.02 | 0.2648 | 0.7572† |
Not significant.
Highly significant.
All treatment plan dosimetric endpoints are shown in Fig. 1. Measured point doses for all the plans with 0.5 cm of MSW had a slightly higher deviation from TPS computed dose of average percentage deviation of 2.96 ± 1.07% and the highest percentage difference for plan with 0.5 cm of MSW was 4.5%. Maximum number of plans with 1.0 cm and 1.5 cm of MSW had a deviation of <3%. The mean and standard deviation of point dose variation between computed and measured dose were 3.0 ± 1.1%, 2.1 ± 0.84% and 1.8 ± 0.9% for the plans with 0.5 cm, 1.0 cm and 1.5 cm of MSW, respectively. Table 2 shows the mean and standard error of the percent difference in each point dose and planar dose measurement for all VMAT SBRT plans. The direction of the point dose deviation measured by MatriXX for different scenarios of VMAT SBRT plans was verified for three patients by A16 microchamber point dose measurement. Similar to 2D array results, point dose measurement using A16 ion chamber showed a higher deviation for 0.5 cm of MSW plans when compared to other two scenarios as shown in Table 3. Comparison of measured planar dose and TPS calculated dose plan was analyzed with passing criteria of 2% dose difference and 2 mm of distance to agreement. The mean and standard deviation of the passing rate was 95.9 ± 1.1%, 97.2 ± 1.4% and 98.1 ± 1.0% for the plans with 0.5 cm, 1.0 cm and 1.5 cm of MSW, respectively (Fig. 3).
Table 3.
Validation/justification of SBRT QA results of matrix 2D ion chamber array measurement with A16 micro-ion chamber results. The Mean ± SD of percentage deviation of TPS calculated and measured CAX dose for three patients i.e. nine plans.
| MSW 0.5 cm | MSW 1.0 cm | MSW 1.5 cm | |
|---|---|---|---|
| Matrix | 2.96 ± 1.07 | 2.09 ± 0.85 | 1.76 ± 0.91 |
| A16 | 2.57 ± 0.76 | 1.37 ± 0.67 | 0.93 ± 0.64 |
All dosimetric data were expressed in percentage.
Fig. 3.
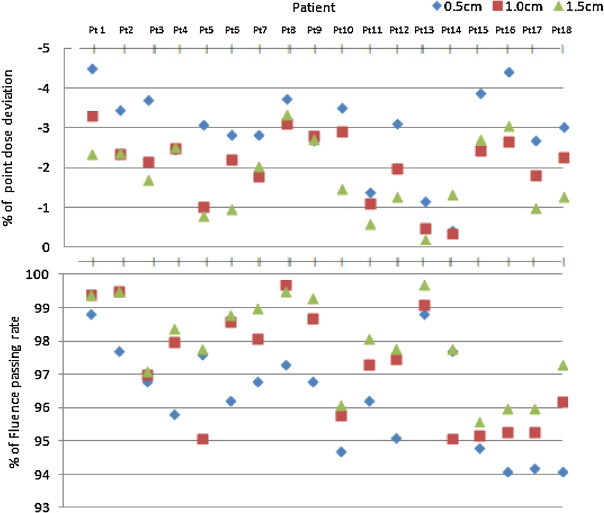
QA results of point dose deviation in CAX and fluence passing rate between TPS calculated and measured data using Matrix 2D ion chamber array for all eighteen patients in three different i.e. 0.5, 1.0, 1.5 cm of minimum segment width plans.
4. Discussion
The study compares three different VMAT SBRT optimization schemes with difference in MSW. We compared the plan quality both by analysing the dose volume histogram and delivery efficiency in terms of total plan MUs and deviation from measured dose to TPS calculated dose. Most of the tumors undergoing SBRT are smaller and regular in dimension and require a very high dose per fraction. The proximity of critical organs and tumor necessitates beam intensity modulation with inverse planning. The plan optimization for all these tumors increased the complexity by forming a large number of smaller and irregular optimized apertures. We investigated the increase in complexity of apertures and its consequent increased monitor units with resultant uncertainties in delivery due to the nature of small fields. The analysis of the VMAT SBRT plans with a different penalty on minimum segment width during segment shape optimization illustrated a significant difference in TPS calculated monitor units and some deviations in measured and TPS calculated dose distribution. The results were 23.2% variation for 1 cm and 32.5% for 1.5 cm of minimum segment width (MSW) with resultant reduction of the number of total monitor units. The decrease in MUs was seen with increasing minimum segment width values to 1.0 cm and 1.5 cm during segment shape optimization. Metrics obtained from the dose volume histogram of differently penalized plans showed similarity in dose distribution with insignificant variation of dose to organs at risk and minimal impact on target coverage. VMAT SBRT plans with 0.5 cm of MSW demonstrated a better dosimetric index with a resultant increase in monitor units and a higher deviation with TPS calculated to measured point dose and fluence passing rate. On the other hand, the plan with 1.5 cm of minimum segment width resulted in a better agreement with TPS calculated for both point dose and fluence verification on delivery with reduced MUs with a degradation in plan quality. At the same time, VMAT SBRT plan generated with 1.0 cm of minimum segment width had a similar dose distribution as plans with 0.5 cm of minimum width, in addition to better plan efficiency of increased delivery accuracy and reduced monitor units.
In a study by Young et al., the reduction in the number of monitor units through aperture regularization minimized the required leaf motion during dynamic delivery thereby potentially reduced the delivery time.17 The use of segment width restrictions, such as increasing the minimum segment width to an extent during optimization, introduces the possibility of reducing redundant modulation and increases delivery efficiency as long as the limits are not so strict as to interfere with the dosimetric plan objectives. Generally, in IMRT treatment planning, intensity distributions created by optimization systems are converted into trajectories of leaves to deliver desired dose distributions to produce two-dimensional non-uniform profiles of arbitrary shape. The beamlet intensity restrictions, smoothing procedures, direct aperture optimization, etc., were proposed by many authors for reducing the beam complexity on treatment planning and delivery for complex intensity patterns while maintaining the dosimetric quality.18–24 The change in the inherent segment sequencing parameter of minimum segment width has the positive effect of reducing the MUs needed for delivering very high dose of uncomplicated intensity patterns. Also, a large decrease in plan modulation not only improves delivery efficiency, but also potentially decreases planning and quality assurance time or difficulty.12–15
The patients undergoing hypofractionated treatment for tumors of the thorax and abdomen are more prone to have respiratory motion. These respiratory motions in the SBRT cases are manageable with the systems like Active Breathing Coordinator, respiratory gating, forced shallow breathing, etc., as per patient's comfort. Decreasing of MU for treatment delivery will reduce the treatment time and increase the comfort for patients, especially for patients undergoing treatment with motion management systems. Many of the VMAT SBRT plans for this study were delivered with the breathhold technique, so an exact determination of the reduction in treatment time is not possible because of the change in patient's capability in the breathhold position. Furthermore, it is well known that the main part of out-of-field doses are due to the linac-head scatter and leakage radiation are proportional to total MUs delivered.16,17 Hypothesis of increasing risk of secondary malignancies would be a sufficient justification to attempt a reduction in the MUs delivered to patients while maintaining a high dose to the target volumes and low dose to critical organ and surrounding normal tissues.
The QA results of each plan with different penalties on segment width were measured and it was shown that for patients calculated with higher penalty on minimum segment width there was a better agreement with the calculated dose from TPS. The mean and standard deviation of percentage difference between calculated and measured point dose showed an increased accuracy for the higher value of minimum segment width. Furthermore, the results of the fluence verification with 2%/2 mm of passing criteria of gamma had an increased passing rate for the plan with less complexity on apertures. However, the plan quality was degraded with increased MSW. For many cases, significant change in the target coverage was observed for 1.5 cm of MSW plans. Increasing the value of minimum segment width to an optimal value during plan optimization can help to prevent the creation of very small and complex segments for tumors with regular dimensions, especially for targets taken for the hypofractionation. Meanwhile with attempt of increasing the minimum segment width during plan optimization for irregular and concave shaped tumors, significant variation in target coverage and dose distribution was observed.
5. Conclusion
We planned stereotactic body radiotherapy with volumetric modulated arc for extracranial lesions with three different scenarios creating a change in minimum segment width during plan optimization. Different penalties were investigated for range of tumors of the lung, liver and abdomen. In an overall view among the calculated MUs, delivery accuracy and plan quality, the plans with optimally increased value of 1 cm of minimum segment width show a clear merit in trade off between better plan quality and delivery efficiency for stereotactic body radiotherapy. Reducing MUs by controlling redundant modulation for VMAT SBRT plans with optimal values of minimum segment width will also increase the comfort for patients undergoing treatment with motion management system.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Matuszak M.M., Yan D., Grills I., Martinez A. Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;77:608–616. doi: 10.1016/j.ijrobp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 2.McGrath S.D., Matuszak M.M., Yan D., Kestin L.L., Martinez A.A., Grills I.S. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: a dosimetric and treatment efficiency analysis. Radiother Oncol. 2010;95:153–157. doi: 10.1016/j.radonc.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Kuijper I.T., Dahele M., Senan S., Verbakel W.F.A.R. Volumetric modulated arc therapy versus conventional intensity modulated radiation therapy for stereotactic spine radiotherapy: a planning study and early clinical data. Radiother Oncol. 2010;94:224–228. doi: 10.1016/j.radonc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Baisden J.M., Romney D.A., Reish A.G. Dose as a function of lung volume and planned treatment volume in helical tomotherapy intensity-modulated radiation therapy-based stereotactic body radiation therapy for small lung tumors. Int J Radiat Oncol Biol Phys. 2007;68:1229–1237. doi: 10.1016/j.ijrobp.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Nagata Y., Takayama K., Matsuo Y. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body adiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia T., Li H., Sun Q. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–125. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Teoh M., Clark C.H., Wood K., Whitaker S., Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84:967–996. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diot Q., Kavanagh B., Timmerman R., Miften M. Biological-based optimization and volumetric modulated arc therapy delivery for stereotactic body radiation therapy. Med Phys. 2012;39:237–245. doi: 10.1118/1.3668059. [DOI] [PubMed] [Google Scholar]
- 10.Rao M., Wu J., Cao D. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using image-modulated radiotherapy and volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2012;83:251–256. doi: 10.1016/j.ijrobp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Das I.J., Ding G.X., Ahnesjo A. Small fields: nonequilibrium radiation dosimetry. Med Phys. 2008;35:206–215. doi: 10.1118/1.2815356. [DOI] [PubMed] [Google Scholar]
- 12.Young, Matuszak M.M., Moran J.M., McShan D.L., Fraass B.A., Roberts D.A. Penalization of aperture complexity in inversely planned volumetric modulated arc therapy. Med Phys. 2012;39:7160–7170. doi: 10.1118/1.4762566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coselmon M.M., Moran J.M., Radawski J., Fraass B.A. Improving IMRT delivery efficiency by applying intensity limits during inverse planning. Med Phys. 2005;32:1234–1245. doi: 10.1118/1.1895545. [DOI] [PubMed] [Google Scholar]
- 14.Sun X., Xia P., Yu N. Effects of the intensity levels and beam map resolutions on static IMRT plans. Med Phys. 2004;31:2402–2411. doi: 10.1118/1.1783551. [DOI] [PubMed] [Google Scholar]
- 15.Sun X., Xia P. A new smoothing procedure to reduce delivery segments for static MLC-based IMRT planning. Med Phys. 2004;31:1158–1165. doi: 10.1118/1.1713279. [DOI] [PubMed] [Google Scholar]
- 16.Seco J., Evans P.M., Webb S. An optimization algorithm that incorporates IMRT delivery constraints. Phys Med Biol. 2002;47:899–915. [PubMed] [Google Scholar]
- 17.Ma L. Smoothing intensity-modulated treatment delivery under hardware constraints. Med Phys. 2002;29:2295–2937. doi: 10.1118/1.1521121. [DOI] [PubMed] [Google Scholar]
- 18.Spirou S., Fournier-Bidoz N., Yang J., Chui C., Ling C. Smoothing intensity-modulated beam profiles to improve the efficiency of delivery. Med Phys. 2001;28:2105–2112. doi: 10.1118/1.1406522. [DOI] [PubMed] [Google Scholar]
- 19.Mohan R., Arnfield M., Tong S., Wu Q., Siebers J. The impact of fluctuations in intensity patterns on the number of monitor units and the quality and accuracy of intensity modulated radiotherapy. Med Phys. 2000;27:1226–1237. doi: 10.1118/1.599000. [DOI] [PubMed] [Google Scholar]
- 20.Matuszak M.M., Larsen E.W., Fraass B.A. Reduction of IMRT beam complexity through the use of beam modulation penalties in the objective function. Med Phys. 2007;34:501–510. doi: 10.1118/1.2409749. [DOI] [PubMed] [Google Scholar]
- 21.Langer M., Thai V., Papiez L. Improved leaf sequencing reduces segments or monitor units needed to deliver IMRT using multileaf collimators. Med Phys. 2001;28:2450–2458. doi: 10.1118/1.1420392. [DOI] [PubMed] [Google Scholar]
- 22.Buckey C.R., Stathakis S., Papanikolaou N. The inter- and intrafraction reproducibilities of three common IMRT delivery techniques. Med Phys. 2010;37:4854–4860. doi: 10.1118/1.3476413. [DOI] [PubMed] [Google Scholar]
- 23.Palma D., Vollans E., James K. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Paddick I., Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2009;105:194–201. doi: 10.3171/sup.2006.105.7.194. [DOI] [PubMed] [Google Scholar]


