Abstract
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, a microorganism that usually affects skin and nerves. Although it is usually well-controlled by multidrug therapy (MDT), the disease may be aggravated by acute inflammatory reaction episodes that cause permanent tissue damage particularly to peripheral nerves. Tuberculosis is predominantly a disease of the lungs; however, it may spread to other organs and cause an extrapulmonary infection. Both mycobacterial infections are endemic in developing countries including Brazil, and cases of coinfection have been reported in the last decade. Nevertheless, simultaneous occurrence of perianal cutaneous tuberculosis and erythema nodosum leprosum is very rare, even in countries where both mycobacterial infections are endemic.
Keywords: Mycobacteria, Leprosy, Tuberculosis, Erythema nodosum leprosum
Background
Mycobacterium leprae, the causative agent of leprosy that affects the skin and peripheral nerves, shows tropism for macrophages and Schwann cells. The disease presents a wide clinical spectrum that is closely related to the patient specific immune response. The benign type, referred as tuberculoid or paucibacillary, is characterized by a Th1 immune response and production of type 1 cytokines including IFN-γ, IL-2, IL-12, IL-15 and TNF-α, which are typical of strong cell-mediated immunity. In lepromatous or multibacillary leprosy, the main characteristics comprise high levels of Th2-type cytokines such as IL-4, IL-5 and IL-10, high bacillary loads in skin lesions and reduced specific cellular immunity [1,2].
Tuberculosis is caused by Mycobacterium tuberculosis and is predominantly a disease of the lungs, with pulmonary tuberculosis accounting for 70% of cases [3]. M. tuberculosis can disseminate to other organs, including lymph nodes, bones, meninges and other extrapulmonary locations [3]. There are 9 million cases of active tuberculosis being reported annually and one third of the world’s population is supposed to be infected with Mycobacterium tuberculosis, although asymptomatically. Of these latent individuals, only 5-10% will develop active tuberculosis in their lifetime [3-5]. Similarly, in the natural history of leprosy, less than 5% of the people exposed to M. leprae will develop clinical disease [6,7].
Infections with intracellular pathogens such as M. leprae and M. tuberculosis, in most cases, are controlled by the cell-mediated immune response, based on CD4+ Th1 cells [8]. Leprosy is a more prevalent cause of cutaneous infections than tuberculosis and, even in endemic countries, the coinfection is uncommon in both diseases. We report in this paper a case of perianal tuberculosis in a patient whose lepromatous leprosy status was diagnosed as a type 2 leprosy reaction.
Case report
A 59-year-old male patient was admitted to our university hospital (Botucatu, SP, Brazil) complaining of a perianal suppurative ulcer developed two months before the first medical consultation. He was under therapy with prednisone 40 mg/day for six months due to a presumed diagnosis of a drug reaction. On physical examination, a clean 10-cm diameter phagedenic perianal ulcer (Figure 1) and two longitudinal suppurative ulcers on the inguino-crural region were observed. During investigation, and soon after reducing the corticosteroid dose, disseminated erythematous papules and nodules were observed in the upper limbs (Figure 2).Histopathology of the perianal ulcer revealed a dense histiocytic dermal infiltrate and a granulomatous inflammatory response with central caseous necrosis (Figure 3). Fite-Faraco staining showed a few acid-fast bacilli and the hypothesis of cutaneous tuberculosis was raised. The histopathology of upper limbs papular and nodular lesions showed foamy macrophages with globi of bacilli, suggesting lepromatous leprosy (Figure 4). The perianal and inguinal ulcers were considered uncommon manifestations of type 2 leprosy developed when corticosteroid was gradually diminished. The patient was treated as for multibacillary leprosy with multidrug therapy and thalidomide.
Figure 1.
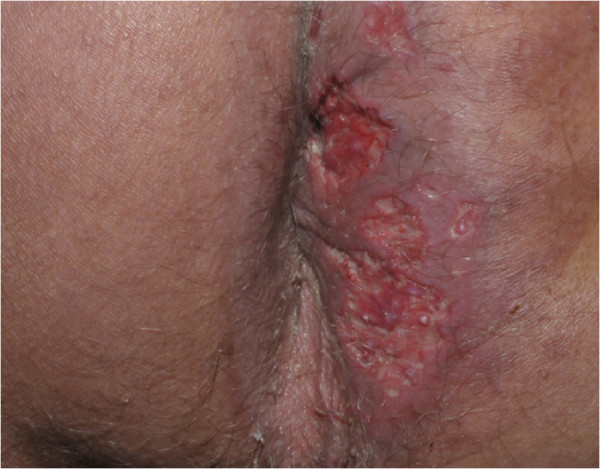
Cutaneous tuberculosis: fagedenic perianal ulcer.
Figure 2.
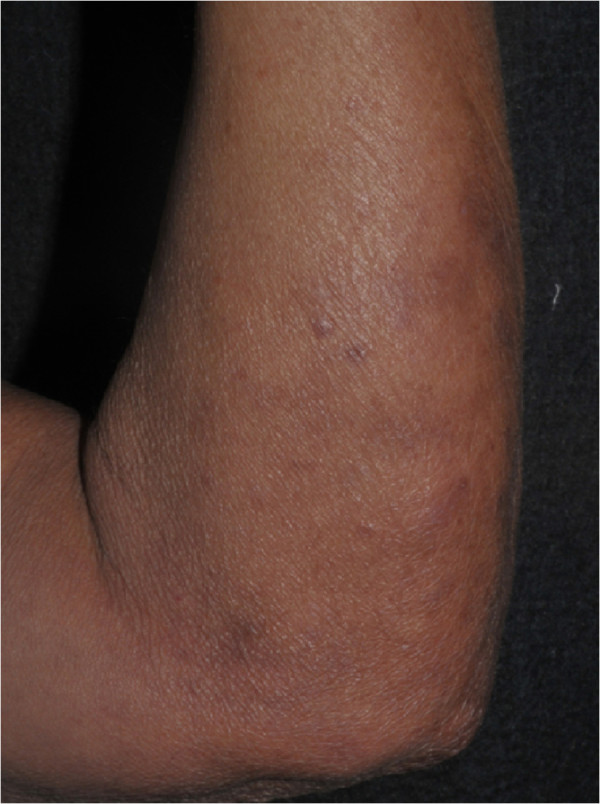
Lepromatous leprosy: erythematous papules and nodules on the upper limbs.
Figure 3.
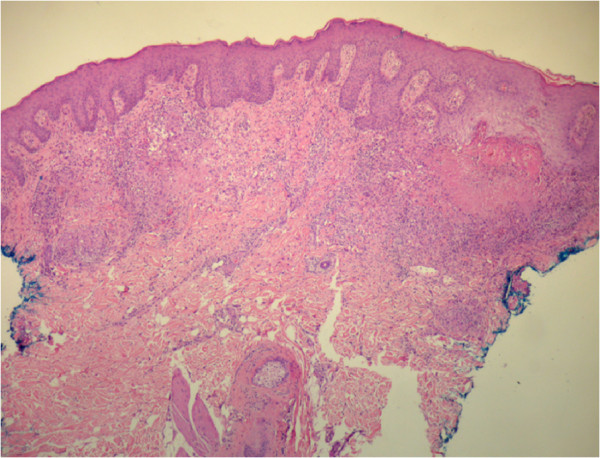
Histopathological exam of the perianal ulcer showing a dense histiocytic infiltrate and central caseous necrosis.
Figure 4.
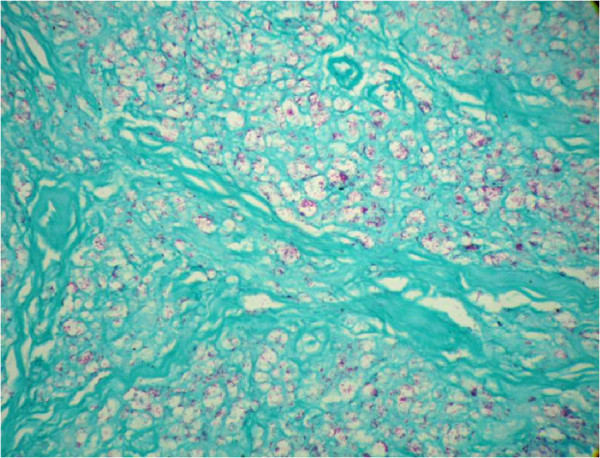
Skin nodule consisting of foamy macrophages with globi of bacilli. Fite-Faraco staining revealed the presence of lepromatous leprosy acid-fast bacilli.
Four weeks later, the patient had fever, weight loss, asthenia and worsening of the perianal ulcer (Figure 5), but without erythema nodosum leprosum. With this clinical picture, new investigation was developed. The tuberculin skin test was positive (20 × 20 mm) and culture of M. tuberculosis – using a biopsy sample from the perianal ulcer – on Löwenstein-Jensen medium was positive (Figure 6). Polymerase chain reaction (PCR) and DNA analysis from upper limb skin samples were positive for M. leprae (Figure 7). With the new information the patient was finally diagnosed as presenting simultaneously lepromatous leprosy – type 2 reaction – and cutaneous tuberculosis expressed by the perianal ulcer. Screening for HIV, HBV or HCV infection as well as lung, renal or colon compromising were negative. Hence, the patient was treated with isoniazid, ethambutol and rifampin for nine months with complete healing of the perianal and inguinal lesions after two months (Figure 8). After the tuberculosis treatment, the patient was submitted to additional multidrug therapy for 12 months with dapsone, rifampin and clofazimine with complete cure of leprosy.
Figure 5.
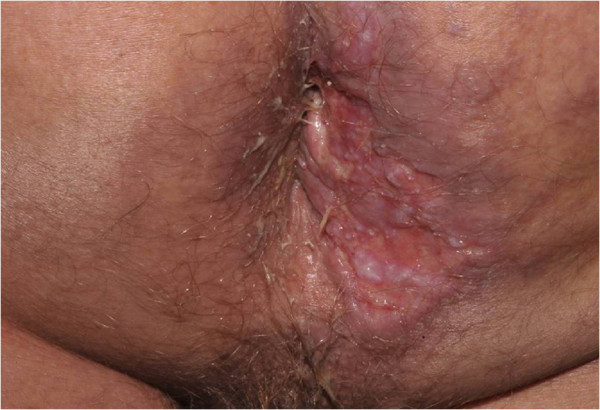
Cutaneous tuberculosis: phagedenic perianal ulcer showing worsening of the perianal ulcer after treatment for leprosy lesion, for two months.
Figure 6.

M. tuberculosis, positive culture on Löwenstein-Jensen medium at 37°C.
Figure 7.
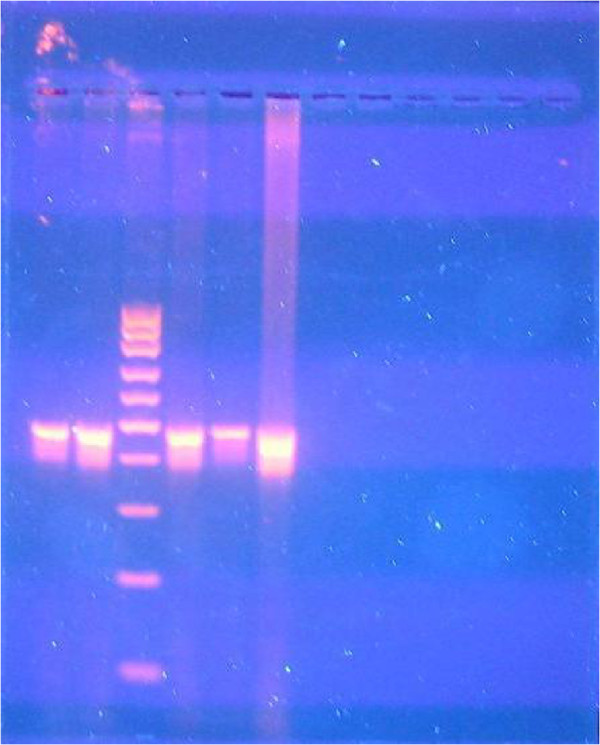
M. leprae , positive polymerase chain reaction DNA analysis.
Figure 8.
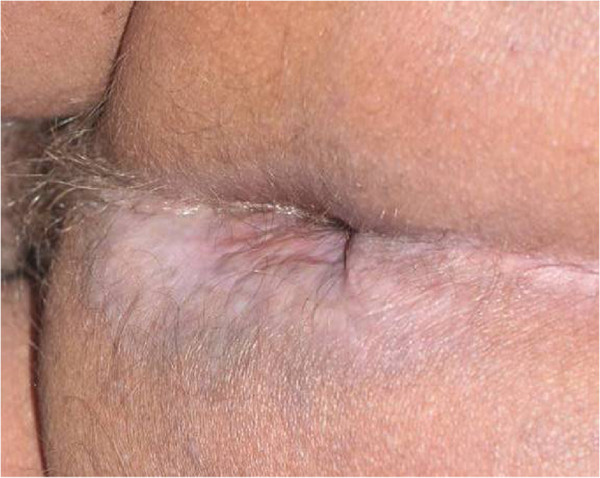
Perianal ulcer completely healed with antituberculous treatment for two months.
Discussion
Tuberculosis is one of the most important health concerns in the world, which causes relevant levels of morbidity and mortality, particularly in many developing countries, in spite of this being the era of human immunodeficiency virus infection [9]. The number of tuberculosis cases in industrialized and developing countries has increased in recent years, followed by increasing multidrug resistance [10].
Multidrug resistance and HIV infection are predisposing factors for the disease, since the immune response of the patient plays a significant role on clinical manifestation of tuberculosis [10-12]. Extrapulmonary tuberculosis accounts for 13% of all cases [4]. Nevertheless, cutaneous tuberculosis is uncommon and is reported to occur in less than 1.5% of the cases of extrapulmonary tuberculosis [13].
The present study reports a clinical case of perianal tuberculosis and lepromatous leprosy with type 2 reaction coinfection without any detected active pulmonary or gastrointestinal infection. The association between lepromatous leprosy and perianal tuberculosis with erythema nodosum leprosum demonstrates an extremely uncommon occurrence.
Perianal tuberculosis may manifest in the following forms: ulcer, fistula, abscess, lupoid infiltration or miliary. The most common type is the ulcerative lesion, which is likely to have well-defined boundaries and is characterized by mucopurulent discharge [14]. The lesion observed in the present report was ulcerative with many bacilli, signaling an immunosuppressive status probably related to corticosteroid therapy.
Perianal tuberculosis has rarely been reported, even in countries where the disease is endemic, and the diagnosis is impaired due to the lack of a pulmonary focus. The possibility of an association with intestinal tuberculosis should be further investigated [15,16].
Coinfection with pulmonary tuberculosis and leprosy type 1 reaction has been reported and the leprosy type 1 reaction was observed during the treatment of pulmonary tuberculosis [17]. According to Trindade et al. [17], a review of patients between 2004 and 2011 showed that only two patients with leprosy were diagnosed as coinfected with pulmonary tuberculosis. The occurrence of both tuberculosis and leprosy in the same individual is not an unusual clinical condition, but it is scarcely reported in the literature, even in countries where both diseases are endemic [18].
Delobel et al.[19] reported a triple association of American cutaneous leishmaniasis, lepromatous leprosy and pulmonary tuberculosis. The authors suggested that the unresponsiveness of patient’s T cells to IL-12 in vitro, stimulated by either L. guyanensis, M. bovis BCG or M. leprae antigens, could be the evidence of patient’s T cells failure to produce an appropriate Th cell response. This was responsible for the triple reported coinfection. There are other reports in the literature of coinfection of leprosy and pulmonary tuberculosis as well as leprosy and disseminated tuberculosis in HIV-infected patients [20-24].
Leprosy is a disease of poverty. Its diagnosis remains based on clinical signs and symptoms. Delayed diagnosis associated with nerve impairment and physical deformities is still common in several endemic areas throughout the globe. Improving the medical attention and infrastructure and promoting sanitary education will provide earlier diagnosis and specific treatment, which are crucial to help interrupt the M. leprae transmission chain [25,26]. Both leprosy and tuberculosis pose significant health risks, making it important for physicians to diagnose accurately and provide appropriate treatment for patients [27].
CD4+ T cells, as well as the cytokines IL-12, IFN-γ and TNF-α, are critical in the control of M. tuberculosis and M. leprae infections, but the host factors that determine why some individuals are protected from infection while others develop the disease are still unclear [28]. Hence, the reason why M. tuberculosis and M. leprae are able to evade host immune surveillance and persist inside the macrophages remains to be understood. These pathogens inhibit phagosome-lysosome fusion and stop phagosome maturation at an early stage, thus allowing escape from microbicidal peptides [29].
Genetic factors of the host and of the pathogen itself may be associated with an increased risk for patients to develop active tuberculosis and leprosy [28]. The immunologic deficits observed in leprosy are specific for M. leprae. Immunosupression has been observed to render individuals susceptible to M. leprae and M. tuberculosis in cases of transplantation, cancer chemotherapy and in treatment with antitumor necrosis factor agents [30-32]. However, the coinfection with lepromatous leprosy and tuberculosis probably depends on multiple factors including low socioeconomic status, poor nutrition, chemotherapy-induced immunosuppression and deficient host immune response [27].
The present report shows a rare case which was probably eased by the immunosuppressive effect of corticosteroid therapy for six months. Cutaneous tuberculosis remains to be one of the most difficult conditions to diagnose in developing countries due to the lack of resources and the necessary observation of clinical and histopathologic findings [11,33,34]. To the best of our knowledge, this is the first case of lepromatous leprosy associated with erythema nodosum leprosum and perianal tuberculosis fully documented.
Conclusion
In the present study, we report an association of lepromatous leprosy with type 2 reaction and perianal ulcerative tuberculosis without previous or active pulmonary infection. Simultaneous occurrence of cutaneous tuberculosis and lepromatous leprosy is extremely infrequent, even in countries where both mycobacterial infections are endemic.
Ethics committee approval
The case report was submitted for analysis and approved by the Research Ethics Committee of the Botucatu Medical School (CEP), in accordance with resolution 466/2012.
Consent
Written informed consent was obtained from the patient for publication of this case report and the figures.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed to the design of the study and manuscript preparation. All authors have read and approved the final manuscript.
Contributor Information
Maria Rita Parise-Fortes, Email: mfortes@fmb.unesp.br.
Joel Carlos Lastória, Email: lastoria@fmb.unesp.br.
Silvio Alencar Marques, Email: smarques@fmb.unesp.br.
Maria Stella Ayres Putinatti, Email: mstellaputinatti@hotmail.com.
Hamilton Ometto Stolf, Email: hstolf@fmb.unesp.br.
Mariângela Ester Alencar Marques, Email: mmarques@fmb.unesp.br.
Vidal Haddad, Email: haddadjr@fmb.unesp.br.
Acknowledgments
The authors would like to thank the photographer of the Department of Dermatology, Eliete Corrêa Soares, for the pictures of the patient.
References
- Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Foss NT. Hanseníase: aspectos clínicos, imunológicos e terapêuticos. An Bras Dermatol. 1999;74(2):113–119. [Google Scholar]
- Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2006. Atlanta, US: Department of Health and Human Services, CDC; 2007. http://www.cdc.gov/tb/statistics/reports/surv2006/pdf/FullReport.pdf. [Google Scholar]
- World Health Organization. Tuberculosis – Global Facts 2011/2102. Geneva: WHO Stop TBDep; ᅟ. http://www.who.int/tb/publications/2011/factsheet_tb_2011.pdf. [Google Scholar]
- Vynnycky E, Fine PE. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol. 2000;152(3):247–263. doi: 10.1093/aje/152.3.247. [DOI] [PubMed] [Google Scholar]
- Borgdorff MW, Sebek M, Geskus RB, Kremer K, Kalisvaart N, Van Soolingen D. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol. 2011;40(4):964–970. doi: 10.1093/ije/dyr058. [DOI] [PubMed] [Google Scholar]
- Worobec SM. Treatment of Leprosy/Hansen’s disease in the early 21st century. Dermatol Ther. 2009;22(6):518–537. doi: 10.1111/j.1529-8019.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Ottenhoff THM. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 2012;20(9):419–428. doi: 10.1016/j.tim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Raviglione MC, editor. World Health Organization. Tuberculosis - The Essentials. 4. Geneva, Switzerland: World Health Organization; 2010. http://www.who.int/tb/features_archive/the_essentials/en/ [Google Scholar]
- Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finalay A, Castro KG, Weyer K. HIV infection and multidrug – resistant tuberculosis: the perfect storm. J Infect Dis. 2007;15(196):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- Handog EB, Gabriel TG, Pineda RT. Management of cutaneous tuberculosis. Dermatol Ther. 2008;21(3):154–161. doi: 10.1111/j.1529-8019.2008.00186.x. [DOI] [PubMed] [Google Scholar]
- Kamil S, Aithal V, Kumaran MS, Abrahana A. Cutaneous tuberculosis of the pinna: a report of two cases. Int J Dermatol. 2013;52(6):714–717. doi: 10.1111/j.1365-4632.2012.05759.x. [DOI] [PubMed] [Google Scholar]
- Yasmeen N, Kanjee A. Cutaneous tuberculosis: a three year prospective study. J Pak Med Assoc. 2005;55(1):10–12. [PubMed] [Google Scholar]
- Akgun E, Tekin F, Ersin S, Osmanoglu H. Isolated perianal tuberculosis. Neth J Med. 2005;63(3):115–117. [PubMed] [Google Scholar]
- Ghiya R, Sharma A, Marfatia YS. Perianal ulcer as a marker of tuberculosis in the HIV infected. Indian J Dermatol Venereol Leprol. 2008;74(4):386–388. [PubMed] [Google Scholar]
- Mathew S. Anal tuberculosis: report of a case and review of literature. Int J Surg. 2008;6(6):e36–e39. doi: 10.1016/j.ijsu.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Trindade MAB, Miyamoto D, Benard G, Sakai-Valente NY, Vasconcelos DM, Naafs B. Leprosy and tuberculosis co-infection: clinical and immunological report of two cases and review of the literature. Am J Trop Med Hyg. 2013;88(2):236–240. doi: 10.4269/ajtmh.2012.12-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace M, Shameemurahman. Coinfection of two age old diseases. Indian J Community Med. 2011;36(3):228–230. doi: 10.4103/0970-0218.86526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel P, Launois P, Djossou F, Sainte-Marie D, Pradinaud R. American cutaneous leishmaniasis, lepromatous leprosy and pulmonary tuberculosis coinfection with downregulation of the T-helper 1 cell response. Clin Infect Dis. 2003;37(5):628–633. doi: 10.1086/376632. [DOI] [PubMed] [Google Scholar]
- Trindade MAB, Manini MIP, Masetti JH, Leite MA, Takahashi MDF, Naafs B. Leprosy and HIV co-infection in five patients. Lepr Rev. 2005;76(2):162–166. [PubMed] [Google Scholar]
- Lockwood DN, Lambert SM. Human immunodeficiency virus and leprosy: an update. Dermatol Clin. 2011;29(1):125–128. doi: 10.1016/j.det.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Inamadar AC, Sampagavi VV. Concomitant occurrence of leprosy, cutaneous tuberculosis and pulmonary tuberculosis – a case report. Lepr Rev. 1994;65(3):282–284. [PubMed] [Google Scholar]
- Prasad R, Verma SK, Singh R, Hosmane G. Concomitant pulmonary tuberculosis and borderline leprosy with type-II lepra reaction in single patient. Lung India. 2010;27(1):19–23. doi: 10.4103/0970-2113.59263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E, Maverakis E, Konia T, Noll E. Lepromatous leprosy in a 26 year-old man with concurrent disseminated tuberculosis. Arch Dermatol. 2012;148(9):1096–1097. doi: 10.1001/archdermatol.2012.970. [DOI] [PubMed] [Google Scholar]
- Sampaio LH, Stefani MAM, Oliveira RM, Sousa ALM, Ireton GC, Reed SG, Duthie MS. Immunologically reactive M. leprae antigens with relevance to diagnosis and vaccine development. BMC Infect Dis. 2011;11:26. doi: 10.1186/1471-2334-11-26. doi:10.1186/1471-2334-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado CG, Barreto JG. Leprosy transmission: still a challenge. Acta Derm Venereol. 2012;92(3):335. doi: 10.2340/00015555-1278. [DOI] [PubMed] [Google Scholar]
- Negrete V, Ida J, Dillig G, Zohrabian N, Feldman J. Concurrent Hansen disease and pulmonary tuberculosis. J Am Dermatol. 2011;64(5):1001–1003. doi: 10.1016/j.jaad.2009.10.027. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263(5147):678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Pieroni F, Stracieri ABPL, Moraes DA, Paton EJA, Saggioro FP, Barros GM, Barros JC, Oliveira MCB, Coutinho MA, Castro NS, Vigoritto AC, Trabasso P, Souza CA, de Souza MP, Mauad MA, Colturato VAR, Simões BP, Foss NP, Voltarelli JC. Six cases of leprosy associated with allogeneic hematopoietic SCT. Bone Marrow Transpl. 2007;40(9):859–863. doi: 10.1038/sj.bmt.1705824. [DOI] [PubMed] [Google Scholar]
- Shih HC, Hung TW, Lian JD, Tsao SM, Hsieh NK, Yang JH. Leprosy in a renal transplant recipient: a case report and literature review. J Dermatol. 2005;32(8):661–663. doi: 10.1111/j.1346-8138.2005.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Scollard DM, Joyce MP, Gillis TP. Development of leprosy and type 1 leprosy reactions after treatment with infliximab: a report of 2 cases. Clin Infect Dis. 2006;43(2):19–22. doi: 10.1086/505222. [DOI] [PubMed] [Google Scholar]
- Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25(2):173–180. doi: 10.1016/j.clindermatol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Sehgal VN, Verma P, Bhattacharya SN, Sharma S, Singh N, Verma N. Cutaneous tuberculosis: a diagnostic dilemma. Skinmed. 2012;10(1):28–33. [PubMed] [Google Scholar]


