Abstract
Objectives:
The objective of this study was to examine the association between convulsive status epilepticus (CSE) and health-related quality of life (HRQL) during a 24-month follow-up in a multisite incident cohort of children with epilepsy.
Methods:
Data were collected in the Health-Related Quality of Life Study in Children with Epilepsy Study from 374 families of children with newly diagnosed epilepsy. The Quality of Life in Childhood Epilepsy (QOLCE) Questionnaire was used to evaluate parent-reported child HRQL. Hierarchical linear regression was used to examine the relationship between CSE and HRQL at 24 months postepilepsy. A total of 359 families completed the 24-month assessment.
Results:
Twenty-two children (6.1%) had experienced CSE during the follow-up. Children with and without CSE were similar, except a larger proportion of children with CSE had partial seizures (p < 0.001). Controlling for clinical, demographic, and family characteristics, CSE was significantly associated with poorer HRQL (β = −4.65, p = 0.031). The final model explained 47% of the variance in QOLCE scores.
Conclusions:
The findings suggested that not only do children with CSE have significantly poorer HRQL compared with their non-CSE counterparts, but that this factor is independent of the effects of demographic and clinical features known to affect HRQL.
The International League Against Epilepsy1 defines convulsive status epilepticus (CSE) as a convulsion lasting ≥30 minutes or recurrent convulsions occurring over a 30-minute period without recovery of consciousness between convulsions. While there has been much research devoted to understanding the neurologic sequelae and clinical prognosis of CSE,2–6 there is little research on health-related quality of life (HRQL) of CSE in childhood. One study demonstrated that use of rectal diazepam for cluster and prolonged seizures was associated with better quality of life.7 Population-based studies of adults who had epilepsy and CSE in childhood showed no association with educational attainment, employment status, and income compared with those who did not have CSE.3,8 These studies shed light on the social functioning aspect of HRQL. It is important to extend assessments to encompass the multidimensionality that is central to HRQL, which is composed of 4 domains: disease state/physical symptoms, functional status, psychological functioning, and social functioning.9
Assessing HRQL in children who have had CSE may enhance understanding of the impact CSE and its treatments have on children with epilepsy, allowing for more informed medical decisions. Regulatory agencies now advocate for the evaluation of patient-reported outcomes, including HRQL,10 and it is well established that a primary goal in managing childhood epilepsy is to optimize HRQL.11 Thus, the objective of this study was to examine the association between CSE and HRQL during a 24-month follow-up in an incident cohort of children newly diagnosed with epilepsy. It was hypothesized that CSE would have a negative impact on HRQL of children.
METHODS
Participants.
The data were collected in the Health-Related Quality of Life Study in Children with Epilepsy Study (HERQULES),12 a multisite cohort study of children 4 to 12 years of age newly diagnosed with epilepsy in Canada. Pediatric neurologists (n = 53) identified eligible children and their families within their practices over a 36-month period. Inclusion criteria were children 4 to 12 years of age with new-onset epilepsy, defined as at least 2 unprovoked seizures, in whom diagnosis had not been confirmed previously, seen for the first time by a pediatric neurologist, and had a parent with sufficient English language skills who was primarily responsible for the child's care for at least 6 months. Children with major comorbid, nonneurologic conditions known to affect HRQL (e.g., asthma requiring daily medication) were excluded.
Standard protocol approvals, registrations, and patient consents.
Parents received a letter of information describing the study and requirements of participation. Parents and neurologists completed 4 questionnaires at postdiagnosis (baseline), and 6, 12, and 24 months. The study protocol received approval from all relevant research ethics boards.
Measures.
Parent report.
Children's HRQL was measured using the 76-item, parent-report, Quality of Life in Children with Epilepsy (QOLCE) Questionnaire.13 The total HRQL score is an unweighted average of the 16 QOLCE subscales (range: 0–100), with higher scores indicating better HRQL. The QOLCE Questionnaire has been found to be valid and reliable13 with a standard error of measurement of 4.01.12 The standard error of measurement of HRQL scales can be interpreted as an indicator of the minimal clinically important difference.14
Parent depression was measured with the Center for Epidemiologic Studies Depression Scale, a 20-item scale that assesses symptoms of depression over the past week.15 Higher scores indicate greater impairment (range: 0–60).
Level of family functioning was measured using the Family Adaptability, Partnership, Growth, Affection, and Resolve, which assesses satisfaction with family relationships.16 It is a valid and reliable, 5-item, 5-point Likert response scale whereby higher scores indicate greater satisfaction (range: 0–20).16
Family demands were measured using the Family Inventory of Life Events and Changes (71 items), which assesses life events experienced by a family during the previous year.17 Higher scores indicate more demands (range: 0–71), and reliability and validity are well established.17 Internal consistency reliabilities for all parent-reported measures have been reported previously.12
Neurologist report.
Neurologists reported whether children had experienced CSE (no/yes), either with the onset of their epilepsy or at any time in the subsequent course, based on standard clinical practice, which typically applies a 30-minute duration as the definition of CSE and has been used in recent investigations.
Severity of epilepsy was assessed with the Global Assessment of Severity of Epilepsy,18 a single-item, 7-point measure used by the neurologists to rate the overall severity at epilepsy outset and at each of child's continuing visits. Lower scores indicate more severe epilepsy. Validity and reliability of the Global Assessment of Severity of Epilepsy has been demonstrated.18
Neurologists also reported on seizure type (generalized; localization-related [partial]; partial onset, secondary generalization; undetermined) and frequency (7-point scale ranging from “not at all frequent” to “extremely frequent” since the last clinic visit), age at onset, and medication information (number of antiepileptic drugs, side effects). Behavior and cognitive problems were assessed clinically and scored by the neurologist using single-item measures as having “severe,” “moderate,” “mild,” or “no problems.” Behavior and cognitive problems were then recoded as either present or absent. Evaluation of child cognitive and behavior problems noted by the neurologist during each visit were not based on any formal diagnostic assessments, unless information was provided by parents or teachers, or neuropsychological records obtained from the school or hospital.
Statistical analysis.
Analysis was done using SAS 9.2 (SAS Institute Inc., Cary, NC). Type I error was set at α = 0.05, and hypothesis tests associated with the effect of CSE on HRQL were 1-sided given that it is unlikely that CSE would improve HRQL.3 Baseline comparisons of children with and without CSE who completed the 24-month follow-up were made using nonparametric methods: Wilcoxon-Mann-Whitney and χ2 tests. For comparisons within these 2 groups (with and without CSE) over time between baseline and 24 months, McNemar tests were used for categorical variables and Wilcoxon signed rank sum and paired t tests were used for continuous variables.
To investigate the main objective of this study, hierarchical, multiple regression was used to quantify the effect of CSE on HRQL at 24 months, controlling for potential confounding factors. Using this approach, unique sets of confounders were sequentially added to the initial unadjusted model. The order of sets of confounders to be added to the model was determined a priori: (1) child demographics (age, sex); (2) clinical characteristics (severity of epilepsy, seizure type, age at onset, number of antiepileptic drugs, side effects of antiepileptic drugs); (3) comorbid conditions (behavior, cognitive problems); and (4) family factors (parent depression, family functioning, and family demands). Multiple imputation was used to account for data assumed to be missing at random.19 Estimates from 5 imputed datasets were combined to obtain unbiased estimates of effect and presented.
RESULTS
Sample characteristics.
Of the 456 eligible children, 374 parents (82%) returned completed postdiagnosis questionnaires. A total of 359 (96%) completed the 24-month questionnaire and 283 (62%) completed all 4 questionnaires. Among children who had CSE, there were no clinical or demographic characteristics associated with missing data. However, among children who did not have CSE, those with missing data were more likely to have cognitive problems (odds ratio [OR] = 1.95, p = 0.0126), parents with elevated symptoms of depression (OR = 1.03, p = 0.022), and families with elevated demands (OR = 1.04, p = 0.030) compared to those with complete data.
Seventeen of 374 children (4.6%) had experienced CSE by the time of the baseline assessment and 5 children additionally experienced CSE within the following 24 months. Overall, 22 of 359 (6.1%) had at least one episode of CSE during the first 24 months of their epilepsy onset. A comparison of baseline clinical and demographic characteristics of children who completed the 24-month follow-up is shown in table 1. There were no significant differences between children with and without CSE regarding clinical, demographic, or family characteristics, with one exception. There was a larger proportion of children with CSE who had partial seizures compared to children without CSE at baseline.
Table 1.
Baseline clinical and demographic characteristics for children completing the 24-month follow-up
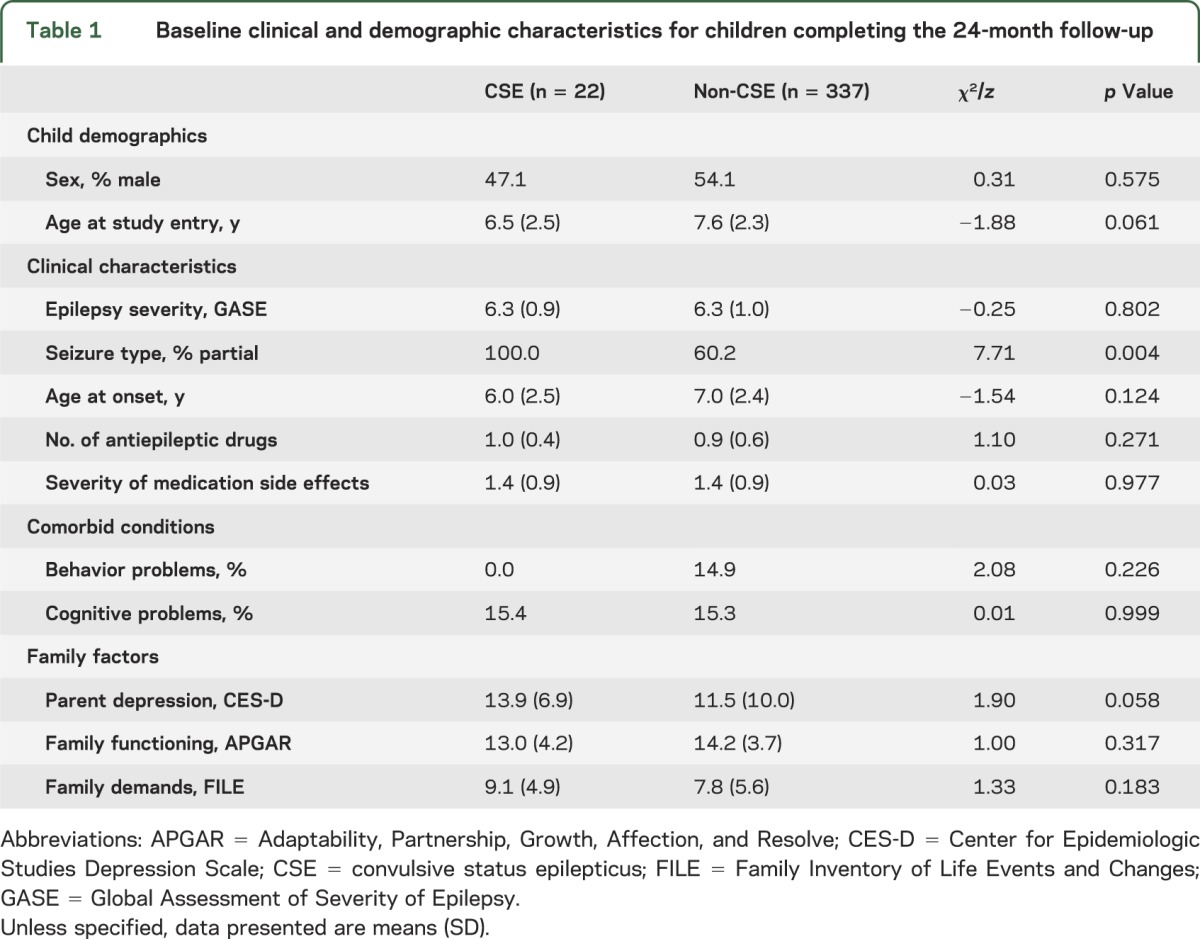
Among children with CSE, clinical and demographic characteristics were relatively stable between assessments, but significant differences were observed for severity of epilepsy and proportion of cognitive problems. Whereas severity of epilepsy lessened from baseline to 24 months (p = 0.008), the proportion of children with CSE and comorbid cognitive problems increased markedly (p = 0.031). In contrast, children without CSE showed improvement in a number of child and family characteristics: HRQL (p < 0.001), severity of epilepsy (p < 0.001), and parent depression (p = 0.016). Co-occurring with these improvements were increases in the number of antiepileptic drugs prescribed (p = 0.001), proportion of children with behavior and cognitive problems (p < 0.001 for both), as well as more family demands (p = 0.002).
Modeling the impact of CSE on HRQL at 24 months.
Each step of the hierarchical multiple regression model is presented in table 2. In the unadjusted model, CSE had a significant negative effect on HRQL at 24 months. In other words, HRQL was significantly poorer among children who had experienced CSE during the 24-month follow-up compared with children who did not have CSE. In the second step, children's demographic characteristics were included in the model. Whereas children's demographic characteristics had no impact on HRQL, CSE remained. In the third step, clinical characteristics were added to the model and more severe epilepsy was found to be associated with poorer HRQL. The effect of CSE continued to be significant and, in fact, its magnitude of effect increased slightly with the addition of clinical characteristics to the model. In the fourth step, comorbid conditions (behavior and cognitive problems) were added to the model and found to be significantly associated with HRQL. While the effect estimate for CSE was reduced, CSE remained a significant risk factor for HRQL. In the fifth step, family factors were added to the model and parent depression, family functioning, and family demands were all found to be significantly associated with HRQL. In this final model, CSE remained significantly associated with HRQL. The model fit the data well with an adjusted R2 = 0.47 and F13,360 = 25.52 (p < 0.001). Results of the regression model are illustrated in the figure.
Table 2.
Hierarchical regression of the influence of CSE on health-related quality of life
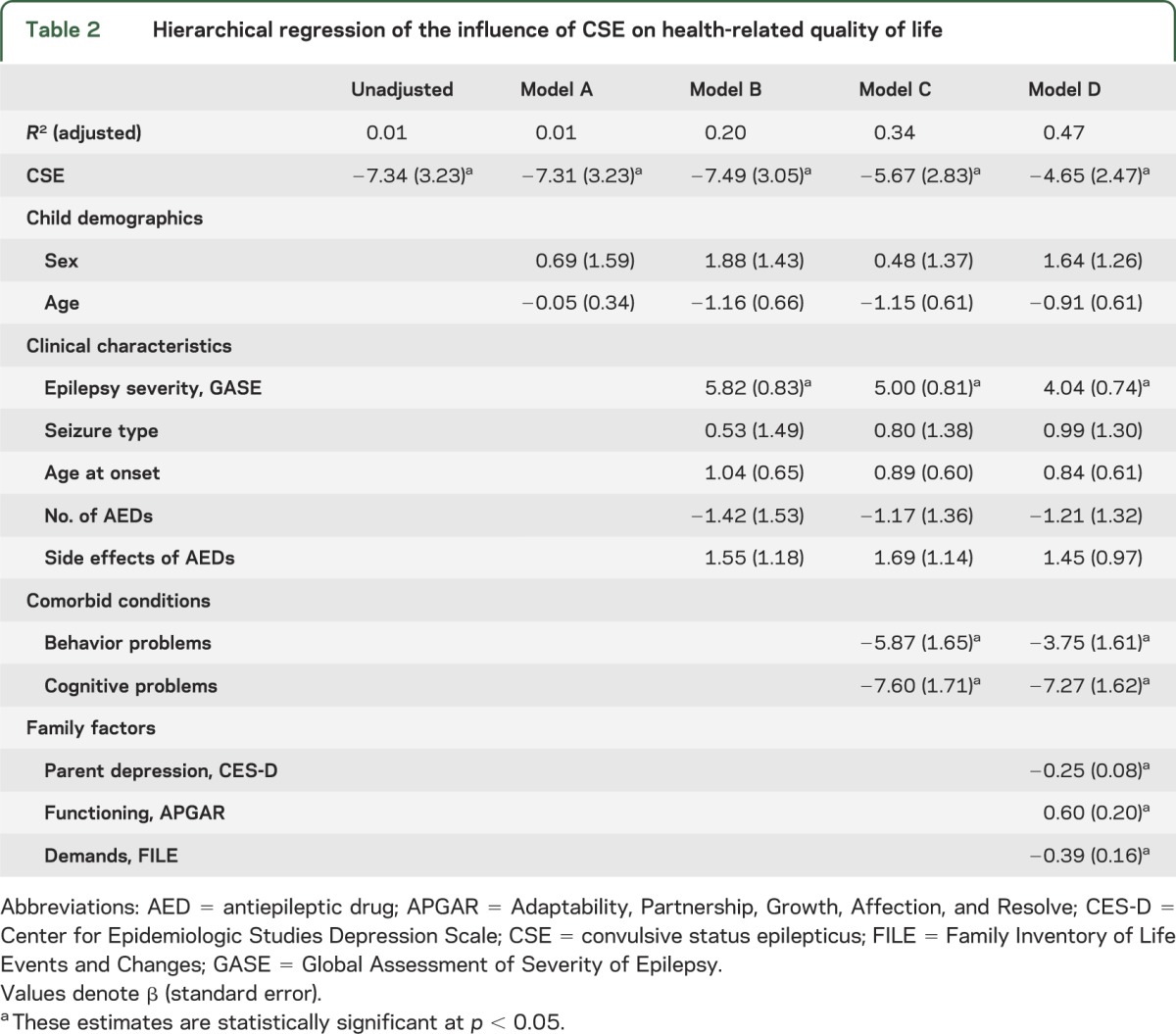
Figure. Impact of CSE on health-related quality of life.
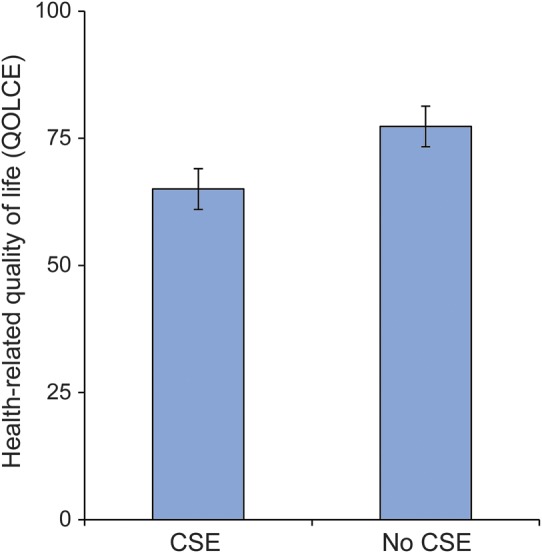
Convulsive status epilepticus (CSE) was coded as absent or present. The error bars represent the 95% confidence intervals surrounding the Quality of Life in Childhood Epilepsy (QOLCE) Questionnaire scores controlling for the confounders described in table 2.
DISCUSSION
Because there is little research on the effect of CSE on HRQL in children with epilepsy, particularly in the first 2 years after diagnosis, it is difficult to place the current findings in the context of what is known in this field. An added challenge is that previous studies in pediatric epilepsy have not investigated HRQL as a multidimensional construct,9 but instead examined particular components of HRQL (i.e., behavior, cognition, and disease state/symptoms). In most studies, CSE appeared to have a negative effect on developmental cognitive outcomes that were assessed,2,4 on average, 1 to 3 years since the diagnosis of epilepsy. For inclusiveness, we conducted post hoc analyses, both unadjusted and adjusted, of the relationship between CSE and behavior and cognitive problems in our sample of children with epilepsy. Our findings did not suggest an association between CSE and behavior or cognitive problems at 24 months (data not shown). These discrepant findings are likely attributable to the differences in the patient profiles and assessment of cognitive outcomes.
Of particular importance is the finding that not only do children with CSE have significantly poorer HRQL compared with their non-CSE counterparts, but that this factor is independent of the effects of demographic and clinical features known to affect HRQL. Our study adds CSE to the list of robust clinical and family risk factors for HRQL in pediatric epilepsy.12,20–22 The hierarchical model presented may have clinical utility in aiding health care professionals to identify which children may be at risk for compromised HRQL, thus potentially informing medical treatment decisions in the context of family-centered care.
While this research contributes to our understanding of the impact of CSE on HRQL in pediatric epilepsy, important questions remain unanswered. It is unknown whether children with CSE will rebound and subsequently report similar HRQL to their counterparts without CSE. Notably, a population-based, long-term prospective study of adults with childhood-onset epilepsy demonstrated no significant differences in educational attainment and employment, marital, and socioeconomic status between those who had CSE in childhood and those who did not.3 That study excluded children with subnormal IQ, however. Because CSE, low IQ, and poor psychosocial outcomes are all associated with remote symptomatic etiology, excluding children with low IQ may have truncated the range over which a true association between CSE and these outcomes may be observed.
It is also noteworthy that similar to previous studies of CSE in children with epilepsy, long-term follow-up studies of adults diagnosed with epilepsy as children are limited by their focus on important qualitative markers of social and educational outcomes to provide insight into the setting and extent of these problems. However, other important quantitative components of HRQL (e.g., disease state/symptoms, psychological functioning) that fall within the spheres of influence of health care professionals and health care systems9 have not been investigated at 2 years postdiagnosis. Long-term prospective studies have suggested little or no association between CSE and individual domains of HRQL such as social outcomes (e.g., educational attainment, employment) and disease state/symptoms (e.g., relapse).3,8,23 In contrast, one study demonstrated that CSE was associated with neurodevelopmental impairments in children with epilepsy at 6 weeks.4 This contrasting evidence indicates that the influence of CSE on HRQL may be quite different within 2 years postdiagnosis vs more than 2 years and warrants further investigation to determine when during the course of childhood epilepsy the effect of CSE on HRQL dissipates. In addition, it is important to address whether a dose-response gradient is present between CSE and HRQL after repeated episodes of CSE. Such gaps in the knowledge base can only be addressed with further longitudinal research that uses robust epidemiologic methodologies.
The following aspects of our study are noteworthy. First, the HERQULES sample, with its large population-based cohort of incident cases of pediatric epilepsy with very good response and retention rates and minimal missing data, offers a unique opportunity to address the clinical association of CSE and HRQL. Second, the incidence of 6.1% for CSE in HERQULES was similar to previous research in children newly diagnosed with epilepsy.24 Third, HRQL was measured using the QOLCE Questionnaire—a valid and comprehensive instrument that is psychometrically sound and widely used. Fourth, an extensive set of confounding variables was measured and adjusted for in the analysis to minimize bias and produce accurate estimates of effect.
Notwithstanding these strengths, there are some limitations of the current study. First, the number of children with CSE was relatively small, potentially reducing the external validity of the findings. Second, as a result of the small number of children with CSE, we were unable to assess how CSE might influence the change in HRQL over time using more complex analytic techniques or a dose-response effect of recurrent episodes of CSE. Third, assessment of HRQL was limited to parent proxy reports. This may be problematic given that previous research has suggested that parents and children have poor agreement on measures of HRQL and may provide different perspectives on HRQL.25 Fourth, important details surrounding CSE—focal or generalized, whether it occurred once or more than once, and whether it occurred before treatment or while on treatment—were not available in the current study.
CSE has a significant detrimental impact on HRQL, which is apparent at 24 months after epilepsy diagnosis in children. Important unanswered questions remain about the nature of the relationship between CSE and HRQL—Do the effects of CSE continue after 24 months post–epilepsy diagnosis and persist into adulthood? Do recurrent episodes of CSE have an additive effect that further reduces HRQL? Research that addresses these questions will be a valuable contribution to understanding HRQL and improve the lives of children with epilepsy and their families.
ACKNOWLEDGMENT
The authors acknowledge the parents and physicians and their staff, whose participation made this study possible. The Canadian Pediatric Epilepsy Network effectively facilitated the participation of physician collaborators across the country.
GLOSSARY
- CSE
convulsive status epilepticus
- HERQULES
Health-Related Quality of Life Study in Children with Epilepsy Study
- HRQL
health-related quality of life
- OR
odds ratio
- QOLCE
Quality of Life in Children with Epilepsy
AUTHOR CONTRIBUTIONS
M.A. Ferro: data analysis and interpretation, drafting manuscript, revising manuscript for content. R.F.M. Chin: data interpretation, revising manuscript for content. C.S. Camfield and S. Wiebe: study design, obtaining funding, revising manuscript for content. S.D. Levin: study concept and design, obtaining funding, acquisition of data. K.N. Speechley: study concept and design, obtaining funding, study supervision, interpretation of data, revising manuscript for content. All authors gave final approval of the manuscript as submitted. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
STUDY FUNDING
HERQULES was funded by Canadian Institutes for Health Research (MOP-64311) to K.N. Speechley (principal investigator), C.S. Camfield, S.D. Levin, M.L. Smith, S. Wiebe, and G.Y. Zou. Research Early Career Award from Hamilton Health Sciences was awarded to M.A. Ferro. The funding source had no role in the conduct of the study or preparation of the manuscript.
DISCLOSURE
M. Ferro reports no disclosures relevant to the manuscript. R. Chin has received travel grants and speaker fees from Special Products Limited and ViroPharma, manufacturers of buccal midazolam. C. Camfield, S. Wiebe, S. Levin, and K. Speechley report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 1993;34:592–596 [DOI] [PubMed] [Google Scholar]
- 2.Singhi PD, Bansal U, Singhi S, Pershad D. Determinants of IQ profile in children with idiopathic generalized epilepsy. Epilepsia 1992;33:1106–1114 [DOI] [PubMed] [Google Scholar]
- 3.Camfield P, Camfield C. Unprovoked status epilepticus: the prognosis for otherwise normal children with focal epilepsy. Pediatrics 2012;130:e501–e506 [DOI] [PubMed] [Google Scholar]
- 4.Martinos MM, Yoong M, Patil S, et al. Early developmental outcomes in children following convulsive status epilepticus: a longitudinal study. Epilepsia 2013;54:1012–1019 [DOI] [PubMed] [Google Scholar]
- 5.Chin RF, Neville BG, Peckham C, et al. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet 2006;368:222–229 [DOI] [PubMed] [Google Scholar]
- 6.Pujar SS, Neville BG, Scott RC, Chin RF; North London Epilepsy Research Network. Death within 8 years after childhood convulsive status epilepticus: a population-based study. Brain 2011;134:2819–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriel RL, Cloyd JC, Hadsall RS, Carlson AM, Floren KL, Jones-Saete CM. Home use of rectal diazepam for cluster and prolonged seizures: efficacy, adverse reactions, quality of life, and cost analysis. Pediatr Neurol 1991;7:13–17 [DOI] [PubMed] [Google Scholar]
- 8.Sillanpaa M, Shinnar S. Status epilepticus in a population-based cohort with childhood-onset epilepsy in Finland. Ann Neurol 2002;52:303–310 [DOI] [PubMed] [Google Scholar]
- 9.Spieth LE, Harris CV. Assessment of health-related quality of life in children and adolescents: an integrative review. J Pediatr Psychol 1996;21:175–193 [DOI] [PubMed] [Google Scholar]
- 10.The PCORI methodology report [online]. Available at: http://www.pcori.org/research-we-support/research-methodology-standards. Accessed December 10, 2013
- 11.Schachter SC. Epilepsy: quality of life and cost of care. Epilepsy Behav 2000;1:120–127 [DOI] [PubMed] [Google Scholar]
- 12.Speechley KN, Ferro MA, Camfield CS, et al. Quality of life in children with new-onset epilepsy: a 2-year prospective cohort study. Neurology 2012;79:1548–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabaz M, Lawson JA, Cairns DR, et al. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav 2003;4:680–691 [DOI] [PubMed] [Google Scholar]
- 14.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861–873 [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 16.Smilkstein G. The family APGAR: a proposal for a family function test and its use by physicians. J Fam Pract 1978;6:1231–1239 [PubMed] [Google Scholar]
- 17.McCubbin HI, Thompson AI, McCubbin MA. FILE: Family Inventory of Life Events and Changes. Family Assessment: Resiliency, Coping and Adaptation Inventories for Research and Practice. Madison: University of Wisconsin Publishers; 1996 [Google Scholar]
- 18.Speechley KN, Sang X, Levin S, et al. Assessing severity of epilepsy in children: preliminary evidence of validity and reliability of a single-item scale. Epilepsy Behav 2008;13:337–342 [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987 [Google Scholar]
- 20.Ferro MA, Landgraf JM, Speechley KN. Factor structure of the Child Health Questionnaire Parent Form-50 and predictors of health-related quality of life in children with epilepsy. Qual Life Res 2013;22:2201–2211 [DOI] [PubMed] [Google Scholar]
- 21.Austin JK, Perkins SM, Johnson CS, et al. Self-esteem and symptoms of depression in children with seizures: relationships with neuropsychological functioning and family variables over time. Epilepsia 2010;51:2074–2083 [DOI] [PubMed] [Google Scholar]
- 22.Rodenburg R, Meijer AM, Dekovic M, Aldenkamp AP. Family factors and psychopathology in children with epilepsy: a literature review. Epilepsy Behav 2005;6:488–503 [DOI] [PubMed] [Google Scholar]
- 23.Berg AT, Shinnar S, Levy SR, et al. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia 2001;42:1553–1562 [DOI] [PubMed] [Google Scholar]
- 24.Berg AT, Shinnar S, Levy SR, Testa FM. Status epilepticus in children with newly diagnosed epilepsy. Ann Neurol 1999;45:618–623 [DOI] [PubMed] [Google Scholar]
- 25.Baca CB, Vickrey BG, Hays RD, Vassar SD, Berg AT. Differences in child versus parent reports of the child's health-related quality of life in children with epilepsy and healthy siblings. Value Health 2010;13:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]


