Abstract
Visual hallucinations are common symptoms of seizures affecting primary and association cortices, and can provide vital information about the ictal onset zone. Epileptic kinetopsia, defined as illusionary movement of stationary objects in the visual field, was reported in a patient with a tumor in the temporal-parietal-occipital (TPO) junction. Intracranial stimulation of TPO junction did not evoke kinetopsia and the site of onset of this phenomenon is unknown.1 We describe a patient with ictal kinetopsia whose seizure onset zone was localized with intracranial EEG.
Visual hallucinations are common symptoms of seizures affecting primary and association cortices, and can provide vital information about the ictal onset zone. Epileptic kinetopsia, defined as illusionary movement of stationary objects in the visual field, was reported in a patient with a tumor in the temporal-parietal-occipital (TPO) junction. Intracranial stimulation of TPO junction did not evoke kinetopsia and the site of onset of this phenomenon is unknown.1 We describe a patient with ictal kinetopsia whose seizure onset zone was localized with intracranial EEG.
Case report.
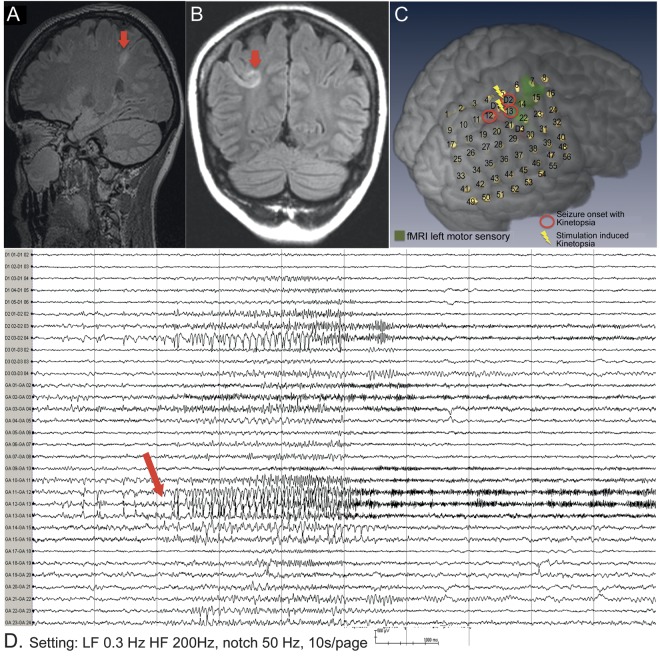
A 33-year-old right-handed woman had had pharmacoresistant focal epilepsy since age 15 months. Her habitual seizures started at age 16 years, characterized by visual perception of stationary objects located on her right side shift to the center or to the left. During these episodes, she described difficulties differentiating near and far objects, without blurring, double vision, scotomas, headache, or light hypersensitivity. This progressed to forceful blinking, tingling sensation of the left cheek and arm, followed by left arm posturing and left foot-jerking movements with preserved awareness. Postictally, visual field and color vision were normal. Scalp EEG revealed interictal epileptiform spikes or polyspikes over right centroparietal region (CP2, CP4, C4>P4, CZ, F4). Brain MRI showed area of cortical dysplasia in the right superior parietal lobule (SPL) and intraparietal sulcus (IPS) with cortical thickening on T1 and fluid-attenuated inversion recovery signal change in the underlying white matter (figure 1, A and B).
Figure 1. Cortical dysplasia of superior parietal cortex and intracranial EEG during seizure with kinetopsia.
MRI brain sagittal (A) and coronal (B) fluid-attenuated inversion recovery images show focal cortical dysplasia (arrows). (C) Multimodal imaging reconstruction with position of subdural grid and depth electrodes. (D) Intracranial EEG of seizure with kinetopsia (bipolar montage, selected channels include depths 1 and 2 [D1, D2] and subdural grid GA 1–24). EEG seizure onset shows increased rapid spikes, maximum GA12, GA13 (arrow), followed by high-frequency fast activity.
Intracranial EEG recording and stimulation.
Intracranial EEG was performed to determine the ictal onset zone and map sensory and motor functions. The patient underwent implantation of 8 × 7 contact subdural grid (10-mm spacing, AD-Tech, Watertown, WI) covering the area of cortical dysplasia and precentral and postcentral gyrus. Three depth electrodes were placed targeting the lesion (anteriorly D1, 1 × 6 contacts 5-mm spaced), posteriorly (D2, 1 × 4 contacts 5-mm spaced), and inferiorly (D3, 1 × 4 contacts 10-mm spaced) (figure 1C). Interictally, frequent spikes were seen in the SPL and IPS. We recorded 51 seizures with kinetopsia at the onset, progressing to bilateral eye blinking and sometimes left hand-tingling sensation. In the postictal period, visual fields, language, and motor skills were normal. Ictal EEG showed stereotypical fast activity in right SPL and IPS (electrode contacts GA12 > GA13 > D2, ∼70 Hz) (figure 1D). Bipolar electrocortical stimulation of contact GA 13 (against distant electrode GA 53) evoked kinetopsia time locked to the electrical stimulus (figure e-1 on the Neurology® Web site at Neurology.org); following stimulation, habitual interictal spikes returned on GA 12, 13, and D2. Resection was carried out at the posterior parietal cortex and pathology revealed Taylor type IIb focal cortical dysplasia. Postoperatively, there were no visual deficits. Transient difficulties to reach for objects with left hand and turning around while walking was noted; this was resolved within a few days. The patient has been seizure-free for 18 months.
Discussion.
Parietal lobe seizures may present with distortions of sensory perception, especially visual illusions,2 as seen in our patient. We elicited kinetopsia with cortical stimulation of contacts in the SPL and IPS regions, time locked to the electrical cortical stimulation and without causing afterdischarges or seizures. This is suggestive of the symptom arising from the site of stimulation. The presence of dysplastic cortex in the IPS raises the possibility that there is atypical localization of kinetopsia in this case; however, it has been shown that dysplastic cortex may bear function.
fMRI studies in normal brains have demonstrated extensive topographic representation of the contralateral visual field in the posterior parietal cortex (Brodmann area 7) fundamental for spatial information processing.3 Seven topographically organized visuospatial association areas are localized in the IPS and SPL.4 Epileptic discharges from or involving these regions can cause distortion of visuospatial perception. We suggest that the mechanism for ictal kinetopsia may involve interhemispheric competition for visual perception. The parietal visual association cortices process information from both visual fields. Inhibitory connections between the left and right cortices reduce the amount of ipsilateral processing performed in each hemisphere.5,6 During interhemispheric competition, synchronized activation of neural assemblies in the cortex causes inhibition of contralateral homologous regions.7 Focal synchronized neuronal activation at seizure onset from these brain regions can augment inhibition of homologous contralateral cortex. In our patient, seizure onset from right parietal cortex may have inhibited visuospatial processing in the homologous contralateral cortex, resulting in illusionary movement of objects in the visual field toward the left. Since we recorded only from right parietal cortex, however, we cannot conclusively prove this hypothesis.
Ictal visual phenomena can provide useful localizing information about the seizure onset or symptomatogenic zone. Seizure onset in SPL and IPS caused kinetopsia and direction of illusionary movement lateralized to the contralateral hemisphere.
Supplementary Material
Footnotes
Supplemental data at Neurology.org
Author contributions: M.B. Perumal: conceptualization, interpretation, and preparation of manuscript. S. Chinnasami: conceptualization, data collection, interpretation of investigations, and preparation of manuscript. Dr. Shah: clinical assessments and preparation of manuscripts. Dr. Rodionov: data collection, brain image reconstruction, and analysis. V. Maglajlija: EEG data collection, analysis, and reporting. Dr. Miserocchi: intracranial placement of electrodes, clinical assessment, and data collection. Dr. McEvoy: intracranial placement of electrodes, investigations, and interpretation of data. Dr. Wehner: conceptualization, interpretation of data, and review of manuscript. Dr. Diehl: conceptualization, clinical assessment, interpretation of investigations, and review of manuscript.
Study funding: This work was undertaken at UCLH/UCL, which receives a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Laff R, Mesad S, Devinsky O. Epileptic kinetopsia: ictal illusory motion perception. Neurology 2003;61:1262–1264 [DOI] [PubMed] [Google Scholar]
- 2.Salanova V. Parietal lobe epilepsy. J Clin Neurophysiol 2012;29:392–396 [DOI] [PubMed] [Google Scholar]
- 3.Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci 2009;13:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 2001;294:1350–1354 [DOI] [PubMed] [Google Scholar]
- 5.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci 1999;354:1325–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. Systematic analysis of deficits in visual attention. J Exp Psychol 1999;128:450–478 [DOI] [PubMed] [Google Scholar]
- 7.Battelli L, Alvarez GA, Carlson T, Pascual-Leone A. The role of the parietal lobe in visual extinction studied with transcranial magnetic stimulation. Cogn Neurosci 2009;21:1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.