Abstract
CONTEXT:
Cancer survival has improved significantly and maintaining fertility is both a major concern and an important factor for the quality of life in cancer patients.
AIMS:
To explore differences in oocyte stimulation for fertility preservation (FP) patients based on cancer diagnosis.
SETTINGS AND DEIGN:
Between 2005 and 2011, 109 patients elected to pursue FP at a single institution.
MATERIALS AND METHOD:
In vitro fertilization (IVF) outcome variables between four cancer diagnostic groups (breast, gynecologic, lymphoma/leukemia and other) and age-matched male factor or tubal factor infertility IVF control group were compared.
STATISTICAL ANALYSIS:
ANOVA and Chi-square analyses were employed to compare variables between the groups that were normally distributed. Kruskal–Wallis with subsequent Mann–Whitney U-test were used for data that were not normally distributed.
RESULTS:
Women with gynecologic malignancies were significantly older than the women in the other three groups, but tended to have a better ovarian response. Women with hematologic malignancies were most likely to have been exposed to chemotherapy and had the longest stimulations with a similar number of oocytes retrieved. The age-matched IVF controls had higher peak estradiol levels, number of oocytes obtained, and fertilization rates when compared to cancer patients with or without a history of prior chemotherapy.
CONCLUSIONS:
Factors including age, type of cancer and chemotherapy exposure, can influence response to ovarian stimulation. Discussing these findings with patients presenting for FP may aid in setting realistic treatment expectations.
KEY WORDS: Cancer, cryopreservation, fertility preservation, in vitro fertilization, oncofertility
INTRODUCTION
An estimated 62,000 women between the ages of 20 and 39 years are expected to be diagnosed with invasive cancer in the United States each year.[1] Cancer survival has improved significantly and maintaining fertility is both a major concern and an important factor for the quality of life in cancer patients.[2,3] Web surveys have found that future fertility is a major concern for young women diagnosed with cancer.[4] Further, informed decision making regarding future fertility has been shown to decrease the patient regret and improve the quality of life.[4] Therefore, a discussion about how cancer treatment can affect fertility, as well as potential ways to preserve fertility should be an integral component of all comprehensive cancer care programs.
There are several fertility preservation (FP) strategies for women undergoing potentially gonadotoxic therapy.[5,6] One option is to undergo a cycle of controlled ovarian hyperstimulation (COH) with subsequent oocyte harvest for oocyte or embryo cryopreservation. Several investigators have reported their results of cancer patients undergoing COH.[7,8,9,10,11,12,13] Quintero et al. compared in vitro fertilization (IVF) cycle data among 32 women who underwent IVF prior to cancer treatment with 21 age-matched male factor or tubal factor infertility IVF controls.[13] They found no differences in the total amount of medication used for ovarian stimulation and number of oocytes retrieved between the two groups.[13] In a subsequent study, these authors found no significant differences between 50 women with cancer and 50 age-matched controls in terms of number of oocytes retrieved and the number of oocytes fertilized.[7] The authors noted, however, that the cancer patients required longer stimulation and greater amounts of medication.[7] Similarly, Knopman et al. reported that among 27 women with breast, uterine, ovarian cancer and Hodgkins lymphoma there were no significant differences with regards to follicle stimulating hormone (FSH), peak estradiol and number of eggs retrieved between the women with cancer and age-matched control group.[9] However, Robertson et al. found significantly lower peak estradiol in the FP patients, but no different in a number of oocytes obtained or embryos created comparing FP and age-matched control patients. Robertson et al. further noted that there were no significant differences in women with localized cancers versus women with systemic disease.[12] In contrast, Klock et al. reported significant differences in peak estradiol, number of oocytes retrieved and cancellation rates between FP and age-matched control patients, but no difference in a number of zygotes created.[14] In addition, women with hormonally sensitive cancers have been shown to have lower estradiol levels, and decreased oocytes retrieved when compared to noncancer patients.[15] Based on these studies it appears that many women with cancer have sufficient response to COH to undergo a successful oocyte harvest, although not necessarily equivalent to age-matched patients undergoing IVF for infertility. Other options for FP include ovarian tissue cryopreservation (OTC), gonadotropin-releasing hormone analog (GnRH) analog treatment prior to chemotherapy, and in vitro oocyte maturation. It is also possible to combine methods.[16,17]
Previous studies of FP have typically grouped many different cancer types together thus limiting the exploration of cancer type-specific responses to COH. Almog et al. explored the differences in ovarian response by specific cancer diagnosis but did not note any differences in the 80 patients studied.[18] We also have reported results of a pilot study demonstrating ovarian stimulation differences among patients with various cancer diagnosis, but these preliminary findings were limited due to the small sample size.[19]
Grouping all malignancies into one cohort may limit the ability to decipher disease specific differences in patients presenting for FP. In addition to the problematic nature of grouping malignancies into one cohort, several studies have excluded women with prior chemotherapy or radiation exposure, despite the potential detrimental effect of such treatments on COH outcomes.[7,12,13] Age is the strongest predictor of IVF success.[20] Chemotherapy has been established to decrease ovarian reserve and response to gonadotropins.[21,22] The purpose of the current study was to explore differences in IVF stimulation characteristics and outcomes based on cancer diagnosis. The second aim of the study was to explore potential differences among women with cancer who have previously been exposed to chemotherapy, women with cancer who had not been exposed to chemotherapy and age-matched male or tubal factor IVF controls. We hypothesize that only age and prior exposure to chemotherapy, and not the type of cancer, would affect stimulation characteristics.
MATERIALS AND METHOD
Fertility preservation program
In 2005, a comprehensive FP program to systematically provide female cancer patients with access to appropriate counseling and a full range of FP options was instituted at our institution. Following a referral from an oncologist, patients undergo an initial consultation with a reproductive endocrinologist. This initial appointment is facilitated by a patient navigator to ensure that these patients are seen quickly. Details of this initial meeting are described elsewhere.[14] Patients are counseled on options available to preserve fertility, which, depending on their particular condition, may include ovarian stimulation with subsequent oocyte harvest for cryopreservation of oocytes or embryos. Patients are routinely offered ovarian reserve testing as well as psychological counseling.
Subjects
Between 2005 and 2011, a total of 334 female patients contacted the FP patient navigator at a single institution. Of the 334 patients, 35 were medically ineligible 124 patients initially elected to pursue FP and are the focus of this study [Figure 1]. Of the 124 patients, 109 began a cycle of COH. Within this group, four were cancelled because of lack of response to gonadotropins and one patient chose to undergo both oocyte cryopreservation as well as OTC. During this same period, 176 age-matched pure male factor or tubal factor infertility controls undergoing their first IVF cycle using an antagonist protocol were identified.
Figure 1.
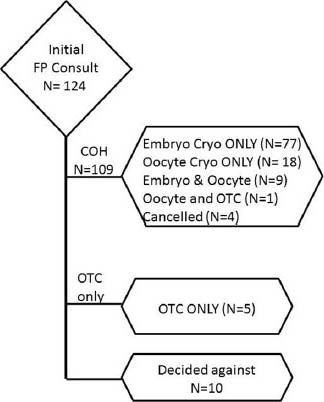
Flow diagram of patients counseled regarding fertility preservation
Procedure
This was a retrospective cohort study. The following information was retrieved from the patients’ medical record: Demographics, pertinent medical, surgical and obstetrics and gynecology history, cancer diagnosis, previous cancer treatment, tobacco use, partner status, ovarian reserve markers, body mass index (BMI), gonadotropin type, dosage and duration and the following COH outcomes measures: Number of mature follicles on ultrasound, stimulation day of antagonist start, peak serum estradiol, number and classification of oocytes retrieved and the number of zygotes created for cryopreservation. For this study, leukemia and lymphoma patients were combined into a single hematologic cancer group due to the similarity in age, a high percentage of chemotherapy exposure, and limited sample size. Prior to combining these patients into one group, demographic, stimulation characteristics and exposure to chemotherapy were examined and found to be similar (data not shown).
Controlled ovarian hyperstimulation
Our protocol has been previously described.[14,23] Briefly, using a GnRH antagonist protocol, COH by injection of recombinant FSH with or without urinary menotropins or recombinant LH was started on the 3rd day of menstruation; the starting dose was decided according to the patient's age and ovarian reserve. When the leading follicle reached 11-12 mm in diameter, patients began to receive a daily injection of 250 μg of ganirelix. Final follicular maturation was triggered by the administration of 250 μg of choriogonadotropin alfa injection when at least three follicles were ≥ 16 mm.
Oocyte retrieval
Transvaginal oocyte retrieval was performed 36 h after choriogonadotropin alfa injection. Oocytes were inseminated 4-6 h after retrieval by co-culture with motile sperm (n = 40 cycles) or by intracytoplasmic sperm injection (n = 61 cycles), depending on semen quality. Fertilization was assessed 15-18 h after insemination. Embryos were cryopreserved at the zygote stage using a slow-freeze protocol. Oocyte cryopreservation was accomplished using either a slow-freeze protocol or vitrification.
Statistical analysis
The data were summarized and ANOVA and Chi-square analyses were employed to compare variables between the groups that were normally distributed. Kruskal–Wallis with subsequent Mann–Whitney U-test was used for data that were not normally distributed. Post hoc testing to adjust for multiple comparisons was not conducted due to the exploratory nature of this study. Analyses are based on available data, sample sizes are provided, and P < 0.05 (two-tailed) was considered to be statistically significant. All statistics were done using SPSS (IBM, Armonk, New York, US). The Institutional Review Board approved the study.
RESULTS
Fertility preservation patient cohort
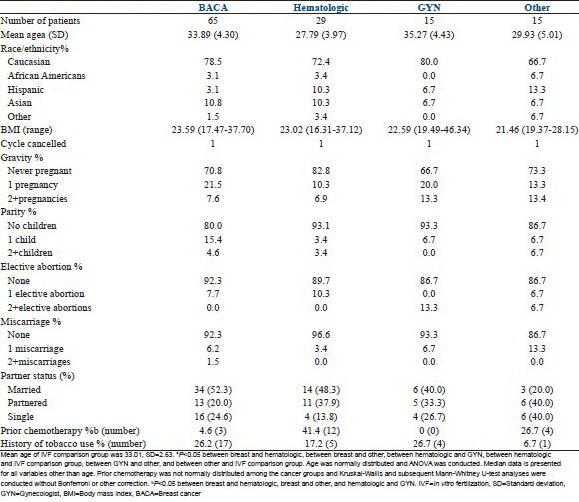
One hundred and twenty-four women initially sought an FP consult (COH cohort). Their demographic data is summarized by cancer diagnosis in supplementary Table 1. Treatment choice of these women is outlined in Figure 1. Briefly, 65 patients had breast cancer, 29 patients had hematologic cancers, 15 patients had gynecologic cancers, and 15 had patients other types of cancers (brain, colon/colorectal, sarcoma, stomach, tonsil, Wilms’ tumor). Within the hematologic malignancies, similar number of patients with prior chemotherapy had a diagnosis of lymphoma (n = 6/20) versus leukemia (n = 6/9). Of note, women with a hematologic malignancy ( = 27.8 ± 4.0 year) were significantly younger than those diagnosed with breast (
= 27.8 ± 4.0 year) were significantly younger than those diagnosed with breast ( =33.7 ± 4.2 year) or gynecologic (
=33.7 ± 4.2 year) or gynecologic ( = 35.2 ± 4.8 year) malignancies. There were significantly more patients in the hematologic group (n = 12) compared to the breast (n = 2) and gynecologic group (n = 0) with a history of prior chemotherapy (P < 0.05). Further, more women in the other group (n = 4/14) had a past history of chemo than women in the BRCA group (n = 2/60). Patients with breast (
= 35.2 ± 4.8 year) malignancies. There were significantly more patients in the hematologic group (n = 12) compared to the breast (n = 2) and gynecologic group (n = 0) with a history of prior chemotherapy (P < 0.05). Further, more women in the other group (n = 4/14) had a past history of chemo than women in the BRCA group (n = 2/60). Patients with breast (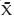 =23.8 ± 3.6) and gynecologic cancers (
=23.8 ± 3.6) and gynecologic cancers (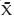 =27.7 ± 9.3) had a higher BMI than those in the “other” group (
=27.7 ± 9.3) had a higher BMI than those in the “other” group (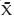 =21.97 ± 2.6). There were no significant differences between groups in terms of ethnicity, tobacco use or reproductive history. Women with a history of chemotherapy exposure had all completed chemotherapy a minimum of 3 months prior to ovarian stimulation. All patients were cleared by their medical oncologist prior to undergoing stimulation.
=21.97 ± 2.6). There were no significant differences between groups in terms of ethnicity, tobacco use or reproductive history. Women with a history of chemotherapy exposure had all completed chemotherapy a minimum of 3 months prior to ovarian stimulation. All patients were cleared by their medical oncologist prior to undergoing stimulation.
Table 1.
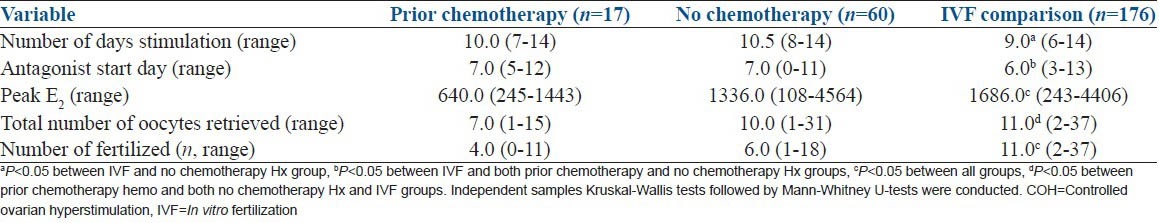
Outcome data for patients (n=75) based on chemotherapy treatment history who completed a cycle of COH for embryo cryopreservation compared to infertile controls. Because fertilization was examined, patients who had oocytes only cryopreserved are not included
Controlled ovarian stimulation findings: Assessing impact of chemotherapy exposure
One hundred and nine (88% of the women who were seen for an FP consult) women opted to undergo controlled ovarian stimulation (COH cohort). One patient had their cycles cancelled because of a lack of appropriate response to the medications. Of the 105 women who completed an IVF cycle, 19 had previously been exposed to chemotherapy, and 86 were chemotherapy naïve. Interestingly, none of the four cancelled patients had been exposed to chemotherapy. As expected, most patients who received chemotherapy prior to COH had hematologic malignancies [Supplementary Table 1].
SUPPLEMENTARY Table 1.
Demographic information for patients who underwent initial consultation in the clinic
In order to better understand the impact of chemotherapy on COH we divided the cancer cohort cryopreserving embryos or a combination of embryos and oocytes (so that we could assess any impact on fertilization; those cryopreserving oocytes only were not included) by exposure history, and compared these groups to age-matched controls with either pure male or tubal factor infertility [Table 1]. As expected, the patients who had been exposed to chemotherapy (n = 17) had a significantly decreased peak estradiol level (772 ± 484 vs. 1498 ± 934 pg/ml) and fewer retrieved oocytes compared with both chemo-naïve cancer patients (n = 60) and the infertile controls (n = 176). Both the control and nonexposed cancer patients had similar total number of oocytes retrieved. Interestingly, infertile controls were stimulated for fewer days and started antagonists sooner than either cancer group [Table 1]. Thus, a current cancer diagnosis, even in the absence of prior chemotherapy exposure, may result in a blunted COH response compared to age-matched infertile controls.
Controlled ovarian hyperstimulation findings: Assessing impact of cancer diagnosis
Next, we explored how a particular cancer diagnosis impacted COH characteristics. This data is summarized in Table 2. We found no significant differences in terms of basal FSH levels. Women with hematologic malignancies had significantly longer stimulation compared to women with breast and gynecologic malignancies and started the antagonist later in the cycle compared to women with all other cancer diagnoses. Interestingly, even though women with breast and gynecologic cancers were significantly older than the women in the other groups, there was a nonstatistical trend for a greater number of oocytes retrieved (breast = 13 and gynecologic = 10 vs. hematologic = 9 and other = 7) and gametes frozen (breast = 18 and gynecologic = 13 vs. hematologic = 16 and other = 10). Finally, there were no differences between the two groups that composed the hematologic malignancies, namely leukemia and lymphoma patients, in any of the reported stimulation outcomes (data not shown).
Table 2.
Outcome data for cancer patients who completed a cycle of COH
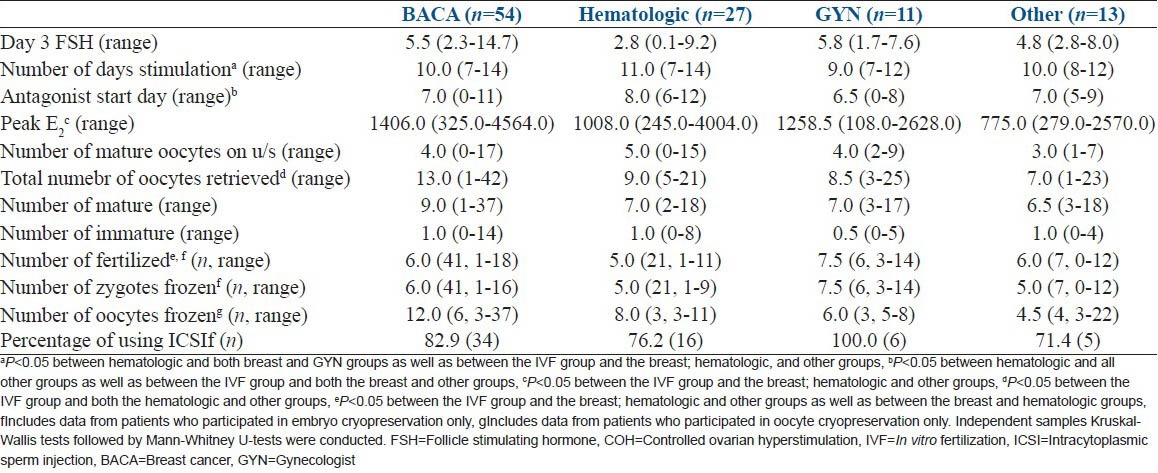
When COH characteristics were compared among patients of various cancer diagnoses and age-matched infertile controls, we noted that the control group required fewer days of stimulation (9, [range 6-14]) compared to breast (10, [range 7-14]), hematologic (11, [range 7-14]), and other (10, [range 8-12]) cancer cohorts. Similarly, the control group's peak estradiol (1686 pg/ml, [range 243-4406 pg/ml]) was significantly higher than the breast (1406 pg/ml, [range 325-4564 pg/ml]), hematologic (1008 pg/ml, [range 245-4004 pg/ml]) and other (775 pg/ml, [range 279-2570 pg/ml]) cancer cohorts. The infertile control groups had significantly higher oocyte yields (11, [range 2-37]) than the hematologic (9, [range 5-21]) and other (7, [range 1-23]) cancer cohorts. Therefore, the gynecologic cancer cohort COH characteristics most closely mirrored that of the age-matched infertile controls. Furthermore, patients with either breast or gynecologic cancer had similar oocyte yields to the control group.
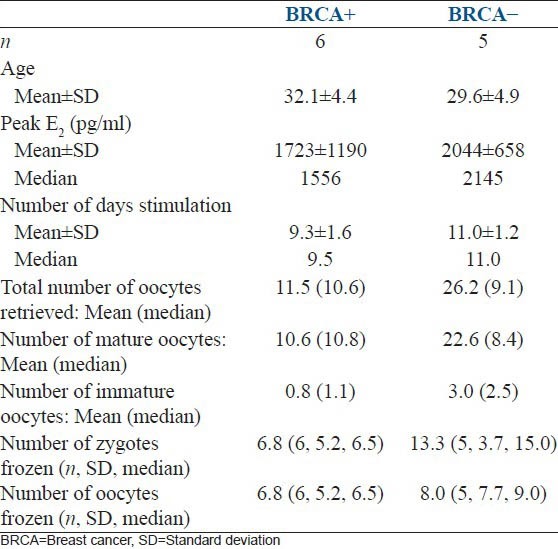
Finally, a preliminary analysis was conducted on the breast cancer patients who underwent breast cancer testing [Table 3]. A total of 11 women were tested, with six being positive and five found to be negative for the mutation. BRCA + woman tended to have lower peak estradiol levels and decreased fertilization rates compared to BRCA-woman. The number of mature oocytes obtained and gametes frozen tended to be higher in the BRCA-cohort.
Table 3.
Description of outcomes among BRCA+ and BRCA– women
DISCUSSION
Most studies of FP patients utilize a heterogeneous group of patients incorporating many cancer diagnoses into one group or are focused solely on patients diagnosed with breast cancer[7,8,9,14,15,21,24] thus limiting the ability to differentiate the impact of a specific cancer diagnosis on ovarian response. Further, many studies have addressed IVF outcomes in patients diagnosed with cancer and compared these patients to other infertility patients.[7,8,9,14,15,21,25] The results from these studies have suggested that FP patients may have a less robust response to gonadotropins compared to age-matched infertile controls, suggesting that cancer and/or its treatments may impact the ovary's ability to respond to gonadotropins. This data is essential for clinicians to understand as they create stimulation protocols for their FP patients; particularly since this group often only has one chance at ovarian stimulation. To the best of our knowledge, the current study is the largest to compare FP outcomes across cancer diagnoses and age-matched infertile controls. We have found that there is significant variation in how patients with different cancer diagnoses respond to ovarian stimulation. We also noted that even chemotherapy-naïve cancer patients do not respond as well as age-matched infertility patients [Table 1]. Thus, in addition to the standard predictors used in infertility patients, specific cancer diagnosis as well as treatment exposures may need to be considered when making stimulation decisions in this patient population. This information is valuable in counseling cancer patients as they decide whether or not to pursue therapy.
Comparing the four cancer groups by demographic characteristics and chemotherapy history, we found significant differences in age, with patients diagnosed with a gynecologic malignancy being the oldest [Supplementary Table 1]. Prior exposure to chemotherapy was also significantly different, as patients with hematologic malignancies were most likely to have been exposed to such treatment. As expected, patients with previous exposure to chemotherapy had decreased peak estradiol levels but similar oocyte yields and gametes frozen. Surprisingly, age-matched infertile controls had higher oocyte yields and fertilization rates compared to cancer patients both with and without a history of chemotherapy exposure [Table 1]. This suggests that patients with cancer may stimulate differently than age-matched controls, even in the absence of exposure to gonadotoxic therapies. FP consultation and stimulation protocols should reflect these findings.
In terms of overall COH outcomes among specific cancer diagnoses, patients with hematologic malignancies had the longest stimulations, although they had an overall similar number of oocytes retrieved [Table 2]. There was a trend in patients with breast or gynecologic malignancies to have a greater number of oocytes retrieved and gametes frozen compared to the other cancer diagnoses suggesting that these hormone-sensitive tumors respond most like the age-matched controls. A total of 11 women underwent ovarian stimulation because of a gynecologic malignancy (diagnoses included borderline ovarian cancer, early endometrial cancer, and uterine rhabdomyosarcoma). At our center, women with a borderline ovarian cancer without surgical evidence of metastasis to the other ovary and women diagnosed with early endometrial cancer following clearance from gynecologic oncology are considered candidates for ovarian stimulation. Despite being the oldest cohort in our study, this group of women with gynecological cancers demonstrated a favorable response to gonadotropin stimulation. Thus this information can provide reassurance to this older group of FP patients as they choose to pursue therapy. However, because many of these women subsequently undergo total abdominal hysterectomy with bilateral salpingo-oophorectomy, appropriate counseling regarding the use of gestational carriers is needed. In addition, these women and their partners need appropriate Federal Drug Administration testing prior to starting ovarian stimulation since this will be required prior to transferring embryos into a gestational carrier.
We found that a diagnosis of breast cancer was the most common reason for undergoing FP. Many patients and physicians question both the use of gonadotropin therapy with the subsequent rise in estradiol and the safety of pregnancy after a diagnosis of breast cancer. We extensively counsel patients that the vast majority of studies examining pregnancy after a diagnosis of breast cancer have demonstrated no adverse effect on survival.[26,27,28] In addition, there is scant evidence suggesting that a short time interval of elevated estradiol levels negatively impacts breast cancer prognosis. While some authors have advocated using aromatase inhibitors during ovarian stimulation to lower estradiol levels, more studies need to be done examining long-term outcomes in women exposed to these agents prior to chemotherapy.[3,8,14] Within the BRCA cohort, preliminary comparisons suggest that BRCA + patients tended to have lower peak estradiol levels, oocyte yields, fertilization rates and gametes frozen compared to BRCA-patients [Table 3]. Due to the small number of patients, statistical analyses were not performed. However, this result is consistent with other studies suggesting that BRCA + patients may have primary occult ovarian insufficiency.[29] Further studies are needed to clarify this association.
A major strength of this paper is that it is the largest series in which patients presenting for FP with a diagnosis of cancer were grouped according their type of cancer and compared to each other in addition to an age-matched infertile control. Most studies only have compared women with a cancer diagnosis to infertile controls. We found that the specific cancer diagnosis and previous treatments impacts ovarian stimulation characteristics. An inherent weakness, however, is that the current study is retrospective in nature.
In this study, we found that the specific cancer diagnosis as well as treatment exposures may need to be taken into account when counseling patients and choosing an ovarian stimulation regimen. This information would be helpful when counseling patients presenting for FP regarding their response to ovarian stimulation. However, there are still many unresolved questions and there is a need of more long-term data. Studies to assess longer term outcome variables including the number of cryopreserved oocytes that were able to be successfully fertilized, the quality of the resulting embryos, and live birth rates from both cryopreserved oocytes as well as embryos are also needed. In addition, long-term follow-up of these cancer patients are necessary to evaluate if and how ovarian stimulation may have affected their cancer prognosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bleyer A, Barr R. Cancer in young adults 20 to 39 years of age: Overview. Semin Oncol. 2009;36:194–206. doi: 10.1053/j.seminoncol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53:281–4. doi: 10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K, Rodriguez-Wallberg K, Schover L. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:2681. [PubMed] [Google Scholar]
- 4.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 5.Kim SS. Fertility preservation in female cancer patients: Current developments and future directions. Fertil Steril. 2006;85:1–11. doi: 10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 6.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–66. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 7.Quintero RB, Helmer A, Huang JQ, Westphal LM. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93:865–8. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: A prospective controlled study. J Clin Oncol. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 9.Knopman JM, Noyes N, Talebian S, Krey LC, Grifo JA, Licciardi F. Women with cancer undergoing ART for fertility preservation: A cohort study of their response to exogenous gonadotropins. Fertil Steril. 2009;91:1476–8. doi: 10.1016/j.fertnstert.2008.07.1727. [DOI] [PubMed] [Google Scholar]
- 10.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–90. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 11.Michaan N, Ben-David G, Ben-Yosef D, Almog B, Many A, Pauzner D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;149:175–7. doi: 10.1016/j.ejogrb.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2011;95:588–91. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Quintero RB, Helmer A, Huang JQ, Westphal LM. Fertility preservation in female cancer patients. Fertil Steril. 2005;84:S70–1. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: Early clinical experience. Fertil Steril. 2010;94:149–55. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Domingo J, Guillén V, Ayllón Y, Martínez M, Muñoz E, Pellicer A, et al. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. 2012;97:930–4. doi: 10.1016/j.fertnstert.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 16.Dittrich R, Maltaris T, Hoffmann I, Oppelt PG, Beckmann MW, Mueller A. Fertility preservation in cancer patients. Minerva Ginecol. 2010;62:63–80. [PubMed] [Google Scholar]
- 17.Dittrich R, Lotz L, Mueller A, Hoffmann I, Wachter DL, Amann KU, et al. Oncofertility: Combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod Biol Endocrinol. 2013;11:19. doi: 10.1186/1477-7827-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almog B, Azem F, Gordon D, Pauzner D, Amit A, Barkan G, et al. Effects of cancer on ovarian response in controlled ovarian stimulation for fertility preservation. Fertil Steril. 2012;98:957–60. doi: 10.1016/j.fertnstert.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Pavone MH, Lawon A, Smith K, Klock S. ART outcomes may differ by cancer diagnosis. Reprod Sci. 2012;19(Suppl 3):337A. doi: 10.4103/0974-1208.138869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padilla SL, Garcia JE. Effect of maternal age and number of in vitro fertilization procedures on pregnancy outcome. Fertil Steril. 1989;52:270–3. doi: 10.1016/s0015-0282(16)60854-3. [DOI] [PubMed] [Google Scholar]
- 21.Barton SE, Missmer SA, Berry KF, Ginsburg ES. Female cancer survivors are low responders and have reduced success compared with other patients undergoing assisted reproductive technologies. Fertil Steril. 2012;97:381–6. doi: 10.1016/j.fertnstert.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–40. doi: 10.1016/j.fertnstert.2011.10.040. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavone ME, Innes J, Hirshfeld-Cytron J, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Hum Reprod Sci. 2011;4:23–8. doi: 10.4103/0974-1208.82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azim A, Oktay K. Letrozole for ovulation induction and fertility preservation by embryo cryopreservation in young women with endometrial carcinoma. Fertil Steril. 2007;88:657–64. doi: 10.1016/j.fertnstert.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 25.Friedler S, Koc O, Gidoni Y, Raziel A, Ron-El R. Ovarian response to stimulation for fertility preservation in women with malignant disease: A systematic review and meta-analysis. Fertil Steril. 2012;97:125–33. doi: 10.1016/j.fertnstert.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 26.de Bree E, Makrigiannakis A, Askoxylakis J, Melissas J, Tsiftsis DD. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol. 2010;101:534–42. doi: 10.1002/jso.21514. [DOI] [PubMed] [Google Scholar]
- 27.Kroman N, Mouridsen HT. Prognostic influence of pregnancy before, around, and after diagnosis of breast cancer. Breast. 2003;12:516–21. doi: 10.1016/s0960-9776(03)00159-0. [DOI] [PubMed] [Google Scholar]
- 28.Azim HA, Jr, Santoro L, Pavlidis N, Gelber S, Kroman N, Azim H, et al. Safety of pregnancy following breast cancer diagnosis: A meta-analysis of 14 studies. Eur J Cancer. 2011;47:74–83. doi: 10.1016/j.ejca.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: A possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–4. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]


