Abstract
AIM:
The aim of the following study is to find out the prevalence of abnormal spermatozoa and associated functional parameters in clinical semen samples of sub-fertile males with the tobacco chewing habit.
SETTINGS AND DESIGN:
Retrospective study was conducted at infertility unit of a tertiary health care center, in a period of 3 years.
MATERIALS AND METHOD:
Semen of 642 males were analyzed; of them 194 men (30.2%) were tobacco chewers and they were grouped according to their intensity of chewing (<10 and ≥ 10 packets/day). Counts, motility, vitality, and morphology of sperms were analyzed.
RESULTS:
In tobacco chewers, 66% of subjects were oligozoospermic, 85% asthenozoospermic and 28% teratozoospermic. Sperm counts (odds ratio [OR] =2.2; 95% confidence interval [CI]: 1.5-3.09), motility (OR = 3.2; 95% CI: 2.05-4.9), and normal morphology (OR = 8.4; 95% CI: 4.9-14.6) were significantly affected (P = 0.001) in tobacco chewers than the non-chewing group. Further, in comparison to the intensity of tobacco chewing, patients with the intensive practice of using ≥10 packets/day had a significant effect on sperm morphology (P = 0.003, OR = 2.7; 95% CI = 1.41-5.08) only. Structural defects in head (P = 0.001) and cytoplasmic residues (P = 0.001) were found to be positively correlated with the intensive chewing, but no significant changes were found in anomalies in mid-piece and tail.
CONCLUSION:
The adverse impact of tobacco chewing on semen parameters was evident even with mild chewers, but with the intensive chewing practice, phenotypes of sperms, mainly defects in the head and cytoplasmic residue were severely affected.
KEY WORDS: Cytoplasmic residues, head defect, motility, sperm count, tobacco chewing
INTRODUCTION
Increasing incidence of infertility has been a global health as well as a social problem, where 35-40% of male partners are solely responsible.[1] Apart from the well-known conventional causes, i.e., disturbance in the endocrine system, anatomy, genetic makeup, varicocele or torsion, occurrence of diabetes and subtle unknown infections, chronic exposure to toxic chemicals and differential unhygienic lifestyle patterns too contribute to male infertility.[2] Tobacco consumption is one of the lifestyle factors that is often detrimental to human health as a whole.[3] Comparing depositions of nicotine in reports of International Agency for Research on Cancer (IARC, France) demonstrated that the consumption of smokeless tobacco 8-10 times versus 30-40 normal cigarettes a day lead to the deposition of almost equal amount of nicotine; in addition, it leads to the incidence of mouth cancer.[4] However, other manifestations of human health were least focused in the study due to tobacco consumption.
Despite strong regulations and campaign, people do consume tobacco products that affect several physiological systems including the reproductive system.[5] Recently, many states of India have banned the sale of chewing tobacco, but practically it has been no effect on consumption in public. Smokeless tobacco products are available in Indian market with many forms, khaini, gutkha, and betel containing lime, catechu and dry leaves of tobacco. The general ingredients of these products vary according to different products.
Tobacco contains more than 30 mutagenic agents among which, nicotine is regarded as the most important component, in the particle phase. Nicotine is quickly absorbed through the respiratory tract, mouth mucosa and skin.[6] Further, of the total nicotine entering the body, an 80-90% is metabolized by the liver and a fraction of nicotine and its degraded products were detected in serum, urine, saliva, milk and seminal plasma.[7] Nicotine affects the sperm plasma membrane and genetic integrity by their powerful oxidizing actions.[8] In animal studies, the impairment of testicular histology and a reduction in diameter of seminiferous tubules as well as, a decrease in the index of Sertoli cells, following 1.5 h of exposure to tobacco, per day (6 days, a week) as cigarette smoke for 10 weeks, were reported.[9] Indeed, the reduced fertility in males with impaired spermatogenesis, sperm motility, deleterious effect on germ cells and embryo development in tobacco exposure were recorded.[10,11] However, the threshold level that damages the integrity of male reproductive system is still obscure. A higher incidence of teratozoospermia in tobacco chewers was demonstrated,[8] but related information on details of morphological anomalies of sperms is still scarce. Moreover, the intracytoplasmic sperm injection technique helps the treatment of severe teratozoospermia by microinjection of sperm to metaphase-II oocyte; nonetheless, failures in many cases due to delayed fertilization, abnormal cleavage rates and spontaneous abortions were known,[12] which could be, a priory, due to defective sperm characteristics, those impeding the normal event of fertilization.[13,14]
As the tobacco chewing habit is quite prevalent among South-East Asia population (53.5%),[15] this retrospective study was undertaken to assess the effect of chewing tobacco on semen quality and specific sperm morphological defects in sub-fertile males undergoing an infertility treatment. This study should help a pertinent analysis of the problem of infertility treatment of male partners, as well as help public awareness in refraining from far-reaching this and other health concerns due to tobacco chewing. This study from the nation of South-east Asia with a sizable ghetto of urban slums and uneducated villagers gives an exemplary mirror of a public health problem of male infertility linked to such a health polluting habit.
MATERIALS AND METHOD
Subjects
Patients, attending the infertility unit of a tertiary health care center, from 2009 to 2012 for infertility treatment, were taken as subjects. Seminal fluid analyses were done for 642 male partners. History of tobacco consumption and other lifestyle patterns were noted. Duration of tobacco chewing in the cohort of subjects was between 2 and 18 years of age. Patients were balkanized accordingly to the habit and the intensity of chewing, i.e., less and more than 10 packets/day into two groups. Subjects were selected on the basis of the following inclusion and exclusion criteria: Inclusion criteria were male partners having the complain of infertility with age, 20-40 years and patients should have 3-5 days of sexual abstinence; exclusion criteria were males with a known/unknown blood borne infectious diseases, patient undergoing an antibiotic treatment, diabetes mellitus, hydrocele, hernia and varicocele, azoospermia cases and addictions other than tobacco chewing.
Sample collection and semen analysis
Semen samples were collected by masturbation into wide-mouth plastic container, in a room close to the andrology laboratory. Semen parameters were analyzed, according to the (World Health Organization [WHO]) standard criteria, i.e., volume ≥2 ml, concentration ≥20 million/ml, total count ≥40 million, progressive motility ≥ 50%, vitality ≥75% and normal morphology >15% (Kruger's criteria).[16] To determine the percentage of motile sperms, an aliquot of 10 μl of gently mixed liquefied semen was observed at ×400 magnification. At least 200 sperms were counted, and the mean value from duplicate measurements was represented. Sperm counts were done by using Neubauer's hemocytometer with requisite dilutions (1:2, 1:5, 1:9, 1:20), as per the WHO criteria.[16] Sperm morphology was assessed in Papanicolau-stained smears (Hematoxylene, orange-G and EA-50 stain) using light microscopy, under the oil immersion at ×1000 magnification. For a few semen samples, sperm morphology was also assessed by atomic force microscopy (AFM), for which the standardization was done by fixation of sperms in 2.5% glutaraldehyde in phosphate buffer saline (PBS), for 1 h and the mixture was centrifuged at 200-300 g for 10 min. Sperm pellet was washed with PBS and smeared on a clean slide with appropriate dilution. AFM images were taken by using the JPK NanoWizard II (Germany), at intermittent contact (air), which is called as tapping mode of operation with an ultrasharp silicon cantilever (k = 40 N/m, resonant frequency = 300 kHz).[17]
Vitality of sperms was estimated by the hypo-osmotic-swelling test by mixing equal volumes of semen and hypo-osmotic solution, prepared from 7.35 g sodium citrate and 13.5 g fructose in 1000 ml distilled water. The mixture was incubated for 30 min at 37°C, from which an aliquot of 10 μl was immediately examined at the ×400 magnification. The percentage of swollen (vital) sperm was assessed by counting a minimum of 200 spermatozoa.
Statistical analysis
Using SPSS, version 20.0 (IBM, USA), logistic regression analysis by odds ratios (ORs) and 95% confidence intervals (CIs) were computed basing on dichotomized value of semen parameters, specified by WHO standard criteria. Pearson's correlation coefficient, r was determined for the intensity of tobacco chewing and sperm anomalies.
RESULTS
From the questionnaire, 30.2% (194/642) subjects were tobacco chewers, from whom, 50.5% of subjects chew gutkha, 27.9% and 21.6% of subjects chew khaini and dried tobacco leaves in betel, respectively. A comparison of semen parameters between tobacco chewers (Group I) and normal subjects, without the habit of tobacco chewing (Group II) is presented [Table 1]. In all parameters tested, Group II subjects had higher mean values than Group I subjects. Further, with respect to sperm count, the mean values declined in 18.9% in Group I subjects; and decrease in mean values for motility, vitality and for normal morphology were 23.4, 8.4 and 28.4%, respectively.
Table 1.
Comparison of semen parameters between tobacco chewers, Group I and non-chewers, Group II with 95% CI*
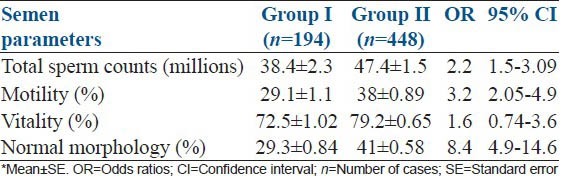
OR values of different semen parameters confirmed significant difference at P = 0.001 between the two groups, I and II [Table 1]. The probability of oligozoospermia in Group I subjects were 2.2 times higher odds than that of Group II subjects (OR = 2.2; 95% CI: 1.5-3.09). Similarly, probability of asthenozoospermia in Group I subjects was 3.2 times higher odds (OR = 3.2; 95% CI: 2.05-4.9) and teratozoospermia 8.4 times was higher odds (OR = 8.4; 95% CI: 4.9-14.6) than those of Group II subjects. However, no significant difference was observed for sperm vitality between the two groups (OR = 1.6; 95% CI: 0.739-3.6).
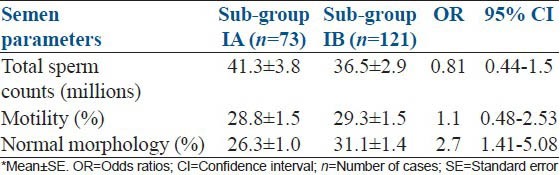
Further, effect of intensity of tobacco chewing on semen quality was analyzed by dividing the Group I subjects into two sub-groups. Sub-group IA was subjects using more than 10 packets of tobacco per day (intensive chewers) and sub-group IB was subjects using less than 10 packets of tobacco per day (non-intensive chewers). Here, only normal morphology of sperm was significantly affected in samples from intensive chewers: With 2.7 times higher odds than the non-intensive chewers (P = 0.003, OR = 2.7; 95% CI = 1.41-5.08). But, no significant difference was found for the total sperm count (P = 0.534, OR = 0.81; 95% CI: 0.441-1.5) and motility values (P = 1.0, OR = 1.1; 95% CI: 0.478-2.5) [Table 2].
Table 2.
Comparison of semen parameters between intensive tobacco chewers, sub-group IA and nonintensive tobacco chewers, sub-group IB*
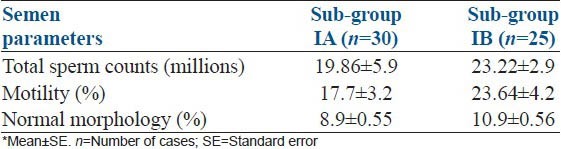
Among tobacco chewers, 28.4% cases were oligoas-thenoteratozoospermic, 59.4% oligoasthenozoospermic and 11.2% were normozoospermic. Mean value of the semen parameters in oligoasthenoteratozoospermia cases were presented in Table 3. The presence of normozoospermic, which contributes to higher mean values as shown in Tables 1 and 2.
Table 3.
comparison of semen parameters in oligoasthenoteratozoospermia cases between intensive tobacco chewers, sub-group IA and non-intensive tobacco chewers, sub-group IB*
As OR value represented 8.4 times higher to be of teratozoospermics in tobacco chewers than non-chewers, this study intensified specific morphological anomalies due to tobacco chewing [Table 4]. Deciphering the sperm morphology, many abnormal features were observed in head, mid-piece and tail [Figure 1]. Double head, pin head, tapered head, bent neck, curved tail and tailless sperms were the several anomalous features abundantly marked. An initial attempt for sperm morphological study by AFM also showed significant abnormal features among tobacco chewers [Figure 2]. However, mean of head defects and cytoplasmic residues were higher in the sub-group IA having an increasing trend from the control group to intensive chewers.
Table 4.
Comparison of morphological abnormalities of spermatozoa between intensive tobacco chewers, sub-group IA and non-intensive tobacco chewers, sub-group IB, in respect to normozoospermic control subjects (% values)*
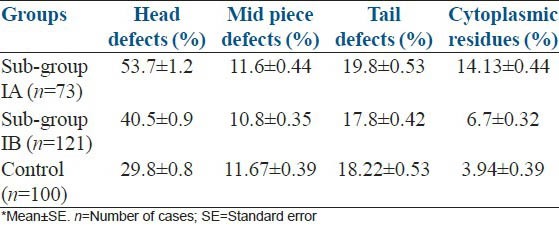
Figure 1.
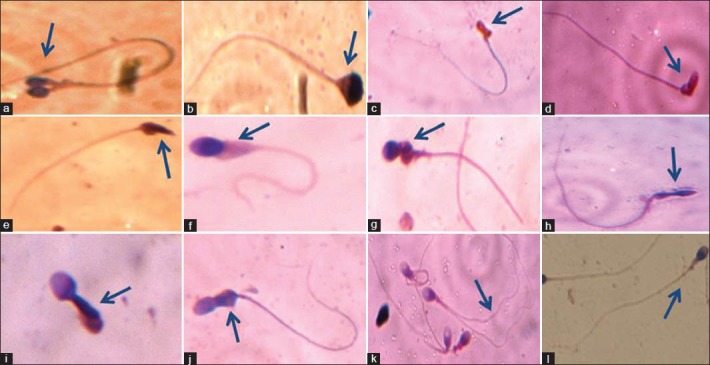
Sperms with different morphological features in studied semen samples: (a) Double head; (b) Pyriform head without acrosome; (c) Abnormal head with irregular acrosome; (d) Bent necked; (e) Cytoplasmic residues with tapered head; (f) Cytoplasmic residues and small acrosome; (g) Round head with abnormal mid-piece; (h) Long amorphous head; (i) Immature spermatozoa; (j) Abnormal mid-piece; (k) Double tailed; (l) Normal spermatozoa
Figure 2.
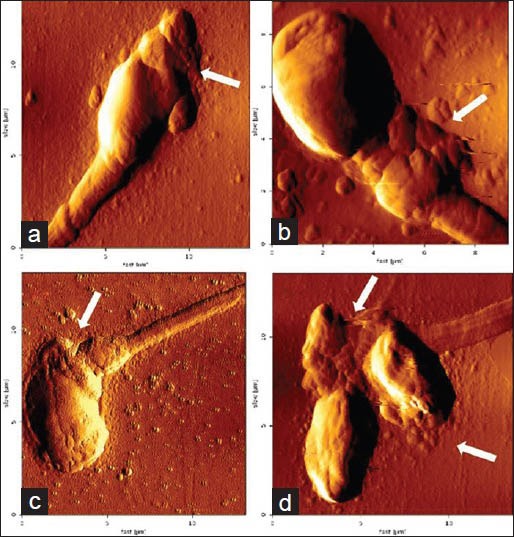
Amplitude images of sperm by atomic force microscopy. The two-dimensional images represent length and width in X and Y-axis, respectively. Each small division represents 2 μ: (a) Abnormal acrosome; (b) Abnormal mid-piece; (c) Bent neck with irregular acrosome; and (d) Double head with abnormal mid-piece
Application of Pearson's correlation also presented the strong positive correlation of head defect (r = 0.400) and cytoplasmic droplet (r = 0.611) with increasing use of the number of chewing tobacco packets (P = 0.001). But no significant change in mid-piece (P = 0.122) and tail defects (P = 0.411) were seen between the three groups [Table 4]. Although cytoplasmic residue was a mid-piece defect,[16] to present the specific defects of sperms, here cytoplasmic residue was analyzed, separately. Hence, the total results depicted the findings on sperm morphological features in addition to clinically assigned semen parameters to find their effects among tobacco chewers.
DISCUSSION
The present retrospective study recorded a decline in quality of semen among the tobacco chewers. Of 642 subjects, 194 (30.2%) had a significant decrease in sperm count, motility and impaired morphology. In tobacco chewers, 66%, 85.5% and 28.4% of subjects were found be below the WHO standard criteria for sperm count, motility and morphology respectively. This depicted significant effect of tobacco chewing as a lifestyle habit leading to several types anomaly in semen quality, consequently male infertility.
Indeed, among the two habits, chewing of tobacco and smoking most get addicted to the former, a priory, due to inebriating role of nicotine. Tobacco either in smoke or chewing forms has a harmful effect on human health causing gum recession, leukoplakia, cardiovascular disease and cancer of the oral cavity or larynx or pharynx.[18] In addition, it has negative effects on male reproductive system in thwarting the production of competent sperms, for the successful fertilization.[7,19,20] These results were corroborated by another similar report on tobacco causing adverse effect on semen quality in Mumbai, India.[8]
Animal studies predicted the concentration-dependent effect of nicotine on function of male reproductive organs, spermatogenesis, litter size, and level of endocrine hormones, eventually fecundity.[21] Nicotine is known to disturb the hypothalamus-pituitary axis by disrupting the testicular microcirculation.[22] As testosterone acts on seminiferous tubules to initiate and maintain spermatogenesis,[23] reduction of this sex hormone level either by impaired Leydig cell function or disturbance in the androgen/estrogen ratio could be a cause of decreased sperm counts.[21,24,25] Further, in vitro studies indicated, nicotine at the concentration 1 mM significantly declined the motility of sperm, and the concentration of 70 ng/ml caused a decrease in the kinematics of sperms.[26,27] Nicotine and other chemicals in tobacco probably cause either damage to mitochondrial genome or/and mitochondrial enzymatic activities or an impairment of function of the seminal vesicle affecting sperm motility. There was no significant effect of chewing tobacco on the function of accessory gland, seen with a small number of 29 patients in a study, but a larger sample size could find a definitive conclusion on the damaging effects of tobacco.[28] However, seminal plasma of non-smokers could retrieve motility of sperms from smokers.[29]
In intensive tobacco chewers, normal morphology was affected more than sperm count and motility, corroborated elsewhere.[8] Even though sperms with irregular acrosome, bent neck and coiled tail were observed in non-intensive tobacco chewers, additional anomaly of head defects and cytoplasm residues were found to be of higher degree in intensive chewing cases. Abnormal development of Golgi proacrosomic vesicles and their improper attachment to nucleus leads to the formation of defective head, either with irregular acrosome or head without acrosome by the elimination of the Golgi body through residual cytoplasm.[30] Here, it could be that certain chemical components interfere the secretory or normal activity of Golgi body during spermiogenesis.
Excess cytoplasmic residues of round spermatids are phagocytosed by Sertoli cells leaving a small cytoplasmic droplet for osmotic balance and final tail elongation during epididymal transit.[31] Epididymal dysfunction due to tobacco chewing may be a direct cause of retaining cytoplasmic residues, by preventing the timely loss of droplet and the development of secondary abnormalities in sperms. Excess cytoplasmic residues are also the source of reactive oxygen species which can cause damage to sperm membrane, proteins and deoxyribonucleic acid (DNA).[32,33,34]
Study on ultrastructure also revealed the presence of Sertoli cells with polymorphic mitochondria with irregular cristae, spermatids with excess cytoplasm, abnormal acrosomes in nicotine treated rats.[35] Another report was also found with curved tail, both bent and curved mid-piece in nicotine treated rats.[21] Moreover, this preliminary study with AFM revealed minute abnormal features of sperm head and neck by representing incandescent resolution and topographic view. However, it takes more time to scan the structure through its cantilever. So, a more intensive study could highlight significant morphological features of sperms to correlate clinically.
Nicotine is absorbed in mouth mucosa 3-4 times faster in chewing cases than smoking. Nicotine levels increase gradually with intensive practice of chewing and residual chemicals remain for a prolonged period than in smoking cases.[36] But, the threshold concentration affecting male reproductive system is yet to be defined. Again the quantity of other chemicals, their specific effects on male reproductive system and pathological concentrations in different individuals is largely unknown. The presence of pesticides, maleic hydrazide, chlordane, dichlorodiphenyl-trichloroethane, dichlorodiphenyldichloroethylene, dieldrin, endrine, heptachlor in smokeless tobacco might be causing these endocrinological disorders and damage of sperm DNA. Carcinogenic compounds in gutkha, khaini and betel with accessories are represented [Table 5].[4] A pictorial depiction of tobacco affecting male reproductive physiology causing infertility is too presented [Figure 3].
Table 5.
Concentration ranges of nicotine and carcinogenic compounds in commercial smokeless chewable tobacco products*
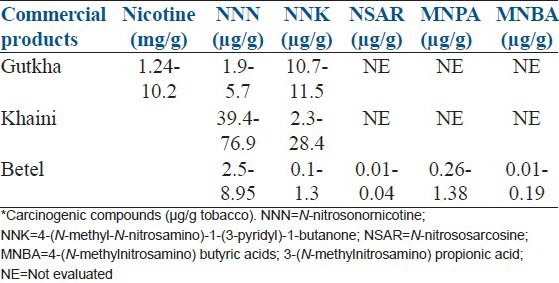
Figure 3.
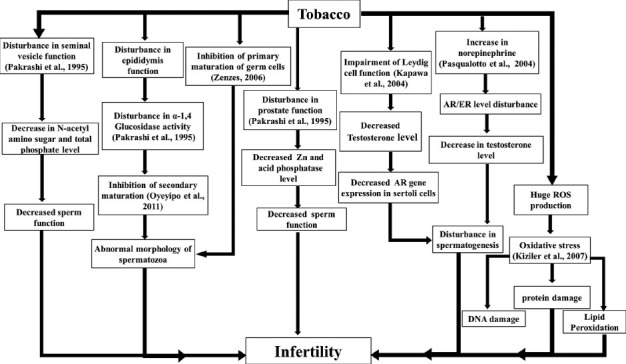
A pictorial depiction of tobacco smoking and chewing affecting male reproductive physiology in causing infertility
In the present study, 38% people were used to chew tobacco intensively, more than 10 packets/day (10-50), which is enough to affect the sperm function. In another point, 13.7% subjects have been chewing tobacco more than 10 times/day since 8 years, still they were normozoospermic; but, the numbers of morphological abnormality cases were higher. Normozoospermic subjects in intensive chewing cases indicated that some unknown protective mechanism is acting in those tobacco exposure cases. Hence, further studies can answer whether, it is the antioxidant level only or any other factors that protects the human male reproductive system from tobacco toxicity during spermatogenesis.
CONCLUSION
It was evident that chewing tobacco had a significant negative effect on the process of spermatogenesis, ultimately affecting sperm count, motility and morphology. Further, intensive chewing alters the normal morphology of sperm either to cause infertility or to cause irreversible epigenetic changes in future offspring. In this context, clinicians and fertility counselors need to be more focused to control male infertility by intimating the awareness of this addiction to enhance the fertility potential; that will be more appealing than waiting for policy decision and implementation.
ACKNOWLEDGEMENTS
SP is supported by a research fellowship from SOA University. Authors are thankful to Prof. DK Roy, Dean for consistent encouragement. Authors are also grateful to Er. Gopabandhu Kar, Managing Member, SOA University and Vice-Chancellor, SOA University for the financial support. The authors thank to Dr. Rupesh Dash, Scientist, Dr. Sanjeeb K. Sahoo, Scientist, Institute of Life Sciences, Bhubaneswar for providing facility for AFM study.
Footnotes
Source of Support: The present work is the in-house grant of Siksha O Anusandhan University, Bhubaneswar, for the partial fulfillment of the PhD work
Conflict of Interest: None declared.
REFERENCES
- 1.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil (Camb) 2010;13:217–25. doi: 10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 3.Koskinen LO, Collin O, Bergh A. Cigarette smoke and hypoxia induce acute changes in the testicular and cerebral microcirculation. Ups J Med Sci. 2000;105:215–26. doi: 10.3109/2000-1967-177. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 89. Lyon, France: IARC; 2007. [Last accessed on 2013 Oct 15]. Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines. Available from: http://www.monographs.iarc.fr/ENG/Monographs/vol89/index.php . [PMC free article] [PubMed] [Google Scholar]
- 5.Stillman RJ, Rosenberg MJ, Sachs BP. Smoking and reproduction. Fertil Steril. 1986;46:545–66. doi: 10.1016/s0015-0282(16)49628-7. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988;319:1318–30. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 7.Künzle R, Mueller MD, Hänggi W, Birkhäuser MH, Drescher H, Bersinger NA. Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003;79:287–91. doi: 10.1016/s0015-0282(02)04664-2. [DOI] [PubMed] [Google Scholar]
- 8.Said TM, Ranga G, Agarwal A. Relationship between semen quality and tobacco chewing in men undergoing infertility evaluation. Fertil Steril. 2005;84:649–53. doi: 10.1016/j.fertnstert.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadnia H, Ghanbari M, Moradi MR, Khaje-Dalouee M. Effect of cigarette smoke on spermatogenesis in rats. Urol J. 2007;4:159–63. [PubMed] [Google Scholar]
- 10.Shefi S, Tarapore PE, Walsh TJ, Croughan M, Turek PJ. Wet heat exposure: A potentially reversible cause of low semen quality in infertile men. Int Braz J Urol. 2007;33:50–6. doi: 10.1590/s1677-55382007000100008. [DOI] [PubMed] [Google Scholar]
- 11.Zenzes MT. Smoking and reproduction: Gene damage to human gametes and embryos. Hum Reprod Update. 2000;6:122–31. doi: 10.1093/humupd/6.2.122. [DOI] [PubMed] [Google Scholar]
- 12.Hamamah S, Fignon A, Lansac J. The effect of male factors in repeated spontaneous abortion: Lesson from in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod Update. 1997;3:393–400. doi: 10.1093/humupd/3.4.393. [DOI] [PubMed] [Google Scholar]
- 13.Chemes HE, Alvarez Sedo C. Tales of the tail and sperm head aches: Changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012;14:14–23. doi: 10.1038/aja.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemes HE. Phenotypes of sperm pathology: Genetic and acquired forms in infertile men. J Androl. 2000;21:799–808. [PubMed] [Google Scholar]
- 15.Rani M, Bonu S, Jha P, Nguyen SN, Jamjoum L. Tobacco use in India: Prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control. 2003;12:e4. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. 4th ed. Geneva, Switzerland: World Health Organisation; 1999. World Health Organization laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. [Google Scholar]
- 17.Kumar S, Chaudhury K, Sen P, Guha SK. Atomic force microscopy: A powerful tool for high-resolution imaging of spermatozoa. J Nanobiotechnology. 2005;3:9. doi: 10.1186/1477-3155-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson DE, Tomar SL, Mowery P, Siegel PZ. Trends in smokeless tobacco use among men in four states, 1988 through 1993. Am J Public Health. 1996;86:1300–3. doi: 10.2105/ajph.86.9.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorfman SF. Tobacco and fertility: Our responsibilities. Fertil Steril. 2008;89:502–4. doi: 10.1016/j.fertnstert.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Gandini L, Lombardo F, Lenzi A, Culasso F, Pacifici R, Zuccaro P, et al. The in-vitro effects of nicotine and cotinine on sperm motility. Hum Reprod. 1997;12:727–33. doi: 10.1093/humrep/12.4.727. [DOI] [PubMed] [Google Scholar]
- 21.Oyeyipo IP, Raji Y, Emikpe BO, Bolarinwa AF. Effects of nicotine on sperm characteristics and fertility profile in adult male rats: A possible role of cessation. J Reprod Infertil. 2011;12:201–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Collin O, Kilter S, Bergh A. Tobacco smoke disrupts testicular microcirculation in the rat. Int J Androl. 1995;18:141–5. doi: 10.1111/j.1365-2605.1995.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe RM, Maddocks S, Millar M, Kerr JB, Saunders PT, McKinnell C. Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J Androl. 1992;13:172–84. [PubMed] [Google Scholar]
- 24.Kapawa A, Giannakis D, Tsoukanelis K, Kanakas N, Baltogiannis D, Agapitos E, et al. Effects of paternal cigarette smoking on testicular function, sperm fertilizing capacity, embryonic development, and blastocyst capacity for implantation in rats. Andrologia. 2004;36:57–68. doi: 10.1111/j.1439-0272.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 25.Pasqualotto FF, Lucon AM, Sobreiro BP, Pasqualotto EB, Arap S. Effects of medical therapy, alcohol, smoking, and endocrine disruptors on male infertility. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:375–82. doi: 10.1590/s0041-87812004000600011. [DOI] [PubMed] [Google Scholar]
- 26.Reddy A, Sood A, Rust PF, Busby JE, Varn E, Mathur RS, et al. The effect of nicotine on in vitro sperm motion characteristics. J Assist Reprod Genet. 1995;12:217–23. doi: 10.1007/BF02211802. [DOI] [PubMed] [Google Scholar]
- 27.Jorsaraei SG, Shibahara H, Ayustawati, Hirano Y, Shiraishi Y, Khalatbari A, et al. The in-vitro effects of nicotine, cotinine and leptin on sperm parameters analyzed by CASA system. Iran J Reprod Med. 2008;6:157–65. [Google Scholar]
- 28.Pakrashi A, Chatterjee S. Effect of tobacco consumption on the function of male accessory sex glands. Int J Androl. 1995;18:232–6. [PubMed] [Google Scholar]
- 29.Zavos PM, Correa JR, Antypas S, Zarmakoupis-Zavos PN, Zarmakoupis CN. Effects of seminal plasma from cigarette smokers on sperm viability and longevity. Fertil Steril. 1998;69:425–9. doi: 10.1016/s0015-0282(97)00540-2. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez Sedó C, Rawe VY, Chemes HE. Acrosomal biogenesis in human globozoospermia: Immunocytochemical, ultrastructural and proteomic studies. Hum Reprod. 2012;27:1912–21. doi: 10.1093/humrep/des126. [DOI] [PubMed] [Google Scholar]
- 31.Cooper TG. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl. 2011;13:130–8. doi: 10.1038/aja.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper TG. Cytoplasmic droplets: The good, the bad or just confusing? Hum Reprod. 2005;20:9–11. doi: 10.1093/humrep/deh555. [DOI] [PubMed] [Google Scholar]
- 33.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79(Suppl 3):1597–605. doi: 10.1016/s0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 34.Kiziler AR, Aydemir B, Onaran I, Alici B, Ozkara H, Gulyasar T, et al. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol Trace Elem Res. 2007;120:82–91. doi: 10.1007/s12011-007-8020-8. [DOI] [PubMed] [Google Scholar]
- 35.Aydos K, Güven MC, Can B, Ergün A. Nicotine toxicity to the ultrastructure of the testis in rats. BJU Int. 2001;88:622–6. doi: 10.1046/j.1464-4096.2001.02384.x. [DOI] [PubMed] [Google Scholar]
- 36.Wong WY, Thomas CM, Merkus HM, Zielhuis GA, Doesburg WH, Steegers-Theunissen RP. Cigarette smoking and the risk of male factor subfertility: Minor association between cotinine in seminal plasma and semen morphology. Fertil Steril. 2000;74:930–5. doi: 10.1016/s0015-0282(00)01567-3. [DOI] [PubMed] [Google Scholar]


